Abstract
Monomolecular crystalline bacterial cell surface layers (S-layers) have broad application potential in nanobiotechnology due to their ability to generate functional supramolecular structures. Here, we report that Bacillus megaterium is an excellent host organism for the heterologous expression and efficient secretion of hemagglutinin (HA) epitope-tagged versions of the S-layer protein SslA from Sporosarcina ureae ATCC 13881. Three chimeric proteins were constructed, comprising the precursor, C-terminally truncated, and N- and C-terminally truncated forms of the S-layer SslA protein tagged with the human influenza hemagglutinin epitope. For secretion of fusion proteins, the open reading frames were cloned into the Escherichia coli-Bacillus megaterium shuttle vector pHIS1525. After transformation of the respective plasmids into Bacillus megaterium protoplasts, the recombinant genes were successfully expressed and the proteins were secreted into the growth medium. The isolated S-layer proteins are able to assemble in vitro into highly ordered, crystalline, sheetlike structures with the fused HA tag accessible to antibody. We further show by fluorescent labeling that the secreted S-layer fusion proteins are also clustered on the cell envelope of Bacillus megaterium, indicating that the cell surface can serve in vivo as a nucleation point for crystallization. Thus, this system can be used as a display system that allows the dense and periodic presentation of S-layer proteins or the fused tags.
INTRODUCTION
The cell envelope of many bacterial and archaeal species is covered by surface layers (S-layers). Typically, they are composed of a single protein or glycoprotein species that can form crystalline arrays exhibiting specific lattice symmetries (34). This regular protein meshwork possesses pores which are well-defined in size and morphology. Most S-layer proteins harbor an N-terminal secretion signal peptide that allows active transport by the Sec-dependent general secretory pathway across the cytoplasmic membrane (7). In Gram-positive bacteria, the S-layers are associated with a heteropolysaccharide called secondary cell wall polymer (SCWP) (30, 35). The N-terminal parts of many S-layer proteins possess highly conserved amino acid sequences, the so-called S-layer homology (SLH) domains, that mediate attachment to the pyruvylated negatively charged SCWPs. Another binding mechanism of S-layer proteins involves a highly conserved N-terminal region comprising neither SLH domains nor SCWPs that consists of N-acetylglucosamine, glucose, and 2,3-dideoxydiacetamido mannosamine uronic acid (12, 20). The structural integrity of S-layers and their adhesion to the underlying cell envelope component are based on noncovalent forces (38). The interaction can be disrupted by high concentrations of chaotropic or metal-chelating agents (34). Upon the removal of the respective agent, the S-layer subunits recrystallize into regular arrays in suspension or on surfaces.
Due to these unique features, S-layers have broad application potentials in biotechnology, nanotechnology, and biomimetics. To date, recombinant S-layer proteins have been generated in Escherichia coli (15, 19, 25, 32), Bacillus subtilis (18, 41), Lactobacillus lactis (27), Lactobacillus casei (3), Lactobacillus brevis (1), Caulobacter crescentus (5, 26), Saccharomyces cerevisiae, and HeLa cells (6, 21).
In the case of some S-layer proteins, it was reported that the cloned genes were not stable when expressed in E. coli or that expression resulted in nonviability of E. coli transformants. Such observations were made for the S-layer proteins of Aeromonas salmonicida (9), B. brevis 47 (46), and L. brevis (43). The instability may be explained by direct repeats within the gene which may facilitate recombination or error-prone replication (9).
Here, we report on the expression of functional hemagglutinin (HA) epitope-tagged SslA derivatives of the Sporosarcina ureae ATCC 13881 S-layer in the Gram-positive Bacillus megaterium. It is commonly accepted that B. megaterium possesses S-layers in its natural environment (4, 37). Due to long term-cultivation, the laboratory strain that we use for expression lost this ability (MoBiTec, personal communication). The B. megaterium expression system may offer an alternative for the heterologous production of S-layer proteins due to several advantages over other expression systems. These include a lack of alkaline protease activities, efficient secretion of heterologous proteins into the medium, structural and segregational stability of recombinant plasmids, and the use of inexpensive substrates (42). Cloning into the E. coli-B. megaterium shuttle vector pHIS1525 allows the translational fusion of the target proteins with the secretion peptide of the extracellular esterase LipA (SPlipA), resulting in secretion of the respective proteins.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Sporosarcina ureae ATCC 13881 cells (Max-Planck Institute for Biochemistry, Martinsried, Germany) were grown at 30°C in LB medium (1% peptone, 0.5% yeast extract, 0.5% NaCl). E. coli Top10 [F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacC74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG] (Invitrogen, United States) was used for cloning of target genes. The E. coli strain was grown at 37°C in LB medium (pH 7.4) with 1.5% agar for plates containing 100 μg/ml ampicillin to select for plasmid-bearing cells. B. megaterium WH320 and MS941 (MoBiTec GmbH, Germany) were used for recombinant expression of three S-layer variants of S. ureae ATCC 13881 S-layer SslA. B. megaterium cells were cultured at 37°C in enriched LB medium (1% peptone, 0.5% yeast extract, 1% NaCl, pH 7.5) with 1.5% agar for plates supplemented with 10 μg/ml tetracycline.
Constructs and cloning.
Cloning of the S-layer fusion proteins (Fig. 1) was performed in two steps. Gene sequences encoding the full-length (amino acids [aa] 1 to 1099) recombinant SslA protein [rSslA(aa1-1099)] and its truncated variants rSslA(aa32-928) and rSslA(aa341-928) were PCR amplified from S. ureae ATCC 13881 chromosomal DNA using primers listed in Table 1. The restriction sites for NarI and SphI were introduced via PCR at the 5′ and 3′ ends, respectively. The purified PCR fragments, as well as the vector pHIS1525 (MoBiTec GmbH, Germany) containing the strong xylA promoter of B. megaterium, were digested with the restriction endonucleases NarI and SphI and cloned in frame to the lipase A secretion peptide present in the vector. For the production of proteins lacking a secretion signal, the gene sequence encoding the N- and C-terminally truncated variant iSslA(aa341-928), lacking a secretion signal (and therefore accumulating in the host cell), was cloned into the vector pHIS1522 (MoBiTec GmbH, Germany). PCR using primers listed in Table 1 introduced the restriction sites for KpnI and SphI at the 5′ and 3′ ends. After ligation, the plasmids were established in E. coli TOP10. The gene sequence encoding an HA tag was amplified by PCR (primers listed in Table 1), inserting the restriction site for SphI at the 5′ end and for AgeI at the 3′ end, respectively. PCR fragments, as well as pHIS1525 and pHIS1522 carrying the coding sequences for the SslA variants, were digested with the restriction enzymes SphI and AgeI. After ligation, pHIS1525 and pHIS1522 carrying the recombinant constructs were established in E. coli TOP10.
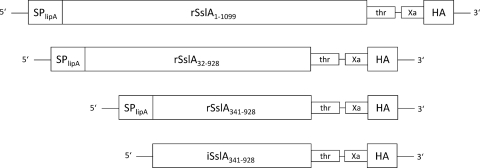
Fig 1.
Scheme of constructed chimeric genes. The chimeric genes encoding the precursor, the C-terminally truncated, and the N- and C-terminally truncated forms of the S-layer protein SslA from Sporosarcina ureae ATCC 13881 and three copies of the epitope of the hemagglutinin protein (HA) were fused via two protease recognition sites (thrombin [thr] and factor Xa) and a (Gly)4Ser linker. Cloning in the E. coli-B. megaterium shuttle vector pHIS1525 allows translational fusion with the secretion peptide of the extracellular esterase LipA (SPlipA). Intracellular accumulation of recombinant proteins is achieved by cloning of the respective reading frames in the E. coli-B. megaterium shuttle vector pHIS1522.
Table 1.
Oligonucleotide primers
| PCR fragment | Primer, direction, and sequence (5′ to 3′)a |
Cloning site | |||
|---|---|---|---|---|---|
| No. | Forward | No. | Reverse | ||
| rSslA(aa1-1099) | 1 | TAT TAT GGC GCC GCT AAC CAA CCA ACG AAA TA | 5 | TAT TAT GCA TGC CCG GAT CCA CGC GGA ACC AGT TTA GAA GTT ACT TTT ATA ACA GG | NarI SphI |
| rSslA(aa32-928) | 2 | TAT TAT GGC GCC GCT GAA TTC ACA GAT GTA AAA GAC | 6 | TAT TAT GCA TGC CCG GAT CCA CGC GGA ACC AGC GAA CTA ATA ACT AAT GCA TTT GC | NarI SphI |
| rSslA(aa341-928) | 3 | TAT TAT GGC GCC ACT GGC GTT AAA AAA GCA GGA AT | 6 | TAT TAT GCA TGC CCG GAT CCA CGC GGA ACC AGC GAA CTA ATA ACT AAT GCA TTT GC | NarI SphI |
| iSslA(aa341-928) | 4 | TAT TAT GGT ACC GGA TGA CTG GCG TTA AAA AAG CAG GAA T | 6 | TAT TAT GCA TGC CCG GAT CCA CGC GGA ACC AGC GAA CTA ATA ACT AAT GCA TTT GC | KpnI SphI |
| HA tag | 7 | TAT ATA GCA TGC CAT CGA AGG TCG TGG CGG C | 8 | TAT TAT ACC GGT CTA TTA GCG GCC GCA CTG AGC AG | SphI AgeI |
Cloning sites are underlined (NarI/KpnI at the 5′ end and SphI at the 3′ end of the coding sequence for S-layer derivatives and SphI at the 5′ end and AgeI at the 3′ end of the coding sequence for the hemagglutinin tag). Primer sequences were derived from the corrected sslA sequence (GenBank accession no. AM085153).
Expression of recombinant S-layers.
The transformation of B. megaterium protoplasts was performed according to the manufacturer's instructions (MoBiTec). The transformed B. megaterium colonies were picked and grown overnight in LB medium containing tetracycline. Overnight cultures were diluted in LB medium supplemented with tetracycline to an optical density at 600 nm (OD600) of ∼0.4. For induction of protein expression, 0.5% (wt/vol) xylose was added. Bacteria were harvested by centrifugation 7 h and 24 h following induction. The culture supernatant was also collected.
Immunolabeling of self-assembly products.
For investigation of the accessibility of the HA tag at the C terminus of the S-layer fusion protein, self-assembly products formed on transmission electron microscopy (TEM) grids were incubated with ZnO-labeled anti-HA antibodies (0.04% ZnO with 32 μg/ml anti-HA antibodies in phosphate-buffered saline [PBS]; Roche) for 1 h at 20°C. Subsequently, unbound ZnO-labeled antibody was removed by placing TEM grids on a drop of water. After drying, samples were subjected to TEM analysis. As a control, the same procedure was carried out with authentic SslA.
For the dot blot assay, in vitro-assembled fusion proteins were centrifuged and the pellet was washed twice to remove monomeric proteins. After resuspension of the pellet, the assembled fusion proteins were dried on a polyvinylidene difluoride (PVDF) membrane (Millipore) and incubated with monoclonal anti-HA antibodies (Roche). Detection of bound antibodies was performed with horseradish peroxidase (HRP)-conjugated secondary antibodies (Amersham Bioscience) and the ECL Plus kit (Amersham Pharmacia Biotech).
Electron microscopy.
S-layer-producing B. megaterium cells were incubated with 2 M guanidine hydrochloride (Gua-HCl) to remove the secreted and assembled S-layers from the cell envelope. After dialysis against dH2O for 24 h at 4°C, the assembled S-layers were applied to carbon-coated copper grids for electron microscopy. The preparations were examined with a Morgagni 268 electron microscope operated at 80 kV.
Microscopy.
For light and fluorescence microscopy, B. megaterium cells expressing S-layer fusion constructs were harvested at different time points, washed with PBS, and adjusted to an OD600 of 1. The cells were treated with 100 μg/ml bovine serum albumin (BSA) in PBS and incubated for 1 h with anti-hemagglutinin-Alexa Fluor 488 conjugates (Invitrogen). After washing with PBS, samples were analyzed in a fluorescence microscope (Keyence, Neu-Isenburg, Germany).
Protein analysis.
Overnight cultures were harvested and shifted into fresh medium containing 0.5% xylose to induce the expression of various SslA constructs. Samples were taken before induction and at 7 h and 24 h after induction. Cells were washed three times with PBS, and 1 × 109 cells were incubated with 2 M Gua-HCl for 1 h at room temperature. Secreted proteins from the growth medium, as well as proteins extracted with Gua-HCl, were precipitated using the methanol-chloroform method (44). Precipitated proteins were dissolved in Laemmli buffer, separated by SDS-PAGE (22), and blotted onto PVDF membrane (Millipore). As a control, 50 μg of whole-cell lysate was analyzed.
For Western blot analysis, HA-specific monoclonal antibodies (Roche) and HRP-conjugated anti-mouse IgG (Amersham Bioscience) were used as primary and secondary antibodies, respectively. Detection of bound antibodies was performed by an enhanced chemiluminescence assay (ECL; Amersham Bioscience).
RESULTS
Expression of the genes encoding the three S-layer fusion proteins rSslA(aa1-1099)-HA, rSslA(aa32-928)-HA, and rSslA(aa341-928)-HA.
The heterologous expression of genes encoding three different S-layer fusion proteins (Fig. 1) in B. megaterium was investigated. SslA of S. ureae ATCC 13881 possesses an N-terminal secretion signal of 31 amino acids. It was shown that the N-terminal 340 amino acids and the C-terminal 171 amino acids can be deleted without interfering with the self-assembly process (32). Three fusion proteins consisting of the full-length [rSslA(aa1-1099)], the C-terminally truncated [rSslA(aa32-928)], and the N- and C-terminally truncated [rSslA(aa341-928)] SslA protein and the fused hemagglutinin (HA) epitope tag were constructed (see Materials and Methods; also see Fig. S1 to S3 in the supplemental material). The S-layer fusion constructs were cloned into the E. coli-B. megaterium shuttle vector pHIS1525. This vector includes, in addition to the strong xylA promoter of B. megaterium, the signal peptide sequence of the extracellular esterase LipA (SPlipA), which allows the secretion of the recombinant proteins into the culture broth (31).
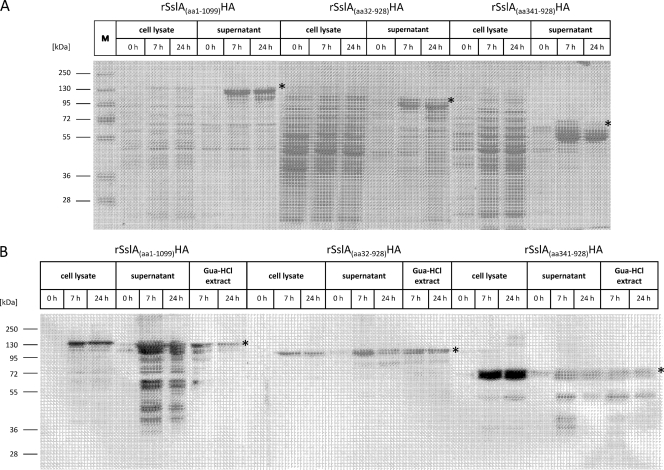
After the induction of expression by the addition of xylose, cells were harvested at various time points and subjected to SDS-PAGE analysis. Both the whole-cell lysates and concentrated growth medium from B. megaterium WH320 cultures with plasmids encoding the constructs rSslA(aa1-1099)-HA, rSslA(aa32-928)-HA, and rSslA(aa341-928)-HA showed additional high-molecular-mass protein bands on SDS gels in comparison to the results for noninduced B. megaterium cells (Fig. 2). The apparent molecular masses of 130 kDa, 110 kDa, and 70 kDa of these protein bands correspond well with the theoretical molecular masses estimated for the three fusion proteins. Obviously, all three S-layer fusion proteins are efficiently expressed in B. megaterium and secreted into growth medium. A number of low-molecular-mass protein bands possibly representing degradation products were also observed. Western blot analysis using specific antibodies against the HA tag identified the chimeric proteins in B. megaterium cell lysate and in the culture supernatant. Only the supernatant fraction showed low-molecular-mass protein bands (Fig. 2B), indicating that protein degradation is preferentially mediated by extracellular proteases. In order to test whether the major extracellular protease NprM (45) is responsible for the observed degradation, the S-layer fusion constructs were expressed in the NprM-deficient B. megaterium strain MS941. However, the degradation pattern of the secreted proteins was unchanged (see Fig. S4 in the supplemental material), indicating that the proteolytic activity is due to another extracellular protease(s).
Fig 2.
Expression and secretion of recombinant S-layer fusion proteins in B. megaterium. Before the addition of 0.5% xylose for the induction of protein expression, transformed B. megaterium WH320 cells were grown until the OD600 reached ∼0.4. Samples were taken at 0, 7, and 24 h after induction. (A) An amount of 50 μg of whole-cell lysate and proteins from 300 μl of cell-free growth medium were precipitated, analyzed by SDS-PAGE, and stained with Coomassie brilliant blue R250. (B) For Western blot analysis with anti-HA antibody, Gua-HCl extracts of whole B. megaterium cells were prepared as described in Materials and Methods. The respective proteins are indicated by asterisks. Lane M shows prestained protein plus ladder (Fermentas GmbH, Germany).
Localization of recombinant S-layer proteins in B. megaterium cells.
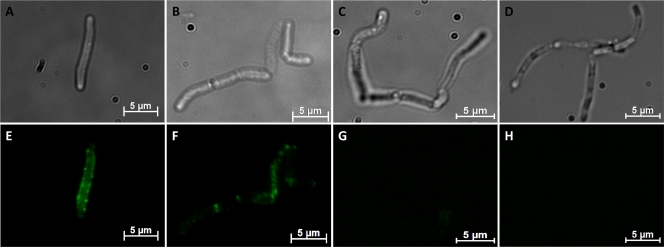
It was discussed that B. megaterium cells carry S-layer proteins in their natural environment (4, 37), but long-term cultivation of expression strains under laboratory conditions can lead to the loss of this property. We were interested in whether the outer surface of such host cells may provide nucleation points for the assembly of secreted recombinant S-layer fusion proteins. Transformed B. megaterium cells were harvested 7 h after induction, washed to remove proteins from the culture medium, and treated with 2 M guanidine hydrochloride (Gua-HCl). The chaotropic agent is able to remove noncovalently associated proteins like S-layers from the cell wall. Incubation with low concentrations of chaotropic agents maintains cell integrity, whereas higher concentrations (5 M Gua-HCl) lead to cell damage (data not shown). Western blot analysis of extracted proteins with anti-HA antibody showed a strong signal at the expected molecular mass of the fusion proteins, indicating that the recombinant S-layer fusion proteins were located on the cell surface of B. megaterium. Fluorescence microscopy of whole cells that were harvested 7 h after induction and incubated for 1 h with Alexa Fluor dye-labeled mouse anti-HA antibodies revealed a homogeneous distribution of green fluorescence around B. megaterium cells expressing the full-length S-layer fusion construct rSslA(aa1-1099)-HA (Fig. 3E), while the expression of the C-terminally truncated form rSslA(aa32-928)-HA led to spotlike structures surrounding the whole cell (Fig. 3F). These fluorescent dots are mainly found at the cell poles and at regions of septum formation. The expression of the N- and C-terminally truncated form rSslA(aa341-928)-HA resulted in a weak fluorescent signal surrounding the cells (Fig. 3G). B. megaterium cells that expressed the N- and C-terminally truncated S-layer fusion protein iSslA(aa341-928)-HA that lacks the secretion signal were used as a control. These cells did not exhibit any fluorescence after treatment with Alexa Fluor-labeled mouse anti-HA antibodies. To analyze whether a concentration of 2 M Gua-HCl is sufficient to remove S-layer proteins from the cell surface completely, the cells were treated with the respective concentration and washed twice with PBS. After incubation with Alexa Fluor-labeled mouse anti-HA antibodies and fluorescence microscopic analysis, cells still exhibited fluorescence, indicating that 2 M Gua-HCl is not sufficient to completely remove S-layer proteins associated with the cell wall (data not shown).
Fig 3.
Optical (A, B, C, D) and fluorescent (E, F, G, H) images of B. megaterium cells expressing S-layer fusion proteins. B. megaterium cells expressing and secreting rSslA(aa1-1099)-HA (A, E), rSslA(aa32-928)-HA (B, F), or rSslA(aa341-928)-HA (C, G) were cultivated in LB medium and harvested 7 h after induction with xylose, washed with PBS, and blocked with BSA. The cells were incubated with anti-hemagglutinin-Alexa Fluor 488 conjugates (Invitrogen) for 1 h and analyzed by fluorescence microscopy. As a control, intracellular iSslA(aa341-928)-HA (D, H) was prepared in the same manner.
Isolation and formation of self-assembly products by rSslA(aa1-1099)-HA and the truncated variants.
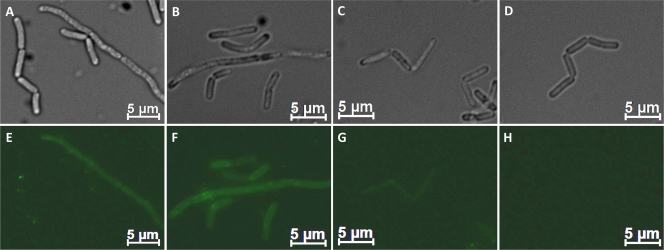
As concluded from our Western blot analysis and fluorescence microscopy data, the secreted recombinant S-layer fusion proteins were partially bound on the cell surface of B. megaterium. To investigate whether the chimeric proteins are able to self-assemble in vitro, the proteins were extracted from the cell wall with 2 M Gua-HCl and dialyzed against distilled water (dH2O). Wild-type B. megaterium cells were incubated in the respective S-layer monomer-containing solution for 2 h. Then, the cells were washed two times with PBS. Treatment with Alexa Fluor-labeled mouse anti-HA antibody and subsequent fluorescence microscopy revealed that the B. megaterium cells were covered with S-layer fusion proteins. In the case of the full-length S-layer fusion protein and the C-terminally truncated form, a homogeneous distribution of green fluorescence could be observed (Fig. 4). In contrast, incubation with the N- and C-terminally truncated form rSslA(aa341-928)-HA did not result in fluorescent staining of the cell envelope (Fig. 4G).
Fig 4.
Optical (A, B, C, D) and fluorescent (E, F, G, H) images of reassembled S-layer fusion proteins on B. megaterium cells. B. megaterium cells were resuspended in monomer containing S-layer fusion protein solutions of rSslA(aa1-1099)-HA (A, E), rSslA(aa32-928)-HA (B, F), or rSslA(aa341-928)-HA (C, G). After 2 h of incubation, the cells were harvested and washed twice with PBS. For detection of assembled S-layer fusion proteins on the surface, cells were incubated with anti-hemagglutinin-Alexa Fluor 488 conjugates (Invitrogen) for 1 h and analyzed by fluorescence microscopy. As a control, wild-type B. megaterium cells (D, H) were prepared in the same manner.
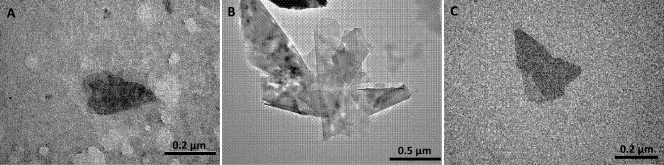
An aliquot of isolated S-layer fusion proteins (see Materials and Methods) was used for TEM analysis. Unstained assemblies of S-layer variants revealed flat double and multilayer sheets (Fig. 5). The size of multilayer structures formed in suspension was between 0.1 and 0.5 μm2. In line with multilayer structures, we occasionally observed moiré patterns, as shown in Fig. 5B, for the C-terminally truncated S-layer fusion protein.
Fig 5.
TEM analysis of recrystallized recombinant S-layer fusion proteins. Recombinant rSslA(aa1-1099)-HA (A), rSslA(aa32-928)-HA (B), and rSslA(aa341-928)-HA (C) were isolated with 2 M Gua-HCl from the cell surface of B. megaterium transformants. After dialysis against dH2O, the assembled structures were subjected to TEM analysis.
Investigation of accessibility of the fused HA tag.
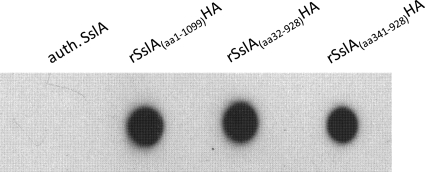
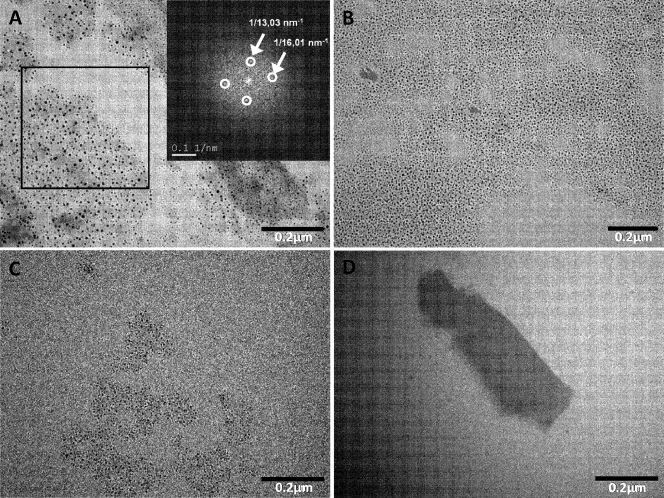
The accessibility and functionality of the fused hemagglutinin tag of recrystallized S-layer fusion proteins was first tested in dot blot assays. All three fusion proteins yielded a strong signal with the anti-HA antibody (Fig. 6). This result indicates the accessibility of the fused HA tag for the respective antibodies. Native SslA that was used as a control showed no reaction with the antibody. The functionality of the fused peptide was further investigated by labeling with anti-HA ZnO conjugates. As shown in Fig. 7A to C, self-assembly products of all three S-layer fusion proteins were densely labeled with the colloidal ZnO conjugates. Diffraction spots obtained by fast Fourier transform analysis (Fig. 7B, inset) revealed that the observed ordered structure corresponds to the lattice spacing of 13.2 nm of the native SslA (32). Self-assembly products formed by authentic SslA remained nearly completely unlabeled (Fig. 7D).
Fig 6.
Dot blot assay of self-assembly products of S-layer fusion proteins and authentic (auth.) SslA. Isolated and dialyzed recombinant S-layer fusion proteins showed strong signals in immunological detection using a monoclonal anti-HA antibody. Authentic SslA that was used as a control showed no reaction with the antibody.
Fig 7.
Immunolabeling of self-assembly products formed by rSslA(aa1-1099)-HA (A), rSslA(aa32-928)-HA (B), and rSslA(aa341-928)-HA (C). Recombinant rSslA(aa1-1099)-HA (A), rSslA(aa32-928)-HA (B), and rSslA(aa341-928)-HA (C) were isolated with 5 M Gua-HCl from the cell surface of B. megaterium transformants. After dialysis against dH2O, the assembled structures were detected by ZnO-labeled anti-HA antibodies. The assembled and labeled structures were characterized by Fourier transformation analysis. The analyzed area is indicated by an inset. The diffraction spots correspond to lattice spacings of authentic SslA. As a control, authentic SslA assemblies incubated with ZnO-labeled anti-HA antibodies were used (D).
DISCUSSION
S-layer proteins and their genetically engineered derivatives have broad potential for biotechnological applications (17, 28, 33, 36, 39, 40). As outlined in the introduction, different prokaryotic expression systems, e.g., E. coli, B. subtilis, and L. casei, as well as the eukaryotic model organisms S. cerevisiae and HeLa cells, have been used for heterologous expression of S-layer proteins. However, structural instabilities and low levels of synthesis affect the yields of these gene expression systems. Here, we report on the high-level expression and secretion of functional S-layer derivatives in the Gram-positive bacterium B. megaterium.
Upon induction of the xylA promoter by the addition of xylose, the proteins were efficiently produced and secreted into the growth medium. Low or no proteolytic activity could be observed for intracellular S-layer fusion proteins. However, the expression of sslA fusion constructs in other expression systems, e.g., S. cerevisiae, leads to protein degradation (personal communication, N. Korkmaz). In the case of B. megaterium, comparison of the cell-associated and the secreted fusion proteins shows significantly more degradation products in the latter fraction. The use of the NprM protease-deficient strain B. megaterium MS941 did not affect the degradation of secreted S-layer fusion proteins. Alternative explanations for the presence of N-terminally truncated fusion proteins, such as the presence of reading frame internal translation start sites, can be ruled out. Probably, the presence of an unknown extracellular protease(s) may explain the observed degradation of secreted proteins.
As described for several truncated versions of SslA (32), the isolated S-layer fusion proteins were able to form highly ordered, crystalline sheetlike structures in vitro. Due to the structure size and the formation of multilayers, no reliable Fourier transformation analysis could be performed. The moiré pattern observed in TEM images of rSslA(aa32-928)-HA (Fig. 5B), however, points to the formation of highly ordered, crystalline structures. The observation that the anti-HA antibody binds to monolayers formed by the S-layer fusion proteins on solid supports (Fig. 7) indicates that the C-terminal HA tag is exposed to the ambient environment, and Fourier transformation analysis revealed the formation of lattices with lattice spacing corresponding to that of native SslA of 13.2 nm (32). The results obtained by immunolabeling of self-assembly products are in good agreement with studies on various S-layer fusion proteins that exhibit C-terminally fused functional domains (e.g., streptavidin [2, 25], birch pollen allergen [8, 15, 18], camel antibody [29], single amino acids [2], and enhanced green fluorescent protein [eGFP] [16]). Studies on structure-function relationships revealed that the middle and, in some cases, the C-terminal parts of S-layer proteins are responsible for self-assembly into 2-dimensional protein arrays. The insertion of functional peptide sequences or single amino acids had no or only slight effects on the formation of S-layer sheets. In Gram-positive bacteria, the N-terminal parts of the S-layer proteins, comprising the SLH domain, are involved in the attachment to the underlying cell wall. Recent studies revealed that SslA contains three N-terminal SLH domains (see Fig. S5 in the supplemental material) (11). In the present study, we could demonstrate that the B. megaterium cell wall, which is believed to be covered by S-layers in its natural environment, can serve as a substrate for S-layer assembly. As shown by fluorescence microscopy of immunolabeled B. megaterium cells, coverage with fluorescent proteins is not homogenous. There are two possible explanations for this observation: either the HA tag of a subpopulation of S-layer fusion proteins is not accessible for the antibodies or the cell wall is only partially covered by S-layers. In Gram-positive bacteria, the anchoring of S-layer subunits to the underlying rigid cell envelope layer occurs by binding to secondary cell wall polymers (SCWPs) (30, 35). This binding can involve SLH domains and pyruvylated SCWPs or occurs via carbohydrate binding of serine, tyrosine, and arginine residues in the N-terminal part of S-layer proteins to an SCWP that contains N-acetylglucosamine and N-acetylmannosamine (30). SPR measurements showed that binding between SLH domains and pyruvylated SCWPs is highly specific (14, 23, 24), and therefore, interactions between amino acids containing hydroxyl, amide, and amino groups and the SCWPs were supposed. While the contents of amino acid residues predicted to form hydrogen bonds or dipole-dipole interactions are nearly the same in the N-terminal and middle part of the S-layer protein, there are marked differences in secondary structure. The central part of the SslA fusion protein is characterized by β-sheet structures, whereas the N-terminal part, mostly arranged in the SLH and signal peptide sequences, is predicted to possess a high content of serine-rich α-helices (see Fig. S5 in the supplemental material) (10, 13). Possibly, the more homogeneous distribution of green fluorescence surrounding B. megaterium cells expressing the full-length and the C-terminally truncated S-layer fusion construct is based on the presence of α-helices and serine residues that can interact with the underlying cell envelope component via van der Waals forces. The disintegration of the surface layer by treatment with Gua-HCl indicates weak interactions, e.g., hydrogen bonds or electrostatic interaction, between the secreted S-layer proteins and cell wall components of B. megaterium. The observed bright fluorescence in septal regions (Fig. 3F) may indicate the local accumulation of specific molecules with a high binding affinity to the S-layer proteins in these areas. However, microscopical artifacts cannot be completely excluded.
Conclusion.
In this study, we show that fusion proteins consisting of the precursor S-layer protein SslA, a C-terminally truncated SslA protein, and an N- and C-terminally truncated SslA protein of S. ureae fused with a hemagglutinin tag were efficiently expressed and secreted by B. megaterium cells. The secreted proteins were assembled on the cell wall. When removed by treatment with chaotropic agents, the isolated S-layer proteins are able to crystallize in vitro to highly ordered structures with an accessible fused HA tag. Combining the self-assembly property of S-layer proteins with novel functions has a great potential for applications in biotechnology; for example, for the bacterial cell surface display of tailor-made S-layer proteins presenting functional epitopes in high density and precise orientation.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the German Federal Ministry of Education and Research (BMBF; grant no. 03WKBH1A).
Footnotes
Published ahead of print 18 November 2011
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Åvall-Jääskeläinen S, Kylä-Nikkilä K, Kahala M, Miikkulainen-Lahti T, Palva A. 2002. Surface display of foreign epitopes on the Lactobacillus brevis S-layer. Appl. Environ. Microbiol. 68:5943–5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badelt-Lichtblau H, et al. 2009. Genetic engineering of the S-layer protein SbpA of Lysinibacillus sphaericus CCM 2177 for the generation of functionalized nanoarrays. Bioconjug. Chem. 20:895–903 [DOI] [PubMed] [Google Scholar]
- 3. Bahl H, et al. 1997. Molecular biology of S-layers. FEMS Microbiol. Rev. 20:47–98 [DOI] [PubMed] [Google Scholar]
- 4. Baumann-Grace JB, Tomcsik J. 1957. The surface structure and serological typing of Bacillus megaterium. J. Gen. Microbiol. 17:227–237 [DOI] [PubMed] [Google Scholar]
- 5. Bingle WH, Nomellini JF, Smit J. 1997. Cell-surface display of a Pseudomonas aeruginosa strain K pilin peptide within the paracrystalline S-layer of Caulobacter crescentus. Mol. Microbiol. 26:277–288 [DOI] [PubMed] [Google Scholar]
- 6. Blecha A, Zarschler K, Sjollema KA, Veenhuis M, Rödel G. 2005. Expression and cytosolic assembly of the S-layer fusion protein mSbsC-EGFP in eukaryotic cells. Microb. Cell Fact. 4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boot HJ, Pouwels PH. 1996. Expression, secretion and antigenic variation of bacterial S-layer proteins. Mol. Microbiol. 21:1117–1123 [DOI] [PubMed] [Google Scholar]
- 8. Breitwieser A, et al. 2002. A recombinant bacterial cell surface (S-layer)-major birch pollen allergen-fusion protein (rSbsC/Bet v1) maintains the ability to self-assemble into regularly structured monomolecular lattices and the functionality of the allergen. Protein Eng. 15:243–249 [DOI] [PubMed] [Google Scholar]
- 9. Chu S, et al. 1991. Structure of the tetragonal surface virulence array protein and gene of Aeromonas salmonicida. J. Biol. Chem. 266:15258–15265 [PubMed] [Google Scholar]
- 10. Cole C, Barber JD, Barton GJ. 2008. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36:W197–W201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Castro E, et al. 2006. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 34:W362–W365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Egelseer EM, et al. 1998. The S-layer proteins of two Bacillus stearothermophilus wild-type strains are bound via their N-terminal region to a secondary cell wall polymer of identical chemical composition. J. Bacteriol. 180:1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engelhardt H, Peters J. 1998. Structural research on surface layers: a focus on stability, surface layer homology domains, and surface layer-cell wall interactions. J. Struct. Biol. 124:276–302 [DOI] [PubMed] [Google Scholar]
- 14. Huber C, Egelseer EM, Ilk N, Sleytr UB, Sára M. 2006. S-layer streptavidin fusion proteins and S-layer-specific heteropolysaccharides as part of a biomolecular construction kit for application in nanobiotechnology. Microelectron Eng. 83:1589–1593 [Google Scholar]
- 15. Ilk N, et al. 2002. Molecular characterization of the S-layer gene, sbpA, of Bacillus sphaericus CCM 2177 and production of a functional S-layer fusion protein with the ability to recrystallize in a defined orientation while presenting the fused allergen. Appl. Environ. Microbiol. 68:3251–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ilk N, et al. 2004. A functional chimaeric S-layer-enhanced green fluorescent protein to follow the uptake of S-layer-coated liposomes into eukaryotic cells. Biochem. J. 379:441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ilk N, et al. 2008. Surfaces functionalized with self-assembling S-layer fusion proteins for nanobiotechnological applications. Colloids Surf. A Physicochem. Eng. Asp. 321:163–167 [Google Scholar]
- 18. Ilk N, Schumi C-T, Bohle B, Egelseer EM, Sleytr UB. 2011. Expression of an endotoxin-free S-layer/allergen fusion protein in gram-negative Bacillus subtilis 1012 for the potential application as vaccines for immunotherapy of atopic allergy. Microb. Cell Fact. 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jarosch M, Egelseer E, Mattanovich D, Sleytr UB, Sára M. 2000. S-layer gene sbsC of Bacillus stearothermophilus ATCC 12980: molecular characterization and heterologous expression in Escherichia coli. Microbiology 146:273–281 [DOI] [PubMed] [Google Scholar]
- 20. Jarosch M, et al. 2001. Analysis of the structure function relationship of the S-layer protein SbsC of Bacillus stearothermophilus ATCC 12980 by producing truncated forms. Microbiology 147:1353–1363 [DOI] [PubMed] [Google Scholar]
- 21. Korkmaz N, Ostermann K, Rödel G. 2011. Expression and assembly of recombinant surface layer proteins in Saccharomyces cerevisiae. Curr. Microbiol. 62:366–373 [DOI] [PubMed] [Google Scholar]
- 22. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 23. Li J, Hu X, Yan J, Yuan Z. 2009. Species-specific cell wall binding affinity of the S-layer proteins of mosquitocidal bacterium Bacillus sphaericus C3-41. Appl. Environ. Microbiol. 75:3891–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mader C, Huber C, Moll D, Sleytr UB, Sára M. 2004. Interaction of the crystalline bacterial cell surface layer protein SbsB and the secondary cell wall polymer of Geobacillus stearothermophilus PV72 assessed by real-time surface plasmon resonance biosensor technology. J. Bacteriol. 186:1758–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moll D, et al. 2002. S-layer-streptavidin fusion proteins as template for nanopatterned molecular arrays. Proc. Natl. Acad. Sci. U. S. A. 99:14646–14651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nomellini JF, Duncan G, Dorocicz IR, Smit J. 2007. S-layer-mediated display of the immunoglobulin G-binding domain of streptococcal protein G on the surface of Caulobacter crescentus: development of an immunoactive reagent. Appl. Environ. Microbiol. 73:3245–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Novotny R, Scheberl A, Giry-Laterriere M, Messner P, Schäffer C. 2005. Gene cloning, functional expression and secretion of the S-layer protein SgsE from Geobacillus stearothermophilus NRS 2004/3a in Lactococcus lactis. FEMS Microbiol. Lett. 242:27–35 [DOI] [PubMed] [Google Scholar]
- 28. Park TJ, et al. 2011. Characterization of a bacterial self-assembly surface layer protein and its application as an electrical nanobiosensor. J. Nanosci. Nanotechnol. 11:402–407 [DOI] [PubMed] [Google Scholar]
- 29. Pleschberger M, et al. 2003. Generation of a functional monomolecular protein lattice consisting of an S-layer fusion protein comprising the variable domain of a camel heavy chain antibody. Bioconjug. Chem. 14:440–448 [DOI] [PubMed] [Google Scholar]
- 30. Ries W, Hotzy C, Schocher I, Sleytr UB, Sára M. 1997. Evidence that the N-terminal part of the S-layer protein from Bacillus stearothermophilus PV72/p2 recognizes a secondary cell wall polymer. J. Bacteriol. 179:3892–3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruiz C, Blanco A, Pastor FIJ, Diaz P. 2002. Analysis of Bacillus megaterium lipolytic system and cloning of LipA, a novel subfamily I.4 bacterial lipase. FEMS Microbiol. Lett. 217:263–267 [DOI] [PubMed] [Google Scholar]
- 32. Ryzhkov P, Ostermann K, Blüher A, Mertig M, Rödel G. 2007. Formation of self-assembly nanotemplates in vitro by native SslA protein and its truncation analysis. Phys. Stat. Sol. (a) 6:1863–1869 [Google Scholar]
- 33. Sára M, Pum D, Schuster B, Sleytr UB. 2005. S-layer as patterning elements for applications in nanobiotechnology. J. Nanosci. Nanotechnol. 5:1939–1953 [DOI] [PubMed] [Google Scholar]
- 34. Sára M, Sleytr UB. 2000. S-layer proteins. J. Bacteriol. 182:859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schäffer C, Messner P. 2005. The structure of secondary cell wall polymers: how Gram-positive bacteria stick their cell walls together. Microbiology 151:643–651 [DOI] [PubMed] [Google Scholar]
- 36. Schrems A, et al. 2011. Bilayer lipid membrane formation on a chemically modified S-layer lattice. Langmuir 27:3731–3738 [DOI] [PubMed] [Google Scholar]
- 37. Sidhu MS, Olsen I. 1997. S-layers of Bacillus species. Microbiology 143:1039–1052 [DOI] [PubMed] [Google Scholar]
- 38. Sleytr UB, Messner P. 1983. Crystalline surface layers on bacteria. Annu. Rev. Microbiol. 37:311–339 [DOI] [PubMed] [Google Scholar]
- 39. Tang J, et al. 2010. Mapping short affinity tags on bacterial S-layer with an antibody. Chemphyschem 11:2323–2326 [DOI] [PubMed] [Google Scholar]
- 40. Tschiggerl H, Casey JL, Parisi K, Foley M, Sleytr UB. 2008. Display of a peptide mimotope on a crystalline bacterial cell surface layer (S-layer) lattice for diagnosis of Epstein-Barr virus infection. Bioconjug. Chem. 19:860–865 [DOI] [PubMed] [Google Scholar]
- 41. Tsuboi A, et al. 1989. In vitro reconstruction of a hexagonal array with a surface layer protein synthesized by Bacillus subtilis harboring the surface layer protein gene from Bacillus brevis 47. J. Bacteriol. 171:6747–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vary PS, et al. 2007. Bacillus megaterium—from simple soil bacterium to industrial protein production host. Appl. Microbiol. Biotechnol. 76:957–967 [DOI] [PubMed] [Google Scholar]
- 43. Vidgrén G, Palva I, Pakkanen R, Lounatmaa K, Palva A. 1992. S-layer protein gene of Lactobacillus brevis: cloning by polymerase chain reaction and determination of the nucleotide sequence. J. Bacteriol. 174:7419–7427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wessel D, Flügge UI. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138:141–143 [DOI] [PubMed] [Google Scholar]
- 45. Wittchen KD, Meinhardt F. 1995. Inactivation of the major extracellular protease from Bacillus megaterium DSM319 by gene replacement. Appl. Microbiol. Biotechnol. 42:871–877 [DOI] [PubMed] [Google Scholar]
- 46. Yamagata H, et al. 1987. Cloning and characterization of the 5′ region of the cell wall protein gene operon in Bacillus brevis 47. J. Bacteriol. 169:1239–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.