Abstract
Enterococcus species composition was determined each hour for 72 h at a polluted marine beach in Avalon, Santa Catalina Island, CA. Species composition during the day was significantly different from that at night, based on an analysis of similarity. Enterococcus faecium and E. faecalis were more prevalent at night than during the day, while E. hirae and other Enterococcus species were more prevalent during the day than the night. Enterococcus spp. containing a yellow pigment were more common during the day than the night, suggesting that the pigmented phenotype may offer a competitive advantage under sunlit conditions. A laboratory microcosm experiment established that the pigmented E. casseliflavus isolate and a pigmented E. faecalis isolate recovered from the field site decay slower than a nonpigmented E. faecalis isolate in a solar simulator in simulated, clear seawater. This further supports the idea that the yellow carotenoid pigment in Enterococcus provides protection under sunlit conditions. The findings are in accordance with previous work with other carotenoid-containing nonphotosynthetic and photosynthetic bacteria that suggests that the carotenoid is able to quench reactive oxygen species capable of causing photoinactivation and photostress. The results suggest that using enterococcal species composition as a microbial source tracking tool may be hindered by the differential environmental persistence of pigmented and nonpigmented enterococci.
INTRODUCTION
Enterococci are fecal indicator organisms used to assess coastal water quality around the world. Their concentrations in marine coastal waters correlate to health risk in swimmers, based on data from epidemiology studies (33). There are dozens of enterococcal species (10). Recently, it has been suggested that a specific subset of these species can be characterized as environmental rather than fecal; specifically, Enterococcus gallinarum, E. casseliflavus, and E. mundtii have been proposed to be environmental strains due to their documented associations with plants, soils, and nonhuman animal hosts (1, 22), while E. faecalis and E. faecium have been suggested to represent fecal strains due to their prevalence in human feces (12, 22).
Photoinactivation of enterococci in marine waters is well documented in both field settings (4, 5, 27) and laboratory microcosms (28, 29). Enterococci can be inactivated via several different mechanisms. Direct photoinactivation results from the direct damage of cellular chromophores by photons; the most common example is UVB damage of DNA (20). Indirect photoinactivation results from the damage of cellular machinery and macromolecules via reactive species generated by photon-excited endogenous (intracellular) or exogenous (extracellular) sensitizers, such as colored dissolved organic matter and humic acids, the most important of which are believed to be reactive oxygen species (ROS), such as singlet oxygen, hydrogen peroxide, superoxide, and hydroxyl radical. There is presently a lack of knowledge about the relative importance of direct versus indirect photoinactivation and the relative importance of UVA, UVB, and visible radiation (2, 13, 20) in causing photodamage of fecal organisms in marine waters (27).
Some enterococcal species, particularly those thought to be associated with soil and nonhuman hosts (1), contain yellow pigments, including E. casseliflavus, E. mundtii, E. pallens, E. gilvus, and E. sulfureus (10, 21). The pigments of E. casseliflavus were investigated by Taylor et al. (30) and determined to be carotenoids. Pigments in E. sulfureus and E. mundtii were also identified to be carotenoids (6). Carotenoids function as protectors against photodamage, as they are able to quench ROS (15, 19). At this time, the effect of the carotenoid pigment in modulating photoinactivation of enterococci has not been studied. Experiments with Staphylococcus aureus, which also contains carotenoids, have shown that gene-knockout mutants that lack carotenoid synthesis capabilities are more susceptible to photodamage and death from ROS, including hydrogen peroxide, superoxide, and hydroxyl radical (8). Thus, there is reason to suspect that carotenoids in enterococci may provide protection against photooxidative damage and subsequent death.
The present study investigates the potential role of pigments in modulating enterococcal photoinactivation using field and laboratory data. For the purpose of this study, we define inactivation as the loss of culturability of bacteria. Hourly enterococcal species composition data were collected for 72 h at a polluted marine beach; these data include the proportion of pigmented enterococci. In addition, a microcosm experiment was carried out to test the relative photoinactivation rates of pigmented and nonpigmented Enterococcus strains. Results suggest that pigmented enterococci are protected from solar inactivation and can persist for extended periods of time in marine water relative to nonpigmented species, leading to differential environmental persistence of pigmented and nonpigmented enterococci.
MATERIALS AND METHODS
Field site.
Avalon Beach is located on Santa Catalina Island in the Southern California Bight. Waterborne enterococci at the beach are thought to emanate mainly from leaking sewer lines (3). Other enterococcal sources at the beach include sands and sediment, urban runoff, and wild animals, including birds (3).
Water sampling.
Water was collected every hour from 0400 h on 19 August to 0300 h on 22 August 2008 10 m north of the Pleasure Pier at Avalon Beach (33°20.9′N, 118°19.5′W). Samples were collected at ankle depth on an incoming wave in an ethanol-sterilized, triple-rinsed bucket and transferred to a 20-liter 10% hydrochloric acid-washed, triple-rinsed cube container. The water was stored in the dark in a cooler and was processed within 6 h of collection.
Irradiance and tidal predictions.
Simple Model of the Atmospheric Radiative Transfer of Sunshine (SMARTS) (16) was used to estimate UVB intensity each hour of the study. “Day” was used to describe times when there was sunlight, and “night” was used when there was no sunlight. Tidal predictions relative to mean sea level were obtained from online tidal calculators for Avalon, Santa Catalina Island, CA (http://tbone.biol.sc.edu/tide/). When the tide relative to mean sea level was greater than or equal to 0.8 m, it was deemed “high,” and all other tides were deemed “low.”
Enterococcal enumeration and species identification.
Enterococci were enumerated using EPA Method 1600 (32). The method uses membrane filtration and membrane-Enterococcus indoxyl-beta-d-glucoside (mEI) agar. Ten milliliters and 100 ml of each sample were filtered to obtain a countable number of colonies. Enterococcus concentrations obtained with this method over the 72 h are reported elsewhere (5). Ten presumptive enterococcus colonies from each time point were picked from mEI medium for species identification and subcultured onto tryptic soy agar (TSA) agar with 5% sheep blood (Northeast Laboratory, Winslow, ME) (here referred to as blood agar plates [BAPs]). In some cases, less than 10 colonies were archived due to lack of colony availability. Of the 72 time points, 10 colonies were obtained at 66 time points, 6 colonies at 2 time points, 5 colonies at 3 time points, and 3 colonies at 1 time point. After 24 h of incubation at 35°C, BAPs were assessed for strain purity by confirming that colonies were morphologically identical. Pure strains were subcultured from BAPs onto TSA slants (Northeast Laboratory, Winslow, ME) and incubated at 35°C for 24 h. TSA slants were subsequently stored at 4°C until species identification could be performed.
Isolates selected for species identification were subcultured from TSA slants onto BAPs 24 h prior to species identification. All isolates were first tested using the Vitek microbial identification system (bioMérieux, St. Louis, MO). Isolates identified as Enterococcus species but at low discrimination (<80% confidence level) were further tested using API 20S (bioMérieux, St. Louis, MO), an alternate species identification system used for Gram-positive cocci. Identifications by Vitek that were nondiscriminatory (e.g., E. casseliflavus/E. gallinarum) were tested using supplemental biochemical analyses, including motility, pigment production, and fermentation of arabinose, sucrose, and mannitol as per Facklam and Collins (11) and Ferguson et al. (12). These methods for enterococcus species identification are well established and have been shown to provide good agreement (>90%) with species identifications obtained using partial 16S rRNA sequencing (23). The presence or absence of pigment was determined for each isolate. The tip of a sterile cotton-tip swab was touched to the surface of a single colony grown on BPA and held under a lamp. Yellow colonies were characterized as pigmented, as described by Facklam and Collins (11).
Microcosm experiments.
The photoinactivation of nonpigmented Enterococcus faecalis (ATCC 19433), pigmented Enterococcus casseliflavus (ATCC 25788), and a lightly pigmented environmental isolate from Avalon Beach (identified as Enterococcus faecalis using the species identification procedures described previously and here referred to as E. faecalis AB) was tested. Experiments were performed in simulated seawater at pH 8.1. Simulated seawater was prepared in deionized water containing all major anions and cations, including carbonates (9, 14), and filter sterilized prior to use. All salts used were reagent grade or better.
Enterococcus spp. were grown in tryptic soy broth (E. faecalis and E. faecalis AB) or brain heart infusion broth (E. casseliflavus) to stationary phase at 37°C, as measured via spectrophotometry at a single wavelength of 650 nm. The density of cells at stationary phase was approximately 109 CFU/ml. Cells were then harvested by centrifuging and washed in phosphate-buffered saline (PBS; pH 7.32) three times. Cells were then resuspended in PBS (referred to as “seed stock”).
Fifty milliliters of simulated seawater and 0.5 ml of Enterococcus seed stock were placed in 100-ml sterile beakers wrapped in black electrical tape. No exogenous sensitizers (e.g., humic acid) were added because previous research indicated that the seawater at Avalon Beach was quite clear with few sensitizers (5). Beakers were placed in a recirculating water bath to maintain their temperature at 15°C, the temperature of seawater in central California, in a solar simulator (Altas Suntest CPS+; Linsengericht-Altenhaßlau, Germany) equipped with a coated quartz filter and a UV special glass filter to block the transmission of wavelengths below 290 nm to simulate natural sunlight (passing wavelength, 290 nm < λ < 800 nm) (26). The irradiance was set at 400 W/m2. The resulting light spectrum was measured using a spectroradiometer (RPS 200 and 380; International Light, Peabody, MA) and compared favorably to the light spectrum at Avalon Beach at 0900 h on 19 August 2008, as estimated using SMARTS. Stir bars in the beakers ensured that the solutions were well mixed throughout the experiment.
One-milliliter samples were withdrawn from the beakers every 15 to 30 min. Appropriate sampling time intervals were determined using data from pilot experiments. Dark controls were run; seeded simulated seawater was stored in the dark at 15°C, and samples were collected every 30 to 60 min.
Enterococcus colonies were enumerated in each sample by spread plating appropriate dilutions in duplicate on TSA for E. faecalis and E. faecalis AB and on brain heart infusion agar for E. casseliflavus. Agar plates were incubated at 37°C, and colonies were counted after 24 h.
Photoinactivation experiments were performed in duplicate for E. faecalis and in triplicate for E. faecalis AB and E. casseliflavus. Dark controls were performed in duplicate for E. faecalis and once for E. faecalis AB and E. casseliflavus. Data from all replicate experiments are presented and used without averaging in the determination of decay rates (see below).
Statistical methods.
Species composition was compared between day and night, as well high and low tides, using the PRIMER program, version 5 (PRIMER-E Ltd., Ivybridge, United Kingdom). Bray-Curtis similarity matrices were created from square-root-transformed standardized species composition data. Analysis of similarity (ANOSIM) was used to determine whether there were significant differences in species composition between samples collected during the day/night or high/low tides. Differences in the occurrence of specific enterococcal species, as well as pigmented versus nonpigmented enterococci during day versus night, were assessed using χ2 tests (IBM SPSS Statistics, version 19.0; Chicago, IL).
For the inactivation experiment, concentrations of Enterococcus at each time point were normalized by the initial concentrations at time zero (∼107 CFU/ml for all experimental conditions), and the natural logarithm of the normalized concentration was plotted as a function of time and fluence. Inactivation rate constants were calculated assuming Chick's law applied using linear least-squares regression on the portion of data where exponential decay was observed, following the approach taken by others (17, 27, 28). The decay rates (slopes of the lines) were compared for the three organisms using multiple linear regression (25) to determine if they were significantly different. When replicate experiments were run, data were not averaged prior to curve fitting.
RESULTS
Field observations.
The 72-h time series of presumptive enterococci measured using mEI medium was previously published by Boehm et al. (5). The data showed a statistically significant diurnal trend, with concentrations being lower during the day relative to the night, and subsequent modeling confirmed the influence of photoinactivation on their concentrations.
The species of a total of 690 presumptive enterococci (∼10 from each time point) were determined to be E. faecium, E. faecalis, E. gallinarum, E. hirae, E. casseliflavus, E. cecorum, E. durans, Aerococcus viridans, other enterococci, and nonenterococci (Table 1). The most common species was E. faecalis (308/690, 45%). The second most common species was the nonenterococcus Aerococcus viridans (119/690, 17%). Two of the isolates could not be identified and were designated as such. Of the isolates whose species were determined (n = 688), 82% were confirmed to be members of the genus Enterococcus.
Table 1.
Identification of isolates
| Species | No. of isolates | Simplified designationa |
|---|---|---|
| Enterococcus faecalis | 308 | Enterococcus faecalis |
| Aerococcus viridans | 119 | Aerococcus viridans |
| Enterococcus faecium | 88 | Enterococcus faecium |
| Enterococcus hirae | 70 | Enterococcus hirae |
| Enterococcus gallinarum | 56 | Enterococcus gallinarum |
| Enterococcus casseliflavus | 17 | Other enterococci |
| Enterococcus species | 8 | Other enterococci |
| Enterococcus durans | 8 | Other enterococci |
| Enterococcus cecorum | 6 | Other enterococci |
| Enterococcus mundtii | 4 | Other enterococci |
| Unidentified | 2 | Unidentified |
| Streptococcus mutans | 1 | Other nonenterococci |
| Streptococcus gallolyticus subsp. gallolyticus | 1 | Other nonenterococci |
| Leuconostoc spp. | 1 | Other nonenterococci |
| Pediococcus acidilactici | 1 | Other nonenterococci |
The simplified designations are those used in Fig. 1.
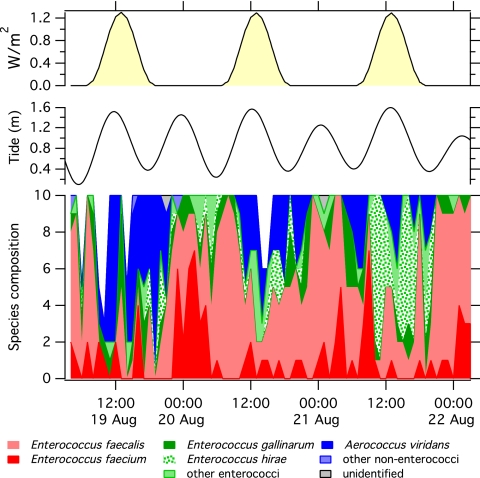
The time series of raw species composition relative to solar intensity and tide level is shown in Fig. 1. To simplify data display, less common organisms (those where n was ≤8) were grouped into categories of “other enterococci” and “other nonenterococci,” as specified in Table 1. A diurnal pattern in both presumptive and confirmed enterococcus species composition was observed. When all species identified at the 72 time points were considered, there was a significant difference in species composition between day and night samples (ANOSIM global R = 0.147, P = 0.002) but no difference in samples collected at high versus low tides (ANOSIM global R = −0.007, P = 0.54). Results were similar when only confirmed enterococcal species were considered; enterococcal species composition was significantly different in samples collected during the day versus the night (ANOSIM global R = 0.055, P = 0.02) but not different in samples collected during high versus low tide (ANOSIM global R = −0.002, P = 0.45).
Fig 1.
(Top) Tide level and UVB irradiance during the study; (bottom) number of each species (height of colored section at each time point) of the total number of isolates examined (designated by the height of the stacked colors).
Variation in the occurrence of specific enterococcal species or groups (Table 1) during day and night was examined using χ2 tests and contingency tables. E. faecium and E. faecalis constituted a greater proportion of the confirmed enterococci isolated during the night versus the day (χ2 test, P < 0.01 for both), while E. hirae and the other enterococci group (see Table 1 for definition) made up a greater proportion of the enterococci isolated during the day than the night (χ2 test, P < 0.05 for both).
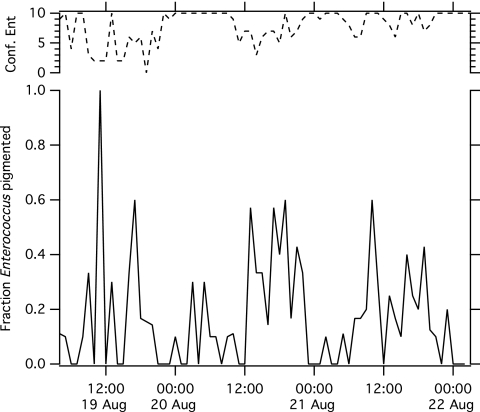
The presence of a yellow pigment was assessed for each isolate, and occurrence of pigmented species was compared between day and night. Of all 690 isolates examined, 151 were pigmented. The species of 42% of these were subsequently found to be A. viridans, and 20% were E. gallinarum, 13% E. hirae, 11% E. casseliflavus, 7% E. faecalis, 3% E. cecorum, and 1% E. faecium. Eighty-eight of the pigmented isolates were confirmed to be Enterococcus. The fraction of confirmed Enterococcus isolates displaying a pigmented phenotype at each time point is provided in Fig. 2. The proportion of pigmented Enterococcus isolates was higher during the day (24% of confirmed Enterococcus species) than the night (8% of confirmed Enterococcus species) (χ2 test, P < 0.0001). If we include all isolates, even nonenterococcal ones, a greater proportion of isolates were pigmented during the day (31% of isolates) than the night (12% of isolates) (χ2 tests, P < 0.0001).
Fig 2.
Fraction of confirmed pigmented Enterococcus strains at each time point. The top line is the total number of confirmed Enterococcus isolates of the ∼10 isolates examined.
Photoinactivation experiment.
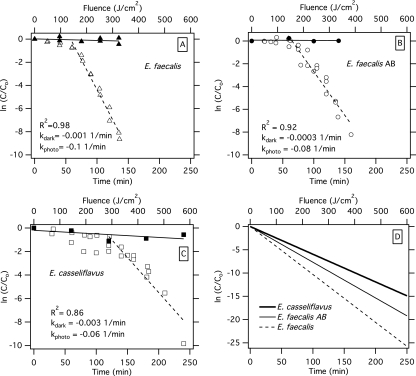
The photoinactivation of E. faecalis, E. faecalis AB, and E. casseliflavus in simulated seawater was compared using a solar simulator. When all three organisms were exposed to identical simulated sunlight, an initial period of limited inactivation (shouldering) when decline was similar to that observed in the dark controls was observed (Fig. 3). For the two E. faecalis strains, this lag period lasted 60 min; for the E. casseliflavus strain, the lag period lasted 120 min. Thereafter, exponential decay was observed for all three organisms. E. faecalis, E. faecalis AB, and E. casseliflavus had first-order decay rates (± standard deviation) of −0.1 ± 0.005, −0.08 ± 0.006, and −0.06 ± 0.007 min−1, respectively. These rates may also be expressed as a function of fluence (27, 31) as −0.04 ± 0.002, −0.03 ± 0.002, and −0.02 ± 0.003 cm2/J, respectively. Multiple linear regression indicated that the decay of the nonpigmented E. faecalis strains was significantly faster than the decay of the pigmented strains (P < 0.05 for all comparisons). There was limited decline of the microorganisms in the dark controls over the course of the experiments (Fig. 3).
Fig 3.
Decay of Enterococcus faecalis (A), Enterococcus faecalis AB (B), and Enterococcus casseliflavus (C) in simulated seawater in a solar simulator as a function of time (bottom axis) and fluence (top axis). Dark control data are provided. The best-fit line is provided for the experimental data after the shoulder (solid line, dark control; dashed line, photoinactivation treatment), and an r2 value is provided for the curve fit for the photoinactivation treatment. First-order decay rates (in units of 1/min) for the dark control (kdark) and photoinactivation treatment (kphoto) are provided. (D) Best-fit line for photoinactivation of the three organisms together on the same plot. C, concentration of Enterococcus measured at each time point; Co, initial concentration.
DISCUSSION
A diurnal pattern in enterococcal species composition was observed in hourly samples collected for 72 h at a marine beach in Avalon, Santa Catalina Island, CA. Fecal enterococcal species E. faecalis and E. faecium made up a greater fraction of the confirmed Enterococcus species at night than the day, while E. hirae and the other enterococci group (includes Enterococcus spp., E. casseliflavus, E. durans, E. cecorum, and E. mundtii) made up a greater fraction of the confirmed Enterococcus species during the day than the night. Although tidal and diel cycles are synchronized over a short period of 3 days, the tide was not an important controller of species composition. While there are certainly other controllers of species composition, including but not limited to temporally variable and differential sources, differential transport, grazing, and interaction with sediment, the results suggests that sunlight exposure influences the composition of enterococcal species at Avalon Beach.
A closer examination of enterococcal phenotypes indicates that a greater fraction of enterococci was pigmented during the day than the night, suggesting that the presence or absence of pigments could be one of the driving forces behind the observed diurnal pattern in species composition. Previous work with Staphylococcus aureus (8) and photosynthetic bacterial (19) mutants indicates that yellow carotenoid pigments, like those present in pigmented Enterococcus spp., are capable of quenching reactive oxygen species and play important roles in protecting organisms from photostress and photooxidation. Our experiments in the solar simulator indicate that a pigmented E. casseliflavus strain and a pigmented E. faecalis strain isolated from Avalon Beach decay more slowly than a nonpigmented E. faecalis strain. There are more differences between the tested organisms than just the presence or absence of a pigment. The nonpigmented and pigmented E. faecalis strains were not confirmed to be isogenic, and the pigmented E. faecalis strain was less pigmented than the E. casseliflavus strain, based on a qualitative evaluation of color. Regardless, the laboratory results suggest that the carotenoid pigment in the environmental E. faecalis AB and E. casseliflavus isolates may be capable of providing protection against photoinactivation.
An initial shouldering in the decay curves of all three tested Enterococcus spp. was observed. A shouldering effect during inactivation experiments has been observed by numerous researchers in experiments involving both viruses and bacteria and has been attributed to shielding of organisms from inactivation by other organisms or particles, the requirement that multiple hits are needed for inactivation, and a threshold effect where a cell can withstand a certain level of stress before death occurs (7, 27, 28, 31). In the present experiment, the shouldering effect lasted 1 h for the E. faecalis strains and 2 h for the E. casseliflavus strain. Additional work will need to be done to investigate the cause of the differential lag periods.
The laboratory study aids in the interpretation of the field results and suggests that the presence of pigments in enterococci affects the fate of these organisms in the environment. The field results are consistent with the following conceptual model. Exogenous or endogenous sources seed both pigmented and nonpigmented enterococci into seawater. Once the sun comes out, enterococci are inactivated due to either direct or indirect photoinactivation; however, the pigmented enterococci do not inactivate as quickly as the nonpigmented due to the protection provided by the carotenoid. The result is that during the day, pigmented enterococci have a competitive advantage over nonpigmented enterococci, and thus, their relative abundance may be higher.
These findings have implications for using enterococcus species identification for microbial source tracking. Environmental and nonhuman enterococcal species are more likely to have pigments than human enterococcal species (1, 24). Assuming that the differential fate of E. faecalis and E. casseliflavus strains observed in our microcosms is representative of the differential fate of nonpigmented and pigmented Enterococcus strains in general, the combined field and microcosm results suggest that species composition in sunlight-exposed water may not reflect that of the enterococcal source. During the dark, however, the ratio of pigmented to nonpigmented enterococci in water may better reflect the ratio of these organisms in their sources.
A secondary finding of this study is that the mEI medium used for the selective cultivation of enterococci allows the growth of Aerococcus viridans at Avalon Beach. Seventeen percent of the 690 screened presumptive enterococci were A. viridans. This is consistent with a study by Moore et al. (22) that also identified the enterococcal species from Avalon Bay during a different time period. However, they found 37% of their presumptive isolates to be A. viridans. The discrepancy in the percentages presented by Moore et al. and those presented herein might be explained by the fact that those authors sampled exclusively during sunlit hours. In the present study, 27% of isolates collected during sunlight hours were A. viridans, while just 6% of those species identified from nighttime samples were A. viridans. The daytime percentage is closer to that reported by Moore et al. Interestingly, 63 (53% of the 119 A. viridans isolates) were pigmented, which might indicate that this organism has a competitive advantage for surviving in sunlit waters.
There are several limitations of this study that should be acknowledged. First, only 10 presumptive isolates per time point were included in the species analysis. It would have been preferable to include more. However, samples in the presented statistical analyses were aggregated (by time of day or tide), so that a large number of isolates (between 400 and 500) were used in exploring various hypotheses. The microcosm studies compared pigmented versus nonpigmented Enterococcus photoinactivation and just begin to explore the potential advantage for Enterococcus to express the carotenoid pigment. More work is warranted to fully understand the role of the pigment in Enterococcus ecology. Additionally, we measured inactivation as loss of culturability; unculturable organisms may be dead (due to cell lysis, for example) but also may remain viable and potentially undergo photorepair. The dynamics of the viable but nonculturable population of enterococci deserves future attention. The results described here apply to clear waters; the presence of particles in water may shield bacteria from sunlight and modulate photoinactivation rates. Finally, Ho et al. (18) suggest that diurnal variation in enterococcus concentrations at Avalon may be due to anthropogenic currents generated by the arrival of ferries in the harbor. Although several lines of evidence presented herein suggest that sunlight plays a role in modulating enterococcal species composition, the potential for diurnal currents or mixing to affect species composition remains a possibility.
ACKNOWLEDGMENTS
A.B.B. and P.A.M. were supported by NSF CBET-0853988. P.A.M. was also supported by an NSF graduate fellowship.
We acknowledge the field assistance of Dave Love, Kara Nelson, Jee Yeon Kim, Kevan Yamahara, Kris McNeill, and Brit Peterson. Kara Nelson and Stanley Grant provided helpful comments on an earlier version of the manuscript. Three anonymous reviewers are acknowledged for their comments that improved the manuscript.
Footnotes
Published ahead of print 11 November 2011
REFERENCES
- 1. Bahirathan M, Puente L, Seyfried P. 1998. Use of yellow pigmented enterococci as a specific indicator of human and non-human sources of fecal pollution. Can. J. Microbiol. 44:1066–1071 [DOI] [PubMed] [Google Scholar]
- 2. Berney M, Weilenmann H-U, Ihssen J, Bassin C, Egli T. 2006. Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl. Environ. Microbiol. 72:2586–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boehm AB, Fuhrman JA, Mrse RD, Grant SB. 2003. Tiered approach for identification of a human fecal pollution source at a recreational beach: case study at Avalon Bay, Catalina Island, California. Environ. Sci. Technol. 37:673–680 [DOI] [PubMed] [Google Scholar]
- 4. Boehm AB, et al. 2002. Decadal and shorter period variability of surf zone water quality at Huntington Beach, California. Environ. Sci. Technol. 36:3885–3892 [DOI] [PubMed] [Google Scholar]
- 5. Boehm AB, et al. 2009. Covariation and photoinactivation of traditional and novel indicator organisms and human viruses at a sewage-impacted marine beach. Environ. Sci. Technol. 43:8046–8052 [DOI] [PubMed] [Google Scholar]
- 6. Breithaupt DE, Schwack W, Wolf G, Hammes WP. 2001. Characterization of the triterpenoid 4,4′-diaponeurosporene and its isomers in food associated bacteria. Eur. Food Res. Technol. 213:231–233 [Google Scholar]
- 7. Cebrián G, Michiels CW, Mañas P, Condón S. 2010. Biological approach to modeling of Staphylococcus aureus high-hydrostatic-pressure inactivation kinetics. Appl. Environ. Microbiol. 76:6982–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clauditz A, Resch A, Wieland K-P, Peschel A, Gotz F. 2006. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 74:4950–4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Department of Energy 1994. Handbook of methods for the analysis of various parameters of the carbon dioxide system in seawater, ORNL/CDIAC-74. Department of Energy, Washington, DC [Google Scholar]
- 10. Facklam RR, Carvalho Md-GS, Teixeira LM. 2002. History, taxonomy, biochemical characteristics and antibiotic susceptibility testing of enterococci. In Gilmore MS. (ed), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC [Google Scholar]
- 11. Facklam RR, Collins MD. 1989. Identification of Enterococcus species from human infections by a conventional test scheme. J. Clin. Microbiol. 27:731–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferguson DM, Moore DF, Getrich MA, Zhowandai MH. 2005. Enumeration and speciation of enterococci found in marine and intertidal sediments and coastal water in southern California. J. Appl. Microbiol. 99:598–608 [DOI] [PubMed] [Google Scholar]
- 13. Gourmelon M, Touati D, Pommepuy M, Cormier M. 1997. Survival of Escherichia coli exposed to visible light in seawater: analysis of rpoS-dependent effects. Can. J. Microbiol. 43:1036–1043 [DOI] [PubMed] [Google Scholar]
- 14. Grebel JE, Pignatello JJ, Song W, Cooper WJ, Mitch WA. 2009. Impact of halides on the photobleaching of dissolved organic matter. Mar. Chem. 115:134–144 [Google Scholar]
- 15. Griffiths M, Sistrom WR, Cohen-Bazire G, Stanier RY. 1955. Function of carotenoids in photosynthesis. Nature 176:1211–1214 [DOI] [PubMed] [Google Scholar]
- 16. Gueymard CA. 2005. Interdisciplinary applications of a versatile spectral solar irradiance model: a review. Energy 30:1551–1576 [Google Scholar]
- 17. Harm W. 1980. Biological effects of UV radiation. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 18. Ho LC, Litton RM, Grant SB. 2011. Anthropogenic currents and shoreline water quality in Avalon Bay, California. Environ. Sci. Technol. 45:2079–2085 [DOI] [PubMed] [Google Scholar]
- 19. Jensen SL. 1965. Biosynthesis and function of carotenoid pigments in microorganisms. Annu. Rev. Microbiol. 19:163–182 [DOI] [PubMed] [Google Scholar]
- 20. Malloy KD, Holman MA, Mitchell D, Detrich HW. 1997. Solar UVB-induced damage and photoenzymatic DNA repair in Antarctic zooplankton. Proc. Natl. Acad. Sci. U. S. A. 94:1258–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martinez-Murcia AJ, Collins MD. 1991. Enterococcus sulfureus, a new yellow-pigmented Enterococcus species. FEMS Microbiol. Lett. 80:69–74 [DOI] [PubMed] [Google Scholar]
- 22. Moore DF, Guzman JA, McGee CM. 2008. Species distribution and antimicrobial resistance of enterococci isolated from surface and ocean water. J. Appl. Microbiol. 105:1017–1025 [DOI] [PubMed] [Google Scholar]
- 23. Moore DF, et al. 2006. Comparison of 16S rRNA sequencing with conventional and commercial phenotypic techniques for identification of enterococci from the marine environment. J. Appl. Microbiol. 100:1272–1281 [DOI] [PubMed] [Google Scholar]
- 24. Mundt JO. 1963. Occurrence of enterococci in animals in a wild environment. Appl. Microbiol. 11:136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neter J, Wasserman W, Kutner MH. 1990. Applied linear statistical methods: regression, analysis of variance, and experimental designs, 3rd ed. Irwin, Homewood, IL [Google Scholar]
- 26. Plumlee MH, Reinhard M. 2007. Photochemical attenuation of N-nitrosodimethylamine (NDMA) and other nitrosamines in surface water. Environ. Sci. Technol. 41:6170–6176 [DOI] [PubMed] [Google Scholar]
- 27. Sinton LW, Davies-Colley RJ, Bell RG. 1994. Inactivation of enterococci and fecal coliforms from sewage and meatworks effluents in seawater chambers. Appl. Environ. Microbiol. 60:2040–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sinton LW, Finlay RK, Lynch PA. 1999. Sunlight inactivation of faecal bacteriophages and bacteria in sewage-polluted seawater. Appl. Environ. Microbiol. 65:3605–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sinton LW, Hall CH, Lynch PA, Davies-Colley RJ. 2002. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68:1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor RF, Ikawa M, Chesbro W. 1971. Carotenoids in yellow-pigmented enterococci. J. Bacteriol. 105:676–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thurston-Enriquez JA, Haas CN, Jacangelo J, Riley K, Gerba CP. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. U.S. Environmental Protection Agency 2006. Method 1600: enterococci in water by membrane filtration using membrane-Enterococcus indoxyl-beta-d-glucoside agar (mEI). EPA 821-R-06-009. Office of Water, U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- 33. Wade TJ, Pai N, Eisenberg J, Colford JM. 2003. Do US EPA water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ. Health Perspect. 111:1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]