Abstract
We report here a novel selectable marker for the hyperthermophilic crenarchaeon Sulfolobus islandicus. The marker cassette is composed of the sac7d promoter and the hmg gene coding for the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (Psac7d-hmg), which confers simvastatin resistance to this crenarchaeon. The basic plasmid vector pSSR was constructed by substituting the pyrEF gene of the expression vector pSeSD for Psac7d-hmg with which the Sulfolobus expression plasmids pSSRlacS, pSSRAherA, and pSSRNherA were constructed. Characterization of Sulfolobus transformants carrying pSSRlacS indicated that the plasmid was properly maintained under selection. High-level expression of the His6-tagged HerA helicase was obtained with the cells harboring pSSRAherA. The establishment of two efficient selectable markers (pyrEF and hmg) was subsequently exploited for genetic analysis. A herA merodiploid strain of S. islandicus was constructed using pyrEF marker and used as the host to obtain pSSRNherA transformant with simvastatin selection. While the gene knockout (ΔherA) cells generated from the herA merodiploid cells failed to form colonies in the presence of 5-fluoroorotic acid (5-FOA), the mutant cells could be rescued by expression of the gene from a plasmid (pSSRNherA), because their transformants formed colonies on a solid medium containing 5-FOA and simvastatin. This demonstrates that HerA is essential for cell viability of S. islandicus. To our knowledge, this is the first application of an antibiotic selectable marker in genetic study for a hyperthermophilic acidophile and in the crenarchaeal lineage.
INTRODUCTION
Organisms of Sulfolobus genus (9) are hyperthermophilic acidophiles thriving in hot springs of high temperature and low pH worldwide. These microbes belong to the crenarchaeal branch of the archaeal domain and serve as model organisms for research of metabolic pathways, transcription, translation, and replication in archaea (33). Numerous biochemical and structural studies have been conducted on Sulfolobus proteins (13, 26, 29, 30, 35) since the publication of the first Sulfolobus genome (32), and these studies have yielded important insights into the molecular mechanisms for the third domain of life. Sulfolobus is also an important model in geomicrobiological study for which genome sequences have been determined for seven strains isolated from hot springs in the United States and Russia (28). Moreover, tools for genetic analysis have been developed for three Sulfolobus species (21), including S. solfataricus, S. acidocaldarius, and S. islandicus, allowing in vivo functional analysis of diverse genes to be conducted (2, 12, 14, 31, 36, 38).
However, all published genetic tools for Sulfolobus species to date rely on the use of an auxotrophic mutant as the host, which is either deficient in pyrimidine synthesis (uracil auxotroph) or in lactose utilization. Genetic selection is then inferred either by the expression of pyrEF coding for orotate phosphoribosyltransferase and orotidine-5′-monophosphate decarboxylase (changing uracil auxotroph to prototroph) or by the expression of lacS coding for β-glycosidase (allowing lactose-dependent growth). In contrast, antibiotic selection represents a general marker that allows genetic analysis to be conducted independent of an auxotrophic mutant.
For Sulfolobus species, it has been reported that a few antibiotics, including chloramphenicol, carbomycin, and streptomycin, influence its growth (27), but these findings have not been exploited for developing genetic selection. Two other general genetic markers were tested at an early stage of Sulfolobus genetic study. These are selection based on the overexpression of an alcohol dehydrogenase gene (5) and selection for hygromycin resistance (10). Unfortunately, these systems lack reproducibility and therefore have not been further developed.
Interestingly, antibiotic selection has successfully been developed for a few archaeal species. This antibiotic marker is based on mevinolin and its derivative simvastatin that are competitive inhibitors of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, an enzyme that is involved in archaeal membrane synthesis (25). Mevinolin was first shown to confer effective genetic selection to haloarchaea (8, 19, 20). Subsequently, its derivative, simvastatin, was established as a selection marker for neutrophilic, hyperthermophilic euryarchaea Thermococcus kodakaraensis (25) and Pyrococcus furiosus (34). However, there has not been any report exploiting simvastatin as a selection marker for a crenarchaeon.
We describe here the construction of an overexpression cassette of an HMG-CoA reductase gene and its application as a selectable marker for shuttle vectors of S. islandicus, a hyperthermophilic, acidophilic crenarchaeon. This genetic selection has successfully been used to rescue lethal deletion mutant cells of herA coding for a bipolar DNA helicase, and this is the first demonstration of rescuing lethal mutant cells for a hyperthermophilic archaeon.
MATERIALS AND METHODS
Strains and growth conditions.
S. islandicus strains (Table 1) were cultivated at 75°C in nutrient-rich medium STV, which contained mineral salts, 0.2% (wt/vol) sucrose, 0.2% (wt/vol) tryptone, and a mixed vitamin solution as described previously (12). Uracil (20 μg/ml) was added to the medium for the cultivation of the ΔpyrEF strains. For target protein expression, 0.2% (wt/vol) sucrose was replaced by 0.2% (wt/vol) arabinose to yield the medium ATV. Tryptone was substituted for Casamino Acids to give the medium SCV for selection of uracil prototrophy, and 5-fluoroorotic acid (5-FOA) was utilized for pyrEF counter selection. The final pH value of each medium was adjusted to ∼3.3 using concentrated sulfuric acid. Phytagel (1.2% [wt/vol]) was added for solidification of the medium. Simvastatin (Hangzhou Deli Chemical, Hangzhou, China) was dissolved in ethanol and sterilized by filtration.
Table 1.
S. islandicus strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| S. islandicus strains | ||
| REY15A | Wild type | 16 |
| E233S | REY15A with ΔpyrEF ΔlacS | 12 |
| E233Sh | E233S double-crossover transformant generated with pMIDherA via herA downstream insertion, harboring the Tg arm pyrEF lacS Out-arm::In-arm | This study |
| E233S ΔherA | E233S with ΔherA, herA on the complementing plasmid pSSR | This study |
| Plasmids | ||
| pHZ2 | Sulfolobus-E. coli shuttle vector, based on pGEM3Z and pRN2, with the pyrEF marker | 12 |
| pSeSD | Expression vector, based on pHZ2 and pZC1, with core araS promoter, multiple cloning sites, and His tag coding sequence | 12, 27a |
| pSSR | Based on pSeSD, with the pyrEF gene replaced by simR at SacI-XmaI sites | This study |
| pSSRlacS | Based on pSSR, with the lacS gene and its promoter region inserted at SphI-SalI sites | This study |
| pSSRAherA | Based on pSSR, with the herA gene inserted at NdeI-SalI sites under the control of the core araS promoter | This study |
| pSSRNherA | Based on pSSRlacS, with the herA gene and its native promoter inserted at XmaI-SalI sites | This study |
| pMID | Gene knockout vector, based on pUC19, with pyrEF+lacS marker | 38 |
| pMIDherA | Based on pMID, with homologous arms In, Out, and Tg inserted for disruption of the herA locus | This study |
General DNA manipulation.
Restriction and modification enzymes were purchased from NEB (Beijing, China) or Takara (Dalian, China). EasyPfu or EasyTaq DNA polymerase from TransGen (Beijing, China) was used as a polymerase for PCR. Plasmid DNA from Escherichia coli or Sulfolobus, DNA fragments from agarose gels, and genomic DNA from Sulfolobus cells were extracted using E.Z.N.A kits (Omega, Norcross, GA). Oligonucleotide synthesis and DNA sequencing were performed by BGI (Beijing, China).
Vector construction.
E. coli strain DH5α was used for vector construction. E. coli cells were cultivated in Luria-Bertani (LB) medium at 37°C. When needed, 100 μg of ampicillin/ml was added to the medium. The plasmids used in the present study are shown in Table 1, and the oligodeoxynucleotide PCR primers used are shown in Table 2.
Table 2.
Oligonucleotides used in this study
| Primer | Sequencea (5′–3′) |
|---|---|
| sac7dp-F-SacI | TAATTGAGCTCCCCTCACTATAACT |
| sac7dp-R | TCTCTTGCATATTAGGTCAAGTTATCT |
| stohmg-F | CTTGACCTAATATGCAAGAGATAATTG |
| stohmg-R-XmaI | TATATACCCGGGATGGTTAAGTTAATT |
| lacS-F-SphI | CGTGCTGCATGCCTCCTCTTATTATTAG |
| lacS-R-SalI | TATATAGTCGACCTAGTGTTGCAAGGCAG |
| herA-F-NdeI | CGCCGCATATGATAATTGGTTATGTAATTGGTC |
| herA-R-SalI | CTAGTCGACATCACCAATTTCCGTTCCAAAG |
| NherA-F-XmaI | TAAGTTCCCGGGGTGAGTATAATAAGGTTG |
| NherA-R-SalI | CTACATGTCGACTCAATCACCAATTTCCGT |
| IN-F-MluI | GACTACGCGTGGTGATTGATGATGGTACAAA |
| IN-R-XhoI | TACTCTCGAGAAAGCATAATACCCAAAACCT |
| OUT-F-SalI | GTACCGTCGACGTAGGTGTTTGTTATGGCTCTC |
| OUT-R-MluI | CATTACGCGTTCTAGTCACGTTTGTTATTAGTCC |
| Tg-F-SphI | TACGCATGCAGATGAAGACTCAATGGATGCAGTA |
| Tg-R-ClaI | GTACCATCGATTCAATCACCAATTTCCGTTCC |
| herAC-F | CAGGATTGGAGTTATTCATCACGCT |
| herAC-R | TCGTGGTAAGTGTAGGTAAGTCCTT |
Restriction sites are indicated in boldface, and sequences for overlap PCR are underlined.
(i) Construction of the hmg overexpression cassette.
Using the genomic DNA of S. acidocaldarius as the template, the sac7d (saci_0064) promoter region (−294 to −1) was amplified with the primers sac7dp-F-SacI and sac7dp-R. Using the genomic DNA of S. tokodaii as the template, the hmg gene (st1352), along with its own putative terminator was amplified with the primers stohmg-F and stohmg-R-XmaI. The primers sac7dp-R and stohmg-F have overhanging ends. The fusion of the sac7d promoter and the hmg gene was done by overlap PCR, resulting in the fusion gene Psac7d-hmg to be used as a marker cassette. The sequence of the marker cassette was confirmed by DNA sequencing.
(ii) Construction of the shuttle plasmids.
The shuttle vector pSSR was constructed by replacing the pyrEF gene on the plasmid pSeSD, an expression vector based on pHZ2 and pZC1 (12, 27a; N. Peng et al., unpublished data), with the hmg marker cassette and used for other shuttle plasmid construction. Three shuttle plasmids were then constructed: pSSRlacS, pSSRAherA, and pSSRNherA. For the first plasmid, S. solfataricus lacS gene (Sso3019) coding for a β-glycosidase was PCR amplified with primers lacS-F-SphI and lacS-R-SalI. The PCR product was digested with SphI and SalI and cloned into pSSR to generate pSSRlacS. For the expression plasmids of S. islandicus herA (SiRe_0064) encoding a bipolar DNA helicase, the gene was amplified with the primers herA-F-NdeI and herA-R-SalI. The obtained gene fragment was then digested with NdeI and SalI and ligated into pSSR to yield pSSRAherA. pSSRNherA was obtained by amplification of the herA gene with its native promoter using the primers NherA-F-XmaI and NherA-R-SalI and then ligated into pSSRlacS.
(iii) Construction of the gene disruption vector.
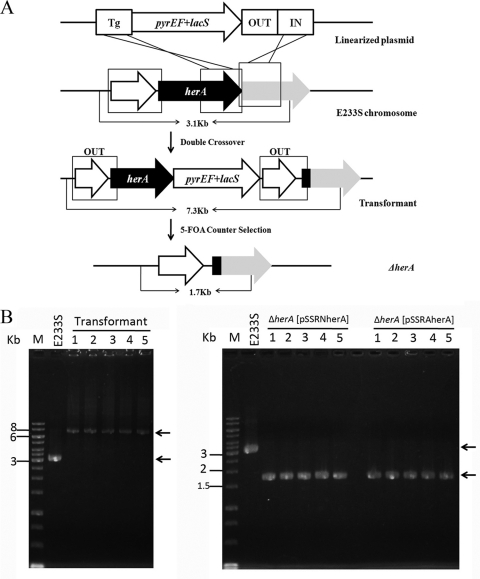
pMIDherA was constructed for herA disruption in S. islandicus as described previously (38). Three homologous sequence arms, namely, target gene (Tg) arm, integration (In) arm, and looping-out (Out) arm were amplified by PCR using the genomic DNA of S. islandicus as the template, and their respective primers. The primers Tg-F-SphI and Tg-R-ClaI were for the Tg arm, OUT-F-SalI and OUT-R-MluI were used for the Out arm, whereas IN-F-MluI and IN-R-XhoI were used for the In arm. The arrangement of these arms in the linear knockout plasmid is shown in Fig. 5A.
Fig 5.
Disruption of the chromosomal herA locus in S. islandicus. (A) Schematic diagram of the gene disruption via marker insertion and target gene deletion (MID). Double-crossover recombination between the linearized plasmid pMIDherA and the host chromosome at the In arm and the Tg arm leads to the marker insertion at the herA locus. Recombination between the two Out arms loops out herA and the marker gene, and the knockout mutants are obtained by 5-FOA counterselection. (B) PCR analyses of the herA gene alleles. Total DNAs were isolated, and PCR was performed with the primers herAC-F and herAC-R. The locations of the primers on the chromosome and the expected sizes of the corresponding products are shown in panel A. DNA size markers were run in lane M. The results of PCR with S. islandicus E233S are shown in lane E233S. The results obtained with five independent colonies of E233Sh, E233S ΔherA [pSSRNherA], or E233S ΔherA [pSSRAherA] are shown in lanes 1 to 5, respectively. The arrowheads to the right of the gels indicate the expected amplified fragments.
Transformation of S. islandicus.
S. islandicus was transformed with plasmids or linearized plasmid DNAs by electroporation as described previously (12). Cell suspension (200 to 500 μl) was plated onto the solid medium STV (uracil was added for S. islandicus E233S) in the presence of 18 μM simvastatin. Colonies appeared on plates after 7- to 8-day incubation. Single colonies were picked and transferred to another solid medium in the presence of 16 μM simvastatin. After 4 days of incubation, the colonies were transferred into liquid medium (30 ml) in the presence of 16 μM simvastatin.
X-Gal assay.
X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) treatment of the cells was performed as previously described (1).
Expression and purification of HerA-His6.
S. islandicus cells containing the shuttle vector pSSRAherA were grown in 250 ml of ATV medium (see Materials and Methods) in the presence of 14 μM simvastatin. After about 3 days of growth (optical density at 600 nm [OD600] of ∼0.7), the cells were harvested and resuspended in buffer A (50 mM Tris-HCl [pH 8.0], 100 mM NaCl). The cells were lysed by sonication on ice, and after centrifugation (10,000 × g for 10 min at 4°C), the supernatant was applied onto a Ni2+-NTA agarose column (Invitrogen, Carlsbad, CA). The column was washed with wash buffer (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 30 mM imidazole), and then the bound proteins were eluted using the elution buffer (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 300 mM imidazole). All fractions were collected and used for SDS-PAGE and Western blot analysis.
Detection of HerA-His6 by Western blot analysis.
Western blot analysis was performed using the His tag-specific antibodies. The fractions after Ni2+ affinity chromatography (obtained as described above) were subjected to SDS-PAGE and then transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, MA) at 30 mA at 4°C for 16 h. The membrane was incubated for 2 h in TBST buffer (20 mM Tris-HCl, 150 mM NaCl, 0.05% [vol/vol] Tween 20) containing 5% (wt/vol) nonfat dried milk (Solarbio, Beijing, China). After blocking, the membrane was incubated with anti-His6 antibodies (Tiangen, Beijing, China) for 2 h. Anti-His6 antibodies were diluted 1:3,000 in TBST buffer. Unbound antibodies were removed by four washes with TBST buffer for 10 min each time. After this, the membrane was incubated with a 1:5,000 dilution of horseradish peroxidase-labeled goat anti-mouse IgG (Tiangen) for 1 h. The membrane was then washed with TBST buffer six times for 5 min. The signals were visualized using an image analyzer (ImageQuant 400; GE Healthcare).
Determination of the loss frequency of pSSRNherA plasmid.
Selection for pSSRNherA in E233S[pSSRNherA] and E233SΔherA[pSSRNherA] was relieved by growth in liquid medium without simvastatin for 20 h after standard inoculation. Aliquots of cells were plated onto the solid medium in the absence of simvastatin. The plates were sprayed with X-Gal when colonies formed. White colonies and blue colonies were counted. The loss frequency of pSSRNherA was calculated as the number of white colonies divided by the total number of colonies.
RESULTS AND DISCUSSION
Effects of simvastatin on the growth of S. islandicus.
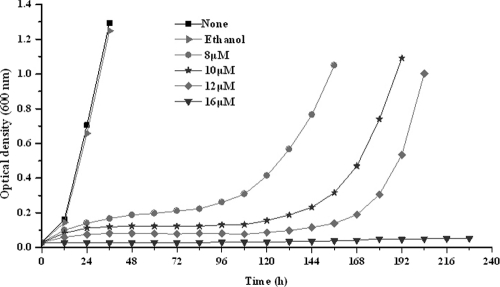
It has been reported that the antibiotic simvastatin inhibits the growth of several neutrophilic archaea (4). To determine whether simvastatin could also affect the growth of S. islandicus, a hyperthermophilic acidophile, the wild-type strain REY15A (16) was cultured in STV or in the same nutrient-rich medium supplemented either with 0.2% (vol/vol) ethanol (the solvent of simvastatin) or with various concentrations of the antibiotic (8 to 16 μM). While 0.2% ethanol did not show any effect on REY15A growth, adding 8 μM simvastatin to the medium greatly retarded the host growth and increasing the concentration to 16 μM stopped the growth completely for more than 10 days (Fig. 1). Testing the simvastatin effect on STV plates showed that 16 μM simvastatin also completely inhibited the growth of S. islandicus REY15A. We performed the same experiments with S. islandicus E233S (ΔpyrEF ΔlacS), an auxotrophic mutant derived from REY15A. Very similar growth curves were obtained (data not shown). Moreover, growth of the auxotrophic host on STV plates was again completely inhibited by 16 μM simvastatin. This indicated that simvastatin exhibited the same inhibition of the growth of S. islandicus wild-type strain and its mutant. Moreover, very similar data were obtained by studying the effect of simvastatin on the growth of S. solfataricus and S. tokodaii, two distantly related Sulfolobus species (data not shown).
Fig 1.
Growth of S. islandicus REY15A in the presence of different concentrations of simvastatin. Cells were cultivated in the nutrient-rich medium STV in the presence of 0.2% (vol/vol) ethanol and 8, 10, 12, or 16 μM simvastatin (dissolved in ethanol). The cell densities (OD600) were measured every 12 h. Each value was the mean based on at least three measurements.
sac7d-hmg fusion gene conferred efficient selection to S. islandicus.
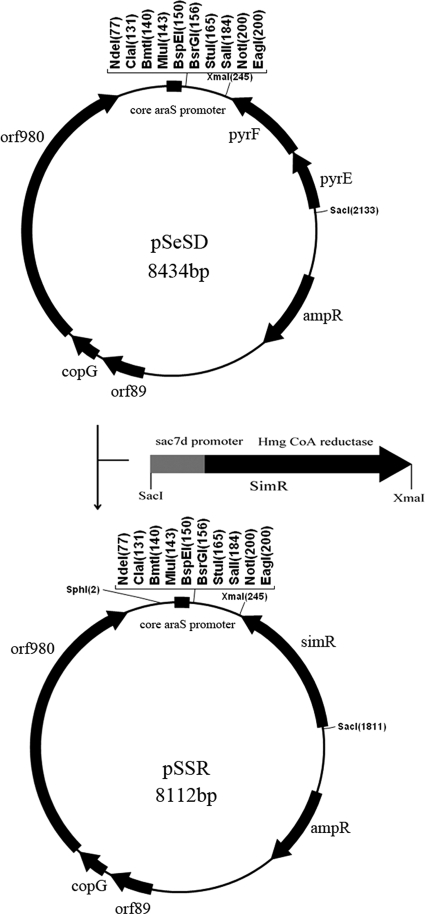
We then aimed at developing simvastatin resistance into a general selection marker for S. islandicus by strongly elevating the expression of HMG-CoA reductase as has been reported for haloarchaea (8, 19), Thermococcus (25), and Pyrococcus (34). Since the strongest constitutive promoter reported for Sulfolobus to date was that of Sac7d (saci_0064) from S. acidocaldarius (7), we fused this promoter to S. tokodaii hmg (st1352) using SOE-PCR (see Materials and Methods). This yielded the overexpression cassette Psac7d-hmg (Fig. 2).
Fig 2.
Schematic diagram of the shuttle vector pSSR. The fusion of the sac7d promoter and the coding region of the Hmg-CoA reductase gene yielded the hmg overexpression cassette Psac7d-hmg. To obtain pSSR, the pyrEF gene on pSeSD was replaced by Psac7d-hmg via SacI and XmaI restriction sites. orf980, copG, and orf89 are from the Sulfolobus plasmid pRN2.
To test whether the hmg overexpression cassette could confer simvastatin resistance to S. islandicus, an hmg-based Sulfolobus-E. coli shuttle vector pSSR was constructed (Fig. 2). This was done by substituting the pyrEF marker on pSeSD, a Sulfolobus expression vector recently constructed in our laboratory (N. Peng and Q. She, unpublished data), for the hmg overexpression cassette. The S. solfataricus lacS gene was then amplified and inserted into pSSR between the SphI and SalI sites to yield pSSRlacS (data not shown).
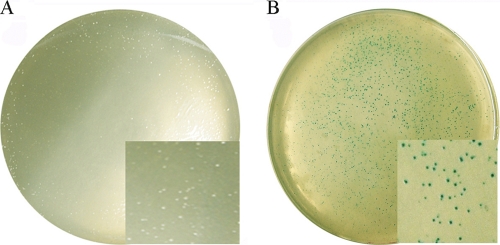
Subsequently, pSSR and pSSRlacS were used to exploit simvastatin resistance as a selection marker. Each plasmid was electroporated into S. islandicus E233S (ΔpyrEF ΔlacS) according to a procedure described previously (12). After incubation for 7 days, colonies appeared on the plates of pSSR and pSSRlacS transformants, and the transformation efficiency was ∼104 colonies/μg of DNA, which is typical for Sulfolobus plasmid transformation. In contrast, no growth was observed for the Sulfolobus cells in samples without DNA transformation. Since lacS activity could be readily detected by an X-Gal assay, the chemical was sprayed onto the colonies, followed by incubation for 1 h. As shown in Fig. 3, although pSSR transformants remained white, the pSSRlacS transformants turned blue. The fact that all colonies formed by the cells transformed with pSSRlacS were blue indicated that they all harbored the plasmid pSSRlacS. This strongly indicates that the hmg marker confers efficient selection to S. islandicus.
Fig 3.
X-Gal staining of the S. islandicus E233S transformants. Colonies of S. islandicus transformants were incubated with X-Gal for 1 h at 75°C. (A) E233S transformed with pSSR; (B) E233S transformed with pSSRlacS.
Interestingly, the genome sequences of a few other thermophilic acidophiles are available, including those of Metallosphaera (6) and Acidianus species (37), as well as Ferroplasma (3), the last of which is a euryarchaeon. Since these archaea are important organisms in biomining, it is very tempting to test for developing genetic systems for these archaea using simvastatin selection.
Overexpression of HerA helicase from pSSR plasmids.
We next tested the pSSR shuttle vector for protein overexpression in Sulfolobus. We chose bipolar DNA helicase HerA for this work. HerA is a highly conserved archaeal P-loop ATPase belonging to the FtsK-HerA superfamily (11, 18), and this protein has been implicated in both cell division and double-stranded DNA break repair (17, 22–24, 39). S. islandicus herA was cloned into pSSR to yield pSSRAherA, which was then transformed into the wild-type strain REY15A. The obtained transformants were used for protein overexpression and purification. Highly purified recombinant HerA protein was obtained by single-step Ni-affinity chromatography. The identity of the band was confirmed by Western blot analysis with His tag-specific antibodies (Fig. 4). Strikingly, ∼1.5 mg of HerA protein was obtained from 1 liter of cell culture.
Fig 4.
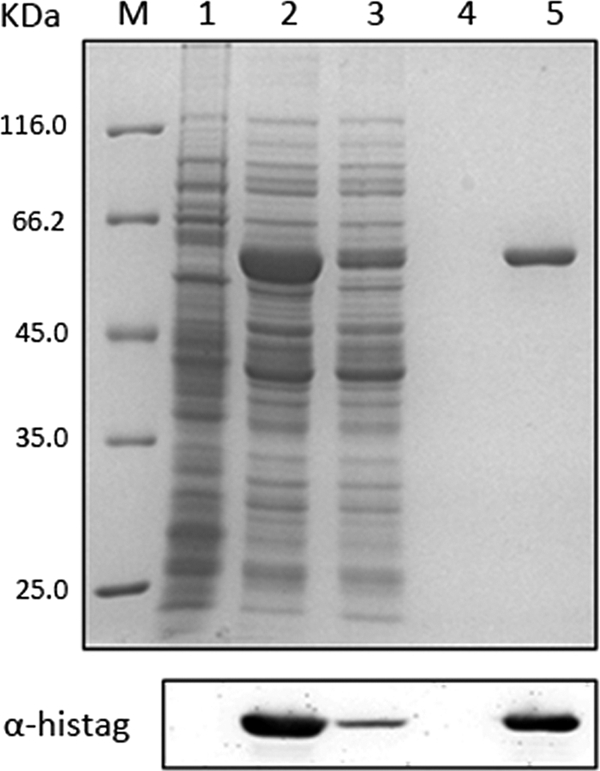
Overexpression, purification, and identification of HerA-His6. The overexpression of HerA-His6 was induced by arabinose in S. islandicus REY15A cells transformed with pSSRAherA. The Ni2+-NTA agarose column was used for the purification, and the fractions were identified by using a Coomassie blue-stained SDS-PAGE gel. The corresponding Western blot of the same samples using His tag-specific antibodies is shown in the lower panel. Lanes: M, molecular mass marker; 1, cell extracts from REY15A cells transformed with pSSR as the control; 2, with pSSRAherA; 3, flowthrough; 4, wash fraction; 5, elution fraction.
It should be noted that transformants grow more slowly in nutrient-rich medium (STV) containing simvastatin than did the wild-type strain in the absence of selection. It usually takes 3 days for E233S[pSSR] to reach an OD600 of 0.6 in the presence of simvastatin, whereas it takes only 1 day for an E233S culture to grow to the same OD without the antibiotic. Because pSSR carries the same backbone of the vector that has been shown to be stably maintained in Sulfolobus cells (12), the growth retardance must be due to the nature of simvastatin selection rather than the maintenance of the plasmid. Thus, one needs to bear this in mind when conducting genetic analysis. This feature does not appear to affect protein overexpression because a large amount of HerA enzyme has been produced using this marker (Fig. 4).
Rescuing ΔherA S. islandicus cells by genetic expression from a pSSR vector.
Our first step toward the study of HerA in vivo function was to generate its gene knockout with S. islandicus. A recently developed knockout scheme, marker insertion and unmarked target gene deletion (MID) (38), was used. As illustrated in Fig. 5A, the gene disruption procedure consists of two steps. First, double-crossover recombination between the plasmid and the host chromosome results in marker gene insertion at the target gene locus. Then, recombination between two copies of the Out arm in the transformants yields knockout mutants that are selectable with 5-FOA if the target gene is nonessential.
While transformation of the disruption plasmid pMIDherA into S. islandicus E233S did yield transformants carrying marker insertion at the herA locus (E233Sh 1 through 5, Fig. 5B, left panel), we failed to recover any herA deletion mutants at the second step, suggesting that ΔherA cells could have lost cell viability. These results are in good agreement with the genetic study of herA in T. kodakaraensis, a hyperthermophilic archaeon, in which a ΔherA mutant was not obtainable (15). We then reasoned that a more direct experiment was required to draw such a drastic conclusion.
Taking advantage of the simvastatin marker, we directly addressed herA gene essentiality by rescuing the mutant cells with pSSRNherA that carried herA with its native promoter. Transformation of E233Sh, the pMIDherA transformant, with each plasmid yielded transformants under simvastatin selection. These transformants—E233Sh strains carrying either pSSR, or pSSRNherA, or pSSRAherA—were grown and plated onto STV solid medium supplemented with 5-FOA, uracil, and 16 μM simvastatin. After 8 days of incubation, transformants harboring either pSSRNherA or pSSRAherA formed colonies, but those containing pSSR, a noncomplementing vector, did not grow, indicating that herA could only be deleted from the chromosome if HerA was to be expressed from a plasmid. Indeed, analysis of the obtained colonies by PCR with primers herAC-F and herAC-R (Fig. 5B, right panel) revealed the expected 1.7-kb DNA fragments, whereas the wild-type herA allele was absent.
Previously, it was shown that Sulfolobus plasmids based on the cryptic plasmid pRN2 were inherently unstable in the absence of selection (12). This prompted us to compare the loss frequency of pSSRNherA in E233S cells versus that in ΔherA cells. After incubation for 20 h without simvastatin selection, a loss rate of 0.52 was found for the herA complementing plasmid in the wild-type strain, i.e., more than half of the population lost the plasmid. In contrast, all 500 colonies formed appeared blue in the X-Gal assay, indicating that all ΔherA cells retained the complementing plasmid pSSRNherA. Thus, if the mutant cells lost the plasmid, they also lost the ability to grow. This reinforces the conclusion that HerA is essential for the cell viability of S. islandicus. It is thus very tempting to demonstrate the essentiality of herA gene in all (hyper)thermophilic archaea.
In conclusion, we have shown that the overexpression of the HMG-CoA reductase in S. islandicus greatly elevates the resistance of the crenarchaeal cells to simvastatin, a competitive inhibitor of the enzyme, and that the strong elevation of the antibiotic tolerance has been successfully developed as a selectable marker. The marker system is based on both the Psac7d-hmg cassette and the pRN2-derived vector. Whether the Psac7d-hmg cassette alone is sufficient to confer simvastatin resistance and can be exploited for direct gene knockout through a marker replacement approach needs further investigation, However, our results indicate that simvastatin is not only a suitable selectable marker for neutral hyperthermophiles (25, 34), it is also a suitable marker for (hyper)thermophilic acidophiles, including other Sulfolobus species, as well as other thermophilic acidophiles, such as Acidianus and Thermoplasma.
ACKNOWLEDGMENTS
We thank George Lipps for providing strain of S. acidocaldarius and Li Huang for strain of S. solfataricus. We also thank Feiyan Wang and Bin Tian for initial work in this study, Zhou Yan for technical assistance, and Wenyuan Han, Ye Hong, Yansheng Li, Mingzhu Chu, and Wenwen Li for helpful discussions.
This study was supported by grants from the National Natural Science Foundation of China (3093002 and 30870046 to Y.S. and 30770050 to J.N.) and by a grant from the Danish Free Research Council/FTP (09-062932) to Q.S.
Footnotes
Published ahead of print 11 November 2011
REFERENCES
- 1. Albers SV, Driessen AJ. 2008. Conditions for gene disruption by homologous recombination of exogenous DNA into the Sulfolobus solfataricus genome. Archaea 2:145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albers SV, et al. 2006. Production of recombinant and tagged proteins in the hyperthermophilic archaeon Sulfolobus solfataricus. Appl. Environ. Microbiol. 72:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen EE, et al. 2007. Genome dynamics in a natural archaeal population. Proc. Natl. Acad. Sci. U. S. A. 104:1883–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allers T, Mevarech M. 2005. Archaeal genetics: the third way. Nat. Rev. Genet. 6:58–73 [DOI] [PubMed] [Google Scholar]
- 5. Aravalli RN, Garrett RA. 1997. Shuttle vectors for hyperthermophilic archaea. Extremophiles 1:183–191 [DOI] [PubMed] [Google Scholar]
- 6. Auernik KS, Maezato Y, Blum PH, Kelly RM. 2008. The genome sequence of the metal-mobilizing, extremely thermoacidophilic archaeon Metallosphaera sedula provides insights into bioleaching-associated metabolism. Appl. Environ. Microbiol. 74:682–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berkner S, Wlodkowski A, Albers SV, Lipps G. 2010. Inducible and constitutive promoters for genetic systems in Sulfolobus acidocaldarius. Extremophiles 14:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blaseio U, Pfeifer F. 1990. Transformation of Halobacterium halobium: development of vectors and investigation of gas vesicle synthesis. Proc. Natl. Acad. Sci. U. S. A. 87:6772–6776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brock TD, Brock KM, Belly RT, Weiss RL. 1972. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Mikrobiol. 84:54–68 [DOI] [PubMed] [Google Scholar]
- 10. Cannio R, Contursi P, Rossi M, Bartolucci S. 1998. An autonomously replicating transforming vector for Sulfolobus solfataricus. J. Bacteriol. 180:3237–3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Constantinesco F, Forterre P, Koonin EV, Aravind L, Elie C. 2004. A bipolar DNA helicase gene, herA, clusters with rad50, mre11, and nurA genes in thermophilic archaea. Nucleic Acids Res. 32:1439–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng L, Zhu H, Chen Z, Liang YX, She Q. 2009. Unmarked gene deletion and host-vector system for the hyperthermophilic crenarchaeon Sulfolobus islandicus. Extremophiles 13:735–746 [DOI] [PubMed] [Google Scholar]
- 13. Dueber EL, Corn JE, Bell SD, Berger JM. 2007. Replication origin recognition and deformation by a heterodimeric archaeal Orc1 complex. Science 317:1210–1213 [DOI] [PubMed] [Google Scholar]
- 14. Ellen AF, Albers SV, Driessen AJ. 2010. Comparative study of the extracellular proteome of Sulfolobus species reveals limited secretion. Extremophiles 14:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujikane R, Ishino S, Ishino Y, Forterre P. 2010. Genetic analysis of DNA repair in the hyperthermophilic archaeon, Thermococcus kodakaraensis. Genes Genet. Syst. 85:243–257 [DOI] [PubMed] [Google Scholar]
- 16. Guo L, et al. 2011. Genome analyses of Icelandic strains of Sulfolobus islandicus, model organisms for genetic and virus-host interaction studies. J. Bacteriol. 193:1672–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hopkins BB, Paull TT. 2008. The Pyrococcus furiosus mre11/rad50 complex promotes 5′ strand resection at a DNA double-strand break. Cell 135:250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iyer LM, Makarova KS, Koonin EV, Aravind L. 2004. Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division, and viral capsid packaging. Nucleic Acids Res. 32:5260–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lam WL, Doolittle WF. 1992. Mevinolin-resistant mutations identify a promoter and the gene for a eukaryote-like 3-hydroxy-3-methylglutaryl-coenzyme A reductase in the archaebacterium Haloferax volcanii. J. Biol. Chem. 267:5829–5834 [PubMed] [Google Scholar]
- 20. Lam WL, Doolittle WF. 1989. Shuttle vectors for the archaebacterium Halobacterium volcanii. Proc. Natl. Acad. Sci. U. S. A. 86:5478–5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leigh JA, Albers SV, Atomi H, Allers T. 2011. Model organisms for genetics in the domain Archaea: methanogens, halophiles, Thermococcales and Sulfolobales. FEMS Microbiol. Rev. 35:577–608 [DOI] [PubMed] [Google Scholar]
- 22. Lundgren M, Bernander R. 2005. Archaeal cell cycle progress. Curr. Opin. Microbiol. 8:662–668 [DOI] [PubMed] [Google Scholar]
- 23. Makarova KS, Koonin EV. 2005. Evolutionary and functional genomics of the Archaea. Curr. Opin. Microbiol. 8:586–594 [DOI] [PubMed] [Google Scholar]
- 24. Manzan A, et al. 2004. MlaA, a hexameric ATPase linked to the Mre11 complex in archaeal genomes. EMBO Rep. 5:54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsumi R, Manabe K, Fukui T, Atomi H, Imanaka T. 2007. Disruption of a sugar transporter gene cluster in a hyperthermophilic archaeon using a host-marker system based on antibiotic resistance. J. Bacteriol. 189:2683–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGeoch AT, Trakselis MA, Laskey RA, Bell SD. 2005. Organization of the archaeal MCM complex on DNA and implications for the helicase mechanism. Nat. Struct. Mol. Biol. 12:756–762 [DOI] [PubMed] [Google Scholar]
- 27. Noll KM, Vargas M. 1997. Recent advances in genetic analyses of hyperthermophilic archaea and bacteria. Arch. Microbiol. 168:73–80 [DOI] [PubMed] [Google Scholar]
- 27a. Peng N, Xia Q, Chen Z, Liang YX, She YX. 2009. An upstream activation element exerting differential transcriptional activation an archaeal promoter. Mol. Microbiol. 74:928–939 [DOI] [PubMed] [Google Scholar]
- 28. Reno ML, Held NL, Fields CJ, Burke PV, Whitaker RJ. 2009. Biogeography of the Sulfolobus islandicus pan-genome. Proc. Natl. Acad. Sci. U. S. A. 106:8605–8610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richards JD, et al. 2008. Structure of the DNA repair helicase hel308 reveals DNA binding and autoinhibitory domains. J. Biol. Chem. 283:5118–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rouillon C, White MF. 2010. The XBP-Bax1 helicase-nuclease complex unwinds and cleaves DNA: implications for eukaryal and archaeal nucleotide excision repair. J. Biol. Chem. 285:11013–11022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schelert J, et al. 2004. Occurrence and characterization of mercury resistance in the hyperthermophilic archaeon Sulfolobus solfataricus by use of gene disruption. J. Bacteriol. 186:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. She Q, et al. 2001. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. U. S. A. 98:7835–7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. She Q, et al. 2009. Genetic analyses in the hyperthermophilic archaeon Sulfolobus islandicus. Biochem. Soc. Trans. 37:92–96 [DOI] [PubMed] [Google Scholar]
- 34. Waege I, Schmid G, Thumann S, Thomm M, Hausner W. 2010. Shuttle vector-based transformation system for Pyrococcus furiosus. Appl. Environ. Microbiol. 76:3308–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wardleworth BN, Russell RJ, Bell SD, Taylor GL, White MF. 2002. Structure of Alba: an archaeal chromatin protein modulated by acetylation. EMBO J. 21:4654–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Worthington P, Hoang V, Perez-Pomares F, Blum P. 2003. Targeted disruption of the alpha-amylase gene in the hyperthermophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 185:482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. You XY, et al. 2011. Genomic analysis of Acidianus hospitalis W1 a host for studying crenarchaeal virus and plasmid life cycles. Extremophiles 15:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang C, et al. 2010. Revealing the essentiality of multiple archaeal PCNA genes using a mutant propagation assay based on an improved knockout method. Microbiology 156:3386–3397 [DOI] [PubMed] [Google Scholar]
- 39. Zhang S, et al. 2008. Archaeal DNA helicase HerA interacts with Mre11 homologue and unwinds blunt-ended double-stranded DNA and recombination intermediates. DNA Repair (Amsterdam) 7:380–391 [DOI] [PubMed] [Google Scholar]