Abstract
Modern sake yeast strains, which produce high concentrations of ethanol, are unexpectedly sensitive to environmental stress during sake brewing. To reveal the underlying mechanism, we investigated a well-characterized yeast stress response mediated by a heat shock element (HSE) and heat shock transcription factor Hsf1p in Saccharomyces cerevisiae sake yeast. The HSE-lacZ activity of sake yeast during sake fermentation and under acute ethanol stress was severely impaired compared to that of laboratory yeast. Moreover, the Hsf1p of modern sake yeast was highly and constitutively hyperphosphorylated, irrespective of the extracellular stress. Since HSF1 allele replacement did not significantly affect the HSE-mediated ethanol stress response or Hsf1p phosphorylation patterns in either sake or laboratory yeast, the regulatory machinery of Hsf1p is presumed to function differently between these types of yeast. To identify phosphatases whose loss affected the control of Hsf1p, we screened a series of phosphatase gene deletion mutants in a laboratory strain background. Among the 29 mutants, a Δppt1 mutant exhibited constitutive hyperphosphorylation of Hsf1p, similarly to the modern sake yeast strains, which lack the entire PPT1 gene locus. We confirmed that the expression of laboratory yeast-derived functional PPT1 recovered the HSE-mediated stress response of sake yeast. In addition, deletion of PPT1 in laboratory yeast resulted in enhanced fermentation ability. Taken together, these data demonstrate that hyperphosphorylation of Hsf1p caused by loss of the PPT1 gene at least partly accounts for the defective stress response and high ethanol productivity of modern sake yeast strains.
INTRODUCTION
Sake yeast strains, which belong to the budding yeast species Saccharomyces cerevisiae, produce much more ethanol during sake fermentation than any other type of S. cerevisiae strain. This property is a critical prerequisite for sake yeast because rapid and high-level ethanol accumulation shortens fermentation periods and inhibits the growth of unwanted microorganisms during sake brewing, in which open fermentation tanks are typically used. Therefore, yeast strains with higher fermentation rates have been historically selected as sake yeast. In particular, modern sake yeast strains (also referred to as the “K7 group” [4]), which were isolated within the last 80 years and are genetically closely related, exhibit high fermentation rates in sake mash.
To identify the molecular mechanisms responsible for the superior fermentation properties of modern sake yeast strains, DNA microarray analyses of sake yeast strain Kyokai no. 701 (K701) and laboratory yeast strain X2180 have been performed during sake fermentation (42, 48). These analyses revealed that K701 is markedly defective in the Msn2p and/or Msn4p (Msn2/4p)-mediated environmental stress response. Consistent with this finding, K701 and other modern sake strains are more sensitive to ethanol stress and heat shock than strain X2180 (40). Loss of the MSN2/4 genes in laboratory strains leads to improvement of the fermentation rate, demonstrating that the dysfunction of Msn2/4p is associated with the superior fermentation characteristics of modern sake yeast (42). However, Msn2/4p might not be the only stress factors defective in K701, since most Msn2/4p target genes are redundantly and/or coordinately regulated by other transcriptional factors, such as Gis1p (5, 45), Yap1p (12, 36), and heat shock factor protein 1 (Hsf1p) (2, 16, 31, 39).
Although Hsf1p was originally isolated as a heat shock transcription factor (33, 46, 47), it responds to a variety of stress conditions, including high temperatures, oxidative stress, glucose starvation, and ethanol stress (2, 15, 26, 37, 38, 49). Hsf1p is constitutively localized in the nucleus, where it is bound to heat shock elements (HSEs), and plays an essential role in cell proliferation, even under normal growth conditions (22, 34, 47). In response to stress, however, Hsf1p becomes highly active via conformational changes (7, 25) and induces the transcription of hundreds of target genes related to protein folding, detoxification, energy generation, carbohydrate metabolism, and cell wall organization (10, 16). Mutants of hsf1 therefore exhibit pleiotropic phenotypes and temperature sensitivity (21, 28, 32, 50).
The phosphorylation state of Hsf1p in response to stress provides an important clue for understanding the regulatory mechanism of this transcription factor. In the absence of stress, Hsf1p is subjected to constitutive low-level phosphorylation, which appears to be negatively controlled by protein kinase A (11). Further phosphorylation by the AMP-activated kinase Snf1p is required for glucose starvation-induced activation of Hsf1p (15). Upon heat shock and oxidative stress, Hsf1p is also extensively phosphorylated, although the responsible kinases are unknown (26, 35). Based on these findings, constitutive hypophosphorylation and stress-responsive hyperphosphorylation of Hsf1p seem closely related to its low basal and stress-induced high activities, respectively.
In contrast to the phosphorylation-induced activation of Hsf1p, serine residues adjacent to the CE2 region of the Kluyveromyces lactis heat shock factor (HSF) are reported to be phosphorylated in its deactivation after heat shock (18). Furthermore, phosphorylation of animal HSF is implicated in both positive and negative regulation (19). Although several kinases have been reported to phosphorylate mammalian HSF on specific residues leading to either enhanced or repressed activities (19), no phosphatases that modulate HSF activity have been identified, with the exception of Xenopus laevis PP5 (8). Taken together, these findings indicate that the phosphorylation states of eukaryotic HSFs are sophisticatedly regulated to fine-tune their activity in response to alterations in the extracellular environment.
In the present study, we investigated the stress response induced by HSEs and Hsf1p to examine the relationship between the defective stress response and high ethanol productivity in modern sake yeast. By focusing on the phosphorylation states of Hsf1p, we found a novel regulatory mechanism of Hsf1p and identified a protein phosphatase responsible for it. Our findings will aid in understanding the sophisticated control of the Hsf1p activity under ethanol stress.
MATERIALS AND METHODS
Strains and plasmids.
Sake yeast strains Kyokai 1, 2, 7, 701, and 10 (K1, K2, K7, K701, and K10, respectively) were provided by the Brewing Society of Japan. Laboratory S. cerevisiae strains X2180, Σ1278b, and W303-1A were provided by the American Type Culture Collection. Twenty-nine phosphatase-gene-disrupted strains and their parental strain (BY4743) were provided by EUROSCARF (Germany). Yeast cells were routinely grown in liquid YPD medium (1% yeast extract, 2% peptone, 2% glucose) at 25°C unless otherwise stated.
For construction of the HSE-pCYC1-lacZ reporter, a part of the SSA3 promoter region containing five consecutive pentameric elements (27) was prepared by annealing the oligonucleotides SSA3-HSE-F (5′-CGCTGTGGAAAGTTATAGAATATTACAGAAGTGCA-3′) and SSA3-HSE-R (5′-CTTCTGTAATATTCTATAACTTTCCACAGCGTGCA-3′), which were then ligated into the Sse8387I site of pAUR-CYC1-lacZ (44). The resultant plasmid, pAUR-HSE-CYC1-lacZ, was digested with StuI and used for transformation to generate yeast strains in which the HSE-pCYC1-lacZ reporter was inserted at the AUR1 locus. Transformants were selected on YPD plates containing aureobasidin A (Takara), and successful insertion of the HSE-pCYC1-lacZ reporter was confirmed by PCR.
For the construction of plasmids pYC140-K7HSF1 and pYC140-ScHSF1, the HSF1 gene was amplified by high-fidelity PCR using KOD Plus version 2 (Toyobo) from K701 or X2180 genomic DNA with the primers HSF1-KpnI-F (5′-GATCAGGTACCTGAACAAACAGTACCGACTAGGACTGT-3′) and HSF1-SacI-R (5′-GATCAGAGCTCGAGGTTGTGTACTGTAGCGGTTTATAC-3′) and cloned into the KpnI-SacI site of pYC140 (17). For the construction of pYC140-PPT1, the PPT1 gene was amplified by high-fidelity PCR from X2180 genomic DNA with the primers PPT1-KpnI-inf-F (5′-GCGTACGCGTCGACGGTACCTGTAGTTGGCGTAATATTCCAGGATTACG-3′) and PPT1-KpnI-inf-R (5′-GTTTAAACGAATTCGGTACCCGTAATTCAACACAAGTGTAATTTCAATAGCTCG-3′) and cloned into the KpnI site of pYC140 using an In-Fusion Advantage PCR cloning kit (Clontech).
Disruption of both copies of the authentic HSF1 genes in K701 [pYC140-K7/ScHSF1] and X2180 [pYC140-K7/ScHSF1] was performed by a PCR-based method (14) with primers HSF1-del-F (5′-GAAGGGAAAGGAAACAAAAAAGACAAAAAGACAGCTGTATTGTTGGCGCCGCCAGATCTGTTTAGCTTGC-3′) and HSF1-del-R (5′-ATACTATATTAAATGATTATATACGCTATTTAATGACCTCGTCCTGTGTATAGTGGATCTGATATCATCG-3′) and plasmids pFA6-kanMX4 and pAG25 (14) as templates to generate strains K701 Δhsf1::kanMX/Δhsf1::natMX [pYC140-K7/ScHSF1] and X2180 Δhsf1::kanMX/Δhsf1::natMX [pYC140-K7/ScHSF1]. Successful disruption of both copies of genomic HSF1 was confirmed by PCR.
HSE-pCYC1-lacZ reporter gene assay.
The reporter gene activity of yeast cells was analyzed in sake brewing tests that included three mashing steps, as described previously (48). Briefly, 15 g of the sake mash was passed through a 1-mm mesh. Yeast cells were collected by serial centrifugation, washed twice with cold distilled water, disrupted in 250 μl of breaking buffer (100 mM Tris-HCl [pH 8.0], 1 mM dithiothreitol, 20% glycerol, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail [Roche]) with glass beads, and centrifuged at 21,900 × g for 10 min. A 100-μl portion of the resulting supernatant was mixed with 300 μl of cold distilled water and 400 μl of 2× Z-buffer (120 mM Na2HPO4, 80 mM NaH2PO4, 20 mM KCl, 2 mM MgSO4, 100 mM 2-mercaptoethanol; pH 7.0) and was then assayed for β-galactosidase activity (48). The expression levels of the lacZ reporter gene were normalized by the protein levels quantified using a Coomassie (Bradford) protein assay kit (Thermo Scientific).
For the reporter gene assay of yeast cells under ethanol stress, yeast cells were precultured overnight in YPD (or YPD containing 300 μg of hygromycin/ml to avoid curing of the pYC140-based plasmid), further cultured in YPD at 25°C until reaching the log phase, and transferred into fresh YPD (control) or YPD containing 8% (vol/vol) ethanol (ethanol stress) for 2 h. For the reporter gene assay of yeast cells under heat shock, log-phase cells prepared as described above were incubated at 25°C (control) or 41°C (heat shock) for 1 h. β-Galactosidase assays were then performed as previously reported (3).
Phosphorylation analysis of Hsf1p.
Yeast cells collected from sake mash (prepared as previously described [48]), YPD medium with or without 8% (vol/vol) ethanol, or YPD medium with or without heat shock (41°C) were frozen in liquid nitrogen and stored at −80°C until use. Upon thawing, glass beads and 175 μl of 20% trichloroacetic acid (TCA) were added to ∼108 yeast cells in a microtube, and the samples were vigorously mixed for 3 min at 4°C. Then, 1 ml of 5% TCA was added to the resulting lysates. Proteins were collected by centrifugation, washed once with ethanol, and dissolved in 40 μl of Laemmli sample buffer (0.125 M Tris-HCl [pH 6.8], 4% sodium dodecyl sulfate [SDS], 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue). The protein levels were quantified a using Coomassie (Bradford) protein assay kit (Thermo Scientific), and 50 μg of protein was then boiled for 5 min, resolved by SDS-PAGE, and blotted onto an Amersham Hybond-P membrane (GE Healthcare). Hsf1p was detected by Western blotting using anti-ScHsf1 polyclonal antiserum (kindly provided by H. Sakurai) and anti-mouse IgG, horseradish peroxidase-linked whole antibody (GE Healthcare) using the Amersham ECL Plus Western blot detection system (GE Healthcare). The signal was quantitated by using a LAS-1000 Plus system (Fujifilm).
For phosphatase treatment, whole-cell extracts were first prepared as described above, and 50 μg of protein was then treated with 25 U of calf intestine alkaline phosphatase (Takara) in 100 μl of alkaline phosphatase buffer (Takara) at 37°C overnight. The samples were concentrated to 10 μl with a Microcon YM-50 filter (Millipore) and subjected to SDS-PAGE and immunoblot analysis as described above.
Monitoring of sake fermentation.
One-step sake fermentation tests were performed as described previously (43). The volume of evolved carbon dioxide during sake fermentation was monitored using a Fermograph II (Atto), as previously reported (43).
RESULTS
Sake yeast exhibits severe defects in HSE-mediated gene expression under ethanol stress.
To examine Hsf1p and HSE-mediated gene expression during sake fermentation, the activity of the HSE-pCYC1-lacZ fusion gene product in strains K701 AUR1::AUR1-C-HSE-pCYC1-lacZ and X2180 AUR1::AUR1-C-HSE-pCYC1-lacZ was monitored during the sake brewing process (Fig. 1A). The X2180-derived strain exhibited elevated β-galactosidase activity from days 3 to 11, suggesting that the increasing ethanol concentration was sufficiently stressful to induce activation of Hsf1p, whereas no significant upregulation of activity was observed in the K701-derived strain throughout the fermentation period. Consistent with this finding, the K701-derived strain also showed a severe defect in HSE-mediated induction of β-galactosidase under acute 8% ethanol stress compared to the X2180-derived strain (Fig. 1B). In contrast, the expression of HSE-pCYC1-lacZ in K701 AUR1::AUR1-C-HSE-pCYC1-lacZ was induced by heat shock (41°C) as strongly as that observed in X2180 AUR1::AUR1-C-HSE-pCYC1-lacZ (Fig. 1C). These results clearly indicated that the Hsf1p and HSE-mediated ethanol stress response was specifically inhibited in K701.
Fig 1.
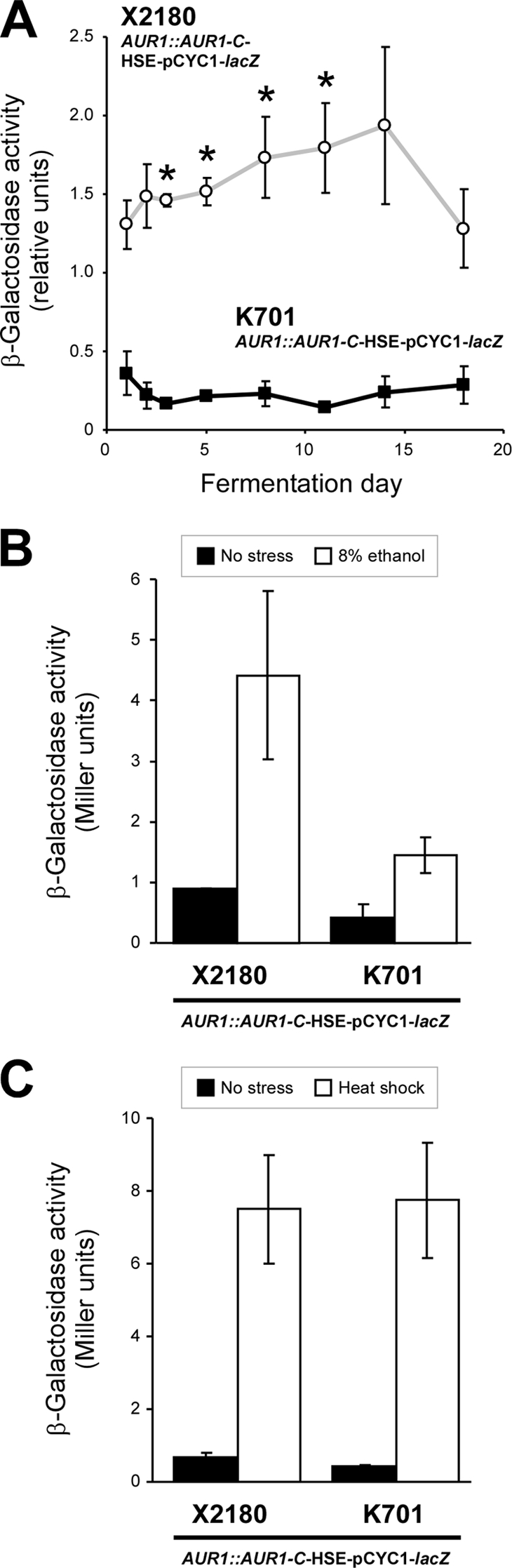
HSE-mediated gene expression activities in laboratory and sake yeast strains. (A) β-Galactosidase activities of the HSE-pCYC1-lacZ reporter in K701 AUR1::AUR1-C-HSE-pCYC1-lacZ (■) and X2180 AUR1::AUR1-C-HSE-pCYC1-lacZ (○) under sake brewing conditions. The data represent average values ± the standard deviations (SD) of two independent experiments. *, significantly higher than the value on day 1 (Student t test, P < 0.05). (B) β-Galactosidase activities of the HSE-pCYC1-lacZ reporter in K701 AUR1::AUR1-C-HSE-pCYC1-lacZ and X2180 AUR1::AUR1-C-HSE-pCYC1-lacZ under nonstress (■) or 8% ethanol stress (□) conditions. The data represent average values ± the SD of four independent experiments. (C) β-Galactosidase activities of the HSE-pCYC1-lacZ reporter in K701 AUR1::AUR1-C-HSE-pCYC1-lacZ and X2180 AUR1::AUR1-C-HSE-pCYC1-lacZ under nonstress (■) or heat shock (41°C, □) conditions. The data represent average values ± the SD of four independent experiments.
Hsf1p of sake yeast is highly and constitutively hyperphosphorylated.
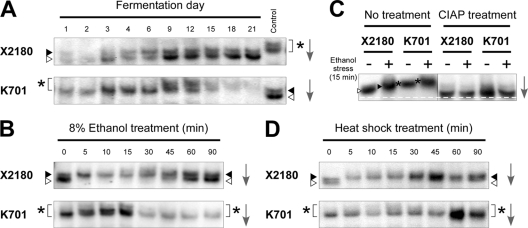
To reveal differences in the regulatory mechanism of Hsf1p between laboratory and sake yeast strains, we examined the phosphorylation state of Hsf1p by Western blot analyses using anti-Hsf1p antiserum. During sake fermentation, Hsf1p of X2180 was almost undetectable until day 3, from which point two bands with different mobilities were observed throughout the fermentation (Fig. 2A). As previously reported, hyperphosphorylated Hsf1p migrates more slowly than hypophosphorylated Hsf1p in denaturing gels (26, 35). Since the appearance of low-mobility Hsf1p coincided with the activation of the HSE-pCYC1-lacZ gene product (Fig. 1A), the two types of Hsf1p likely corresponded to the hypo- and hyperphosphorylated forms of Hsf1p. In contrast, the mobility of K701 Hsf1p was even lower than the low-mobility form of Hsf1p in X2180 throughout the sake fermentation (Fig. 2A). After day 9, K701 Hsf1p migrated even more slowly in the gel and was nearly undetectable from day 15 until the end of the fermentation.
Fig 2.
Immunoblot analyses of Hsf1p in laboratory and sake yeast strains. (A) Phosphorylation states of Hsf1p in X2180 (upper) and K701 (lower) during sake fermentation. The rightmost lanes show the same samples as day 9 of K701 (upper) and day 21 of X2180 (lower) as controls. (B) Phosphorylation states of Hsf1p under 8% ethanol stress in X2180 (upper) and K701 (lower). (C) Effects of phosphatase treatment on Hsf1p. The cell extracts of X2180 and K701 under nonstress and 8% ethanol stress conditions were incubated in the absence (left panel) or presence (right panel) of calf intestinal alkaline phosphatase (CIAP) and subjected to immunoblot analysis. The white dashed line represents the position of furthest migration of the dephosphorylated forms of Hsf1p. (D) Phosphorylation states of Hsf1p under heat shock (41°C) in X2180 (upper) and K701 (lower). White triangles, gray triangles, and asterisks indicate hypophosphorylated, hyperphosphorylated, and superhyperphosphorylated forms of Hsf1p, respectively, whose positions were determined by comparison with the control samples analyzed in the same gel. The arrows indicate the direction of electrophoretic migration.
We also investigated the mobility patterns of Hsf1p in the two yeast strains under 8% ethanol stress (Fig. 2B). Upon acute ethanol stress, X2180 Hsf1p rapidly altered its mobility and gradually returned to the original state, as was previously observed under heat shock or oxidative stress (26, 35). The mobility of K701 Hsf1p was lower than the low-mobility form of X2180 Hsf1p, even under normal growth conditions, and slightly further decreased after being transferred to 8% ethanol. The entire band faded after a 30-min ethanol treatment, as was observed in the sake mash after day 15 (Fig. 2A). The markedly low-mobility form of K701 Hsf1p was also observed under heat shock, although it did not decrease during the treatment (Fig. 2D).
Based on the immunoblot analyses, Hsf1p under nonstress or stress conditions appeared to be in the hypo- or hyperphosphorylated states in X2180. Furthermore, differences in the mobility of Hsf1p between the two strains were no longer observed when the lysates were treated with phosphatase (Fig. 2C), demonstrating that the mobilities were the result of variations in phosphorylation levels. These results, combined with the data of the HSE-pCYC1-lacZ reporter gene assays (Fig. 1), suggested that hyperphosphorylation of Hsf1p in X2180 was closely related to its activation under heat shock or ethanol stress conditions, whereas the marked hyperphosphorylation of Hsf1p in K701 presumably triggered its dysfunction and instability in combination with ethanol stress.
Differences in the Hsf1p-mediated ethanol stress response are not due to mutations in the HSF1 gene of sake yeast.
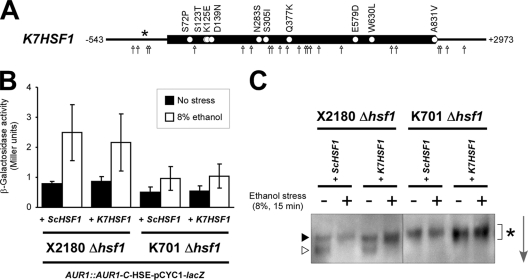
Analysis of the whole-genome sequence of modern sake yeast K7, which is nearly genetically identical to K701 but has a foaming phenotype (29), revealed that the nucleotide and amino acid sequences of the K7-derived HSF1 gene (K7HSF1) are 99% identical to those of the S288c-derived HSF1 gene (ScHSF1) (Sake Yeast Genome Database, http://nribf1.nrib.go.jp/SYGD). We identified 25 single nucleotide polymorphisms (SNPs) in the K7HSF1 open reading frame (ORF) region, 10 of which were nonsynonymous, 8 SNPs in the 5′- and 3′-untranslated regions (UTRs), and a single insertion of an adenine nucleotide at the −200 position of the 5′UTR (Fig. 3A). To determine whether these mutations were responsible for the defective Hsf1p-mediated ethanol stress response in sake yeast, we compared the effects of the expression of K7HSF1 and ScHSF1 in both K701 Δhsf1 and X2180 Δhsf1 backgrounds (Fig. 3B and C). Both K7HSF1 and ScHSF1 were able to function normally in the X2180 Δhsf1 background. In contrast, ScHSF1 in the K701 Δhsf1 background behaved irregularly, similarly to K7HSF1; HSE-mediated gene expression was significantly impaired, and Hsf1p was markedly hyperphosphorylated under acute 8% ethanol stress. These results suggested that the defective ethanol stress response and the marked hyperphosphorylation of Hsf1p, both of which are characteristic to sake yeast, were not caused by the mutations in the K7HSF1 gene, but by sake yeast-specific impairment of Hsf1p regulation.
Fig 3.
Exchange of ScHSF1 or K7HSF1 alleles has no apparent effects on Hsf1p-mediated stress responses. (A) Mutation points in the K7HSF1 allele revealed by whole-genome sequencing of K7 (http://nribf1.nrib.go.jp/SYGD). White circles, arrows, and the asterisk indicate nonsynonymous SNPs, other SNPs, and an adenine-nucleotide insertion, respectively. (B) β-Galactosidase activities of the HSE-pCYC1-lacZ reporter in X2180 Δhsf1 AUR1::AUR1-C-HSE-pCYC1-lacZ and K701 Δhsf1 AUR1::AUR1-C-HSE-pCYC1-lacZ background strains expressing ScHSF1 or K7HSF1 under nonstress (■) and 8% ethanol stress (□) conditions. The data represent average values ± the SD of three or more independent experiments. (C) Phosphorylation states of Hsf1p in X2180 Δhsf1 and K701 Δhsf1 background strains expressing ScHSF1 or K7HSF1 under nonstress and 8% ethanol stress conditions. The same symbols as in Fig. 2 are used to show the phosphorylation states of Hsf1p, whose respective positions were determined by comparison with the control samples analyzed in the same gel. The arrow indicates the direction of electrophoretic migration.
Modern sake yeast-specific loss of PPT1 phosphatase is related to the marked hyperphosphorylation of Hsf1p.
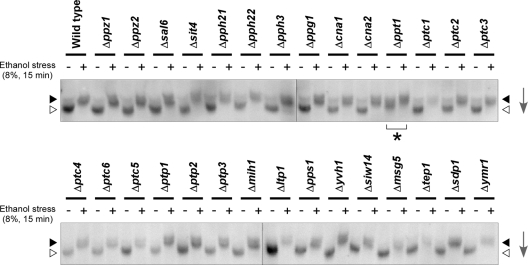
To identify the regulatory factor controlling the phosphorylation state of Hsf1p, we screened nonessential phosphatase-encoding gene disruptants (Fig. 4). Among known S. cerevisiae protein phosphatase genes (30), 29 disruptants in the laboratory strain BY4743 background were subjected to Western blot analysis with anti-Hsf1p antiserum. While most disruptants showed mobilities of Hsf1p similar to that of wild-type cells under both nonstress and stress conditions, Hsf1p in the Δppt1 strain migrated significantly slower, even without ethanol stress, as was observed in K701. From this result, Ppt1p was suggested to be a putative phosphatase that constitutively dephosphorylated Hsf1p and was defective in K701.
Fig 4.
Phosphorylation states of Hsf1p in wild-type cells and the indicated phosphatase gene disruptants in a BY4743 background under nonstress and 8% ethanol stress conditions. The same symbols as in Fig. 2 are used to indicate the phosphorylation states of Hsf1p. The arrows indicate the direction of electrophoretic migration.
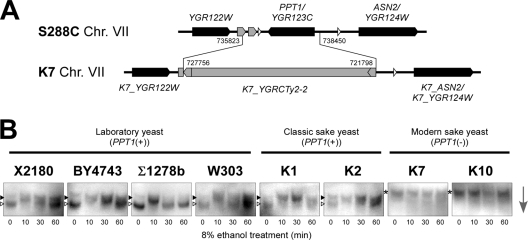
Intriguingly, we found that the PPT1 gene was completely deleted in K7; a 2.6-kb region including the PPT1 gene was replaced by a Ty2 element (Fig. 5A) (1). This type of PPT1-gene loss occurs specifically in modern sake yeast strains, as determined by Southern blot and PCR analyses (our unpublished data). We next examined the Hsf1p phosphorylation states in four laboratory strains, two classic sake strains, and two modern sake strains (Fig. 5B). In the laboratory and classic sake strains that contained PPT1 in their genomes, most Hsf1p proteins displayed reduced mobility after a 10-min treatment with 8% ethanol. In contrast, low-mobility Hsf1p was observed throughout the experiment in modern sake yeast strains K7 and K10, which lack PPT1 in their genomes. These results demonstrated that loss of the PPT1 gene was closely linked to the modern sake yeast-specific constitutive hyperphosphorylation of Hsf1p.
Fig 5.
Loss of PPT1 is related to the marked hyperphosphorylation of Hsf1p. (A) Schematic representation of the PPT1 locus in laboratory and sake strains revealed by whole-genome sequencing of K7 (1). Black, white, and gray symbols indicate ORFs, tRNA genes, and a retrotransposon and LTRs, respectively. Numbers indicate the chromosomal positions of gene replacement by a Ty2 element. (B) Phosphorylation states of Hsf1p under 8% ethanol stress conditions in the laboratory, classic sake, and modern sake strains. The same symbols as in Fig. 2 are used to indicate the phosphorylation states of Hsf1p, whose respective positions were determined by comparison with the control samples analyzed in the same gel. The arrow indicates the direction of electrophoretic migration.
Loss of PPT1 contributes to the features of modern sake yeast.
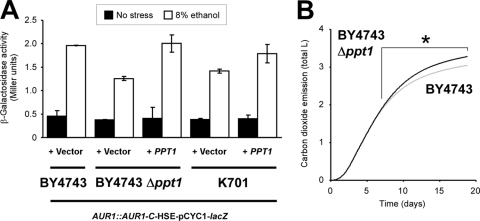
To confirm the impact of Ppt1p on HSE-mediated gene expression under ethanol stress, we performed the HSE-pCYC1-lacZ reporter gene assay in the BY4743, BY4743 Δppt1, and K701-derived strains (Fig. 6A). We note that the BY4743-derived strains showed the much less β-galactosidase activities than the X2180-derived strain (Fig. 1B) probably because of their auxotrophy. Compared to the parental strain, Δppt1 AUR1::AUR1-C-HSE-pCYC1-lacZ exhibited the decreased β-galactosidase activity under acute 8% ethanol stress, whereas this activity was recovered by expression of the PPT1 gene from a low-copy vector (Student t test, P < 0.05). When unstressed, these BY4743-derived strains showed almost the same β-galactosidase activity. Similarly, PPT1 expression in K701 AUR1::AUR1-C-HSE-pCYC1-lacZ also exhibited significantly increased β-galactosidase activity specifically under ethanol stress (Student t test, P < 0.05). These results demonstrated that the loss of PPT1 led to the impairment of the HSE-mediated ethanol stress response in both laboratory and sake yeast.
Fig 6.
Effects of the loss of PPT1 on the HSE-mediated stress response and fermentation properties in strains BY4743, BY4743 Δppt1, and K701. (A) β-Galactosidase activities of the HSE-pCYC1-lacZ reporter in BY4743 AUR1::AUR1-C-HSE-pCYC1-lacZ, BY4743 Δppt1 AUR1::AUR1-C-HSE-pCYC1-lacZ, and K701 AUR1::AUR1-C-HSE-pCYC1-lacZ background strains transformed with a PPT1 expression construct (+ PPT1) or empty vector (+ Vector) under nonstress (■) and 8% ethanol stress (□) conditions. The data represent average values ± the SD of three or more independent experiments. (B) Sake fermentation profiles of BY4743 (gray line) and BY4743 Δppt1 (black line). Total carbon dioxide emission during fermentation progression was monitored using a Fermograph II (Atto), as previously described (42). Averaged data from three independent experiments are shown. *, significantly higher than the wild type (Student t test, P < 0.05).
We recently reported that modern sake yeast strains display increased sensitivity to extracellular stress (40) and that a defective stress response correlates with high ethanol productivity (42). Thus, we next focused on the effects of PPT1 on fermentation properties. Small-scale sake brewing tests using BY4743 and its Δppt1 disruptant revealed that the loss of PPT1 was associated with improved fermentation ability (Fig. 6B). Although carbon dioxide emissions of both strains were similar during the initial 7 days, the sake mash containing Δppt1 exhibited a significantly higher level of carbon dioxide production after this initial period (e.g., 1.87 ± 0.02 l [BY4743] versus 1.90 ± 0.01 l [BY4743 Δppt1] at 7 days [Student t test, P = 0.0540], 2.10 ± 0.02 l versus 2.16 ± 0.01 l at 8 days [P = 0.0081], 2.45 ± 0.03 l versus 2.57 ± 0.01 l at 10 days [P = 0.0010], 3.05 ± 0.03 l versus 3.28 ± 0.01 l at 19 days [P = 0.0002]). We also confirmed that the ethanol concentration was significantly higher in the sake made from the Δppt1 disruptant after the 19-day fermentation (BY4743, 9.55% ± 0.24% [vol/vol]; BY4743 Δppt1, 9.80% ± 0.06% [vol/vol]; Student t test, P < 0.05). At the end of the fermentations, the proportion of dead cells in the Δppt1 strain (6.48%) was slightly higher than in its parental strain (4.73%). Taken together, the above findings demonstrated that the Δppt1 disruptant mimicked every examined phenotypic characteristic of modern sake yeast, including the constitutive hyperphosphorylation of Hsf1p, defective HSE-mediated expression induction under ethanol stress, and superior fermentation ability.
DISCUSSION
Sake yeast strains display superior fermentation properties and have long been considered to possess enhanced stress response machineries, since yeast cells are subjected to multiple extracellular stresses during fermentation. We recently discovered, however, that modern sake yeast strains are unexpectedly defective in the stress response (40, 42), suggesting that high stress tolerance is not required for the efficient ethanol fermentation ability of yeast cells. Consistent with this speculation, we revealed here that the Hsf1p-mediated ethanol stress response in the K701-derived strain was also inhibited during fermentation (Fig. 1). During the sake fermentation process, yeast cells might not need to rapidly acquire ethanol stress tolerance, because the ethanol concentration does not acutely increase in sake mash (maximum of 1 to 2% [vol/vol] per day). Moreover, excess stress responses might have inhibitory effects on glycolysis and ethanol production efficiency by diversifying carbon flux; for example, Hsf1p is reported to activate the expression of several genes involved in aerobic respiration, trehalose synthesis, and the pentose phosphate pathway (16). Thus, the Hsf1p-mediated ethanol stress response may have been incapacitated in the course of selection of modern sake yeast. This finding, together with our previous report on the dysfunction of the stress-responsive transcriptional factors Msn2/4p in modern sake yeast (42), provides novel insight into yeast stress responses as major impediments of effective ethanol fermentation. Therefore, the genetic engineering of stress-responsive transcription machineries represents a potential strategy for improving the ethanol fermentation efficiency of industrial yeast strains.
In modern sake yeast, inactivation of Hsf1p under ethanol stress appears to be related to its marked hyperphosphorylation, which is distinct from the constitutive hypophosphorylation and stress-induced hyperphosphorylation observed in other strains of S. cerevisiae (Fig. 2 and 5). Although it is unknown how phosphorylation inactivates Hsf1p in combination with ethanol stress, this inactivation might be linked to protein instability, since the signals of K701 Hsf1p irreversibly decreased following extensive hyperphosphorylation under conditions of prolonged ethanol stress but not heat shock (Fig. 2). To reveal the complete mechanism and significance of the marked hyperphosphorylation of Hsf1p, it is necessary to identify the responsible kinases, specific phosphorylation sites, and resultant conformational changes of Hsf1p and also to determine the influence of ethanol stress on these factors. In particular, identification of the kinases involved would provide a clue to understand this novel type of yeast Hsf1p regulation. In mammalian cells, several kinases, including GSK-3, ERK, and PKC/JNK, negatively regulate HSF through phosphorylation (19). The effects of disrupting the more than 100 yeast kinase genes identified in budding yeast (20) on the phosphorylation state and ethanol-induced transcriptional activity of Hsf1p should be examined to identify the key molecule(s) involved in this novel type of Hsf1p hyperphosphorylation.
In the present study, we further demonstrated that the modern sake yeast-specific constitutive hyperphosphorylation of Hsf1p is associated with the loss of the PPT1 phosphatase gene (Fig. 4 and 5). Since the Δppt1 strain exhibited a HSE-mediated ethanol stress response and sake fermentation properties similar to those of K701 (Fig. 6), we propose that the loss of PPT1 plays a pivotal role in the establishment of modern sake yeast-specific phenotypes. Because the observed effect of PPT1 on the HSE-mediated ethanol stress response in K701 was partial (Fig. 6A), we recognize that Ppt1p is not the sole factor responsible for the defective ethanol stress response in sake yeast. However, our finding of Ppt1p is of great importance from the viewpoint that this study has directly integrated genomic (1) and phenotypic (40, 42) studies of modern sake yeast. PPT1 was originally identified as a protein phosphatase related to human PP5 (6, 23), and its physiological roles are not well understood. Several lines of evidence indicate that Ppt1p physically interacts with Hsp90 chaperone (13, 24, 41, 51), since deletion of the PPT1 gene leads to hyperphosphorylation of Hsp90 (41). Based on the negative role of Hsp90 on the HSE-dependent stress response (9), Ppt1p might positively influence Hsf1p activity by direct dephosphorylation of Hsf1p and/or through inactivation of Hsp90 via dephosphorylation. In addition, it has been reported that physical interactions between PP5 and HSF-Hsp90 complexes are observed in a Xenopus oocyte model system (8), suggesting that the regulatory mechanism of HSF by PP5/Ppt1p subfamily protein phosphatases might be highly conserved among eukaryotes. Identifying the molecular mechanism by which Ppt1p modulates the ethanol-mediated activity of Hsf1p is necessary for elucidating the full picture of these sophisticatedly regulated yeast stress responses.
ACKNOWLEDGMENTS
We greatly appreciate the gift of anti-ScHsf1 polyclonal antiserum from H. Sakurai (Kanazawa University, Kanazawa, Japan).
This study was supported by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (Japan).
Footnotes
Published ahead of print 4 November 2011
REFERENCES
- 1. Akao T, et al. 2011. Whole-genome sequencing of sake yeast Saccharomyces cerevisiae Kyokai no. 7. DNA Res. 18:423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amorós M, Estruch F. 2001. Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Mol. Microbiol. 39:1523–1532 [DOI] [PubMed] [Google Scholar]
- 3. Araki Y, et al. 2009. Ethanol stress stimulates the Ca2+-mediated calcineurin/Crz1 pathway in Saccharomyces cerevisiae. J. Biosci. Bioeng. 107:1–6 [DOI] [PubMed] [Google Scholar]
- 4. Azumi M, Goto-Yamamoto N. 2001. AFLP analysis of type strains and laboratory and industrial strains of Saccharomyces sensu stricto and its application to phenetic clustering. Yeast 18:1145–1154 [DOI] [PubMed] [Google Scholar]
- 5. Cameroni E, Hulo N, Roosen J, Winderickx J, De Virgillio C. 2004. The novel yeast PAS kinase Rim15 orchestrates G0-associated antioxidant defense mechanisms. Cell Cycle 3:462–468 [PubMed] [Google Scholar]
- 6. Chen MX, et al. 1994. A novel human protein serine/threonine phosphatase, which possesses four tetratricopeptide repeat motifs and localizes to the nucleus. EMBO J. 13:4278–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen T, Parker CS. 2002. Dynamic association of transcriptional activation domains and regulatory regions in Saccharomyces cerevisiae heat shock factor. Proc. Natl. Acad. Sci. U. S. A. 99:1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conde R, Xavier J, McLoughlin C, Chinkers M, Ovsenek N. 2005. Protein phosphatase 5 is a negative modulator of heat shock factor 1. J. Biol. Chem. 280:28989–28996 [DOI] [PubMed] [Google Scholar]
- 9. Duina AA, Kalton HM, Gaber RF. 1998. Requirement for Hsp90 and a CyP-40-type cyclophilin in negative regulation of the heat shock response. J. Biol. Chem. 273:18974–18978 [DOI] [PubMed] [Google Scholar]
- 10. Eastmond DL, Nelson HC. 2006. Genome-wide analysis reveals new roles for the activation domains of the Saccharomyces cerevisiae heat shock transcription factor (Hsf1) during the transient heat shock response. J. Biol. Chem. 281:32909–32921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferguson SB, et al. 2005. Protein kinase A regulates constitutive expression of small heat-shock genes in an Msn2/4p-independent and Hsf1p-dependent manner in Saccharomyces cerevisiae. Genetics 169:1203–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gasch AP, et al. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gavin AC, et al. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141–147 [DOI] [PubMed] [Google Scholar]
- 14. Goldstein AL, McCusker JH. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541–1553 [DOI] [PubMed] [Google Scholar]
- 15. Hahn J-S, Thiele DJ. 2004. Activation of the Saccharomyces cerevisiae heat shock transcription factor under glucose starvation conditions by Snf1 protein kinase. J. Biol. Chem. 279:5169–5176 [DOI] [PubMed] [Google Scholar]
- 16. Hahn J-S, Hu Z, Thiele DJ, Iyer VR. 2004. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell. Biol. 24:5249–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansen J, et al. 2003. Further development of the cassette-based pYC plasmid system by incorporation of the dominant hph, nat, and AUR1-C gene markers and the lacZ reporter system. FEMS Yeast Res. 4:323–327 [DOI] [PubMed] [Google Scholar]
- 18. Høj A, Jakobsen BK. 1994. A short element required for turning off heat shock transcription factor: evidence that phosphorylation enhances deactivation. EMBO J. 13:2617–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holmberg CI, Tran SE, Eriksson JE, Sistonen L. 2002. Multisite phosphorylation provides sophisticated regulation of transcription factors. Trends Biochem. Sci. 27:619–627 [DOI] [PubMed] [Google Scholar]
- 20. Hunter T, Plowman GD. 1997. The protein kinases of budding yeast: six score and more. Trends Biochem. Sci. 22:18–22 [DOI] [PubMed] [Google Scholar]
- 21. Imazu H, Sakurai H. 2005. Saccharomyces cerevisiae heat shock transcription factor regulates cell wall remodeling in response to heat shock. Eukaryot. Cell 4:1050–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jakobsen BK, Pelham HR. 1988. Constitutive binding of yeast heat shock factor to DNA in vivo. Mol. Cell. Biol. 8:5040–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeong JY, Johns J, Sinclair C, Park JM, Rossie S. 2003. Characterization of Saccharomyces cerevisiae protein Ser/Thr phosphatase T1 and comparison to its mammalian homolog PP5. BMC Cell Biol. 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krogan NJ, et al. 2006. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440:637–643 [DOI] [PubMed] [Google Scholar]
- 25. Lee S, et al. 2000. The yeast heat shock transcription factor changes conformation in response to superoxide and temperature. Mol. Biol. Cell 11:1753–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu X-D, Thiele DJ. 1996. Oxidative stress induced heat shock factor phosphorylation and HSF-dependent activation of yeast metallothionein gene transcription. Genes Dev. 10:592–603 [DOI] [PubMed] [Google Scholar]
- 27. Liu X-D, Liu PCC, Santro N, Thiele DJ. 1997. Conservation of a stress response: human heat shock transcription factors functionally substitute for yeast HSF. EMBO J. 16:6466–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morano KA, Santro N, Koch KA, Thiele DJ. 1999. A transactivation domain in yeast heat shock transcription factor is essential for cell cycle progression during stress. Mol. Cell. Biol. 19:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ouchi K, Akiyama H. 1971. Non-foaming mutants of sake yeasts selection by cell agglutination method and by froth flotation method. Agric. Biol. Chem. 35:1024–1032 [Google Scholar]
- 30. Sakumoto N, et al. 1999. A series of protein phosphatase gene disruptants in Saccharomyces cerevisiae. Yeast 15:1669–1679 [DOI] [PubMed] [Google Scholar]
- 31. Simon JR, Treger JM, McEntee K. 1999. Multiple independent regulatory pathways control UBI4 expression after heat shock in Saccharomyces cerevisiae. Mol. Microbiol. 31:823–832 [DOI] [PubMed] [Google Scholar]
- 32. Smith BJ, Yaffe MP. 1991. A mutation in the yeast heat-shock factor gene causes temperature-sensitive defects in both mitochondrial protein import and the cell cycle. Mol. Cell. Biol. 11:2647–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sorger PK, Pelham HR. 1987. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 6:3035–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sorger PK, Lewis MJ, Pelham HR. 1987. Heat shock factor is regulated differently in yeast and HeLa cells. Nature 329:81–84 [DOI] [PubMed] [Google Scholar]
- 35. Sorger PK, Pelham HR. 1988. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54:855–864 [DOI] [PubMed] [Google Scholar]
- 36. Stanhill A, Schick N, Engelberg D. 1999. The yeast ras/cyclic AMP pathway induces invasive growth by suppressing the cellular stress response. Mol. Cell. Biol. 19:7529–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takemori Y, Sakaguchi A, Matsuda S, Mizukami Y, Sakurai H. 2006. Stress-induced transcription of the endoplasmic reticulum oxidoreductin gene ERO1 in the yeast Saccharomyces cerevisiae. Mol. Gen. Genomics 275:89–96 [DOI] [PubMed] [Google Scholar]
- 38. Tamai KT, Liu X, Silar P, Sosinowski T, Thiele DJ. 1994. Heat shock transcription factor activates yeast metallothionein gene expression in response to heat and glucose starvation via distinct signaling pathways. Mol. Cell. Biol. 14:8155–8165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Treger JM, Schmitt AP, Simon JR, McEntee K. 1998. Transcriptional factor mutations reveal regulatory complexities of heat shock and newly identified stress genes in Saccharomyces cerevisiae. J. Biol. Chem. 273:26875–26879 [DOI] [PubMed] [Google Scholar]
- 40. Urbanczyk H, et al. 2011. Sake yeast strains have difficulty in entering a quiescent state after cell growth cessation. J. Biosci. Bioeng. 112:44–48 [DOI] [PubMed] [Google Scholar]
- 41. Wandinger SK, Suhre MH, Wegele H, Buchner J. 2006. The phosphatase Ppt1 is a dedicated regulator of the molecular chaperone Hsp90. EMBO J. 25:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watanabe D, et al. 2011. Enhancement of the initial rate of ethanol fermentation due to dysfunction of yeast stress response components Msn2p and/or Msn4p. Appl. Environ. Microbiol. 77:934–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watanabe D, Ota T, Nitta F, Akao T, Shimoi H. 2011. Automatic measurement of sake fermentation kinetics using a multi-channel gas monitor system. J. Biosci. Bioeng. 112:54–57 [DOI] [PubMed] [Google Scholar]
- 44. Watanabe M, et al. 2007. Elevated expression of genes under the control of stress response element (STRE) and Msn2p in an ethanol-tolerance sake yeast Kyokai no. 11. J. Biosci. Bioeng. 104:163–170 [DOI] [PubMed] [Google Scholar]
- 45. Wei M, et al. 2008. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 4:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiederrecht G, Shuey DJ, Kibbe WA, Parker CS. 1987. The Saccharomyces and Drosophila heat shock transcription factors are identical in size and DNA binding properties. Cell 48:507–515 [DOI] [PubMed] [Google Scholar]
- 47. Wiederrecht G, Seto D, Parker CS. 1988. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell 54:841–853 [DOI] [PubMed] [Google Scholar]
- 48. Wu H, et al. 2006. Global gene expression analysis of yeast cells during sake brewing. Appl. Environ. Microbiol. 72:7353–7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamamoto A, Ueda J, Yamamoto N, Hashikawa N, Sakurai H. 2007. Role of heat shock transcription factor in Saccharomyces cerevisiae oxidative stress response. Eukaryot. Cell 6:1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zarzov P, Boucherie H, Mann C. 1997. A yeast heat shock transcription factor (Hsf1) mutant is defective in both Hsc82/Hsp82 synthesis and spindle pole body duplication. J. Cell Sci. 110:1879–1891 [DOI] [PubMed] [Google Scholar]
- 51. Zhao R, et al. 2005. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120:715–727 [DOI] [PubMed] [Google Scholar]