Abstract
Meningococcal vaccines containing factor H binding protein (fHbp) are in clinical development. fHbp binds human fH, which enables the meningococcus to resist complement-mediated bacteriolysis. Previously, we found that chimeric human IgG1 mouse anti-fHbp monoclonal antibodies (MAbs) had human complement-mediated bactericidal activity only if the MAb inhibited fH binding. Since IgG subclasses differ in their ability to activate complement, we investigated the role of human IgG subclasses on antibody functional activity. We constructed chimeric MAbs in which three different murine fHbp-specific binding domains were each paired with human IgG1, IgG2, or IgG3. Against a wild-type group B isolate, all three IgG3 MAbs, irrespective of their ability to inhibit fH binding, had bactericidal activity that was >5-fold higher than the respective IgG1 MAbs, while the IgG2 MAbs had the least activity. Against a mutant with increased fHbp expression, the anti-fHbp MAbs elicited greater C4b deposition (classical pathway) and greater bactericidal activity than against the wild-type strain, and the IgG1 MAbs had similar or greater activity than the respective IgG3 MAbs. The bactericidal activity against both wild-type and mutant strains also was dependent, in part, on activation of the alternative complement pathway. Thus, at lower epitope density in the wild-type strain, the IgG3 anti-fHbp MAbs had the greatest bactericidal activity. At a higher epitope density in the mutant, the IgG1 MAbs had similar or greater bactericidal activity than the IgG3 MAbs, and the activity was less dependent on the inhibition of fH binding than at a lower epitope density.
INTRODUCTION
Neisseria meningitidis is an important cause of meningitis and sepsis. There are no broadly effective vaccines against group B strains (15, 34), in part because the group B capsular polysaccharide cross-reacts with host antigens (11) and is poorly immunogenic (20). Several protein antigens are under investigation as vaccine candidates for prevention of group B meningococcal disease (9, 15, 34). One of the most promising is factor H binding protein (fHbp) (25), which is present in nearly all meningococcal strains (30). The antigen binds factor H (fH) (22) enables the meningococcus to resist complement activation and evade innate host defenses. Furthermore, binding is specific for human fH (17).
One potential limitation of fHbp vaccines is that the antigen is relatively sparsely exposed on the surface of some meningococcal strains, which increases resistance of the bacteria to anti-fHbp complement-mediated bactericidal activity (10, 32, 33, 38). Further, most murine anti-fHbp monoclonal antibodies (MAbs) lacked bactericidal activity when tested with human complement (3, 39). In a recent study, we constructed chimeric antibodies in which the human IgG1 constant region was paired with three murine fHbp-specific binding domains (14). Although all three MAbs elicited similar C1q-dependent C4b deposition on live bacteria (classical complement pathway), only the two antibodies that inhibited binding of fH to fHbp had bactericidal activity with human complement. Thus, there appeared to be insufficient complement activation by the IgG1 anti-fHbp MAbs for complement-mediated bacteriolysis unless fH binding also was inhibited.
The four human IgG subclasses are known to differ in their ability to activate complement (8, 27). Specifically, IgG1 and IgG3 antibodies activate complement efficiently, whereas IgG2 antibodies are effective mainly at high epitope density, and IgG4 antibodies are ineffective complement activators (12, 27). There is no information available on the effect of human IgG subclasses on fHbp-specific antibody functional activity. To address this question, in the present study we investigated the effect of IgG subclass on functional activity of a panel of chimeric anti-fHbp MAbs in which individual mouse anti-fHbp paratopes were paired with human IgG1, IgG2, and IgG3 constant regions. We also investigated the contribution of the alternative complement pathway and antigen density on antibody function.
MATERIALS AND METHODS
Murine anti-fHbp MAbs.
The murine fHbp-specific MAbs JAR 3 (IgG3), JAR 5 (IgG2b) (5, 38, 39) and MAb502 (IgG2a) (13, 35) have been previously described. JAR 3 and JAR 5 MAbs inhibit binding of each other to fHbp (38) and recognize overlapping epitopes that involved glycine and lysine at positions 121 and 122, respectively, of fHbp (3, 5). MAb502 recognizes a conformational epitope requiring an arginine at position 204 (35), and this MAb does not inhibit binding of JAR 3 or JAR 5 to fHbp. Control MAbs included SEAM 12 (16), which reacts with the group B capsule, and anti-PorA P1.7 (NIBSC code 01/514, which was obtained from the National Institute for Biological Standards and Control, Potters Bar, United Kingdom).
Chimeric human IgG1, IgG2, and IgG3 mouse anti-fHbp MAbs.
The methods used for the preparation, expression, and purification of chimeric human IgG1 mouse anti-fHbp MAbs have been previously described (14). Briefly, sequence-specific primers were designed to facilitate the insertion of the previously described murine VH and VL regions (14, 35) into modified FRT bicistronic eukaryotic expression vectors (Invitrogen) that had been engineered to contain the human IgG1, IgG2, or IgG3 constant regions. The DNA sequences of all constructs were verified prior to transfection into Flp-In CHO cells (Invitrogen). Transfected cells were cultured in serum-free medium (Sigma-Aldrich) and grown for approximately 2 weeks. Antibody from the cell culture supernatant was concentrated and purified using HiTrap protein G columns (GE Healthcare), which was performed as described previously (14). Concentrations of the human IgG-mouse chimeric MAbs were determined by a capture enzyme-linked immunosorbent assay (ELISA) in which goat anti-human κ chain-specific antibody (Biosource) bound to wells of a microtiter plate was used to capture the chimeric antibody, which was then detected by goat anti-human κ chain-specific antibody conjugated with alkaline phosphatase (Biosource). Antibody concentrations of the chimeric human mouse anti-fHbp MAbs were assigned by comparison with binding of human IgG1, IgG2, and IgG3 myeloma standards (Sigma).
Flow cytometry.
Binding of the chimeric MAbs to the surface of live encapsulated bacteria, and the ability of the MAbs to inhibit binding of fH to live bacteria, were measured by flow cytometry as described previously (14). The test strain was H44/76 (B:15:P1.7,16; ST-32), which expresses fHbp ID 1 (38, 39). In some experiments, we also used a mutant of H44/76 (H44/76-OE) in which fHbp was overexpressed (see below). For the flow binding experiments, a fixed concentration of anti-fHbp MAb (4 μg/ml) or, as a negative control, 100 μg of an irrelevant MAb (a monoclonal κ chain antibody from a human myeloma [Sigma])/ml was incubated with ∼107 bacteria/ml. Bound antibody was detected using CF488-conjugated goat anti-human IgG (BioTium). For measurement of inhibition of fH binding to the bacterial surface, bacteria at ∼107 cells/ml were incubated with 50 or 2 μg of anti-fHbp MAb/ml for 30 min at room temperature, followed by the addition of ∼90 μg of human fH/ml. The source of fH was heat-inactivated (30 min at 56°C) 20% human serum that had been depleted of IgG as previously described (4). Bound fH was detected by a sheep polyclonal antiserum to fH (Lifespan Bioscience), followed by washing and incubation with a donkey anti-sheep IgG antibody (Invitrogen) conjugated with Alexa Fluor 488.
Mutant group B strain H44/76 with increased fHbp expression.
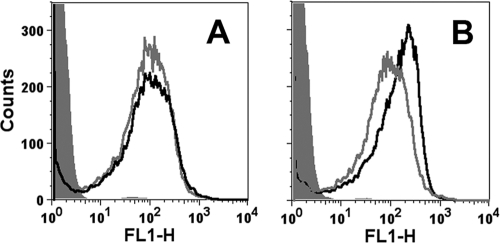
The shuttle vector pFP12, which has an origin of replication from a naturally occurring plasmid in Neisseria gonorrhoeae (31), was used to increase fHbp expression as described previously for N. meningitidis group C strain RM1090 (18). A mutant of H44/76, in which the gene encoding fHbp had been inactivated (38), was transformed with pFP12-fHbp containing the full-length gene encoding fHbp ID 1. The transformation was performed as previously described (21), and transformants were selected on GC agar plates containing 5 μg of chloramphenicol/ml. By flow cytometry, the mutant (designated H44/76-OE) bound ∼3-fold more anti-fHbp MAb than did the parent wild-type strain (Fig. 1).
Fig 1.
Binding of anti-fHbp MAb MAbs to live bacteria of group B strain H44/76 and a mutant (H44/76-OE) with increased fHbp expression. (A) Binding of control murine anticapsular MAb (5 μg/ml). Gray line, wild-type strain; black line, H44/76-OE mutant; filled gray histogram, mutant incubated with 100 μg of an irrelevant MAb/ml. (B) Binding of murine anti-fHbp MAbs (50 μg of JAR 3 + JAR 4/ml). Symbols are as defined in panel A.
Human complement sources.
The complement used to measure bactericidal activity was serum from a healthy adult with normal total hemolytic complement activity and no detectable serum bactericidal antibodies against the test strain. To eliminate nonbactericidal IgG antibodies that might augment or inhibit the activity of the test MAbs, the serum was depleted of IgG using a protein G column (HiTrap Protein G; GE Life Sciences, Piscataway, NJ), which was performed as previously described (4). As described in Results, in some experiments we used a commercial human complement source that had been depleted of C6 (Complement Tech) and that also was depleted of IgG in our laboratory.
Serum bactericidal assay.
Human complement-mediated bactericidal activity was measured as previously described (3, 4) using group B strain H44/76 wild type or the H44/76 mutant described above with increased fHbp expression (H44/76-OE). In brief, the bacterial cells were grown, harvested, and resuspended in buffer as described elsewhere (14). Immediately before the assay was performed, the anti-fHbp MAbs were centrifuged for 2 h at 100,000 × g to remove possible aggregates. The 40-μl bactericidal reaction mixture contained 1 to 100 μg of MAb/ml, ca. 300 to 400 CFU of bacteria, and 20% human complement (see above). The 50% bactericidal activity (BC50) was defined by the anti-fHbp MAb concentration that resulted in a 50% decrease in CFU/ml after a 60-min incubation in the reaction mixture compared to the CFU/ml in negative control wells at time zero. In some experiments, in order to inhibit alternative complement pathway activation, we added 50 μg of a mouse anti-properdin MAb/ml, which blocked properdin function (MAb A233; Quidel Corp.) (1, 2), or 100 μg of a mouse anti-Bb MAb (A227; Quidel Corp.)/ml (19) to the bactericidal reaction mixture. In flow cytometric studies using 15% human serum as a complement source and 10 mM MgCl2 and 10 mM EGTA (to block the classical and lectin complement pathways), the concentrations of the MAb inhibitors used in the bactericidal reaction were >10-fold in excess of those required for inhibition of alternative complement pathway-mediated C3b deposition on zymosan (data not shown).
C4b and C3b deposition on N. meningitidis.
Flow cytometry was used to measure the deposition of human C4b and human C3b on the surfaces of live bacteria of the H44/76 wild-type strain or the H44/76-OE mutant strain (14). In brief, bacteria were grown and harvested as described previously (38), resuspended in D-PBS-BSA (Dulbecco phosphate-buffered saline containing 0.1 g of CaCl2/liter and 0.1 g of MgCl2/liter × 6H20 [Mediatech], pH 7.4, with 1% [wt/vol] bovine serum albumin [Equitech-Bio]) to a density of ∼108 cells/ml. The bacteria were incubated with human complement (10% C6-depleted human serum to prevent bacteriolysis, which also was depleted of IgG as described above). Different concentrations of the chimeric human-mouse anti-fHbp MAbs diluted in D-PBS-BSA were added. After 10 to 15 min incubation at room temperature, bound human C4b or C3b was detected with 1:100 dilutions of fluorescein isothiocyanate-conjugated anti-human C4b or C3c, respectively (Meridian Life Science).
RESULTS
Chimeric human mouse anti-fHbp MAbs with different IgG subclasses have similar respective binding and fH inhibition characteristics.
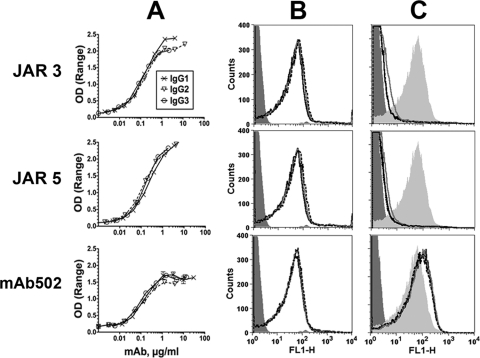
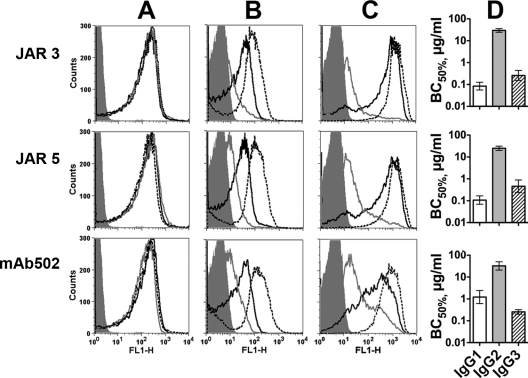
We constructed three sets of human-mouse chimeric anti-fHbp antibodies in which the mouse JAR 3, JAR 5, or MAb502 paratopes were each paired with human IgG1, IgG2, or IgG3 constant regions (total of nine antibodies). For each of the paratopes, the three IgG subclass MAbs showed similar respective binding to recombinant fHbp when measured by ELISA (Fig. 2A), and similar respective binding with live bacteria of group B strain H44/76 when measured by flow cytometry (Fig. 2B). Irrespective of the IgG subclass, as little as 2 μg of JAR 3 or JAR 5/ml inhibited the binding of fH to the surfaces of the live bacterial cells (Fig. 2C). As expected (14, 35), none of the MAb502 chimeric antibodies inhibited the binding of fH (for MAb502, the data in Fig. 2C were obtained with 50 μg of each IgG subclass/ml).
Fig 2.
Binding and fH inhibition by chimeric human IgG subclass mouse anti-fHbp MAbs. (A) Binding to fHbp by ELISA. (B) Binding to live N. meningitidis bacterial cells of strain H44/76 by flow cytometry. The MAbs were tested at 4 μg/ml. IgG1, black solid lines; IgG2, gray solid lines; IgG3, black dashed lines (the respective binding lines are superimposed). An irrelevant human IgG1 MAb (100 μg/ml) was used as a negative control (dark gray filled histograms). (C) Inhibition of fH binding by flow cytometry using 20% IgG-depleted human serum as a source of fH (∼90 μg/ml) and 2 μg (JAR 3 or JAR 5) or 50 μg (MAb502) of chimeric antibody/ml. Light gray filled area, fH without MAb; dark gray filled area, bacteria without fH as a negative control. Symbols for IgG1, IgG2, and IgG3 MAbs are as defined for panel B.
Chimeric human IgG3 mouse anti-fHbp MAbs elicit greater C3b deposition on live N. meningitidis bacteria than IgG1 or IgG2 MAbs.
We measured the deposition of C4b on the surfaces of live N. meningitidis cells of wild-type group B strain H44/76 as a marker of lectin and/or classical complement pathway activation and C3b deposition as a marker of activation of any of the three complement pathways (classical, lectin, or alternative). For each of the paratopes, C4b deposition elicited by the different IgG subclasses was similar, although there was slightly more deposition elicited by each of the IgG3 MAbs (Fig. 3A). For C3b deposition (Fig. 3B), the chimeric IgG3 antibodies elicited greater activation than the corresponding IgG1 MAbs (black dashed lines versus black solid lines, respectively), and both of these subclasses elicited more C3b deposition than the respective IgG2 subclasses (gray solid lines).
Fig 3.
Deposition of human C4b and C3b on live bacterial cells of group B strain H44/76 as measured by flow cytometry. The bacteria were incubated with human complement and 4 μg of chimeric anti-fHbp MAbs/ml. IgG1, black solid lines; IgG2, gray solid lines; and IgG3, black dashed lines. An irrelevant human MAb (4 μg/ml) was used as a negative control (gray filled histograms). (A) Activation of C4b deposition using 10% commercial C6-depleted human serum, which also had been depleted of IgG as described in Materials and Methods. (B) Activation of C3b deposition. The MAb concentrations and complement were the same as those for panel A. Similar respective results were obtained in two independent experiments with C6-depleted human serum as the complement source and in a third experiment with a different human complement source that had not been C6-depleted (data not shown).
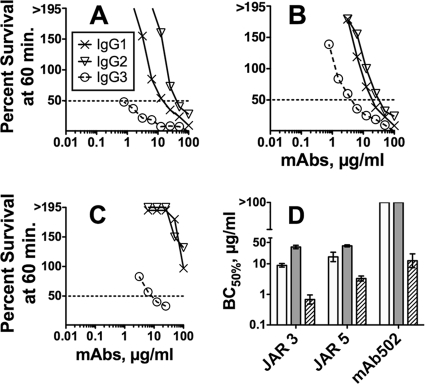
Chimeric IgG3 anti-fHbp MAbs have greater human complement-mediated bactericidal activity than IgG1 or IgG2 MAbs.
All three IgG3 anti-fHbp MAbs had greater human complement-mediated bactericidal activity against group B strain H44/76 than the respective IgG1 or IgG2 MAbs (Fig. 4A to C). For each paratope, the respective geometric mean IgG3 concentrations (in μg/ml) for 50% human complement-mediated bactericidal activity (BC50) in three to four independent assays were significantly lower than those of the IgG1 or IgG2 MAbs (P < 0.001 by analysis of variance [Fig. 4D]). For JAR 3 and JAR 5, the IgG1 concentrations for 50% bacteriolysis also were lower than for the respective IgG2 subclass MAbs (P < 0.0001 and P = 0.01, respectively). (For MAb502, neither the IgG1 nor the IgG2 MAb had activity [Fig. 4C].)
Fig 4.
Complement-mediated bactericidal activity of chimeric anti-fHbp MAbs. (A to C) Survival of wild-type N. meningitidis group B strain H44/76 after 60 min of incubation with anti-fHbp chimeric MAbs and 20% human complement. Crosses with solid line, IgG1; open triangles with solid line, IgG2; open circles with dashed line, IgG3. (A) JAR 3; (B) JAR 5; (C) MAb502. (D) Geometric mean MAb concentrations (μg/ml) for 50% human complement-mediated bactericidal activity in three to four independent assays. Error bars represent two standard errors. For all three paratopes, bacteriolysis occurred at lower concentrations of IgG3 (hatched bars) than the respective IgG2 (gray bars) or IgG1 (white bars; P < 0.0001) subclasses. For JAR 3 and JAR 5, bacteriolysis occurred at lower concentrations of IgG1 than with the respective IgG2 subclass MAbs (P < 0.0001 and P = 0.01, respectively).
Effect of increased fHbp expression on IgG subclass anti-fHbp MAb complement activation and bactericidal activity.
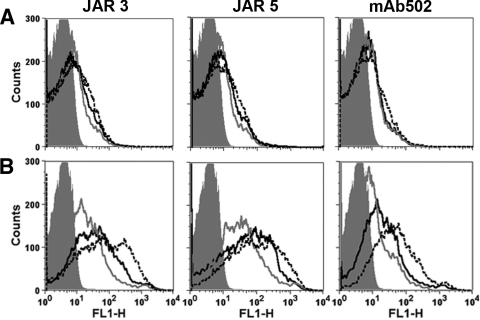
As described in Materials and Methods, the mutant group B strain H44/76-OE bound ∼3-fold more anti-fHbp MAb as determined by flow cytometry than did the parent wild-type strain (Fig. 1). A shown by flow cytometry, the binding of each of the respective chimeric IgG subclass anti-fHbp MAbs to live bacteria of the H44/76-OE mutant strain was similar to each other (Fig. 5A). For all three paratopes, there was greater C4b deposition on the mutant bacteria elicited by the IgG3 MAbs than the respective IgG1 or IgG2 subclasses, while the IgG2 MAbs elicited the lowest C4b deposition (Fig. 5B). The IgG2 subclass also elicited the lowest C3b deposition on the mutant (Fig. 5C). For JAR 3 and JAR 5, however, deposition of C3b on the mutant by the respective IgG3 and IgG1 was similar to each other, while for the MAb502 the IgG3 elicited slightly more C3b than the IgG1 (Fig. 5C).
Fig 5.
Effect of increased fHbp expression on IgG subclass anti-fHbp complement deposition and bactericidal activity. (A to C) Flow cytometry with live bacterial cells of a mutant strain with overexpressed fHbp (H44/76-OE). (A) Binding of 4 μg of chimeric anti-fHbp MAbs/ml (IgG1, solid black lines; IgG2, gray lines; IgG3, dashed lines; the respective lines are superimposed). An irrelevant MAb (100 μg/ml) was used as a negative control (gray filled histograms). (B and C) Deposition of C4b and C3b, respectively, elicited by 4 μg of anti-fHbp MAb/ml and 10% C6-depleted human serum as a complement source, which also had been depleted of IgG. (D) Anti-fHbp MAb complement-mediated bactericidal activity against mutant strain H44/76-OE. For JAR 3 and JAR 5, the geometric IgG1 concentrations required for 50% bactericidal activity (BC50) (white bars) were lower than the respective IgG3 concentrations (black diagonal hatched bars P ≤ 0.014). For MAb502, the geometric mean BC50 of IgG1 was higher than for IgG3 (P = 0.005). For all three paratopes, the IgG2 BC50 values were higher than the respective IgG1 or IgG3 values (P < 0.0001). The data are from three independent experiments. Error bars represent two standard errors.
Regardless of the IgG subclass, the respective concentrations of anti-fHbp MAb required for complement-mediated bactericidal activity were 4- to >10-fold lower against the H44/76-OE mutant than against the wild-type strain (compare Fig. 5D to Fig. 4D). Also, in contrast to the WT strain, which was more susceptible to bactericidal activity by the IgG3 MAbs than the IgG1 MAbs, the reverse pattern was found with the H44/76-OE mutant (i.e., for JAR 3 or JAR 5, the concentrations of the IgG1 MAbs required for 50% bacteriolysis mutant were lower than for the respective IgG3 MAbs; P ≤ 0.014 [Fig. 5D]). These results were unexpected since the respective IgG3 MAbs elicited more C4b deposition on the H44/76-OE mutant than did the IgG1 MAbs (Fig. 5B) and elicited similar C3b deposition to each other (Fig. 5C). For MAb502, the IgG1 and IgG2 MAbs, which were not bactericidal against the H44/76 wild-type strain (BC50 > 100 μg/ml [Fig. 4D]), were bactericidal against the H44/76-OE mutant (Fig. 5D). Thus, overexpression of fHbp markedly increased overall susceptibility to anti-fHbp MAb complement-mediated bactericidal activity, which was particularly evident for the IgG1 and IgG2 MAbs.
Inhibition of the alternative complement pathway decreases anti-fHbp MAb bactericidal activity.
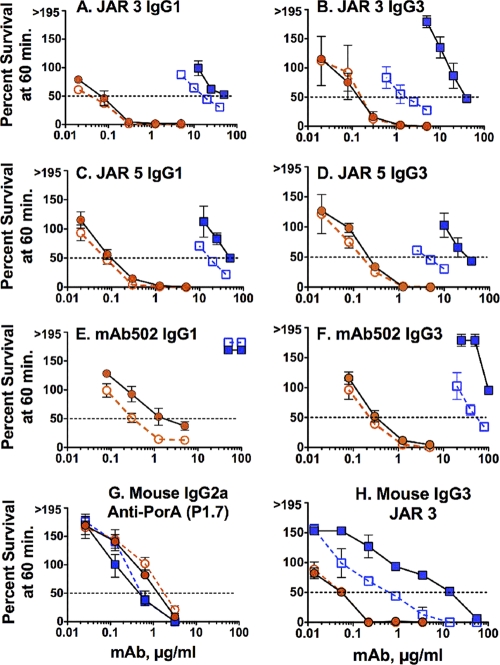
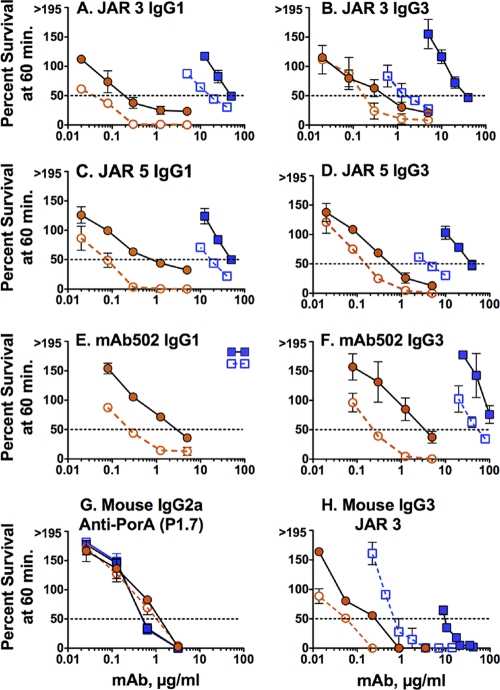
We measured complement-mediated anti-fHbp MAb bactericidal activity in the presence or absence of anti-properdin or anti-Bb MAbs, which were used to block the alternative complement pathway (see Materials and Methods). With the anti-properdin MAb (Fig. 6), there was decreased bactericidal activity of the anti-fHbp MAbs when measured against the wild-type strain (respective lines with blue symbols) but not against the mutant H44/76-OE mutant strain (respective lines with orange symbols). The only exception was MAb502 IgG1, which required properdin for optimal bactericidal activity against the H44/76-OE mutant (Fig. 6E).
Fig 6.
Effect of blocking properdin on anti-fHbp MAb bactericidal activity. Survival of N. meningitidis bacteria incubated for 60 min with 20% IgG-depleted human serum and anti-fHbp MAbs in the presence or absence of 50 μg of an anti-properdin MAb inhibitor/ml. Results for the H44/76 wild-type strain without anti-properdin inhibitor (open blue squares with dashed line), the H44/76 wild-type strain with anti-properdin inhibitor (blue filled squares with black solid line), the H44/76-OE mutant with overexpressed fHbp without inhibitor (open orange circles with dashed line), and the H44/76-OE mutant with anti-properdin inhibitor (orange filled circles with solid black line) are indicated. The data points and error bars represent respective means and ranges from two to three independent experiments.
When properdin was inhibited, there also was a decrease in the bactericidal activity of a control mouse JAR 3 anti-fHbp MAb against the wild-type strain but not against the mutant H44/76-OE strain (Fig. 6H). In contrast, there was no effect of inhibiting properdin on the bactericidal activity of a control mouse anti-PorA P1.7 MAb when measured against the wild-type strain or the H44/76-OE mutant (Fig. 6G). The lack of an effect of blocking properdin on anti-PorA P1.7 MAb bactericidal activity indicated that the role of the alternative complement pathway on bactericidal activity was specific for the anti-fHbp MAbs.
In the presence of the anti-Bb MAb, there was decreased anti-fHbp MAb bactericidal activity against both the wild type and the H44/76-OE mutant (Fig. 7). The effect of inhibiting factor B also was specific for anti-fHbp MAbs, as evidenced by inhibition of the mouse JAR 3 anti-fHbp MAb (Fig. 7H) but not the control mouse anti-PorA P1.7 MAb (Fig. 7G).
Fig 7.
Effect of the addition of an anti-Bb MAb on anti-fHbp MAb bactericidal activity. Survival of N. meningitidis bacteria incubated for 60 min with 20% IgG-depleted human serum and anti-fHbp MAbs in the presence or absence of 100 μg of an anti-Bb MAb inhibitor/ml. The results for the H44/76 wild-type strain without anti-Bb MAb inhibitor (open blue squares with dashed line), the H44/76 wild-type strain with anti-Bb MAb inhibitor (blue filled squares with solid line), the H44/76-OE mutant with overexpressed fHbp without anti-Bb MAb inhibitor (open orange circles with dashed line), and the H44/76-OE mutant with anti-Bb MAb inhibitor (orange filled circles with solid line) are given. The data points and error bars represent respective means and ranges from two to three independent experiments.
DISCUSSION
Most vaccine antigens are relatively abundant on the surface of the pathogen. An exception is fHbp, which was discovered by “genome mining” and is sparsely distributed on many meningococcal strains (32, 33, 38). Low fHbp expression was one reason the protein remained undiscovered until recently. Despite sparseness of the antigen, anti-fHbp antibodies elicit complement-mediated bactericidal activity and confer passive protection in animal models of meningococcal bacteremia (38, 39). Recently, we showed that bactericidal activity of chimeric human IgG1 anti-fHbp MAbs was dependent on their ability to inhibit binding of fH to fHbp (14). In the present study we extended these antibody functional studies to include chimeric IgG2 and IgG3 anti-fHbp MAbs and determined the role of antigen density and complement activation via the alternative pathway.
We confirmed that only the chimeric IgG1 anti-fHbp MAbs, which inhibited fH binding (JAR 3 or JAR 5), had human complement-mediated bactericidal activity against the group B wild-type strain. In contrast, all three chimeric IgG3 anti-fHbp MAbs, including MAb502, which did not inhibit fH binding, had bactericidal activity (Fig. 4). Thus, for the IgG3 subclass inhibition of fH binding was not required for bactericidal activity. Further, the IgG3 MAb activity was 5- to >10-fold higher than the respective IgG1 MAb activity.
All of the chimeric IgG anti-fHbp MAbs, irrespective of IgG subclass, had substantially greater bactericidal activity against a mutant of strain H44/76 with increased fHbp expression than against the wild-type strain. These results underscored the large effect of strain expression of fHbp on susceptibility to anti-fHbp bactericidal activity, which was observed previously in bactericidal assays of polyclonal anti-fHbp antisera when tested against naturally occurring meningococcal isolates with different levels of fHbp expression (32) or isogenic mutants (33). In the present study, two of the chimeric IgG1 anti-fHbp MAbs also had greater bactericidal activity than the respective IgG3 MAbs against the mutant with increased fHbp expression, which was opposite to their respective activity measured against the wild-type strain. These results were consistent with previous studies using hapten (4-hydroxy-3-nitrophenacetyl)-specific recombinant chimeric MAbs and human erythrocytes, which showed better human IgG1 than IgG3 complement-mediated lysis when the hapten concentration on the target red cells was high, whereas the IgG3 subclass was the most effective when the hapten concentration was low (27).
The underlying mechanism for the greater bactericidal activity of the chimeric IgG3 anti-fHbp MAbs than the respective IgG1 anti-fHbp MAbs against the wild-type H44/76 strain, while the reverse was observed against the mutant with increased fHbp, is not known. Human IgG3 has an extended hinge region (63 amino acids, as opposed to 15 amino acids for IgG1) (26), which might allow for greater flexibility of the Fc portion of the IgG3 molecule and more efficient engagement of C1q when fHbp is sparsely exposed. This hypothesis, however, is not supported by studies of mutant human IgG3 molecules with truncated hinge regions where the greater ability of IgG3 than IgG1 to activate complement-mediated lysis of red cells with low hapten density was independent of the hinge region (29). Based upon data from mutant IgG1 and IgG3 molecules with substitutions of single amino acid residues contributing to C1q binding (29), subtle structural differences in the CH2 domain appear to be responsible for the greater ability of IgG3 to engage C1q and activate the classical complement pathway when the target antigen is sparse. At a higher antigen density, these structural differences appear to have much less influence than at a low antigen density, since at a high antigen density both IgG1 and IgG3 have high activity. It is interesting that even under high-antigen-density conditions, the chimeric IgG2 anti-fHbp MAbs had much lower activity than the respective IgG1 or IgG3 chimeric MAbs (Fig. 5D), which likely resulted from a lower ability of human IgG2 to bind C1q (7). Note that for our studies we did not construct chimeric IgG4 anti-fHbp MAbs because in previous studies IgG4 antibodies had minimal or no complement activating properties (27, 37).
The effect of human IgG subclasses on complement-mediated bactericidal activity against group B strain H44/76 was investigated previously using recombinant chimeric MAbs to PorA with different human IgG subclasses and identical mouse V genes. In one study, all three IgG1 anti-PorA MAbs tested had superior bactericidal activity to the respective IgG3 MAbs (28), while in the second study an IgG1 anti-PorA MAb had similar or better activity than the IgG3 MAb (37). The second study also investigated the activity of a chimeric IgG2 anti-PorA MAb, which had less activity than the IgG1 or IgG3 MAbs, while the IgG4 MAb with identical respective V region genes had no activity. Thus, for PorA, the IgG1 MAbs were as effective or more effective for eliciting human complement-mediated bactericidal activity than the respective IgG3 MAbs, whereas with fHbp the IgG3 MAbs were more effective.
PorA is present in the meningococcal outer membrane as a homotrimer or heterotrimer with PorB or Rmp subunits (6, 23, 24, 36). Our hypothesis is that fHbp is present in the meningococcal outer membrane as a monomer. Since activation of the classical complement pathway requires engagement of C1q by at least two bound IgG molecules, failure of an anti-fHbp MAb to activate complement-mediated bacteriolysis could result from too great an average distance between most of the bound IgG molecules to permit optimal C1q engagement. In contrast, two IgG anti-PorA MAbs bound to a PorA homotrimer, or to a heterotrimer containing two PorA subunits, may engage C1q and activate the classical complement pathway more efficiently than a monomeric fHbp. An alternative explanation is that PorA is simply more abundant in the outer membrane than fHbp, which would result in PorA subunits being physically closer to each other and for anti-PorA MAbs to be more efficient than anti-fHbp MAbs for engaging C1q and eliciting complement-mediated bactericidal activity.
By flow cytometry there were minimal differences in C4b deposition elicited on the H44/76 wild-type strain by the chimeric anti-fHbp MAbs with different IgG subclasses (Fig. 3A). The IgG3 MAbs, however, gave greater C3b deposition (Fig. 3B) and had greater bactericidal activity (Fig. 4) than the respective IgG1 or IgG2 MAbs. A previous study of N. gonorrhoeae demonstrated the importance of the alternative complement pathway when the classical complement pathway was activated (1). Specifically, properdin, which stabilizes the alternative pathway C3/C5 convertases, was found to be important for augmenting C3 deposition on the bacteria.
To investigate whether or not optimal anti-fHbp MAb meningococcal bactericidal activity required the alternative complement pathway, we added anti-properdin or anti-human Bb MAbs to the bactericidal reaction mixture. Both alternative pathway inhibitors decreased bactericidal activity of the anti-fHbp MAbs against the wild-type strain, but only the anti-Bb MAb decreased bactericidal activity against the H44/76-OE mutant with increased fHbp expression (Fig. 6 and 7). When properdin, which is a positive regulator of the alternative pathway, is blocked, the alternative complement pathway is decreased but not totally eliminated (2). In contrast, when factor B is blocked, we would expect more complete inhibition of the alternative pathway. Our results therefore suggest that the amount of alternative complement pathway amplification required for optimal anti-fHbp MAb bactericidal activity depends on the extent of classical pathway activation, which in turn is dependent on the amount of strain fHbp expression. With the H44/76-OE mutant with increased fHbp expression, which elicited more anti-fHbp C4b and C3b deposition than the wild-type strain, only a small amount of alternative pathway activity was needed for optimal bactericidal activity. Therefore, blocking properdin had no effect, but blocking factor B had an effect. In contrast, there was less anti-fHbp C4b or C3b deposition on the wild-type strain than with the H44/76-OE mutant. Therefore, more alternative pathway activation was required for optimal anti-fHbp bactericidal activity against the wild-type strain than against the H44/76-OE mutant strain. Even a partial decrease in alternative pathway activity caused by the anti-properdin MAb had an effect with the wild-type strain.
The model described above also may explain why only the chimeric IgG1 and IgG2 anti-fHbp MAbs that blocked fH binding had bactericidal activity against the wild-type strain. With fH binding blocked, the alternative pathway convertases were stable for sufficient time to permit increased C3b deposition and formation of a functional membrane attack complex. With MAb502, which did not inhibit fH, bound fH would function to destabilize the alternative pathway convertases to the extent that there was less C3b deposition, and IgG1 and IgG2 bactericidal activity was not achieved. Why the MAb502 IgG3, which did not block fH binding, was bactericidal against the H44/76 wild-type strain is not known since only minor differences were observed in activation of the classical pathway by the respective chimeric IgG1, IgG2, or IgG3 MAb502 antibodies (C4b deposition [Fig. 3A]). Conceivably, the flow cytometric assay used for detection of C4b deposition on the live bacteria was insensitive for detecting small differences in classical complement pathway activation by the different IgG subclass MAbs and that once a threshold of C4b deposition was achieved by the chimeric IgG3 MAb502, anti-bacteriolysis proceeded without a requirement for inhibition of fH binding.
In summary, our data extend previous observations that a critical determinant of susceptibility of different meningococcal strains to anti-fHbp bactericidal activity is relative expression of fHbp on the bacterial surface (10, 32, 33, 38). Further, our data showed that anti-fHbp MAb bactericidal activity is affected by IgG subclass, the ability of the MAbs to inhibit binding of fH, and regulation of the alternative pathway. The results provide insights on how anti-fHbp antibodies with different human IgG subclasses interact with complement at the meningococcal cell surface and elicit bactericidal activity, which is critical for protection against disease.
ACKNOWLEDGMENTS
This study was supported in part by Public Health Service grants R01 AI 046464 and AI 082263 (to D.M.G.) and AI57932 (to D.C.R.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). S.G. is a Ph.D. student at the University Vita-Salute San Raffaele, Milan, Italy. Her work at Children's Hospital Oakland Research Institute was supported in part by a grant from Novartis Vaccines, Siena, Italy. The laboratory work at Children's Hospital Oakland Research Institute was performed in a facility funded by Research Facilities Improvement Program grant C06 RR 016226 from the National Center for Research Resources, NIH. D.M.G. was supported in part by an endowment established by The Clorox Company, Oakland, CA.
We are grateful to Sanjay Ram, University of Massachusetts Medical Center, for providing critical comments on the manuscript.
Footnotes
Published ahead of print 7 November 2011
REFERENCES
- 1. Agarwal S, et al. 2010. An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae. J. Immunol. 185: 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agarwal S, et al. 2011. Linkage specificity and role of properdin in activation of the alternative complement pathway by fungal glycans. mBio 2: e00178–e00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beernink PT, Lopasso C, Angiolillo A, Felici F, Granoff D. 2009. A region of the N-terminal domain of meningococcal factor H-binding protein that elicits bactericidal antibody across antigenic variant groups. Mol. Immunol. 46: 1647–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beernink PT, et al. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186: 3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beernink PT, et al. 2008. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate, factor H-binding protein. Infect. Immun. 76: 4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blasius R, Strittmatter W, Crowe B, Achtman M. 1990. Two distinct outer membrane serotype subcomplexes of Neisseria meningitidis serogroup A. Infect. Immun. 58: 2008–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruggemann M, et al. 1987. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J. Exp. Med. 166: 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burton DR, Gregory L, Jefferis R. 1986. Aspects of the molecular structure of IgG subclasses. Monogr. Allergy 19: 7–35 [PubMed] [Google Scholar]
- 9. Callaghan MJ, et al. 2011. Potential of recombinant opa proteins as vaccine candidates against hyperinvasive meningococci. Infect. Immun. 79: 2810–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donnelly J, et al. 2010. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc. Natl. Acad. Sci. U. S. A. 107: 19490–19495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finne J, Leinonen M, Makela PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis: implications for vaccine development and pathogenesis. Lancet ii: 355–357 [DOI] [PubMed] [Google Scholar]
- 12. Garred P, Michaelsen TE, Aase A. 1989. The IgG subclass pattern of complement activation depends on epitope density and antibody and complement concentration. Scand. J. Immunol. 30: 379–382 [DOI] [PubMed] [Google Scholar]
- 13. Giuliani MM, et al. 2005. The region comprising amino acids 100 to 255 of Neisseria meningitidis lipoprotein GNA 1870 elicits bactericidal antibodies. Infect. Immun. 73: 1151–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giuntini S, Reason DC, Granoff DM. 2011. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect. Immun. 79: 3751–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Granoff DM. 2010. Review of meningococcal group B vaccines. Clin. Infect. Dis. 50(Suppl. 2): S54–S65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Granoff DM, et al. 1998. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J. Immunol. 160: 5028–5036 [PubMed] [Google Scholar]
- 17. Granoff DM, Welsch JA, Ram S. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect. Immun. 77: 764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hou VC, Koeberling O, Welsch JA, Granoff DM. 2005. Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. J. Infect. Dis. 192: 580–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hourcade DE, Wagner LM, Oglesby TJ. 1995. Analysis of the short consensus repeats of human complement factor B by site-directed mutagenesis. J. Biol. Chem. 270: 19716–19722 [DOI] [PubMed] [Google Scholar]
- 20. Jennings HJ, Lugowski C. 1981. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J. Immunol. 127: 1011–1018 [PubMed] [Google Scholar]
- 21. Koeberling O, Welsch JA, Granoff DM. 2007. Improved immunogenicity of a H44/76 group B outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. Vaccine 25: 1912–1920 [DOI] [PubMed] [Google Scholar]
- 22. Madico G, et al. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177: 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marzoa J, et al. 2009. Analysis of outer membrane porin complexes of Neisseria meningitidis in wild-type and specific knockout mutant strains. Proteomics 9: 648–656 [DOI] [PubMed] [Google Scholar]
- 24. Marzoa J, Sanchez S, Ferreiros CM, Criado MT. 2010. Identification of Neisseria meningitidis outer membrane vesicle complexes using 2-D high resolution clear native/SDS-PAGE. J. Proteome Res. 9: 611–619 [DOI] [PubMed] [Google Scholar]
- 25. Masignani V, et al. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197: 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michaelsen TE, Frangione B, Franklin EC. 1977. Primary structure of the “hinge” region of human IgG3. Probable quadruplication of a 15-amino acid residue basic unit. J. Biol. Chem. 252: 883–889 [PubMed] [Google Scholar]
- 27. Michaelsen TE, Garred P, Aase A. 1991. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur. J. Immunol. 21: 11–16 [DOI] [PubMed] [Google Scholar]
- 28. Michaelsen TE, et al. 2003. Binding properties and antibacterial activities of V-region identical, human IgG and IgM antibodies, against group B Neisseria meningitidis. Biochem. Soc. Trans. 31: 1032–1035 [DOI] [PubMed] [Google Scholar]
- 29. Michaelsen TE, Sandlie I, Bratlie DB, Sandin RH, Ihle O. 2009. Structural difference in the complement activation site of human IgG1 and IgG3. Scand. J. Immunol. 70: 553–564 [DOI] [PubMed] [Google Scholar]
- 30. Murphy E, et al. 2009. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J. Infect. Dis. 200: 379–389 [DOI] [PubMed] [Google Scholar]
- 31. Pagotto FJ, Salimnia H, Totten PA, Dillon JR. 2000. Stable shuttle vectors for Neisseria gonorrhoeae, Haemophilus spp. and other bacteria based on a single origin of replication. Gene 244: 13–19 [DOI] [PubMed] [Google Scholar]
- 32. Pajon R, Beernink PT, Harrison LH, Granoff DM. 2010. Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine 28: 2122–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pajon R, Fergus AM, Koeberling O, Caugant DA, Granoff DM. 2011. Meningococcal factor H binding proteins in epidemic strains from Africa: implications for vaccine development. PLoS Negl. Trop. Dis. 5: e1302 doi:10.1371/journal.pntd.0001302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sadarangani M, Pollard AJ. 2010. Serogroup B meningococcal vaccines-an unfinished story. Lancet Infect. Dis. 10: 112–124 [DOI] [PubMed] [Google Scholar]
- 35. Scarselli M, et al. 2009. Epitope mapping of a bactericidal monoclonal antibody against the factor H binding protein of Neisseria meningitidis. J. Mol. Biol. 386: 97–108 [DOI] [PubMed] [Google Scholar]
- 36. Song J, Minetti CA, Blake MS, Colombini M. 1999. Meningococcal PorA/C1, a channel that combines high conductance and high selectivity. Biophys. J. 76: 804–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vidarsson G, et al. 2001. Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J. Immunol. 166: 6250–6256 [DOI] [PubMed] [Google Scholar]
- 38. Welsch JA, Ram S, Koeberling O, Granoff DM. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J. Infect. Dis. 197: 1053–1061 [DOI] [PubMed] [Google Scholar]
- 39. Welsch JA, Rossi R, Comanducci M, Granoff DM. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J. Immunol. 172: 5606–5615 [DOI] [PubMed] [Google Scholar]