Abstract
Past surveys of feral house fly populations have shown that Musca domestica salivary gland hypertrophy virus (MdSGHV) has a worldwide distribution, with an average prevalence varying between 0.5% and 10%. How this adult-specific virus persists in nature is unknown. In the present study, experiments were conducted to examine short-term transmission efficiency and long-term persistence of symptomatic MdSGHV infections in confined house fly populations. Average rates of disease transmission from virus-infected to healthy flies in small populations of 50 or 100 flies ranged from 3% to 24% and did not vary between three tested geographical strains that originated from different continents. Introduction of an initial proportion of 40% infected flies into fly populations did not result in epizootics. Instead, long-term observations demonstrated that MdSGHV infection levels declined over time, resulting in a 10% infection rate after passing through 10 filial generations. In all experiments, induced disease rates were significantly higher in male flies than in female flies and might be explained by male-specific behaviors that increased contact with viremic flies and/or virus-contaminated surfaces.
INTRODUCTION
Musca domestica salivary gland hypertrophy virus (MdSGHV) is a globally distributed, insect-pathogenic virus that infects adult house flies exclusively (13, 21, 31). This enveloped, nonoccluded, double-stranded DNA (dsDNA) virus causes symptomatic salivary gland hypertrophy (SGH) in both genders of M. domestica flies and suppresses ovarian development in infected females (7, 22). Thus, it has the potential to act as a sterilizing agent upon introduction into fly populations. The prevalence of symptomatic MdSGHV infection in house fly populations generally ranges from 0.5% to 10%, but occasional peaks as high as 34% have been reported (12). It is unknown how MdSGHV persists over time in nature. Our past research has demonstrated that MdSGHV is acquired only by adult flies, replicates in the salivary glands, and is transmitted horizontally during feeding and after exposure to virus-contaminated surfaces (12, 23). During one short feeding event (lasting 2 to 3 s), an infected fly releases roughly 106 virions, and the released virus is infectious when consumed by healthy flies (12, 23). However, flies subjected to force-feeding assays develop resistance to oral infection within 24 h after eclosion (31). Because feral flies are unlikely to commence foraging and feeding activities during this time window, the per os infection route alone does not appear to explain viral transmission and disease manifestation under natural conditions. Hitherto unidentified behaviors of and interactions between healthy and viremic flies may play a significant role in viral transmission and maintenance of viremia in fly populations.
It is possible that gender-specific behaviors also influence the transmission efficiency of MdSGHV. Our past research has shown that both genders of M. domestica flies are equally susceptible to oral infection with MdSGHV in force-feeding assays (23) and to infection via intrahemocoelic injection with viral inoculum (22). However, in several surveyed field populations, the incidence of infection was up to 2-fold higher in male than in female flies (7, 12). The former results indicate that there are no gender-specific differences in susceptibility to viral entry or suitability as a host for successful establishment of viral infection. The latter observations suggest various possible scenarios, such as higher mortality rates of infected females than males in the field, a higher chance of capturing infected males than females, and/or a higher transmission efficiency of MdSGHV to males than to females, potentially regulated by gender-specific behaviors. In vertebrates, hormone-mediated differences between genders can render males more susceptible to parasitism than females, a bias that may be masked by gender-specific behaviors that reduce the chance of male exposure to parasites (30, 33, 41). In contrast, little is known about gender biases in parasite or pathogen infections of invertebrates. Sheridan et al. (35) conducted a meta-analysis of 61 available data sets that compared gender-specific prevalences of infection of invertebrates (including crustaceans, ticks, and several insect orders) by various parasite taxa (including protozoans, fungi, helminths, and arthropods). Their results indicated the absence of a consistent, universal gender bias in infections among the examined invertebrate taxa; male-biased and female-biased prevalences of infection occurred at equal frequencies and under both natural and experimental conditions.
Another open question is whether MdSGHV, by sterilizing infected females, significantly hampers the intrinsic rate of increase in fly populations. If transmission and disease spread occur at sufficiently high rates, introduction of MdSGHV into fly populations may lead to an epizootic and result in a population collapse. However, our field surveys have not indicated such dynamics but instead suggest that MdSGHV infections are of a chronic (i.e., long-lasting) nature, accounting for persistent (i.e., long-term) low-level infection rates in fly populations (12, 13, 31).
To address some of the unknown features of MdSGHV-house fly interactions, we examined disease transmission dynamics as well as persistence of MdSGHV in confined fly populations. Our objective was to answer the following specific questions. Is symptomatic MdSGHV infection transmitted from infected flies to healthy flies within small, undisturbed populations? Does the gender of donor and/or exposed flies impact resulting infection rates? Finally, will MdSGHV persist in confined house fly populations over multiple generations?
MATERIALS AND METHODS
Fly rearing and general experimental conditions.
House flies were obtained from an established colony originating from flies collected near Orlando, FL. This virus-free colony is maintained at the United States Department of Agriculture, Center for Medical, Agricultural, and Veterinary Entomology, Gainesville, FL. Larvae were reared on a mixture of wheat bran, alfalfa, cornmeal, and water (14). Adult food consisted of a 6:6:1 mixture (by weight) of nonfat powdered milk, sugar, and powdered egg. Water for adults was provided in sealed plastic cups with inserted cotton wicks and exchanged when necessary. Rearing and experiments were carried out under constant conditions (26°C, 12-h–12-h light-dark cycle, 40% relative humidity).
Virus preparation and production of viremic donor flies.
Most experiments were conducted using the Florida type virus strain (MdSGHV03); in experiments examining transmission within small fly populations, additional strains from Denmark and New Zealand (MdSGHV15 and MdSGHV07, respectively) were included (31). These strains are maintained as part of the Insect Pathology Lab Virus Collection at the University of Florida, Entomology and Nematology Department, Gainesville, FL, and are stored in ready-to-use aliquots at −70°C (31). To produce cohorts of synchronously infected donor flies, newly emerged adults were cold immobilized and injected in the prothorax with 2.5 μl of virus inoculum (at 10−5 infected gland pair equivalent per microliter) as described by Lietze et al. (23). This virus dosage will induce SGH in 100% of challenged flies within 4 days postinjection (dpi). For transmission experiments, injected flies were used either unmarked or marked with a spot of yellow craft paint (Apple Barrel Colors) on the dorsal thorax.
MdSGHV transmission within small laboratory fly populations.
Two bioassays were conducted to examine transmission of MdSGHV from infected donor flies to healthy exposed flies within small, undisturbed fly populations. Bioassay 1 compared transmission of MdSGHV03 at two different ratios of infected donor to uninfected exposed flies (40:60 and 10:90). Bioassay 2 compared transmission of three different geographical strains, MdSGHV03 (Florida), MdSGHV07 (New Zealand), and MdSGHV15 (Denmark), reported previously to express different levels of infectivity (31), at a 40:60 ratio of donor flies to exposed flies. For both bioassays, marked, injected donor flies (n = 40 or 10 flies at a 1:1 gender ratio) were transferred to a wire-screened cage (30 × 30 × 20 cm3) containing water and food. Three days later, pupae (n = 63 or 95) that were expected to emerge within 2 to 3 days were added to each cage. With an estimated pupal mortality of 5%, the total number of adults per cage was expected to be 100. The outlined timing ensured full expression of SGH by the donor flies at the time that healthy exposed adults emerged from the pupae. Cages were maintained under constant conditions (defined above) and inspected daily to record and remove dead marked donor flies or unmarked exposed flies. Seven days after adult emergence from the pupae, all unmarked exposed flies and a subsample (maximum of 10 flies) of the marked donor flies were dissected to record symptoms of SGH. Three or four replicates per bioassay were conducted over time.
Gender-specific virus transmission.
Twenty marked, virus-injected (test) or saline-injected (control) donor flies were released in a plastic cage (12 × 12 × 16 cm3) containing water and food and maintained under constant conditions as described before. Each donor group consisted of either only females, only males, or an equal mix of both genders. Five days after introduction of marked donor flies, 30 newly emerged virgin flies (females, males, or both genders) were added to each cage, which then held a final fly population of 50 flies. Dead marked and unmarked flies were recorded and removed daily. Seven days after introduction of unmarked healthy flies, all unmarked flies and a subsample (n = 10) of marked donor flies were dissected to record symptoms of SGH. Each possible gender combination (total of nine per treatment) (see Table 2) was replicated over time (six times in tests and twice in controls).
Table 2.
Transmission of MdSGHV infection from infected donor to healthy exposed flies within 7 daysa
| Combination | Gender of viremic donor flies | Gender of healthy exposed fliesb | Total no. of flies dissected | % Transmissionc | % Survival | % Survival in controlsd |
|---|---|---|---|---|---|---|
| 1 | Mixed | Mixed | 173 | 14 ± 3 | 96 ± 2 | 93 ± 3 |
| Mixed | Mixed-female | 88 | 8 ± 3 | 98 ± 1 | 100 ± 0 | |
| Mixed | Mixed-male | 85 | 20 ± 4 | 94 ± 3 | 87 ± 7 | |
| 2 | Mixed | Female | 177 | 7 ± 3 | 98 ± 1 | 100 ± 0 |
| 3 | Mixed | Male | 163 | 24 ± 5 | 91 ± 5 | 95 ± 5 |
| 4 | Female | Mixed | 177 | 15 ± 3 | 98 ± 1 | 97 ± 3 |
| Female | Mixed-female | 88 | 5 ± 2 | 98 ± 1 | 100 ± 0 | |
| Female | Mixed-male | 89 | 25 ± 4 | 99 ± 1 | 93 ± 7 | |
| 5 | Female | Female | 176 | 9 ± 2 | 98 ± 2 | 98 ± 2 |
| 6 | Female | Male | 157 | 17 ± 6 | 87 ± 6 | 95 ± 2 |
| 7 | Male | Mixed | 163 | 10 ± 4 | 91 ± 5 | 95 ± 5 |
| Male | Mixed-female | 85 | 7 ± 2 | 94 ± 4 | 97 ± 3 | |
| Male | Mixed-male | 78 | 14 ± 5 | 87 ± 7 | 93 ± 7 | |
| 8 | Male | Female | 177 | 7 ± 2 | 98 ± 1 | 98 ± 2 |
| 9 | Male | Male | 177 | 12 ± 2 | 98 ± 2 | 88 ± 5 |
Results are based on six replicate assays per combination.
Mixed-female/mixed-male, females/males of a mixed-gender group.
Statistical comparisons are presented in the text.
Results for controls are based on two replicate assays per combination.
Persistence of MdSGHV in large laboratory fly populations.
To examine whether MdSGHV infection persisted over several generations in confined house fly populations, tests were set up in small, dome-shaped tents measuring approximately 1 cubic meter (Bugdorm cages; Megaview Science). The initial fly population consisted of 800 virus-injected and 1,200 healthy flies, making up a total population of 2,000 flies at an equal gender ratio and giving an initial infection rate of 40%. Control populations consisted of 2,000 healthy flies. At the time of introduction, all flies were between 24 and 54 h old. Each tent was provisioned with food and water supplies that were inspected twice per week and exchanged when necessary. Once a week, dead flies were removed, and a container with oviposition substrate (1:1 mix of fresh and spent house fly rearing medium) was introduced for 24 h to enable females to deposit eggs. The substrate was then removed and transferred to fresh medium to allow continued development of progeny larvae under constant rearing conditions. Larval medium was adjusted (larvae culled or additional medium added) to allow production of 2,000 to 3,000 pupae from each tent. Pupae were harvested after 7 days, and approximately 1,000 pupae were reintroduced into their tent of origin at the first sign of adult emergence. Upon completion of adult emergence (within 48 h of introduction), the remaining pupae were removed to prevent oviposition by gravid females around empty puparia. This procedure maintained a constant population size of approximately 2,000 flies. For diagnostics, a weekly random sample of 100 flies was removed by aspiration and dissected to record gender and SGH symptoms. The experiment was conducted in three replicates, and data were collected over a period of 12 weeks.
Statistical analyses.
Statistical analyses were conducted using the Statistical Analysis System (SAS) for Windows (32). For short-term experiments, transmission rates (i.e., the ratio of infected flies to the total number of flies examined after a 7-day exposure to virus-infected flies) and mortality rates (number of dead flies per total number of exposed flies) were analyzed by logistic regression using the Genmod procedure of SAS, with “strain,” “treatment,” “gender,” and/or “gender combination” as class variables; means were separated using the least-squares statement of SAS (28). For the long-term, multigenerational experiment, differences between infection rates of males and females per time interval were analyzed by t test comparisons of square-root-transformed percent data, using the t test procedure of SAS (6, 20). All data are presented as untransformed means ± standard errors.
RESULTS
MdSGHV transmission within small laboratory fly populations.
Dissection of virus-injected donor flies verified 100% infection (n = 254), whereas none of the saline-injected donor flies showed SGH symptoms (n = 31). None of the flies that were exposed to saline-injected control flies showed SGH symptoms when dissected 7 days after emergence (Table 1). The average transmission rates of MdSGHV from infected donor flies to exposed healthy flies ranged from 3% ± 2% to 22% ± 6% (Table 1). With the exception of strain MdSGHV07, transmission of MdSGHV to male flies was significantly higher than that to female flies in both bioassays (P ≤ 0.0494; χ2 ≥ 3.86; df = 1). In bioassay 1, transmission of MdSGHV03 occurred at a higher rate when 40% of the population was initially infected and was lower (reduced by half) when donor flies accounted for 10% of the population (Table 1). These differences were statistically significant for comparing infection rates of both genders and males (P = 0.0044 and 0.0197, respectively; χ2 = 8.11 and 5.44, respectively; df = 1). In bioassay 2, transmission of MdSGHV03 and MdSGHV15 occurred at similar rates and was higher than the transmission of MdSGHV07. Significantly fewer males became infected when exposed to flies infected with MdSGHV07 than when exposed to flies infected with MdSGHV15 (P = 0.0375; χ2 = 4.33; df = 1).
Table 1.
Transmission of MdSGHV strains from infected donor flies to healthy exposed flies in small laboratory fly populationsa
| Bioassay | MdSGHV strain | % Donor fliesb | Gender of exposed healthy fliesc | Mean transmission ± SEd | Total no. of flies dissected |
|---|---|---|---|---|---|
| 1 | Saline (control) | 10 | Female + male | 0 ± 0a | 356 |
| Female | 0 ± 0a | 204 | |||
| Male | 0 ± 0a | 152 | |||
| MdSGHV03 | 10 | Female + male | 6 ± 1bc | 270 | |
| Female | 3 ± 2ab | 158 | |||
| Male | 10 ± 1cd | 112 | |||
| 40 | Female + male | 13 ± 3d | 238 | ||
| Female | 6 ± 1bc | 131 | |||
| Male | 22 ± 6e | 107 | |||
| 2 | MdSGHV03 | 40 | Female + male | 8 ± 3ab | 175 |
| Female | 4 ± 1a | 96 | |||
| Male | 15 ± 8bc | 79 | |||
| MdSGHV07 | 40 | Female + male | 5 ± 2a | 173 | |
| Female | 4 ± 3a | 88 | |||
| Male | 6 ± 2ab | 85 | |||
| MdSGHV15 | 40 | Female + male | 10 ± 4abc | 178 | |
| Female | 3 ± 2a | 92 | |||
| Male | 17 ± 7c | 86 |
Healthy flies were introduced as pupae at 3 dpi of the donor flies and emerged at 5 dpi.
Total number of flies per cage = 100.
Exposed healthy flies were dissected 7 days after eclosion to record SGH symptoms.
Numbers per bioassay followed by different letters are significantly different (P ≤ 0.05; SAS Proc Genmod with least-squares mean statement).
Mortality rates of the exposed flies were low, with an average of 94% ± 1% (minimum, 87%; maximum, 97%; N = 20 assays; n = 1,484 exposed flies) of the flies surviving the 7-day duration of the assays. There was no significant difference in mortality between any of the virus and control treatments in these bioassays (for bioassay 1, P ≥ 0.5680, χ2 ≤ 0.33, and df = 1; for bioassay 2, P ≥ 0.3206, χ2 ≤ 0.99, and df = 1; and for both bioassays 1 and 2, P ≥ 0.1711, χ2 ≤ 1.87, and df = 1).
Gender-specific virus transmission.
Dissection of virus-injected donor flies verified 100% infection (n = 508), whereas none of the saline-injected donor flies showed SGH symptoms (n = 178). None of the flies that were exposed to saline-injected control flies showed SGH symptoms when dissected 7 days after initial exposure (n = 516). The average rates of transmission of MdSGHV from infected donor flies to exposed healthy flies ranged from 5% ± 2% to 24% ± 5% (Table 2). In all nine tested combinations, transmission of MdSGHV to male flies was higher than that to female flies (Table 2), and except for the combinations with only male donor flies, these differences were statistically significant (P ≤ 0.0263; χ2 ≥ 4.93; df = 1).
Comparing the transmission of MdSGHV from donor groups composed of both females and males (mixed gender), females only, or males only to a specific group of exposed flies (mixed gender, females only, females in a mixed-gender group, males only, or males in a mixed-gender group), transmission was not influenced by the gender (or gender combination) of the donor flies when the exposed flies were mixed-gender groups (P ≥ 0.2398; χ2 ≤ 1.38; df = 1), females only (P ≥ 0.5386; χ2 ≤ 0.38; df = 1), females in mixed-gender groups (P ≥ 0.3561; χ2 ≤ 0.85; df = 1), or males in mixed-gender groups (P ≥ 0.0891; χ2 ≤ 2.89; df = 1). When the exposed flies were males only, transmission from a male-only donor group (12% ± 2%) was significantly lower than that from a mixed-gender donor group (24% ± 5%; P ≤ 0.0061; χ2 ≥ 7.51; df = 1), whereas transmission from a female-only donor group (17% ± 6%) was intermediate and not significantly different from either of the aforementioned scenarios (P ≥ 0.0935; χ2 ≤ 2.81; df = 1).
Mortality rates of the exposed flies were low, with an average of 95% ± 1% (minimum, 57%; maximum, 100%; N = 72 assays; n = 2,160 exposed flies) of the flies surviving the 7-day duration of the assays. There was no significant difference in mortality between any of the treatment and control assays in these experiments (P = 0.6891; χ2 = 0.16; df = 1).
Persistence of MdSGHV in large laboratory fly populations.
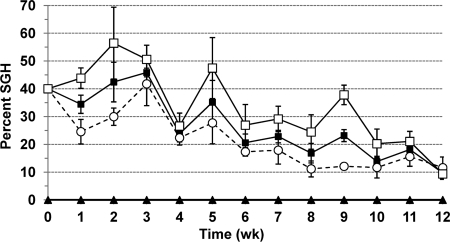
Throughout the 12-week duration of the experiment, MdSGHV was detected continuously in sampled flies from the tents initially stocked with viremic flies and was passed through a total of 10 filial generations. The initial infection rate of the parental generation in each replicate population (2,000 flies) was 40%. One week after setup of the experiment, infection rates were 0% and 34% ± 3% in control and viremic populations, respectively (Fig. 1). During the 12-week observation period, no infection was recorded in control populations, whereas infection persisted in viremic populations (Fig. 1). Infection rates fluctuated but declined steadily to an average of 10% ± 3% after 12 weeks. As shown in Fig. 1, infection rates were consistently higher in males than in females, with differences being statistically significant at weeks 1 and 9 (P = 0.0359 and 0.0011, respectively; t = −3.11 and −8.48, respectively; df = 4). At the end of the experiment, 1,605 ± 112 and 1,356 ± 124 flies were alive in the control and viremic populations, respectively.
Fig 1.
Persistence of SGH symptoms in house fly populations of approximately 2,000 flies maintained and reared in small 1-m3 tents. The initial population consisted of 40% MdSGHV-injected (virus) or uninjected (control) flies and 60% uninjected flies. Percent SGH was determined weekly by dissecting samples of 100 flies per replicate tent. Eggs of filial generations were removed, reared to pupae, and reintroduced into the tents before adult emergence to maintain a constant population size. Solid squares and triangles represent average infection rates of mixed-gender samples from virus and control treatments, respectively. Empty circles and squares show average infection rates of dissected females and males, respectively, from viremic populations. Bars indicate standard errors.
DISCUSSION
All experiments demonstrated that in undisturbed, confined house fly populations, MdSGHV was transmitted from infected to healthy flies and subsequent infection rates were higher in exposed male than female flies. Gender biases (male or female) in invertebrate infections have been reported for a range of host and parasite taxa (35). These biases, if discussed at all, were attributed to sexual selection pressures (39) or physiological and/or morphological differences between male and female hosts (17, 18, 29). Our results indicate that behavioral differences, e.g., the frequency and/or intensity of interactions between infected and healthy flies of the same or opposite gender, may also impact the probability of a host to acquire infection. Differences in susceptibility to infections and suitability as a host for MdSGHV may be ruled out for M. domestica, based on results from force-feeding assays and intrahemocoelic injections (22, 23). MdSGHV, unlike other entomopathogenic viruses, does not cause asymptomatic or covert infections (21), and therefore we determined a successful disease transmission from infected flies to conspecifics by recording the presence or absence of hypertrophied salivary glands in the exposed flies. In the following discussion, the terms “transmission” and “transmission efficiency” indicate MdSGHV transmission that led to manifestation of the disease (SGH) and imply successful pathogenesis after entering the host.
One of our experiments specifically addressed the gender-biased transmission efficiency of MdSGHV from infected donor flies to exposed healthy flies and confirmed that symptomatic MdSGHV infection was transmitted at significantly higher rates to males than to females. Furthermore, when exposed groups were composed of males only, the rate of disease transmission from mixed-gender donor groups was 2-fold higher than that from male-only donor groups, while the rate of transmission from female-only donor groups was intermediate. If interactions between flies play an important role in viral transmission, these results indicate that (i) healthy males interacted more with conspecifics than healthy females did, (ii) they interacted more with infected females than with infected males, and (iii) they interacted more with infected females when other (infected) males were present.
Male house flies are more gregarious and avid than females and, especially when young, attempt to copulate with any nearby flying or inanimate object (36), a behavior that would increase the chance of male flies coming into contact with any of the infected donor flies in the transmission experiments. Previous mating experiments conducted with MdSGHV-infected and healthy M. domestica flies clearly showed that MdSGHV was not transmitted during copulation (22); in these experiments, each copulation lasted between 53 min and 82 min (V.-U. Lietze, unpublished data), and afterwards, flies were separated and maintained individually. Interactions between infected and healthy flies in the present study may have involved repeated mounting, grooming, wounding, cofeeding on the food substrate and water source, and consumption of secretions and excretions (vomit and fecal spots) deposited by conspecifics.
Another possible explanation for more efficient virus transmission to males than to females could be gender-specific differences in feeding behavior resulting in more uptake by males of virus particles from food that was contaminated with MdSGHV by infected donor flies. It is known that MdSGHV-infected flies, regardless of gender, release copious levels of infectious virus particles when they salivate onto food substrates (12, 23). However, fly age seems to play a critical role in susceptibility of adults to oral MdSGHV infection, and resistance increases drastically within 6 h after emergence (31). Substantial feeding within this short time window after emergence is highly unlikely to occur for either gender. Besides oral administration or injection of MdSGHV preparations, it is also possible to infect house flies by submersion in homogenates of infected flies and by topical application of viremic salivary gland homogenates by using a Potter spray tower, but the mechanisms involved in these infection processes are not understood (13). Taking into account all of the above considerations, we propose that grooming behaviors and activities resulting in cuticular damage (wounding) may play an as yet unidentified but significant role in MdSGHV transmission.
The dynamics of virus transmission and dissemination within insect host populations may be influenced by various factors (4, 10, 19), including susceptibility of the host; behavior and movements of susceptible hosts (partially discussed above); infectivity and pathogenicity of the virus; geographic origin (genetic variability) of the virus and/or host; size and density of the susceptible host population; virus density, i.e., number of infected hosts (or free-living infectious organisms) per number of susceptible hosts; distribution of the virus (infected hosts) and/or susceptible hosts within the population; and a number of environmental conditions.
Individual house flies are known to display an inherent, hitherto unexplained variability in susceptibility to oral infection with MdSGHV. Force-feeding of newly emerged flies with viral inoculum (administered at 1 × 10−4 infected salivary gland pair equivalent per fly) never produced 100% infection but resulted in rates ranging from 30% to 79%, with no difference between treated females and males (23, 31). Although genetic variability is known to impact the insect immune system, it should be noted that the flies used in this and previous studies originated from a highly inbred, genetically homogenous colony that has been in culture for over 30 years. With regard to horizontal per os transmission of viruses within insect populations, other factors that may influence host susceptibility would include starvation or food stress, variation in food quality, and variation in age or developmental stage (5). The house flies in the present experiments were unlikely to have suffered from starvation, food stress, or exposure to variable food quality, because the same highly nutritious food was provided ad libitum in all experiments. Adult age, on the other hand, may have played a role in MdSGHV transmission because, as mentioned above, M. domestica adults do develop resistance to oral MdSGHV infection soon after emergence. In this study, all of the exposed flies were introduced as pupae or newly emerged flies to cages or small tents containing a group of infected flies, and it is possible that MdSGHV transmission within these confined fly populations occurred only during a short time window. Future experiments in which infected and exposed flies are separated after different exposure times could help to define such a time window.
Differences in biological activity of geographical isolates or strains have been documented for other entomopathogenic viruses (10, 16, 34, 37). We previously performed phylogenetic analyses of 16 MdSGHV strains by comparing 600-bp nucleotide sequences from each of five open reading frames that have homology to genes encoding DNA polymerase and partial homology to the genes encoding four per os infectivity factor proteins (p74, pif-1, pif-2, and pif-3). The results showed that nucleotide sequences from all strains were highly homologous and that detected polymorphisms were correlated with geographic source (31). While strains MdSGHV03 (Florida) and MdSGHV07 (New Zealand) clustered together, MdSGHV15 (Denmark) clearly belonged to a separate clade. Parallel force-feeding assays showed different infectivities of various strains against newly emerged and 24-h-old flies (31). Significantly, strains MdSGHV07 and MdSGHV15 induced much higher average infection rates in older flies (16% and 26%, respectively) than did the Florida type strain, MdSGHV03 (3%). However, when overall transmission rates from infected to healthy flies in mixed-gender groups were compared in the transmission experiments presented herein, all three geographical strains appeared equally infective.
Early models of disease dynamics relied on the basic “mass action” assumption that the rate of horizontal pathogen transmission in a host population is directly proportional to the product of the densities of the pathogen and susceptible hosts (2, 3). This assumption has been challenged by several researchers (8, 9, 19, 26). A linear relationship between transmission efficiency and densities of virus and hosts may exist only within a limited range of densities. The above-cited researchers tested the mass action assumption experimentally by using different insect-virus model systems, such as the nucleopolyhedrovirus (LdNPV) of the gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae) (8, 9), the granulovirus (PiGV) of the Indian meal moth, Plodia interpunctella (Lepidoptera: Pyralidae) (19), and the invertebrate iridescent virus (IIV6) of the mosquito Aedes aegypti (Diptera: Culicidae) (26). Their studies established that changes in virus or host density caused changes in behavioral and/or physiological host traits that would modulate either host susceptibility or distribution patterns of either the virus or the host. Thus, disease dynamics could not be explained by the basic mass action assumption. In the present study, increasing the initial proportion of infected flies 4-fold, from 10% to 40%, while maintaining the same population size, increased MdSGHV transmission about 2-fold (from 6% to 13%). During random observations, flies were often aggregated around the small food and water sources, a behavior that theoretically could enhance the spread of an orally transmitted virus.
Introducing a large proportion of infected virus donors (40%) into a confined population under crowded conditions was expected to enhance disease transmission and lead to an epizootic. Knowing that MdSGHV infection reduces longevity and completely sterilizes female flies (22), we also hypothesized that a confined population with a large proportion of infected flies might eventually collapse. In contrast, the long-term experiment involving large confined laboratory populations showed that MdSGHV persisted over multiple fly generations. Populations maintained sufficient numbers of healthy adults to produce offspring. It should be noted that eggs were removed, reared to pupation, and reintroduced weekly to maintain a constant population size of about 2,000 flies. The observed increase of the infection rate above 40% within the first 3 weeks of the experiment (despite the weekly “dilution” of infected flies with newly introduced healthy flies) may be explained by an increased likelihood to aspirate older, infected flies during sampling. Aging infected flies (>10 days old) move slower than healthy flies and can readily be captured by hand (V.-U. Lietze, personal observation). Based on available mortality data (22), all of the initially infected flies should have died after 3 to 4 weeks. The prevalence of MdSGHV persisted but declined over time, resulting in a 10% infection rate after passing through 10 filial generations. This decline approached the levels of infection observed in the field, which generally range from 0.5% to 10% (7, 12, 13).
The closest known relative of MdSGHV is a virus (GpSGHV) that infects the hematophagous tsetse flies Glossina pallidipes and Glossina morsitans (Diptera: Glossinidae) (11). This virus is transmitted horizontally and vertically and sterilizes both genders of the host. Unlike MdSGHV, GpSGHV persists in tsetse fly populations in an asymptomatic state but can be activated to the symptomatic state by unknown triggers, causing a complete collapse of laboratory colonies (1). In field populations of tsetse flies, the prevalence of symptomatic GpSGHV infection is generally low and comparable to that of MdSGHV infection in feral house flies (21). While the vertical transmission route may prevail in field populations, Abd-Alla et al. (1) demonstrated that laboratory rearing conditions that involved simultaneous or successive cofeeding on blood sources significantly enhanced horizontal virus transmission and that consumption of virus-contaminated blood meals activated virus production in previously asymptomatic flies.
A compelling example of efficient dissemination within field populations is the lethal viremia caused by the enveloped, nonoccluded dsDNA Oryctes virus (OrV) that infects the coconut palm rhinoceros beetle, Oryctes rhinoceros (Coleoptera: Scarabaeidae) (15). Adult beetles become infected by consuming virus-contaminated excretions of infected beetles, which they encounter during mating, cofeeding on the host plant, or visits to larval breeding sites (27, 40). Within larval breeding sites, the virus is transmitted to both larvae and adults via contact with adult feces or virus-killed larval cadavers (40). Owing to its efficient autonomous epizootic spread, inoculative releases of OrV-infected adults have been very successful in reducing O. rhinoceros populations in coconut plantations on a number of South Pacific islands (15). In contrast, other nonoccluded DNA viruses, such as mosquito iridescent viruses (MIVs), show very low oral transmission efficiencies on susceptible host larvae (24, 25, 38). Undeen and Fukuda (38) were able to increase per os infection rates significantly by administering virus in combination with abrasive materials, suggesting that MIV of Aedes (Ochleratatus) taeniorhynchus (Diptera: Culicidae) has no active means of penetrating the host and invades only through random breaks in the peritrophic matrix or cuticle.
In summary, the results of this study demonstrate that (i) MdSGHV is transmitted from infected to healthy flies within small, undisturbed populations; (ii) induced infection rates are higher in exposed male than female flies and in certain cases are influenced by the gender or gender combination of the donor flies; and (iii) introduction of MdSGHV-infected flies into confined populations does not produce epizootics but results in a persistent, although declining, prevalence of viral infection during an observation period of 12 weeks. Key elements of MdSGHV transmission and persistence in house fly populations still remain unknown. Future investigations are needed to identify the mechanisms regulating host resistance to oral infection, examine behavioral and physiological factors responsible for higher infection rates in males than in females, and explain how epizootics and eradication of fly populations by MdSGHV are prevented under natural conditions.
ACKNOWLEDGMENT
We acknowledge partial financial support by the USDA National Research Initiative (NRI 2007-35302-18127).
Footnotes
Published ahead of print 4 November 2011
REFERENCES
- 1. Abd-Alla AMM, et al. 2010. Dynamics of the salivary gland hypertrophy virus in laboratory colonies of Glossina pallidipes (Diptera: Glossinidae). Virus Res. 150:103–110 [DOI] [PubMed] [Google Scholar]
- 2. Anderson RM, May RM. 1979. Population biology of infectious diseases. Nature 280:361–367 [DOI] [PubMed] [Google Scholar]
- 3. Anderson RM, May RM. 1981. The population dynamics of microparasites and their invertebrate hosts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 291:451–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andreadis TG. 1987. Transmission, p 159–176. In Fuxa JR, Tanada Y. (ed), Epizootiology of insect diseases. John Wiley & Sons, New York, NY [Google Scholar]
- 5. Beisner BE, Myers JH. 1999. Population density and transmission of virus in experimental populations of the western tent caterpillar (Lepidoptera: Lasiocampidae). Environ. Entomol. 28:1107–1113 [Google Scholar]
- 6. Cody RP, Smith JK. Applied statistics and the SAS programming language. Pearson Prentice Hall, Upper Saddle River, NJ. 2006 [Google Scholar]
- 7. Coler RR, et al. 1993. Characterization and description of a virus causing salivary gland hyperplasia in the housefly, Musca domestica. Med. Vet. Entomol. 7:275–282 [DOI] [PubMed] [Google Scholar]
- 8. D'Amico V, Elkinton JS, Dwyer G, Burand JP, Buonaccorsi JP. 1996. Virus transmission in gypsy moths is not a simple mass action process. Ecology 77:201–206 [Google Scholar]
- 9. Dwyer G, Elkinton JS, Buonaccorsi JP. 1997. Host heterogeneity in susceptibility and disease dynamics: tests of a mathematical model. Am. Nat. 150:685–707 [DOI] [PubMed] [Google Scholar]
- 10. Escribano A, et al. 1999. Selection of a nucleopolyhedrovirus for control of Spodoptera frugiperda (Lepidoptera: Noctuidae): structural, genetic, and biological comparison of four isolates from the Americas. J. Econ. Entomol. 92:1079–1085 [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Maruniak A, et al. 2009. Two viruses that cause salivary gland hypertrophy in Glossina pallidipes and Musca domestica are related and form a distinct phylogenetic clade. J. Gen. Virol. 90:334–346 [DOI] [PubMed] [Google Scholar]
- 12. Geden CJ, Lietze V-U, Boucias D. 2008. Seasonal prevalence and transmission of salivary gland hypertrophy virus of house flies (Diptera: Muscidae). J. Med. Entomol. 45:42–51 [DOI] [PubMed] [Google Scholar]
- 13. Geden CJ, Steenberg T, Lietze V-U, Boucias DG. Salivary gland hypertrophy virus of house flies in Denmark: prevalence, host range, and comparison with a Florida isolate. J. Vector Ecol., in press [DOI] [PubMed] [Google Scholar]
- 14. Hogsette JA. 1992. New diets for production of house flies and stable flies (Diptera: Muscidae) in the laboratory. J. Econ. Entomol. 85:2291–2294 [DOI] [PubMed] [Google Scholar]
- 15. Huger AM. 2005. The Oryctes virus: its detection, identification, and implementation in biological control of the coconut palm rhinoceros beetle, Oryctes rhinoceros (Coleoptera: Scarabaeidae). J. Invertebr. Pathol. 89:78–84 [DOI] [PubMed] [Google Scholar]
- 16. Hughes PR, Gettig RR, McCarthy WJ. 1983. Comparison of the time-mortality response of Heliothis zea to 14 isolates of Heliothis nuclear polyhedrosis virus. J. Invertebr. Pathol. 41:256–261 [Google Scholar]
- 17. Kaaya GP. 1989. Glossina morsitans morsitans: mortalities caused in adults by experimental infection with entomopathogenic fungi. Acta Trop. 46:107–114 [DOI] [PubMed] [Google Scholar]
- 18. Kaaya GP, Darji N. 1988. The humoral defense system in tsetse—differences in response due to age, sex and antigen types. Dev. Comp. Immunol. 12:255–268 [DOI] [PubMed] [Google Scholar]
- 19. Knell RJ, Begon M, Thompson DJ. 1998. Transmission of Plodia interpunctella granulosis virus does not conform to the mass action model. J. Anim. Ecol. 67:592–599 [Google Scholar]
- 20. Köhler W, Schachtel G, Voleske P. 1992. Biostatistik. Einführung in die Biometrie für Biologen und Agrarwissenschaftler. Springer, Berlin, Germany [Google Scholar]
- 21. Lietze V-U, Abd-Alla AMM, Vreysen MJB, Geden CJ, Boucias DG. 2011. Salivary gland hypertrophy viruses: a novel group of insect pathogenic viruses. Annu. Rev. Entomol. 56:63–80 [DOI] [PubMed] [Google Scholar]
- 22. Lietze V-U, Geden CJ, Blackburn P, Boucias DG. 2007. Effects of salivary gland hypertrophy virus on the reproductive behavior of the housefly, Musca domestica. Appl. Environ. Microbiol. 73:6811–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lietze V-U, Sims KR, Salem TZ, Geden CJ, Boucias DG. 2009. Transmission of MdSGHV among adult house flies, Musca domestica (Diptera: Muscidae), occurs via salivary secretions and excreta. J. Invertebr. Pathol. 101:49–55 [DOI] [PubMed] [Google Scholar]
- 24. Linley JR, Nielsen HT. 1968. Transmission of a mosquito iridescent virus in Aedes taeniorhynchus. II. Experiments related to transmission in nature. J. Invertebr. Pathol. 12:17–24 [DOI] [PubMed] [Google Scholar]
- 25. Linley JR, Nielsen HT. 1968. Transmission of a mosquito iridescent virus in Aedes taeniorhynchus. I. Laboratory experiments. J. Invertebr. Pathol. 12:7–16 [DOI] [PubMed] [Google Scholar]
- 26. Marina CF, et al. 2005. Transmission dynamics of an iridescent virus in an experimental mosquito population: the role of host density. Ecol. Entomol. 30:376–382 [Google Scholar]
- 27. Monsarrat P, Veyrunes JC. 1976. Evidence of Oryctes virus in adult feces and new data for virus characterization. J. Invertebr. Pathol. 27:387–389 [Google Scholar]
- 28. Neter J, Wasserman W, Kutner MH. 1990. Applied linear statistical models: regression, analysis of variance, and experimental designs. Richard Irwin Inc., Boston, MA [Google Scholar]
- 29. Nigam Y, Maudlin I, Welburn S, Ratcliffe NA. 1997. Detection of phenoloxidase activity in the hemolymph of tsetse flies, refractory and susceptible to infection with Trypanosoma brucei rhodesiense. J. Invertebr. Pathol. 69:279–281 [DOI] [PubMed] [Google Scholar]
- 30. Poulin R. 1996. Sexual inequalities in helminth infections: a cost of being a male? Am. Nat. 147:287–295 [Google Scholar]
- 31. Prompiboon P, et al. 2010. Musca domestica salivary gland hypertrophy virus: a globally distributed insect virus that infects and sterilizes female houseflies. Appl. Environ. Microbiol. 76:994–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. SAS 2004. User's guide, version 9.1. SAS Institute, Cary, NC [Google Scholar]
- 33. Schalk G, Forbes MR. 1997. Male biases in parasitism of mammals: effects of study type, host age, and parasite taxon. Oikos 78:67–74 [Google Scholar]
- 34. Shapiro M, Robertson JL. 1991. Natural variability of three geographic isolates of gypsy moth (Lepidoptera: Lymantriidae) nuclear polyhedrosis virus. J. Econ. Entomol. 84:71–75 [Google Scholar]
- 35. Sheridan LAD, Poulin R, Ward DF, Zuk M. 2000. Sex differences in parasitic infections among arthropod hosts: is there a male bias? Oikos 88:327–334 [Google Scholar]
- 36. Tobin EN, Stoffolano JG. 1973. The courtship of Musca species found in North America. 1. The house fly Musca domestica. Ann. Entomol. Soc. Am. 66:1249–1257 [Google Scholar]
- 37. Toprak U, Bayram S, Gurkan OM. 2006. Comparative biological activities of a plaque-purified variant and a Turkish native isolate of SpliNPV-B against Spodoptera littoralis (Lepidoptera: Noctuidae). Pest Manag. Sci. 62:57–63 [DOI] [PubMed] [Google Scholar]
- 38. Undeen AH, Fukuda T. 1994. Effects of host resistance and injury on the susceptibility of Aedes taeniorhynchus to mosquito iridescent virus. J. Am. Mosq. Control Assoc. 10:64–66 [PubMed] [Google Scholar]
- 39. Wedekind C, Jakobsen PJ. 1998. Male-biased susceptibility to helminth infection: an experimental test with a copepod. Oikos 81:458–462 [Google Scholar]
- 40. Zelazny B. 1976. Transmission of a baculovirus in populations of Oryctes rhinoceros. J. Invertebr. Pathol. 27:221–227 [Google Scholar]
- 41. Zuk M, McKean KA. 1996. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 26:1009–1023 [PubMed] [Google Scholar]