Abstract
Edema disease (ED) in piglets is caused by Shiga toxin Stx2e-producing Escherichia coli. We show that a genetically disarmed Stx2e toxoid is a safe antigen that generates antiserum protecting piglets against the Stx2e toxin. Immunization of suckling piglets with the Stx2e toxoid was safe, had no adverse effects on growth of the piglets, and resulted in effective prevention of edema disease clinical symptoms after challenge with the Stx2e toxin. Our data showed that maternal immunity against the Stx2e toxoid can be transmitted from the vaccinated sows to the piglets via the colostrum. Very high levels of Stx2e-specific serum antibodies persisted in these piglets until 1 month postweaning, bridging the critical period in which the weaned piglets are most susceptible to edema infection. Challenge with Stx2e toxin resulted in clinical signs of edema disease and death of all control piglets from nonimmunized sows, whereas none of the piglets from immunized sows developed clinical signs of ED.
INTRODUCTION
Edema disease (ED), a frequently fatal disease in newly weaned piglets, is caused by Shiga toxin-producing Escherichia coli (STEC). The Shiga toxins (Stx) are assembled protein toxins consisting of one enzymatically active A subunit and five receptor binding B subunits. The A subunit has N-glycosidase activity that removes a specific adenine base from the 28S rRNA of the 60S ribosomal subunits, shutting down protein synthesis in the targeted cells. The B subunit of Stx2e binds to globotetraosylceramide present on the surface of host cells. Signs of edema include swollen eyelids, ataxia, recumbence, convulsions, paralysis, or sudden death (13). Damage to the vascular endothelium results in edema, hemorrhage, and microthrombosis and ultimately results in high mortality in STEC-infected pigs (5, 17, 20). These STEC strains mediate colonization via F18 fimbriae (12, 24). The F18 fimbrial adhesin recognizes and binds to specific glycosphingolipids, having blood group ABH determinants on the type 1 core present on enterocytes of weaned piglets (4).
ED is distributed worldwide (1), and the STEC causative agent has been extensively characterized (1, 6, 8, 18, 21). Although control of ED is mainly based on antimicrobial therapy, it comes usually too late for piglets with visible clinical signs, as toxin has been produced in the gut with uptake and systemic spread throughout the body. Moreover, excess use of antibiotics in swine production and the increase of antibiotic-resistant porcine STEC isolates are becoming a major concern.
Therefore, different approaches for immunoprophylaxis against ED have been attempted. Passive transfer by injecting piglets with Stx2e-specific antiserum or feeding them with spray-dried sow blood plasma or egg yolk of chickens immunized with F18 showed evidence of protection against colonization and spread of the toxin in the body (14, 16, 22). Immunization of piglets with F18 could not induce effective protection against F18+ E. coli colonization (25). Oral administration of an Stx2e mutant-producing E. coli strain to piglets induced protective immunity against challenge with the wild-type Stx2e E. coli strain. However, the applicability of this approach is limited since the Stx2e mutant strain has to be administered daily within a capsule for 4 successive days (19). Conversely, several studies have shown protection of piglets against edema disease by immunization with inactivated Stx2e toxin (2, 11, 15, 16). However, to date no commercial vaccine or effective therapy exists to treat edema disease.
In this study, we used an Stx2e toxin double mutant, altered in the A subunit at two amino acid positions (Y77S, E167Q) in the enzymatic active site, to immunize piglets actively and/or passively. Immunizing piglets with that toxoid induced high levels of anti-Stx2e antibodies and protected all weaned piglets against Stx2e toxin challenge. Immunizing pregnant sows induced high levels of anti-Stx2e antibodies in serum and protected offspring piglets through colostrum uptake against Stx2 toxin without any clinical signs.
MATERIALS AND METHODS
Stx2e toxin and Stx2e toxoid production.
Total genomic DNA from E. coli strain 107/86 (12) was used as template to amplify the wild-type Stx2e operon (pEXP133) (see the supplemental material). Site-specific mutations (Y77S and E167Q) were introduced in the stx2eA gene of the stx2e operon (pEXP132) using two consecutive, overlap extension PCRs (see the supplemental material).
The Stx2e toxin and Stx2e toxoid (E167Q, Y77S) were purified from E. coli strains C43 DE3(pExp133) and C43 DE3(pEXP132), respectively (see the supplemental material). The average yields of purified Stx2e toxin or toxoid were around 1 mg per liter of induced culture.
In vitro cytotoxicity of the Stx2e toxin or Stx2e toxoid was tested on Vero cells (10). The Stx2e toxin was highly cytotoxic for Vero cells with a 50% cytotoxicity level (CD50) for Vero cells of 2.4 pg/ml. In contrast, the double mutant Stx2e toxoid did not show any toxic effect on Vero cells, even when used undiluted. Moreover, protein A-purified anti-Stx2e antibodies protected the Vero cells against the action of the Stx2e toxin at 24 pg/ml (10× CD50) and did not affect the growth of the Vero cells, while the same dilution of toxin without antibody killed all Vero cells.
The in vivo toxicity assay was performed on mice by intraperitoneal injection with 1 μg of the purified Stx2e toxin. All mice died upon injection with the toxin while all mice injected with the same dose of the purified Stx2e toxoid survived without showing any clinical symptoms.
Experimental animals.
The piglets and sows in this study were raised on a conventional farm or in experimental isolation units of the Zootechnical Centre of the Katholieke Universiteit Leuven. All animal experiments were carried out in accordance with the guidelines of the Ethical Committee of the Katholieke Universiteit Leuven and approved by the Ethical Committee of the Katholieke Universiteit Leuven.
Toxicity testing of Stx2e toxin for piglets.
In order to define the appropriate doses for toxin challenge of piglets, the toxicity of our Stx2e toxin was tested on piglets. Initially, blood samples were collected from crossbred (Hypor × Piétrain) piglets and tested for Stx2e antibodies. Accordingly, six piglets (35 days of age) that had low levels of serum Stx2e antibodies were selected for experimental injection with the Stx2e toxin. Two piglets per group (groups I, II, and III) were injected intravenously with a different dose of the Stx2e toxin: 5 ng/kg, 50 ng/kg, and 500 ng/kg of body weight, respectively. These doses of the toxin were estimated based on the CD50 on Vero cells. Animals were monitored for clinical signs, including edema of eyelids and face, ataxia, recumbence, convulsion, padding legs, paralysis, dyspnea, or sudden death, for 7 days postinjection.
Immunization and challenge of piglets.
Seven days before the actual experiment, blood samples were collected from 5 sows (Zootechnical Centre, Katholieke Universiteit Leuven) and examined for antibodies against Stx2e. Nine crossbred (Hypor × Piétrain) piglets delivered from the 2 sows that had low levels of Stx2e antibodies were selected for the experiment. Those piglets were divided into 2 groups: an immunization group (n = 6) and a control group (n = 3). In the immunization group, the piglets were immunized intramuscularly at 13 and 26 days of age with 50 μg and 75 μg Stx2e toxoid per piglet, respectively. The immunizing solution was prepared by mixing the Stx2e toxoid saline solution (1 mg/ml) with an equal volume of incomplete Freund's adjuvant (IFA) (Sigma). The control piglets received a suspension of saline and IFA in the same manner. The piglets were weaned at 30 days of age and transferred to the experimental isolation unit.
At 40 days of age, the piglets in both groups were challenged intravenously with 50 ng Stx2e toxin/kg of body weight and observed for the clinical signs of ED for 7 days. During the follow-up period, piglets were euthanized after recumbence for 2 days or at 7 days postchallenge. The lesions were recorded, focusing on edema of stomach (cardiac and pyloric), mesentery (small intestinal, spiral colon, and terminal colon), and mesenteric lymph nodes and hemorrhages in stomach wall, ileum, spiral colon, terminal colon, and cecum.
Blood samples were collected for Stx2e-specific antibody analysis immediately before immunization, 13 days after the first immunization (26 days of age), 10 days after the second immunization (36 days of age), and 7 days after toxin challenge (47 days of age).
The piglets were weighed at 14, 26, and 36 days of age.
Immunization of sows and challenge of offspring piglets.
Prior to the experiment, 10 pregnant sows, breed Hypor, around 2 years old were bled to check for Stx2e antibodies. The sows with low serum levels of Stx2e-specific antibodies were selected for the experiment.
Three sows (immunization group) were intramuscularly administered 200 and 250 μg Stx2e toxoid 5 and 3 weeks prepartum, respectively. The immunizing solution was prepared by mixing Stx2e toxoid solution (1 mg/ml) in saline with an equal volume of IFA (Sigma). One of the 2 sows in the control group was placebo immunized with 2 ml of an IFA-saline suspension while the other was left unimmunized. Blood samples were collected from immunized and control sows for Stx2e-specific antibody analysis before immunization, 14 days after the first immunization, and 28 days after the second immunization (4 days postpartum).
Piglets delivered from immunized sows and nonimmunized sows were bled at 4, 14, 24, 39 (13 days after weaning), and 53 (27 days after weaning) days of age for serum Stx2e-specific antibody evaluation. Each time, 6 piglets (2 piglets/sow, mostly different piglets) from the immunized sows and 6 (only 2 for day 4 of age) piglets (2 piglets/sow, mostly different piglets) from nonimmunized sows were selected.
At weaning (26 days of age), 9 piglets born to immunized sows (3 piglets/sow) and 6 piglets born to nonimmunized sows (2 piglets/sow) were randomly selected and transported to the experimental isolation unit for toxin challenge. Five days after weaning (31 days of age), they were challenged by intravenous injection of 65 ng Stx2e toxin/kg of body weight and observed for 8 days for clinical signs of ED. The piglets were euthanized after 2 days of recumbence or at 8 days after challenge. The clinical signs and lesions were recorded as before. Blood samples were collected from the piglets just before euthanasia (39 days of age) for Stx2e-specific antibody analysis.
Indirect ELISA for Stx2e-specific pig antibodies.
An indirect enzyme-linked immunosorbent assay (ELISA) was developed to detect Stx2e-specific antibodies in serum. Blood was taken from the jugular vein. After 18 h of incubation at room temperature, serum was collected, inactivated at 56°C for 30 min, and treated with kaolin (1 volume of serum was mixed in 4 volumes of 25% kaolin) for 30 min at room temperature.
Stx2e toxoid was applied as a coating at a concentration of 2 μg/ml in coating buffer (carbonate-bicarbonate buffer, pH 9.6) on a 96-well microtiter plate. After 2 h of incubation at 37°C, the remaining binding sites were blocked overnight at 4°C with phosphate-buffered saline (PBS) supplemented with 0.2% (vol/vol) Tween 80. The serum was diluted 1/40 in ELISA dilution buffer (PBS plus 0.05% [vol/vol] Tween 20 plus 3% [wt/vol] bovine serum albumin [BSA]). The diluted serum samples were applied and incubated for 1 h at 37°C. Thereafter, an optimal dilution of conjugate (goat anti-pig IgG horseradish peroxidase [HRP] from Bethyl Laboratories, Inc.) in dilution buffer was added to the wells for 1 h at 37°C. The optical density (OD) was measured at 405 nm in an ELISA plate reader after addition of ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] solution for 20 min.
Statistical analysis.
Data analyses were performed using the statistical analysis system (SAS Institute, Inc., Cary, NC). The mean of antibody OD values, the weight, and average daily weight gain (DWG) between the immunization and control group were evaluated by Tukey's multiple comparison test. Significant differences are reported at a 95% confidence level.
RESULTS
Toxicity testing of the Stx2e toxin on piglets.
Three groups (I, II, and III) of piglets were injected intravenously with 5 ng, 50 ng, and 500 ng of Stx2e toxin/kg of body weight, respectively. One day after toxin injection, all piglets of group III died without clinical signs. In group II, piglets had clinical signs of ED and all piglets died after 3 days. Piglets of group I remained normal for 4 days after toxin injection. From these data, a toxin dose of 50 ng Stx2e toxin/kg body weight was selected for challenge.
Immunization and challenge of piglets. (i) Weight gain.
To evaluate whether Stx2e toxoid immunization had negative effects on growth of piglets, the average weight and daily weight gain (DWG) were measured in the immunization and control groups after immunization with Stx2e toxoid. The results are summarized in Table 1.
Table 1.
Weight gain of immunized and nonimmunized pigletsa
| Piglet group | Day 14, wt | Day 26 |
Day 36 |
||
|---|---|---|---|---|---|
| Wt | DWG | Wt | DWG | ||
| Immunized (n = 6) | 5.02 ± 0.17 | 8.61 ± 0.39 | 0.30 ± 0.02 | 10.59 ± 0.60 | 0.25 ± 0.02 |
| Control (n = 3) | 4.95 ± 0.09 | 8.13 ± 0.12 | 0.27 ± 0.01 | 9.95 ± 0.69 | 0.23 ± 0.03 |
| P | >0.78 | >0.44 | >0.15 | >0.53 | >0.50 |
Data are mean weights (kg) and daily weight gains (DWG; kg/day) of piglets (±SEM) at 14, 26, and 36 days of age. Day 14 is 1 day after first immunization, day 26 is the day of booster immunization (13 days after first immunization), and day 36 is 10 days after booster immunization.
The initial weights (just a day after the first immunization) were almost similar between the two groups. Thirteen days after the first immunization (26 days of age) and 10 days after booster immunization (36 days of age), no statistical differences between the groups were observed. Similarly, the DWGs did not differ significantly between the two groups.
(ii) Stx2e antibody response in piglets after immunization with Stx2e toxoid.
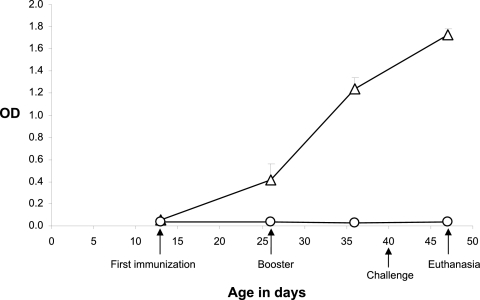
Before immunization with Stx2e toxoid, Stx2e-specific antibody levels in sera of experimental piglets were low. The levels of Stx2e-specific antibodies started rising after primary immunization and were significantly higher than those in the control group at 10 days after booster immunization when piglets had been weaned for 6 days (36 days of age) (P < 0.0001). Furthermore, Stx2e antibody responses of immunized piglets continued to increase 7 days after challenge with Stx2e toxin (47 days of age or 17 days postweaning) (Fig. 1). The OD values of Stx2e antibodies for the control group remained consistently low during the whole experiment (Fig. 1).
Fig 1.
Mean OD value of Stx2e-specific antibodies (±SEM) in serum of piglets at 13 days (first immunization), 26 days (booster immunization), 36 days (10 days after the booster immunization), and 47 days (7 days after challenge) of age. ▵, immunization group (n = 6); ○, control group (n = 3). Piglets were weaned at the age of 30 days.
(iii) Clinical signs and gross lesions after challenge.
Ten days after weaning (40 days of age), all piglets were challenged with Stx2e toxin. During 7 days postchallenge, none of the 6 piglets in the immunization group developed clinical signs of ED. In contrast, all control piglets had clinical signs of ED: one piglet died 1 day postchallenge, another one showed distinct clinical signs of ED and was euthanized after 2 days of recumbence, and the remaining piglet had mild signs of ED with slight edema of eyelids and mild ataxia for 2 days. The latter and all piglets of the immunization group were euthanized 7 days after challenge. Postmortem examination was done in all piglets. Hemorrhages in ileum, spiral colon, cecum, and terminal colon were observed in the 3 piglets of the control group, and edema of mesentery and mesenteric lymph node was present in 2 piglets of the control group. In the immunization group, only slight hemorrhages in the cecum of 3 of the 6 piglets were seen.
Immunization of sows and challenge of offspring piglets. (i) Stx2e maternal antibodies in sows.
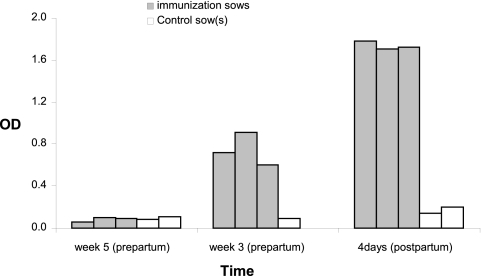
Pregnant sows with low levels of serum Stx2e antibodies were immunized with Stx2e toxoid at 5 weeks prepartum and boosted 2 weeks thereafter. Following the first immunization, high levels of specific Stx2e antibodies were detected in serum of all immunized sows at 3 weeks prepartum but not in serum from a control sow (Fig. 2). Twenty-eight days after the booster immunization (4 days postpartum), immunized sows had OD values for serum Stx2e-specific antibodies 10 times higher than those of control sows (Fig. 2). Thus, the Stx2e toxoid induced significantly high levels of maternal Stx2e-specific antibodies in the sows after two immunizations with the Stx2e toxoid (P < 0.05).
Fig 2.
Presence of maternal Stx2e antibodies in serum of sows 5 weeks prepartum (time of first immunization), 3 weeks prepartum (time of booster immunization and 14 days after the first immunization), and 4 days postpartum.
(ii) Anti-Stx2e antibodies in offspring piglets.
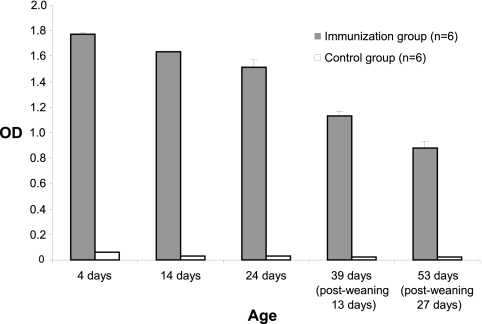
Piglets from Stx2e toxoid-immunized and nonimmunized sows were randomly bled for serum Stx2e-specific antibody analysis. As shown in Fig. 3, all piglets (unchallenged) born to immunized sows had very high ODs for Stx2e-specific serum antibodies until 27 days postweaning (53 days of age). In contrast, piglets born to nonimmunized sows persistently displayed low levels of Stx2e serum antibodies until termination of the study.
Fig 3.
Mean OD value of Stx2e antibodies in serum of unchallenged piglets from immunized sows (immunization group; n = 6) and nonimmunized sows (control group; n = 6; n = 2 only for 4 days of age).
(iii) Protection of offspring piglets after challenge with Stx2e toxin.
After challenge with Stx2e toxin, all 6 piglets born to nonimmunized sows developed typical clinical signs of ED and all died after 2 days of challenge. Gross lesions with edema in mesentery and mesenteric lymph node and mild hemorrhages of spiral colon, cecum, and terminal colon were observed in all these piglets. In contrast, none of the 9 piglets derived from Stx2e-immunized sows had clinical signs of ED. At 8 days postchallenge (39 days of age), these piglets were euthanized and only slight hemorrhages in cecum and spiral colon were present in 5 of the 9 piglets of this group. At the moment of euthanasia, blood samples were collected. Analysis of the serum samples showed that the mean OD value of Stx2e serum antibodies of these piglets (1.13 ± 0.05 [standard error of the mean {SEM}]) was similar to that of the age-matched nonchallenged piglets of the same nests of the immunized sows (with the mean OD value of 1.13 ± 0.04 [SEM]).
DISCUSSION
In the present study, we constructed a genetic mutant toxin Stx2e (Y77S, E167Q), designated Stx2e toxoid, and we demonstrated its protective efficacy in both active and passive immunization against challenge with the Stx2e toxin. We obtained high yields of pure Stx2e toxoid and toxin by a single ion-exchange chromatography purification protocol. The pure Stx2e toxoid was safe in mice and rabbits and generated anti-Stx2e toxoid polyclonal antiserum that protects Vero cells against the purified Stx2e toxin. This purified Stx2e toxin killed all the mice, while the Stx2e toxoid did not induce any disease symptoms.
In a first experiment of our study, the Stx2e toxoid was used to immunize suckling piglets. In accordance with the results of Johansen et al. (15), Stx2e toxoid immunization induced strong antibody responses as indicated by high ELISA OD values in 1:40-diluted serum samples. Moreover, immunization with the toxoid effectively prevented development of clinical signs and lesions in pigs after challenge with Stx2e toxin. Moreover, the toxoid immunization itself had no negative effects on production as no significant differences in body weight and daily weight gain had been observed between control and immunized piglets. Therefore, in agreement with the previous reports (2, 15, 16), we demonstrated that the Stx2e toxoid immunization protected against ED.
ED mainly occurs within the first 2 weeks postweaning with high mortality among infected piglets (1). Although there are a number of reports indicating protective efficacy of Stx2e vaccination in piglets, to date no commercial vaccine is available. Stx2e-producing E. coli strains are frequently isolated from feces of late finisher pigs and sows (7). High seroprevalence for Stx2e-positive E. coli in breeding sows (unpublished data) might result in mild passive protective immunity for preweaned piglets but might impair active vaccination of the piglets. On the other hand, natural passive immunity was noted to decline usually rapidly below protective levels after weaning (3). The results of field trials with the Stx2e toxoid vaccination from Bosworth and colleagues (2) showed that serum antibody responses after vaccination varied among piglets and were lower than those obtained in another study in which vaccinated piglets were selected from the Stx2e antibody-negative ones, although vaccination protocols in these two studies were identical. Moreover, the immune system of piglets at birth is functionally not completely mature and several weeks are needed for it to become fully developed (9). All these factors could account for constraints in the development of active vaccination of piglets against ED in the field because, ideally, such a vaccine should provide protection at an early susceptible age, i.e., around weaning and prior to a decline of their passive systemic immunity.
Maternal vaccination of sows has been used in the field to protect piglets and pigs from neonatal colibacillosis, necrotizing diarrhea, erysipelas, atrophic rhinitis, swine influenza, and Aujesky's disease (23). The results of our study disclosed for the first time that maternal passive immunity derived from the sows immunized with Stx2e toxoid can protect weaned piglets from lethal and disease effects of Stx2e toxin. In fact, after immunization with the Stx2e toxoid of the pregnant sows, high levels of specific Stx2e antibodies developed in all immunized sows. Low levels of Stx2e antibody were present in nonimmunized sows throughout the study. Moreover, significantly higher levels of specific Stx2e antibodies were detected in the piglets from the Stx2e toxoid-immunized sows and persisted for at least 1 month postweaning, indicating that maternal antibodies had successfully been transferred. Challenge with Stx2e toxin led to clinical signs of ED and death in all control piglets, whereas none of the piglets from immunized sows developed clinical signs of ED.
Our results revealed the applicable alternative approach of maternal passive immunity against ED by passive protection of piglets: immunizing pregnant sows could overcome the problems of natural passive immunity interference with active vaccination and immunological immaturity of the neonate. High levels of passive Stx2e antibodies in serum persisted 1 month postweaning and provide sufficient immunity to piglets for protection against ED. Additionally, sow vaccination versus piglet vaccination reduces cost and labor.
In conclusion, it was possible to demonstrate that active immunization with the Stx2e toxoid induced strong immune responses in piglets and sows, as well as providing high levels of passive antibodies in piglets. The latter was confirmed by complete protection of the piglets from lethal doses of Stx2e toxin. The findings of our study look promising for combating ED in the pig.
Supplementary Material
ACKNOWLEDGMENTS
Thi Kim Nguyen Oanh was supported by a Ph.D. grant from the Vlaamse Interuniversitaire Raad (VLIR)-Belgium. The project was funded by the VLIR-OI project on porcine colibacillosis in Vietnam.
We thank Bert Driessen for technical assistance in bleeding the animals.
Footnotes
Published ahead of print 14 November 2011
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Bertschinger HU. 1999. Postweaning Escherichia coli diarrhea and edema disease, p 441–454 In Straw BE, D'Allaire S, Mengeling WL, Taylor DJ. (ed), Diseases of swine. Iowa State University Press, Ames, IA: [Google Scholar]
- 2. Bosworth BT, et al. 1996. Vaccination with genetically modified Shiga-like toxin IIe prevents edema disease in swine. Infect. Immun. 64: 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butler J, et al. 2009. The piglet as a model for B cell and immune system development. Vet. Immunol. Immunopathol. 128: 147–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coddens A, et al. 2009. Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli. J. Biol. Chem. 284: 9713–9726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cornick NA, Matise I, Samuel JE, Bosworth BT, Moon HW. 2000. Shiga toxin-producing Escherichia coli infection: temporal and quantitative relationships among colonization, toxin production, and systemic disease. J. Infect. Dis. 181: 242–251 [DOI] [PubMed] [Google Scholar]
- 6. da Silva AS, Valadares GF, Penatti MP, Brito BG, da Silva Leite D. 2001. Escherichia coli strains from edema disease: O serogroups, and genes for Shiga toxin, enterotoxins, and F18 fimbriae. Vet. Microbiol. 80: 227–233 [DOI] [PubMed] [Google Scholar]
- 7. Fratamico PM, Bagi LK, Bush EJ, Solow BT. 2004. Prevalence and characterization of Shiga toxin-producing Escherichia coli in swine feces recovered in the National Animal Health Monitoring System's Swine 2000 study. Appl. Environ. Microbiol. 70: 7173–7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frydendahl K. 2002. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet. Microbiol. 85: 169–182 [DOI] [PubMed] [Google Scholar]
- 9. Gaskins HR. 1998. Immunological development and mucosal defence in the pig intestine, p 81–102 In Wiseman J, Varley MA, Chadwick JP. (ed), Progress in pig science. Nottingham University Press, Nottingham, United Kingdom [Google Scholar]
- 10. Gentry MK, Dalrymple JM. 1980. Quantitative microtiter cytotoxicity assay for Shigella toxin. J. Clin. Microbiol. 12: 361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gordon VM, Whipp SC, Moon HW, O'Brien AD, Samuel JE. 1992. An enzymatic mutant of Shiga-like toxin II variant is a vaccine candidate for edema disease of swine. Infect. Immun. 60: 485–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imberechts H, et al. 1992. Characterization of F107 fimbriae of Escherichia coli 107/86, which causes edema disease in pigs, and nucleotide sequence of the F107 major fimbrial subunit gene, fedA. Infect. Immun. 60: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imberechts H, De Greve H, Lintermans P. 1992. The pathogenesis of edema disease in pigs. A review. Vet. Microbiol. 31: 221–233 [DOI] [PubMed] [Google Scholar]
- 14. Imberechts H, Deprez P, Van Driessche E, Pohl P. 1997. Chicken egg yolk antibodies against F18ab fimbriae of Escherichia coli inhibit shedding of F18 positive Escherichia coli by experimentally infected pigs. Vet. Microbiol. 54: 329–341 [DOI] [PubMed] [Google Scholar]
- 15. Johansen M, et al. 1997. Prevention of edema disease in pigs by vaccination with verotoxin 2e toxoid. Can. J. Vet. Res. 61: 280–285 [PMC free article] [PubMed] [Google Scholar]
- 16. MacLeod DL, Gyles CL. 1991. Immunization of pigs with a purified Shiga-like toxin II variant toxoid. Vet. Microbiol. 29: 309–318 [DOI] [PubMed] [Google Scholar]
- 17. MacLeod DL, Gyles CL, Wilcock BP. 1991. Reproduction of edema disease of swine with purified Shiga-like toxin-II variant. Vet. Pathol. 28: 66–73 [DOI] [PubMed] [Google Scholar]
- 18. Mainil JG, Jacquemin E, Pohl P, Kaeckenbeeck A, Benz I. 2002. DNA sequences coding for the F18 fimbriae and AIDA adhesin are localised on the same plasmid in Escherichia coli isolates from piglets. Vet. Microbiol. 86: 303–311 [DOI] [PubMed] [Google Scholar]
- 19. Makino SI, et al. 2001. Genetically modified Shiga toxin 2e (Stx2e) producing Escherichia coli is a vaccine candidate for porcine edema disease. Microb. Pathog. 31: 1–8 [DOI] [PubMed] [Google Scholar]
- 20. Matise I, Cornick NA, Samuel JE, Moon HW. 2003. Binding of Shiga toxin 2e to porcine erythrocytes in vivo and in vitro. Infect. Immun. 71: 5194–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niewerth U, et al. 2001. The AIDA autotransporter system is associated with F18 and Stx2e in Escherichia coli isolates from pigs diagnosed with edema disease and postweaning diarrhea. Clin. Diagn. Lab. Immunol. 8: 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nollet H, Deprez P, Van Driessche E, Muylle E. 1999. Protection of just weaned pigs against infection with F18 Escherichia coli by non-immune plasma powder. Vet. Microbiol. 65: 37–45 [DOI] [PubMed] [Google Scholar]
- 23. Pravieux JJ, Poulet H, Charreyre C, Juillard V. 2007. Protection of newborn animals through maternal immunization. J. Comp. Pathol. 137 (Suppl. 1): S32–S34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smeds A, et al. 2001. Characterization of the adhesin of Escherichia coli F18 fimbriae. Infect. Immun. 69: 7941–7945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verdonck F, et al. 2007. Mucosal immunization of piglets with purified F18 fimbriae does not protect against F18+ Escherichia coli infection. Vet. Immunol. Immunopathol. 120: 69–79 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.