Abstract
Strain JAU4234, identified as Streptomyces padanus, was isolated from soil collected in Jiangxi Province, China. It produced actinomycin X2, fungichromin, and a new polyene macrolide compound with antifungal activity, antifungalmycin 702. Antifungalmycin 702 had good general antifungal activity and may have potential future agricultural and/or clinical applications.
TEXT
Antibiotic discovery for the treatment of pathogen infections is one of the greatest public health achievements of the 20th century. However, pathogenic microbes are rapidly adapting to the antibiotics, making them ineffective and leading to the emergence of resistance (18). Of particular concern are multiple-drug-resistant pathogens such as New Delhi metallo-beta-lactamase 1 (NDM-1)-positive bacteria (6). Despite this threat and ever increasing public awareness regarding “superbug” infections, treatment options, unfortunately, continue to be limited. One direct course of action to treat drug-resistant pathogenic infections and avoid an epidemic is to discover new antibiotics or to modify the structures of known antibiotics as drug leads.
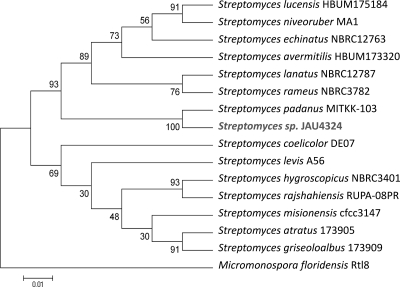
The Streptomyces genus is a remarkably rich source of natural products, accounting for the production of two-thirds of the commercially available antibiotics (1). It is noteworthy that this genus still produces a larger number and a wider variety of new antibiotics than the members of any other genus (e.g., meroparamycin, oligomycins, neopeptins, and salaceyin A) and seems to be an almost inexhaustible reservoir of novel antibiotics (2). In the course of our screening program for new antibiotics, strain JAU4234 was isolated from a soil sample collected at the campus of Jiangxi Agricultural University (Nanchang, Jiangxi province, China), where two new antibiotics, nanchangmycin and meilingmycin, have been discovered in “Streptomyces nanchangensis” NS3226 (14–16). Screening showed that strain JAU4234 possessed antimicrobial activity against Gram-positive and Gram-negative bacteria (Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Bacillus thuringiensis, Bacillus mycoides), yeasts (Saccharomyces cerevisiae, Candida utilis, Candida albicans), and molds (Penicillium citrinum, Trichoderma viride, Mucor sufu, Aspergillus niger, Aspergillus oryzae, Aspergillus flavus, Rhizopus nigricans, Absidia orchidis, Geotrichum candidum) (data not shown). 16S rRNA gene sequence analysis (GenBank accession number JF701605) indicated that this strain is a member of the genus Streptomyces and closely related to Streptomyces padanus (Fig. 1). Strain JAU4234 has the same culture and phenotypic characteristics as S. padanus MITKK-103 (see Fig. S1 in the supplemental material for the morphological characteristics of strain JAU4234 observed by optical and scanning electron microscopy) (7). Therefore, strain JAU4234 was identified as S. padanus JAU4234. Other strains of this species have been widely used as biocontrol agents in China and Taiwan (12) and are known to produce fungichromin (an antifungal antibiotic) and actinomycin X2 (an antitumor antibiotic) (7, 12).
Fig 1.
Neighbor-joining phylogenetic tree for Streptomyces sp. strain JAU4234 based on the 16S rRNA gene sequences with Micromonospora floridensis as the outgroup. Bootstrap values are based on 1,000 replicates. The scale bar represents 1 base substitution per 100 bases.
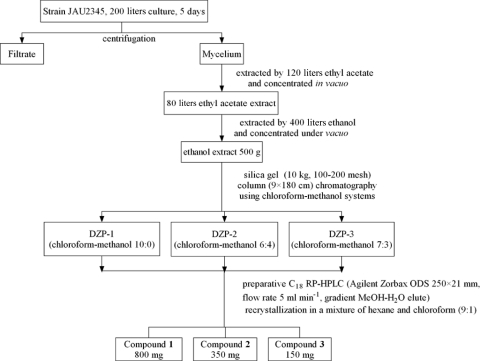
To identify antimicrobially bioactive compounds produced by this strain, 200 liters of fermentation broth was separated into filtrate and mycelium by centrifugation. For the medium and culture conditions used in this work, see the supplemental material. Antimicrobial activity was observed only in the mycelia extract. Figure 2 depicts the separation and purification scheme of bioactive compounds from S. padanus JAU4234. Bioactivity-guided purification resulted in bioactive compounds 1 (800 mg), 2 (350 mg), and 3 (150 mg), obtained from the mycelium. Compound 1 showed strong activity against bacteria but no activity against fungi. Compound 2 and compound 3 exhibited marked antifungal activity but no antibacterial activity.
Fig 2.
Bioassay-guided separation and purification scheme of bioactive compounds from S. padanus JAU4234.
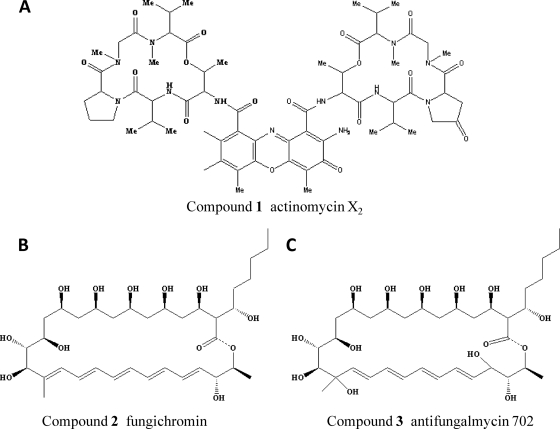
The chemical structures of compounds 1, 2, and 3 were elucidated (Fig. 3) on the basis of spectroscopic data from fast atom bombardment mass spectrometry (FAB-MS), infrared (IR), UV, 1H nuclear magnetic resonance (NMR), 13C NMR, and two-dimensional (2D) NMR analyses. The UV spectrum of compound 1 had a typical actinomycin class pattern with an absorption maximum at 444 nm. The spectral patterns of compounds 2 and 3 are characteristic of polyene macrolides (compound 2, λmax 356, 340, 337, and 320 nm; compound 3, λmax 318, 303, 290, and 211 nm) (see Fig. S2 in the supplemental material).
Fig 3.
Chemical structures of actinomycin X2 (A), fungichromin (B), and antifungalmycin 702 (C).
High-resolution FAB-MS analysis suggested their molecular masses to be 1,269.65 (compound 1), 670.83 (compound 2), and 704.39 (compound 3) Da and revealed their molecular formulas to be C62H84N12O17, C35H58O12, and C35H60O14, according to the 1H,13C NMR, and distortionless enhancement by polarization transfer spectral data. A search of the SciFinder database (American Chemical Society) showed that seven congeners of actinomycin are known to have a mass of 1,269 Da, elizabethin and fungichromin are the only known polyene antibiotics with a mass of 670, and no known polyene antibiotics have a mass of 704. Therefore, compound 3 could be a new compound.
2D NMR spectrum, including double-quantum filtered-correlated spectroscopy, heteronuclear multiple-quantum correlation, and heteronuclear multiple bond correlation indicated the presence of proline and 4-oxoproline in compound 1. Only actinomycin X2 has both proline and 4-oxoproline in the molecule, suggesting that compound 1 is actinomycin X2 (8, 13). On the basis of 1D and 2D NMR spectral data, the chemical shift of compound 2 matched that of fungichromin, indicating that compound 2 is likely fungichromin (5, 10, 12). Compound 3 had a structure similar to that of compound 2, the main distinction being the absence of two olefinic methine carbons and the presence of two oxygen-bearing carbons (one oxygen-bearing methine and one oxygen-bearing quaternary carbon) in compound 3. 2D NMR data suggested that the two hydroxyl groups are located at C-16 and C-25 and that an ester link exists between C-1 and C-27 (see Table S1 in the supplemental material). The putative structure of compound 3 (as elucidated in Fig. S3 in the supplemental material) was compared with all known compounds through a SciFinder database search. The search indicated that compound 3 is a novel polyene macrolide antibiotic we designated antifungalmycin 702.
Spectral data for the novel compound antifungalmycin 702 (compound 3, Fig. 3C) are as follows: C35H60O14, yellow powder; mp 170 to 173.5 °C; [α]D23 = +4.34° (c 0.1, MeOH), UV λmax (MeOH): 318, 303, 290, 211 nm; IR (KBr) νmax: 3,506 (OH), 2,926, 2,855, 1,721, 1,637, 1,384, 1,241, 1,099 cm−1; negative FAB-MS m/z (%): 703 [M-H]− (100); negative HR-FAB-MS m/z: 703.3903 ([M-H]−, calcd 703.3904). For 1H and 13C NMR spectral data, see Table S1 in the supplemental material.
The in vitro antimicrobial activities of these three compounds were evaluated by determining their MICs (12, 18). Although compound 1 has been previously isolated and identified, MIC data for the compound are not available. We therefore determined the MICs at which it inhibited 50 and 90% of a selection of Gram-positive and Gram-negative bacteria (MIC50 and MIC90, respectively). Compound 1 had strong antibacterial activity against all of the Gram-positive and Gram-negative bacteria examined (Table 1) and was especially effective against S. aureus (MIC50, 0.002 μg/ml), indicating that compound 1 could have expanded application as a potential candidate for the treatment of bacterial pathogens such as methicillin-resistant S. aureus and NDM-1-positive bacteria (11). It should, however, be noted that compound 1 induces greater cytotoxicity in cultured human leukemia cells (HL-60) than actinomycin D, through the stimulation of apoptotic pathways (7). Compound 2, isolated in the 1950s, has been well studied and is widely used as a fungicidal drug for infectious vaginitis (3, 4, 17). Compound 3 exhibited a broad antifungal activity on 8 plant pathogens at low MICs (Table 2) corresponding to but slightly higher than the MICs of compound 2. In preliminary experiments, compound 3 did not induce acute lethal toxicity at 1,500 mg/kg by the intraperitoneal and oral routes of administration in mice, suggesting low toxicity to mammal cells (data not shown). This is in contrast to the median lethal dose of compound 2, which is only 33.3 mg/kg on intraperitoneal administration to mice (data from SciFinder database). Hence, compound 3 has significant potential as a new antifungal biopesticide or drug lead for applications in the agricultural and medical fields.
Table 1.
MICs of actinomycin X2 (compound 1) against bacteria
| Bacterium | MIC50 (μg/ml) | MIC90 (μg/ml) |
|---|---|---|
| Staphylococcus aureus | 0.002 | 0.017 |
| Bacillus cereus | 0.02 | 0.19 |
| Bacillus megaterium | 0.08 | 0.44 |
| Shigella flexneri | 0.17 | 1.87 |
| Pseudomonas solanacearum | 0.26 | 2.3 |
| Escherichia coli | 0.73 | 4.49 |
| Xanthomonas citri | 0.73 | 5.0 |
| Xanthomonas oryzae | 124 | 226 |
Table 2.
MICs of fungichromin and antifungalmycin 702 against plant pathogens
| Fungus | MIC50 (μg/ml) |
MIC90 (μg/ml) |
||
|---|---|---|---|---|
| Fungichromin | Antifungalmycin | Fungichromin | Antifungalmycin | |
| Rhizoctonia solani | 1.47 | 6.20 | 41.02 | 15.94 |
| Helminthosporium sigmoideum Cav | 2.53 | 11.05 | 18.49 | 26.72 |
| Magnaporthe grisea | 3.45 | 15.24 | 10.47 | 37.72 |
| Penicillium notatum | 1.33 | 18.24 | 3.88 | 66.48 |
| Plantain head blight fungus | 3.17 | 23.40 | 10.65 | 94.66 |
| Gibberella zeae | 2.21 | 26.71 | 14.50 | 92.34 |
| Mucor rouxianus | 2.11 | 28.80 | 5.22 | 94.07 |
| Ustilaginoidea virens | 0.21 | 26.72 | 133.1 | 128.27 |
Like the fungicidal activities of compound 2 and other polyene macrolide antibiotics, that of compound 3 may involve binding to cell membrane sterols, resulting in an altered cell membrane, leakage of cell constituents, and cell death (9). The members of the polyene antibiotic family usually possess a macrocyclic lactone ring formed from acetate and propionate by a polyketide pathway (5). The structure shared by compounds 2 and 3 suggests that compound 3 is no exception, likely being derived, as is compound 2, through the condensation of a propionate unit, an octanoate unit, and multiple acetate units, as is typical of polyketide biosynthesis (10). In summary, because of the encouraging results described above, our future work will focus on elucidating the antifungal action and biosynthetic pathway of compound 3.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grants 30960011 and 31071724) and the Natural Science Foundation of Jiangxi Province, China (grants 2009GZN0030 and 2010GZN0037).
Footnotes
Published ahead of print 4 November 2011
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Bentley SD, et al. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147. [DOI] [PubMed] [Google Scholar]
- 2. Bérdy J. 2005. Bioactive microbial metabolites. J. Antibiot. 58:1–26 [DOI] [PubMed] [Google Scholar]
- 3. Cope AC, Johnson HE. 1958. Fungichromin. Determination of the structure of the pentaene chromophore. J. Am. Chem. Soc. 80:1504–1506 [Google Scholar]
- 4. Frey Tirri B, Bitzer J, Geudelin B, Drewe J. 2010. Safety, tolerability and pharmacokinetics of intravaginal pentamycin. Chemotherapy 56:190–196 [DOI] [PubMed] [Google Scholar]
- 5. Harrison PH, Noguchi H, Vederas JC. 1986. Biosynthesis of polyene antibiotics: intact incorporation of 13carbon-labeled octanoate into fungichromin by Streptomyces cellulosae. J. Am. Chem. Soc. 108:3833–3834 [Google Scholar]
- 6. Kumarasamy KK, Toleman MA, Walsh TR. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurosawa K, et al. 2006. Characterization of Streptomyces MITKK-103, a newly isolated actinomycin X2-producer. Appl. Microbiol. Biotechnol. 72:145–154 [DOI] [PubMed] [Google Scholar]
- 8. Mirau PA, Shafer RH. 1982. High-resolution proton nuclear magnetic resonance analysis of conformational properties of biosynthetic actinomycin analogues. Biochemistry 21:2622–2626 [DOI] [PubMed] [Google Scholar]
- 9. Mulks MH, Nair MG, Putnam AR. 1990. In vitro antibacterial activity of faeriefungin, a new broad-spectrum polyene macrolide antibiotic. Antimicrob. Agents Chemother. 34:1762–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noguchi H, et al. 1988. Biosynthesis and full NMR assignment of fungichromin, a polyene antibiotic from Streptomyces cellulose. J. Am. Chem. Soc. 110:2938–2945 [Google Scholar]
- 11. Rahman H, et al. 2010. Novel anti-infective compounds from marine bacteria. Mar. Drugs 8:498–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shih HD, et al. 2003. Fungichromin: a substance from Streptomyces padanus with inhibitory effects on Rhizoctonia solani. J. Agric. Food Chem. 51:95–99 [DOI] [PubMed] [Google Scholar]
- 13. Shimizu M, Igarashi Y, Furumai T, Onaka H, Kunoh H. 2004. Identification of endophytic Streptomyces sp. R-5 and analysis of its antimicrobial metabolites. J. Gen. Plant Pathol. 70:66–68 [Google Scholar]
- 14. Sun Y, et al. 2003. A complete gene cluster from Streptomyces nanchangensis NS3226 encoding biosynthesis of the polyether ionophore nanchangmycin. Chem. Biol. 10:431–441 [DOI] [PubMed] [Google Scholar]
- 15. Sun Y, et al. 2002. ‘Streptomyces nanchangensis’, a producer of the insecticidal polyether antibiotic nanchangmycin and the antiparasitic macrolide meilingmycin, contains multiple polyketide gene clusters. Microbiology 148:361–371 [DOI] [PubMed] [Google Scholar]
- 16. Sun Y, Zhou X, Tu G, Deng Z. 2003. Identification of a gene cluster encoding meilingmycin biosynthesis among multiple polyketide synthase contigs isolated from Streptomyces nanchangensis NS3226. Arch. Microbiol. 180:101–107 [DOI] [PubMed] [Google Scholar]
- 17. Umezawa S, Tanaka Y, Ooka M, Shiotsu S. 1958. A new antifungal antibiotic, pentamycin. J. Antibiot. 11:26–29 [PubMed] [Google Scholar]
- 18. Zhang C, et al. 2008. Isolation, structure, and antibacterial activity of philipimycin, a thiazolyl peptide discovered from Actinoplanes philippinensis MA7347. J. Am. Chem. Soc. 130:12102–12110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.