Abstract
Borrelia burgdorferi, a tick-borne bacterial pathogen, causes a disseminated infection involving multiple organs known as Lyme disease. Surface proteins can directly participate in microbial virulence by facilitating pathogen dissemination via interaction with host factors. We show here that a fraction of the B. burgdorferi chromosomal gene product BB0337, annotated as enolase or phosphopyruvate dehydratase, is associated with spirochete outer membrane and is surface exposed. B. burgdorferi enolase, either in a recombinant form or as a membrane-bound native antigen, displays enzymatic activities intrinsic to the glycolytic pathway. However, the protein also interacts with host plasminogen, potentially leading to its activation and resulting in B. burgdorferi-induced fibrinolysis. As expected, enolase displayed consistent expression in vivo, however, with a variable temporal and spatial expression during spirochete infection in mice and ticks. Despite an extracellular exposure of the antigen and a potential role in host-pathogen interaction, active immunization of mice with recombinant enolase failed to evoke protective immunity against subsequent B. burgdorferi infection. In contrast, enolase immunization of murine hosts significantly reduced the acquisition of spirochetes by feeding ticks, suggesting that the protein could have a stage-specific role in B. burgdorferi survival in the feeding vector. Strategies to interfere with the function of surface enolase could contribute to the development of novel preventive measures to interrupt the spirochete infection cycle and reduce the incidences of Lyme disease.
INTRODUCTION
A group of tick-borne spirochetes belonging to Borrelia burgdorferi sensu lato complex causes a frequent and multisystem illness known as Lyme disease or Lyme borreliosis (41, 49). The bacteria exist in a complex enzootic infectious cycle consisting of Ixodes scapularis ticks and a mammalian reservoir host, usually small rodents. As the infected tick successfully attaches onto a host, the pathogen begins to multiply within the feeding tick gut. A fraction of resident spirochetes then cross the gut epithelial barrier and gain entry into the hemocoel, swiftly migrate to the salivary glands, and finally invade the host dermis (13, 14, 16, 42). B. burgdorferi replicates locally in the dermis and then spreads to distant skin areas or hematogenously disseminates to distant organs, including joints, heart, or brain tissues, causing varied clinical manifestations such as arthritis, carditis, and meningitis (2). Since its identification nearly 3 decades ago, the incidence of Lyme disease is still on the rise in many parts of United States, Europe, and Asia (40, 49). Preventive measures, such as a vaccine to combat the incidence of Lyme disease in humans, are currently unavailable.
To persist in a complex enzootic cycle, B. burgdorferi is required to disseminate, invade, and colonize in a wide range of host and vector tissues. In vertebrates, the movement of spirochetes through the skin or through the basement membrane of endothelium is likely to require the production of proteases that assist the pathogen in the degradation of extracellular matrices (8, 10–12, 51). Plasminogen (Pg) is the proenzyme of a broad-spectrum serine protease known as plasmin, which is very abundant in plasma and certain tissues. Conversion of Pg to active plasmin is mediated by proteolytic activation cascades induced by specific activators, such as tissue-type Pg activator (tPA) and urokinase (uPA). Once activated, plasmin is involved in intravascular fibrinolysis or degradation of extracellular matrix material, which is relevant for cell dissemination or invasion (45, 50). Invasive pathogenic spirochetes including Borrelia and Leptospira are known to expresses multiple Pg-binding surface proteins that likely assist in pathogen dissemination through host tissues (11, 12, 22, 25, 28, 31, 52–54). However, the precise reasons why spirochetes express several Pg-binding proteins, whether one serves as primary Pg receptor or relative contributions of multiple Pg-binding microbial proteins in enzootic life cycle of infectious spirochetes remains an enigma.
Enolase, also known as phosphopyruvate hydratase, is an integral enzyme of the glycolysis and gluconeogenesis pathways, catalyzes the reversible interconversion of 2-phosphoglycerate and phosphoenolpyruvate. Although likely evolved as a enzyme involved in sugar metabolism, enolase is a multifunctional protein in both prokaryote and eukaryotes and can be found in both the cytosol and the cell membrane (37). Specifically, surface enolase is shown to act as a Pg receptor in certain tumor cells, a condition often linked to the initiation of disease processes in eukaryotes (37, 38). Enolase is also localized on the cell surface in many microorganisms (4, 30, 33, 43, 44), where it interacts with Pg and assists in microbial dissemination within hosts. The B. burgdorferi genome encodes for all known components of glycolytic pathway, including enolase (21). In agreement with a previous mass spectrometry-based study indicating detection of enolase in isolated B. burgdorferi outer membrane vesicles (35), we show here that enolase is readily exposed on the B. burgdorferi surface. We further show that despite retaining its enzymatic activity, the protein acts as a Pg receptor and contributes to spirochete survival in feeding ticks.
MATERIALS AND METHODS
Mice, Borrelia organisms, and ticks.
A fully infectious isolate of B. burgdorferi, clone B31-A3, was used throughout this study (17). Four- to six-week-old female C3H/HeN mice were purchased from the National Institutes of Health. The ticks used in the present study were reared in the laboratory as described elsewhere (29). All animal experiments were performed in accordance with the guidelines of the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee.
Production of recombinant enolase and antibody.
The B. burgdorferi gene BB0337 encoding enolase was amplified by PCR using specific primers: BB0337 sense (5′-CGG AAT TCC GGT TTT CAC ATT TAT GAA AT-3′) and BB0337 antisense (5′-CCG CTC GAG TTT TTG TTT AAT AGA ATA AA-3′). Recombinant enolase was produced in Escherichia coli using the bacterial expression vector pET302 (Invitrogen), and protein expression was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) to a final concentration of 1 mM. The His-enolase protein was affinity purified by using ProBond (Invitrogen). The generation of murine polyclonal antibodies against recombinant BB0337 and immunoblotting assays were performed as described earlier (29).
Plasminogen binding assays.
Cellular assays were performed as detailed previously (33). Briefly, the wells of microtiter plates were coated with B. burgdorferi cells in the presence of glutaraldehyde, which facilitates the immobilization of cells without compromising the membrane permeability. B. burgdorferi (107 cells/well) were incubated in 50 μl of phosphate-buffered saline (PBS) for 1 h at 37°C, followed by a 10-min incubation with 1% (vol/vol) glutaraldehyde. After subsequent blocking with 1% (wt/vol) bovine serum albumin in PBS, varied amounts (0.5 to 2 μg/well) of human plasminogen (hPg; Sigma) were added to the microtiter plate, followed by incubation for 1 h at 37°C. The cells were then washed three times, and bound protein was detected using anti-Pg monoclonal antibody (R&D Systems) and horseradish peroxidase (HRP)-conjugated secondary antibodies. Competition experiments included the addition of enolase or BBA52 antibodies prior to the addition of hPg. In additional experiments, wells were also coated with recombinant enolase (1 μg/well), and the binding of hPg (1 to 3 μg/well) was determined, as described above.
Degradation of fibrin in jellified matrices.
Fibrinolysis was assayed as described previously (27), with minor modifications. Briefly, 107 B. burgdorferi cells were preincubated with hPg (50 μg) for 3 h in the presence or absence of tPA (50 ng) in a final volume of 1 ml. Thereafter, the mixtures were washed three times with PBS to remove free hPg. The resulting cell pellets were placed in the wells of a fibrin substrate matrix gel that contained 1.25% low-melting-temperature agarose, hPg (100 μg), fibrinogen (4 mg), and thrombin in a final volume of 2 ml. Controls consisted of untreated cells (no hPg incubation) or incubation without cells. The jellified matrix was incubated in a humidified chamber at 37°C for 8 h. Plasmin activity was detected by the observation of clear hydrolysis haloes within the opaque jellified fibrin containing matrix and recorded with a Canon Revel T2 digital camera.
Triton X-114 phase partitioning.
Triton-X-114 phase-partitioning assays were performed as previously described (56). Briefly, 109 spirochetes were resuspended in PBS (pH 7.4) and incubated overnight at 4°C with agitation with 2% (vol/vol) Triton X-114 (Sigma). After incubation, phase-partitioned lysates were centrifuged at 13,000 × g for 15 min at 4°C to remove insoluble cell debris. The resulting supernatant was removed and placed at 37°C to allow for phase separation. The resulting aqueous and detergent-enriched phases were washed three times mixed with 10 volumes of ice-cold acetone, followed by centrifugation at 13,000 × g for 15 min to precipitate proteins. Protein pellets were finally resuspended in PBS and subjected to SDS-PAGE and immunoblot analysis with enolase antibody or antibodies against known amphiphilic protein (OspA), as detailed elsewhere (56).
PK accessibility assay.
Proteinase K (PK) accessibility assays were performed as described previously (9). Briefly, B. burgdorferi (2 × 108) was washed three times in 1 ml of PBS (pH 7.4) and collected by centrifugation at 4,000 × g for 4 min. The cell pellet was resuspended in 1 ml of PBS and split into two equal 500-μl volumes. One aliquot received 200 μg of PK (Sigma), while the other aliquot received an equal volume of PBS without PK. Both aliquots were incubated for 20 min at room temperature before the addition of 10 μl of phenylmethylsulfonyl fluoride (Sigma) to stop PK activity. Spirochete suspensions were subsequently pelleted by centrifugation at 10,000 × g for 10 min and resuspended in PBS for immunoblot analysis with antibodies against BB0337, FlaB, or OspA. Densitometric analysis of immunoblots was performed using a gel documentation system (ChemiDoc XRS; Bio-Rad).
Measurement of the enolase activity.
Enolase activity was determined by measuring the conversion of NADH·H+ to NAD+, as described previously (6, 38). Briefly, the enzymatic reactions were performed at 25°C in 81 mM HEPES buffer (pH 7.4) containing 25 mM MgSO4 with 100 mM KCl, 1.9 mM 2-phosphoglycerate solution (2-PGE; Sigma), 0.24 mM β-NADH (Sigma), 1.3 mM ADP (Sigma), lactate dehydrogenase/pyruvate kinase (PK/LDH Enzyme Solution; Sigma), and 1.6 μg of the protein/well in a final reaction volume of 200 μl. The enolase activity was measured in terms of the rate of reduction in the absorbance at 340 nm (i.e., increase in the production of NAD+ from NADH). For kinetic studies, varied concentrations of 2-PGE (1 to 6 mM) were used.
Enolase activity on the surface of intact B. burgdorferi.
The enolase activity of intact B. burgdorferi cells was measured by a direct assay, as described previously for pathogenic streptococci (38). Briefly, 108 B. burgdorferi cells were washed three times with the reaction buffer (100 mM HEPES [pH 7.0], 10 mM MgSO4, and 7.7 mM KCl), centrifuged, and finally resuspended in a volume of 400 μl. Twofold serial dilution of the cells were mixed with equal volume of the reaction buffer in presence or absence of 6 mM 2-PGE (Sigma), followed by incubation at 37°C for 5 min. The bacteria were removed by centrifugation (10,000 rpm for 1 min) after incubation, and the supernatants were measured for the production of phosphoenolpyruvate at A240. To ensure that the cell permeability is not compromised during the assay, the viability of the B. burgdorferi cells was determined before and after the enzymatic assay by using vital fluorescence labeling (Live/Dead BacLight viability kit; Invitrogen), as detailed previously (55).
Quantitative PCR.
Quantitative reverse transcriptase PCR (qRT-PCR) analysis was performed as previously described (55). RNA samples were extracted from murine tissues using TRIzol reagent (Invitrogen), treated with DNase I (Invitrogen), and finally purified using an RNeasy kit (Qiagen). RNA was used as a template for RT-PCR using an AffinityScript cDNA synthesis kit (Stratagene). qRT-PCR analysis was performed using iQ Sybr green Supermix (Bio-Rad). For quantitative analysis of gene expression, the target transcripts were normalized to the number of flaB transcripts. Since there is probably no suitable gene, or method, to accurately quantify spirochete levels in vivo, we used flaB RNA-based qRT-PCR to measure spirochete burdens which also produces similar patterns in the differences of the tissue burdens of B. burgdorferi when using DNA-based qPCR (47). For quantitative measurement of B. burgdorferi burden in infected tissues, flaB transcripts were normalized to mouse or tick β-actin levels. All qRT-PCR results were checked for specificity by melting-curve analysis.
Active immunization and infection studies.
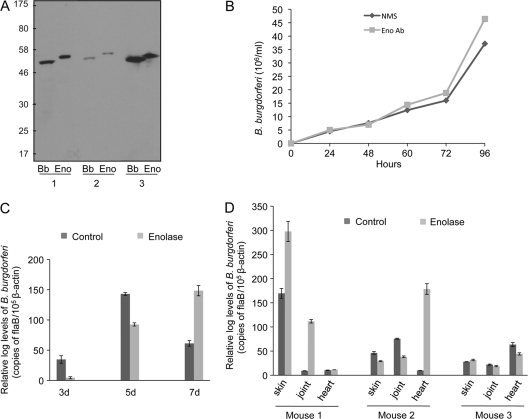
Groups of mice (three animals/group) were immunized with adjuvant containing either recombinant enolase, or PBS containing the same volume of adjuvant. Ten days after the final boost, mice were infected with a subcutaneous injection of B. burgdorferi (105 spirochetes/mouse). Blood was collected at day 3, 5, and 7 after initial spirochete challenge. The mice were sacrificed 12 days after infection. Heart, tibiotarsal joint, and skin samples were collected and frozen in liquid nitrogen. RNA was isolated from infected tissues and B. burgdorferi burden was measured using qRT-PCR (55). To verify whether enolase was involved in tick acquisition of B. burgdorferi, separate groups of mice (three animals/group) were immunized with recombinant enolase or PBS (using equal volume of adjuvants), as described above and, 10 days after the last boost, the mice were infected by a single intradermal injection with B. burgdorferi (105 spirochetes/mouse). After 12 days, sterile I. scapularis nymphs (15 ticks/mouse) were allowed to engorge on the mice. Partially fed or fully engorged ticks were removed from mice at 5, 48, and 72 h during feeding and assessed for pathogen burden using qRT-PCR, as described above. Animal infection studies, including immunization and B. burgdorferi challenge studies, were independently repeated two times.
In vitro bactericidal assay.
In vitro bactericidal assay was performed as described previously (56). Normal mice serum or serum samples from mice immunized with enolase were used for the bactericidal assays. At 24 h after the addition of antiserum (undiluted sera without complement inactivation), 1 μl of medium containing spirochetes was added to 1 ml of fresh BSK-H medium to assess the spirochetes' ability to regrow in the culture. Spirochetes were incubated at 33°C and enumerated by dark-field microscopy at 24, 48, 60, 72, and 96 h, as described previously (36).
Bioinformatics and statistical analysis.
Predictions of Pg-binding domain and catalytic motif in primary enolase sequences were performed as detailed earlier (1). Multiple sequence alignment was achieved using CLUSTAL W alignment (DNASTAR, Inc.). The results were presented as means ± the standard errors of the mean (SEM). Statistical comparisons were performed by using a Student t test. Statistical significance was accepted for P < 0.05.
RESULTS
B. burgdorferi binds plasminogen via enolase.
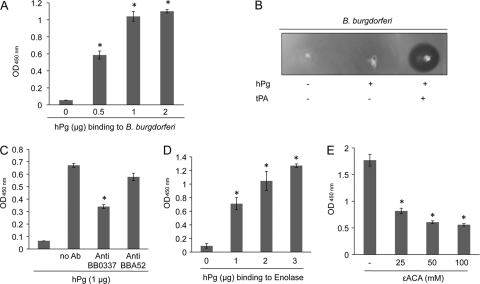
We first assessed whether the infectious B. burgdorferi B31 isolate binds Pg and induces fibrinolysis and whether enolase participates in the spirochete-Pg interaction. Intact fixed B. burgdorferi cells were immobilized onto microtiter wells and incubated with human Pg (hPg), and bound proteins were detected using HRP-conjugated secondary antibodies. The results show that B. burgdorferi cells were bound to hPg in a dose-dependent fashion (Fig. 1A). Next, jellified matrices containing fibrinogen were used to examine plasmin activity in vitro, as described previously (27). As demonstrated in Fig. 1B, the association of B. burgdorferi with Pg promoted increased fibrinolysis (Fig. 1B, lane 3). These experiments indicate an interaction of hPg and B. burgdorferi cells, which likely triggers the activation of plasmin eventually leading to the degradation of fibrinogen.
Fig 1.
Plasminogen interacts with B. burgdorferi and involvement of enolase. The data represent means plus the SEM from three independent experiments. (A) Plasminogen-B. burgdorferi interaction. B. burgdorferi were fixed onto the microtiter plates with glutaraldehyde and incubated with human plasminogen (hPg) in a concentration-dependent manner. Binding was detected using anti-plasminogen and secondary antibodies. The differences between hPg (0.05 to 2) and control (no hPg) are significant (*, P < 0.05). (B) Fibrinolytic activity of plasminogen-bound B. burgdorferi. Spirochetes were incubated in the absence (lane 1) or presence of plasminogen (lane 2), or together with plasminogen and tPA (lane 3), spotted into the Matrigel, and incubated for the appearance of halo areas indicative of fibrinolytic activity. (C) Enolase antibodies competitively reduce B. burgdorferi-plasminogen interaction. B. burgdorferi were coated onto microtiter wells as detailed in Fig. 1A and incubated with anti-enolase or anti-BBA52 antibodies prior to incubation with hPg. The difference between anti-enolase antibodies and control (no Ab) is significant (*, P < 0.05). (D) Plasminogen binds to recombinant enolase. Enolase (1 μg) was immobilized on microtiter well plates and incubated with hPg in a concentration-dependent manner. Bound proteins were detected using anti-plasminogen and secondary antibodies. The differences between hPg (1 to 3 μg) and no hPg (0) wells are significant (*, P < 0.05). (E) A lysine analog inhibits enolase-Pg interaction. Immobilized enolase (1 μg) was incubated with hPg (1 μg) in the presence of different concentrations of inhibitor (εACA). The differences between wells incubated with εACA or without (−) are significant. * P < 0.05.
Since enolase is one of the well-known Pg-binding proteins detected in many pathogenic organisms (18, 27, 33, 34, 37, 38), we next assessed whether enolase is involved in B. burgdorferi-Pg interaction. To accomplish this, we used enolase antibodies and specific inhibitors, as extensively used in previous studies (18, 22, 23). Unlike antibodies against a control B. burgdorferi membrane protein, BBA52 (29), antibodies raised against recombinant enolase were able to decrease Pg binding to B. burgdorferi cells (Fig. 1C). Increasing concentrations of hPg directly bound to immobilized enolase in a dose-dependent fashion (Fig. 1D). Other B. burgdorferi membrane proteins, such as BBA52 (29) or BBA57 (9) did not bind hPg (data not shown). The enolase-Pg interaction was significantly inhibited by a known inhibitor (18, 33), the lysine analogue ε-aminocaproic acid (εACA) (Fig. 1E), further supporting the specificity of the interaction and suggesting that, similar to other organisms, an exposed lysine residue(s) in B. burgdorferi enolase is likely responsible for this binding activity.
Enolase is exposed on the B. burgdorferi surface.
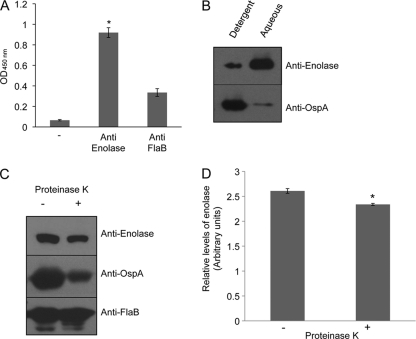
To serve as a Pg-binding protein, enolase must localize on the microbial surface. The B. burgdorferi genome encodes for a single enolase protein without an identifiable signal peptide; however, the enzyme has been detected on the surface of many other pathogens. In agreement with a previous study showing the presence of enolase in isolated B. burgdorferi outer membrane vesicles (35), enolase antibody is able to bind to the surface of intact immobilized spirochetes with greater efficiency than antibodies against a control subsurface protein, FlaB (Fig. 2A). To corroborate this finding further, we used Triton X-114 to separate B. burgdorferi cells into aqueous (soluble) or detergent (membrane-associated) fractions and assessed the localization of enolase by using immunoblotting. The results showed that enolase partitioned into primarily soluble, but also membrane-associated fractions (Fig. 2B). As expected, a control membrane protein, outer surface protein A (OspA), was predominantly localized into the membrane fraction. We further assessed whether enolase is exposed on the microbial surface using a more direct assay. To achieve this, intact spirochetes were subjected to a controlled PK digestion and then assessed by immunoblot analysis with antisera against enolase or known surface (OspA) or subsurface (FlaB) antigens. The results indicated that PK digestion reduced the amount of enolase, suggesting that the antigen is exposed on the borrelial surface (Fig. 2C). Densitometric analysis of representative immunoblots (Fig. 2C) indicated that, unlike FlaB, the levels of enolase is significantly reduced after proteinase K treatment (P < 0.05, Fig. 2D).
Fig 2.
Enolase is surface exposed on B. burgdorferi cells. (A) Anti-enolase antibody binds to the surfaces of intact spirochetes. B. burgdorferi cells were incubated using glutaraldehyde, which immobilizes the cells onto the microtiter wells without compromising membrane permeability. Cells were incubated with anti-enolase or anti-FlaB antibodies, and bound antibodies were detected using secondary antibodies. The data represent the means with the SEM from three independent experiments. The differences between anti-enolase antibodies and controls are significant (*, P < 0.05). (B) Enolase is associated with borrelial membrane fraction. Triton X-114-extractable membrane (detergent-phase) or soluble (aqueous-phase) proteins were immunoblotted by antibodies against enolase or known membrane protein (OspA). (C) Enolase is sensitive to proteinase K-mediated degradation of B. burgdorferi surface proteins. Viable spirochetes were incubated in the absence (−) or presence (+) of proteinase K for removal of protease sensitive surface proteins and processed for immunoblot analysis using anti-enolase antibodies. B. burgdorferi OspA and FlaB antibodies were utilized as controls for surface-exposed and subsurface proteins, respectively. (D) Densitometric analysis of proteinase K-mediated degradation of enolase. Relative densities of B. burgdorferi enolase in the absence or presence of proteinase K, as determined by immunoblot analysis with anti-enolase antibodies shown in Fig. 2C, were determined by a densitometric scan. Differences between the levels of enolase in the absence and presence of proteinase K treatment are significant (*, P < 0.05).
Recombinant enolase or surface-exposed native B. burgdorferi protein retains enzymatic activity.
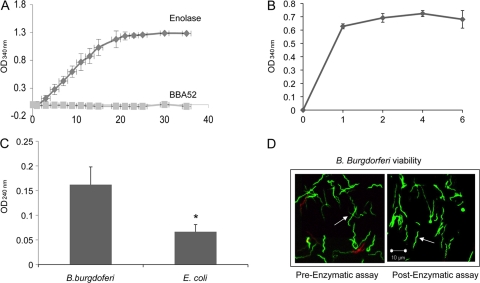
We next assessed whether recombinant enolase or the native protein on intact B. burgdorferi surface retains the enzymatic activity. The enolase activity was assessed by examining catalysis of NADH·H+ to NAD+, resulting from the conversion of 2-phosphoglycerate to phosphoenolpyruvate, as described earlier (38). The results show the saturation of enolase activity either over time- or substrate-dependent manners (Fig. 3A and B), suggesting specific catalytic activities of recombinant enolase. To determine whether the native enolase is enzymatically active on spirochete surface, we adopted an assay originally developed for determining surface enolase activities of pathogenic streptococci (38). The assay relies upon measurement of phosphoenolpyruvate production in the presence of intact bacterial cells. The results indicated detectable enolase activity in B. burgdorferi cells but not in control Gram-negative bacteria, E. coli (Fig. 3C). The extracellular enolase activity is produced by intact B. burgdorferi cells, since a comparison of spirochetes before and after the enzymatic reaction using a bacterial viability assay (56) did not show detectable differences in viable spirochete numbers (Fig. 3D).
Fig 3.
Enzymatic activities of recombinant and membrane bound B. burgdorferi enolase. (A) Activity of the recombinant enolase. Enzyme activity was measured by a coupled enzymatic assay by measurement of catalysis of 2-phosphoglycerate to phosphoenolpyruvate for a period of 35 min using 1.6 μg of recombinant enolase or a control B. burgdorferi membrane protein BBA52. (B) Substrate saturation by recombinant enolase. Different concentrations of the substrate 2-phosphoglycerate (1 to 6 mM) were incubated with fixed amount of enolase (2 μg). (C) Enolase activity on the surface of intact B. burgdorferi. The conversion of 2-phosphoglycerate to phosphoenolpyruvate was used to measure the enolase activity in B. burgdorferi cells and E. coli. (D) Enolase activity assay conditions did not affect B. burgdorferi viability. Aliquots of B. burgdorferi were isolated before or after enzymatic assays as described in panel C and subjected to spirochete viability analysis. A representative fluorescence labeling of live and dead spirochetes before or after enzyme treatment, as assessed under a laser confocal microscope, is presented. Live B. burgdorferi was stained with Syto 9 (green, arrows), and the dead spirochetes were stained with propidium iodide (red).
Enolase expression during the tick-mouse infection cycle.
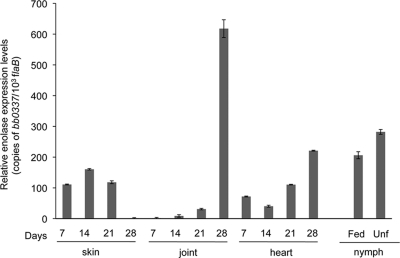
Although a consistent expression of BB0337 encoding enolase is expected for the viability of B. burgdorferi, we assessed its expression in vivo to determine whether the protein level is altered during specific phases of spirochete infection in mice or in ticks due to its potential role as a plasminogen-binding protein. To accomplish this, C3H mice were infected with B. burgdorferi, and skin, heart, and joint samples were collected 7, 14, 21, and 28 days after infection. Larval and nymphal ticks were fed on parallel groups of 15-day-infected mice (25 ticks/mouse), and fully engorged ticks were isolated after 3 days of repletion. Batches of infected fed larvae were allowed to molt and then collected as unfed nymphs. Total RNA was prepared from murine and tick samples and subjected to qRT-PCR to measure the enolase transcript levels. The results indicated that enolase is constitutively expressed in vivo with relatively enhanced transcription in ticks, as well as in certain tissues and phases of infection in mice (Fig. 4).
Fig 4.
Enolase expression in vivo. The relative expression levels of enolase in the murine hosts and in representative life stages of ticks were analyzed, and the results are presented as copies of enolase transcript per 1,000 copies of flaB transcripts. Total RNA was isolated from multiple tissues of B. burgdorferi-infected mice (six mice/group) at days 7, 14, 21, and 28 after challenge, unfed nymphs following larval molting (Unf), and fed nymphs (Fed) after 3 days of feeding. BB0337 transcripts were measured using qRT-PCR. Transcripts encoding enolase were abundant in all murine tissues tested and were detected in these stages of the ticks. Bars denote the mean ± the standard error of four representative qPCR measurements from two independent infection experiments. The enolase transcript level in joints at day 28 is significantly different from that of other time points or tissue locations (P < 0.05).
Active immunization of mice with enolase failed to evoke protective immunity.
Enolase has detectable surface exposure and is expressed at all tested murine tissue locations, so we next assessed whether the immunization of mice using recombinant enolase could elicit protective immunity and influence the outcome of Lyme disease. To accomplish this, C3H mice (three animals/group) were immunized with purified recombinant enolase. As a control, separate groups of mice were also immunized with PBS mixed with similar volume of adjuvant. Ten days after final immunization, mice were tested for development of enolase antibody (Fig. 5A). Despite the facts that enolase is surface exposed (Fig. 2C) and anti-enolase antibody binds to the B. burgdorferi cells (Fig. 2A), no significant bactericidal activity was found when spirochetes were exposed to enolase antibodies (Fig. 5B). To determine whether enolase antibody still confers protection of mice against B. burgdorferi infection, the enolase-immunized mice were inoculated with B. burgdorferi (105 spirochetes/mouse). Blood samples were collected from infected mice 3, 5, and 7 days after challenge. At 12 days after infection, the mice were sacrificed, and skin, joint, and heart samples were collected. The pathogen levels were measured by qRT-PCR. In parallel, another group of mice were maintained for 4 weeks and monitored for joint inflammation by measurement of ankle swelling at weekly intervals. The results indicated that compared to control, immunization with enolase failed to exert a consistent influence on spirochete burden in the blood (Fig. 5C) or any of the tested tissues (Fig. 5D). Although enolase expression is upregulated in most tissues during late infection, such as in joints between the third and fourth weeks of infection (Fig. 4), mice immunized with enolase developed ankle swelling similar to that seen in the controls (data not shown).
Fig 5.
Enolase antibody lacks bactericidal properties and does not interfere with B. burgdorferi infectivity. (A) Detection of antibodies against enolase in immunized mice. Groups of mice (three animals/group) were immunized with recombinant enolase or PBS (control) mixed with adjuvant. B. burgdorferi lysates (Bb) and recombinant protein (Eno) immunoblotted with serum were collected from individual enolase-immunized mice (indicated as 1, 2, and 3). Note that the recombinant His tag protein migrates as a slightly higher molecular protein compared to the native protein. (B) Borreliacidal activities of enolase antibodies in vitro as assessed by the growth of spirochetes following antiserum treatment. B. burgdorferi was treated with enolase antiserum (Eno Ab) or normal mouse serum (NMS) as a control. After 24 h, 1 μl of medium was inoculated into 2 ml of fresh medium, and the growth of the spirochetes was assessed using dark-field microscopy at the indicated time periods. (C and D) Effect of enolase immunization on B. burgdorferi infection in mice. Mice were immunized with enolase as detailed in Fig. 5A, and 10 days after the final immunization the mice were infected with B. burgdorferi (105 spirochetes/mouse). The spirochete burdens in both groups of mice were assessed by qRT-PCR analysis by measuring the copies of the B. burgdorferi flaB that normalized with murine β-actin transcript levels. B. burgdorferi levels were examined in blood collected 3, 5, and 7 days after challenge (C) or from skin, joint, and heart locations (D) at 12 days after infection.
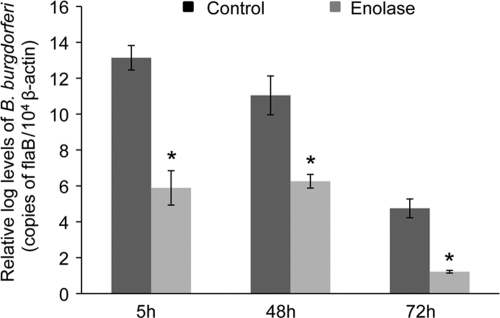
Enolase antibodies interfere with B. burgdorferi acquisition by ticks.
A previous study indicated that while ticks apparently lack the endogenous capacity to activate host Pg, the blood meal in feeding ticks contains components of the host Pg activation system, which influences B. burgdorferi acquisition by larval ticks (11). We therefore assessed whether antibodies against enolase, as a Pg receptor, influences spirochete acquisition by ticks. To accomplish this, mice were actively immunized with enolase or PBS-adjuvant mixture (control) prior to B. burgdorferi infection as described above, and after 12 days, naive nymphs were allowed to parasitize the mice. Partially fed nymphs were forcibly removed from mice at 5 and 48 h after the onset of feeding. Fully engorged nymphs were also collected after 72 h of feeding. The spirochete burden was assessed by qRT-PCR analysis of flaB transcripts normalized against tick β-actin levels. The results indicated that the levels of B. burgdorferi in ticks that parasitized enolase-immunized mice were significantly lower than the respective control groups for all tested time points of feeding (Fig. 6), suggesting that enolase antibody is able to interfere with spirochete persistence in feeding nymphs.
Fig 6.
Active immunization of mice with enolase reduces B. burgdorferi acquisition by Ixodes nymphs. Mice were immunized with enolase or PBS and infected with B. burgdorferi. After 12 days, nymphs (15 ticks/mouse) were allowed to engorge on the mice and were collected after 5, 48, and 72 h of feeding. Bars denote the mean value ± the SEM of four representative quantitative PCR measurements from two independent animal infection experiments. Differences between mice immunized with enolase and controls at all time points were significant (*, P < 0.05).
DISCUSSION
B. burgdorferi has evolved a remarkable ability to disseminate through a diverse range of host tissues—a property that facilitates spirochete maintenance in a complex enzootic cycle. The pathogen has a strong affinity for a diverse array of host extracellular matrix (ECM) components, including fibronectin, integrins, collagen, proteoglycans, and laminin (3, 7, 8, 10, 20, 39, 46). The host ECM likely provides a protective niche for B. burgdorferi, allowing the spirochete to persist in the host despite humoral and cellular immune responses. However, under normal conditions, such as in healthy humans, certain components of the ECM may not be exposed and thus may not be accessible for interaction with bacteria, which, however, could be exposed after a tissue trauma or after an infection (15). The proteolytic activity achieved by certain pathogens via subversion of specific host proteases, such as plasmin, plays an important role in the establishment of infections (32). Plasmin, the active form of plasminogen (Pg), is a broad-spectrum serine protease and a key component of the fibrinolytic system (45). The Pg-binding property of many bacteria, including pathogenic spirochetes, has been suggested to be a contributing factor in tissue invasion and survival in the hosts (11, 19, 52–54). B. burgdorferi binds Pg (11, 12, 22, 25), and activated plasmin facilitates the invasiveness of the pathogen (11, 12). Although not essential for spirochete infectivity in mice, Pg has been shown to facilitate the dissemination of B. burgdorferi within the tick (11). Here, we show that a surface enolase participates in B. burgdorferi-Pg interaction and is potentially important for spirochete survival during B. burgdorferi acquisition in feeding ticks.
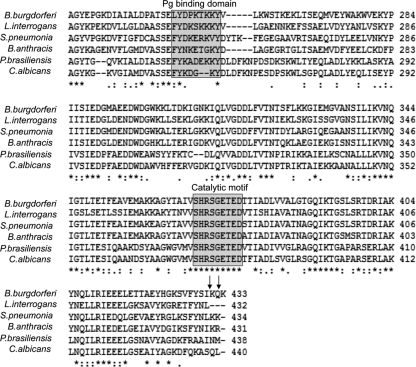
In agreement with a previous study (35), our data suggest that a fraction of B. burgdorferi enolase exists as a cell surface protein, although how protoplasmic enolase is translocated across the cellular membranes or is associated with it remains unknown. Similar to other organisms, B. burgdorferi enolase lacks classical membrane localization sequences, a transmembrane domain, or lipidation motifs. In eukaryotes, however, certain scaffold proteins bind enolase through its PDZ domain and translocate the enzyme to the plasma membrane (24). Although the mechanism of enolase transport awaits further study, it is a well-known fact that many pathogens including Trichomonas vaginalis (33), Candida albicans (27), Paracoccidioides brasiliensis (34), or Streptococcus suis (18) transport enolase to the microbial surface, where it interacts with host Pg. The Pg-enolase interaction is mediated by two C-terminal lysine residues of enolase and highly conserved kringle domains of Pg (5). However, as shown for S. pneumoniae, enolase possesses an additional internal Pg-binding site, a nine-residue motif FYDKERKVY, although full conservation of this motif is not required for the binding (5, 26). Currently, the three-dimensional structure of B. burgdorferi enolase is not known; however, primary sequence analysis and CLUSTAL W alignment has shown that B. burgdorferi enolase also possesses a conserved internal Pg-binding motif LYDPKTKKY located between amino acids 248 and 258, which does not nonoverlap with the catalytic motif SHRSGETED located between amino acids 368 and 376 (Fig. 7). Therefore, conservation of known Pg-binding motifs in borrelial enolase and the fact that the lysine analogue εACA or enolase antibodies significantly inhibited Pg binding to recombinant enolase or B. burgdorferi, along with the additional data presented here, justify our conclusion that the Lyme disease pathogen binds Pg via surface enolase, which facilitates pathogen survival in the host.
Fig 7.
Partial alignment of amino acid sequences from different microorganisms showing positions of potential Pg-binding domain and catalytic motif in the primary sequence of enolase. The shaded boxes represent the Pg-binding domain and catalytic motif, whereas the positions of two extreme C-terminal lysine residues that also mediate Pg binding are denoted by arrows. The GenBank accession numbers for the enolase from respective organisms are as follows: Borrelia burgdorferi B31, AAC66719.1; Leptospira interrogans serovar Lai strain 56601, AAN49150.1; Streptococcus pneumoniae, AJ303085.1; Bacillus anthracis strain Ames Ancestor, AAT34498.1; Paracoccidioides brasiliensis 01, EF558735.1; and Candida albicans, L10290.1.
Virulence roles of microbial antigens can be effectively studied by molecular genetics approaches. However, our attempts to create enolase-deficient spirochetes were unsuccessful (data not shown), probably due to its indispensable involvement in glycolysis, the only energy generation pathway in B. burgdorferi (21). Through an alternate approach, our antibody-blocking studies provided indirect evidence for the role of surface enolase in spirochete persistence, as a potential Pg receptor in vivo, at least in feeding ticks. Although our findings suggest that the total numbers of spirochetes, when normalized to tick β-actin transcript levels, are reduced at the late feeding stage (72 h) compared to the early feeding stages (5 or 48 h), enolase antibodies reduced B. burgdorferi levels at all tested stages of feeding. B. burgdorferi-Pg interaction in vivo was highlighted in a previous elegant study in which host-derived Pg was shown to be important for the acquisition of B. burgdorferi by larval ticks, as well as for its systemic dissemination within feeding nymphs (11). Currently, we do not know exactly how enolase antibody influences the spirochete acquisition in feeding ticks, but such effects may not be due to the bactericidal action of the antibody. As the tick gut undergoes dramatic physiological and structural remodeling during feeding (48), surface enolase, by facilitating pathogen-Pg interaction, likely contributes to B. burgdorferi entry or survival during blood meal engorgement. Therefore, the antibody binding could potentially interfere with the function of surface-exposed enolase in feeding ticks, including its possible interaction with Pg. In contrast, although plasminogen has been previously reported to be important for spirochete dissemination through host blood (11), in our immunization experiments enolase antibodies were unable to reduce pathogen levels in murine blood. Thus, the role of surface enolase during hematogenous dissemination of spirochetes could be functionally redundant, possibly due to the presence of other Pg-binding proteins on the spirochete surface (22, 25, 31). We were unable to accomplish a complete inhibition of B. burgdorferi-Pg interaction, even using polyclonal enolase antibodies (Fig. 1C). This suggests the occurrence of additional Pg receptor(s) in spirochetes. Nevertheless, enolase immunization interfered with pathogen survival in feeding ticks that underscored its role as a functional Pg receptor in vivo and also reinforced the importance of B. burgdorferi-Pg interaction during borrelial infection. A better understanding of Pg-enolase interaction may shed new light into complex interaction between the pathogen and hosts and likely to contribute to the development of novel measures to combat B. burgdorferi infections.
ACKNOWLEDGMENTS
We thank Adam Coleman, Toru Kariu, Xiuli Yang, and Brian Backstedt for their assistance with this study.
This research was supported by funding from NIH/NIAID (award AI080615 to U.P.).
Footnotes
Published ahead of print 24 October 2011
REFERENCES
- 1. Agarwal S, Kulshreshtha P, Bambah Mukku D, Bhatnagar R. 2008. α-Enolase binds to human plasminogen on the surface of Bacillus anthracis. Biochim. Biophys. Acta 1784: 986–994 . [DOI] [PubMed] [Google Scholar]
- 2. Barthold SW, Diego C, Philipp MT. 2010. Animal models of borreliosis, p 353–405 In Samuels DS, Radolf JD. (ed), Borrelia, molecular biology, host interaction and pathogenesis. Caister Academic Press, Norfolk, United Kingdom: . [Google Scholar]
- 3. Benoit VM, Fischer JR, Lin YP, Parveen N, Leong JM. 2011. Allelic variation of the Lyme disease spirochete adhesin DbpA influences spirochetal binding to decorin, dermatan sulfate, and mammalian cells. Infect. Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bercic RL, et al. 2008. Identification of major immunogenic proteins of Mycoplasma synoviae isolates. Vet. Microbiol. 127: 147–154 . [DOI] [PubMed] [Google Scholar]
- 5. Bergmann S, et al. 2003. Identification of a novel plasmin(ogen)-binding motif in surface displayed alpha-enolase of Streptococcus pneumoniae. Mol. Microbiol. 49: 411–423 . [DOI] [PubMed] [Google Scholar]
- 6. Bergmeyer HU. 1974. Methods of enzymatic analysis, 2nd ed, vol I Verlag Chemie, Weinheim, Germany: . [Google Scholar]
- 7. Brissette CA, Bykowski T, Cooley AE, Bowman A, Stevenson B. 2009. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect. Immun. 77: 2802–2812 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brissette CA, Verma A, Bowman A, Cooley AE, Stevenson B. 2009. The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin. Microbiology 155: 863–872 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brooks CS, Vuppala SR, Jett AM, Akins DR. 2006. Identification of Borrelia burgdorferi outer surface proteins. Infect. Immun. 74: 296–304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coburn J, Fischer JR, Leong JM. 2005. Solving a sticky problem: new genetic approaches to host cell adhesion by the Lyme disease spirochete. Mol. Microbiol. 57: 1182–1195 . [DOI] [PubMed] [Google Scholar]
- 11. Coleman JL, et al. 1997. Plasminogen is required for efficient dissemination of Borrelia burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89: 1111–1119 . [DOI] [PubMed] [Google Scholar]
- 12. Coleman JL, et al. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 63: 2478–2484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Silva AM, Fikrig E. 1995. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 53: 397–404 . [DOI] [PubMed] [Google Scholar]
- 14. de Silva AM, Tyson KR, Pal U. 2009. Molecular characterization of the tick-Borrelia interface. Front. Biosci. 14: 3051–3063 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubreuil JD, Giudice GD, Rappuoli R. 2002. Helicobacter pylori interactions with host serum and extracellular matrix proteins: potential role in the infectious process. Microbiol. Mol. Biol. Rev. 66: 617–629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dunham-Ems SM, et al. 2009. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J. Clin. Invest. 119: 3652–3665 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elias AF, et al. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70: 2139–2150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esgleas M, et al. 2008. Isolation and characterization of alpha-enolase, a novel fibronectin-binding protein from Streptococcus suis. Microbiology 154: 2668–2679 . [DOI] [PubMed] [Google Scholar]
- 19. Fenno JC, et al. 2000. Identification of a Treponema denticola OppA homologue that binds host proteins present in the subgingival environment. Infect. Immun. 68: 1884–1892 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer JR, LeBlanc KT, Leong JM. 2006. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 74: 435–441 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fraser CM, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390: 580–586 . [DOI] [PubMed] [Google Scholar]
- 22. Fuchs H, Wallich R, Simon MM, Kramer MD. 1994. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc. Natl. Acad. Sci. USA U. S. A. 91: 12594–12598 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ge J, Catt DM, Gregory RL. 2004. Streptococcus mutans surface alpha-enolase binds salivary mucin MG2 and human plasminogen. Infect. Immun. 72: 6748–6752 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hafner A, Obermajer N, Kos J. 2010. Gamma-1-syntrophin mediates trafficking of gamma-enolase towards the plasma membrane and enhances its neurotrophic activity. Neurosignals 18: 246–258 . [DOI] [PubMed] [Google Scholar]
- 25. Hu LT, et al. 1997. Isolation, cloning, and expression of a 70-kilodalton plasminogen binding protein of Borrelia burgdorferi. Infect. Immun. 65: 4989–4995 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Itzek A, et al. 2010. Contribution of plasminogen activation toward the pathogenic potential of oral streptococci. PLoS One 5: e13826 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jong AY, et al. 2003. Binding of Candida albicans enolase to plasmin(ogen) results in enhanced invasion of human brain microvascular endothelial cells. J. Med. Microbiol. 52: 615–622 . [DOI] [PubMed] [Google Scholar]
- 28. Klempner MS, Noring R, Epstein MP, McCloud B, Rogers RA. 1996. Binding of human urokinase type plasminogen activator and plasminogen to Borrelia species. J. Infect. Dis. 174: 97–104 . [DOI] [PubMed] [Google Scholar]
- 29. Kumar M, Yang X, Coleman AS, Pal U. 2010. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J. Infect. Dis. 201: 1084–1095 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lafrentz BR, Shoemaker CA, Klesius PH. 2011. Immunoproteomic analysis of the antibody response obtained in Nile tilapia following vaccination with a Streptococcus iniae vaccine. Vet. Microbiol. 152: 346–352 . [DOI] [PubMed] [Google Scholar]
- 31. Lagal V, Portnoi D, Faure G, Postic D, Baranton G. 2006. Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes Infect. 8: 645–652 . [DOI] [PubMed] [Google Scholar]
- 32. Lahteenmaki K, Kuusela P, Korhonen TK. 2001. Bacterial plasminogen activators and receptors. FEMS Microbiol. Rev. 25: 531–552 . [DOI] [PubMed] [Google Scholar]
- 33. Mundodi V, Kucknoor AS, Alderete JF. 2008. Immunogenic and plasminogen-binding surface-associated alpha-enolase of Trichomonas vaginalis. Infect. Immun. 76: 523–531 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nogueira SV, et al. 2010. Paracoccidioides brasiliensis enolase is a surface protein that binds plasminogen and mediates interaction of yeast forms with host cells. Infect. Immun. 78: 4040–4050 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nowalk AJ, Nolder C, Clifton DR, Carroll JA. 2006. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 6: 2121–2134 . [DOI] [PubMed] [Google Scholar]
- 36. Pal U, et al. 2001. Inhibition of Borrelia burgdorferi-tick Interactions in vivo by outer surface protein A antibody. J. Immunol. 166: 7398–7403 . [DOI] [PubMed] [Google Scholar]
- 37. Pancholi V. 2001. Multifunctional alpha-enolase: its role in diseases. Cell. Mol. Life Sci. 58: 902–920 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pancholi V, Fischetti VA. 1998. alpha-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 273: 14503–14515 . [DOI] [PubMed] [Google Scholar]
- 39. Parveen N, et al. 2006. Bgp, a secreted glycosaminoglycan-binding protein of Borrelia burgdorferi strain N40, displays nucleosidase activity and is not essential for infection of immunodeficient mice. Infect. Immun. 74: 3016–3020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Piesman J, Eisen L. 2008. Prevention of tick-borne diseases. Annu. Rev. Entomol. 53: 323–343 . [DOI] [PubMed] [Google Scholar]
- 41. Piesman J, Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129 (Suppl): S191–S220 . [DOI] [PubMed] [Google Scholar]
- 42. Piesman J, Schneider BS, Zeidner NS. 2001. Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J. Clin. Microbiol. 39: 4145–4148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pitarch A, et al. 2004. Proteomics-based identification of novel Candida albicans antigens for diagnosis of systemic candidiasis in patients with underlying hematological malignancies. Proteomics 4: 3084–3106 . [DOI] [PubMed] [Google Scholar]
- 44. Pitarch A, Jimenez A, Nombela C, Gil C. 2006. Decoding serological response to Candida cell wall immunome into novel diagnostic, prognostic, and therapeutic candidates for systemic candidiasis by proteomic and bioinformatic analyses. Mol. Cell Proteomics 5: 79–96 . [DOI] [PubMed] [Google Scholar]
- 45. Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. 1995. The cell biology of the plasminogen system. FASEB J. 9: 939–945 . [DOI] [PubMed] [Google Scholar]
- 46. Probert WS, Johnson BJ. 1998. Identification of a 47-kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30: 1003–1015 . [DOI] [PubMed] [Google Scholar]
- 47. Promnares K, et al. 2009. Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Mol. Microbiol. 74: 112–125 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sonenshine DE. 1993. Biology of ticks, vol 1 Oxford University Press, New York, NY: . [Google Scholar]
- 49. Steere AC, Coburn J, Glickstein L. 2004. The emergence of Lyme disease. J. Clin. Invest. 113: 1093–1101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vassalli JD, Sappino AP, Belin D. 1991. The plasminogen activator/plasmin system. J. Clin. Invest. 88: 1067–1072 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Verma A, Brissette CA, Bowman A, Stevenson B. 2009. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect. Immun. 77: 4940–4946 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Verma A, et al. 2010. Leptospiral endostatin-like protein A is a bacterial cell surface receptor for human plasminogen. Infect. Immun. 78: 2053–2059 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vieira ML, et al. 2010. In vitro identification of novel plasminogen-binding receptors of the pathogen Leptospira interrogans. PLoS One 5: e11259 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vieira ML, Vasconcellos SA, Goncales AP, de Morais ZM, Nascimento AL. 2009. Plasminogen acquisition and activation at the surface of leptospira species lead to fibronectin degradation. Infect. Immun. 77: 4092–4101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang X, Coleman AS, Anguita J, Pal U. 2009. A chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog. 5: e1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang X, et al. 2010. Characterization of unique regions of Borrelia burgdorferi surface-located membrane protein 1. Infect. Immun. 78: 4477–4487 . [DOI] [PMC free article] [PubMed] [Google Scholar]