Abstract
Maintaining optimal bone integrity, mass, and strength throughout adult life requires ongoing bone remodeling, which involves coordinated activity between actions of bone-resorbing osteoclasts and bone forming-osteoblasts. Osteoporosis is a disorder of remodeling in which bone resorption outstrips deposition, leading to diminished bone mass and an increased risk of fractures. Here we identify Akt1 as a unique signaling intermediate in osteoblasts that can control both osteoblast and osteoclast differentiation. Targeted knockdown of Akt1 in mouse primary bone marrow stromal cells or in a mesenchymal stem cell line or genetic knockout of Akt1 stimulated osteoblast differentiation secondary to increased expression of the osteogenic transcription factor Runx2. Despite enhanced osteoblast differentiation, coupled osteoclastogenesis in Akt1 deficiency was markedly inhibited, with reduced accumulation of specific osteoclast mRNAs and proteins and impaired fusion to form multinucleated osteoclasts, defects secondary to diminished production of receptor activator of NF-κB ligand (RANKL) and macrophage colony-stimulating factor (m-CSF), critical osteoblast-derived osteoclast differentiation factors. Delivery of recombinant lentiviruses encoding Akt1 but not Akt2 to Akt1-deficient osteoblast progenitors reversed the increased osteoblast differentiation and, by boosting accumulation of RANKL and m-CSF, restored normal osteoclastogenesis, as did the addition of recombinant RANKL to conditioned culture medium from Akt1-deficient osteoblasts. Our results support the idea that targeted inhibition of Akt1 could lead to therapeutically useful net bone acquisition, and they indicate that closely related Akt1 and Akt2 exert distinct effects on cellular differentiation pathways.
INTRODUCTION
Bone growth during childhood and bone remodeling during adult life are dynamic processes that are responsible for reaching and maintaining optimal bone integrity, mass, and strength. In the adult skeleton, bone remodeling consists of two stages (11, 15, 18, 28, 31), resorption by osteoclasts, which are multinucleated cells of hematopoietic origin (11, 15, 18, 28, 31), and new bone deposition by osteoblasts, which are derived from pluripotent mesenchymal stem cells (18, 31). Both phases of remodeling are coupled temporally and spatially (11, 18, 31, 47), and both are controlled by an interplay between local and systemically derived signals mediated by mechanical strain, growth factors, hormones, and other molecules, and developmental programs directed by bone cell-specific transcription factors (18, 31, 47). The most common bone disease is osteoporosis, a disorder of remodeling in which bone resorption outstrips deposition, leading to diminished bone mass and increased risk of fractures. As it has been estimated that over 50 million people worldwide have osteoporosis, accounting for more than 1.5 million fractures per year (18, 47), the biomedical significance of deficient bone remodeling is potentially staggering.
Although current therapy for osteoporosis is directed at both slowing bone loss and enhancing bone accretion, only the former approach has been consistently successful (18, 47). In postmenopausal women, lack of estrogen is associated with increased numbers of osteoclasts, and treatment with estrogen or selective estrogen receptor modulators is effective in decreasing the rate of bone loss (18, 47). Bisphosphonates, analogs of pyrophosphate, concentrate in bone and inhibit resorption by blocking osteoclast activity (18, 47), as can antibodies to receptor activator of NF-κB ligand (RANKL), a potent stimulator of osteoclast differentiation (14). Optimal treatment for osteoporosis also should include agents that stimulate new bone formation and lead to improvements in bone mass and strength. This has been seen to some extent with parathyroid hormone (PTH) or its NH2-terminal fragment, PTH 1-34 (18, 47), although its utility is limited by the need for daily injections and by its lack of therapeutic synergy with anti-resorptive agents (45).
A major impediment in optimizing therapy for osteoporosis is that normally bone formation and resorption are tightly coupled. Osteoblasts produce soluble factors, primarily macrophage colony-stimulating factor (m-CSF) and RANKL (11, 14, 15), that promote osteoclast differentiation and function. Conversely, osteoclasts secrete factors and liberate bioactive molecules from bone matrix that attract osteoblast precursors to sites of bone resorption (11, 15, 28). From a physiological perspective, coupling makes sense as a means to continually monitor and modify bone strength and to maintain integrity in response to mechanical and other stresses (11, 28, 31), but in pathological situations of diminished bone mass it would be better to reduce resorption and simultaneously promote bone acquisition.
Multiple signaling networks activated by different hormones and growth factors have an impact on bone mass and strength by influencing both osteoblast and osteoclast differentiation and function (18, 31). Among these many signaling cascades, there is substantial evidence that the PI3-kinase-Akt pathway is critical for bone development, growth, and integrity (12, 21, 29, 30). For example, mice globally lacking Akt1 and Akt2, two of the three Akts in mammals (3, 22) have a severe bone deficiency phenotype along with major defects in other growth and developmental programs that lead to neonatal death (30), while conversely, loss of the endogenous PI3-kinase pathway inhibitor, Pten, in osteoblast precursors, which leads to enhanced Akt signaling (3, 22) or overexpression of a constitutively active Akt, promotes net bone formation and increased bone mass (21, 32). Moreover, our laboratory has shown that inhibition of PI3-kinase or Akt activity prevented osteoblast differentiation stimulated by the key bone-promoting growth factor, bone morphogenetic protein 2 (BMP2), and impaired longitudinal bone growth (25, 26), and we have found that a constitutively active Akt could restore osteoblast differentiation that had been diminished by blockade of PI3-kinase (25, 26).
The three mammalian Akts are highly related serine-threonine protein kinases that are activated by the many hormones and growth factors that stimulate class Ia PI3-kinases to promote increased production of the signaling intermediate, phosphatidylinositol 3,4,5 triphosphate (3, 22). Despite similarities in their protein structure, kinase activity, and substrates, it has become clear that each Akt has selective and specialized functions in whole-animal physiology and potentially unique actions in different tissues and organs. As an example, the dominant phenotype in mice engineered to globally lack Akt1 is postnatal growth deficiency (7), which is also manifested as impaired bone growth during childhood and mild osteopenia in the adult (17). In contrast, mice lacking Akt3 have defective brain development (10) and mice deficient in Akt2 develop impaired glucose tolerance (6), but neither has been reported to show defects in growth or in bone formation (6, 10).
In recent studies we found that Akt2 was required for BMP2-initiated osteoblast differentiation of mouse mesenchymal stem cells in culture but that Akt1 was dispensable (27). Here we define distinct functions for Akt1 in bone cells and show that it acts to restrain osteogenic differentiation but to promote osteoblast-mediated osteoclast development. Thus, loss of Akt1 in osteoblasts appears to dissociate coupling between osteoblasts and osteoclasts, raising the possibility that its targeted inhibition could lead to therapeutically useful net bone accretion.
MATERIALS AND METHODS
Reagents.
Dulbecco's modified Eagle's medium (DMEM), alpha-minimum essential media (α-MEM), fetal calf serum, penicillin-streptomycin, phosphate-buffered saline (PBS), trypsin/EDTA solution, and Superscript III first-strand synthesis kit were from Invitrogen (Carlsbad, CA). Okadaic acid was from Alexis Biochemicals (San Diego, CA). Protease inhibitor tablets and Nitro Blue Tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP) tablets were purchased from Roche Applied Sciences (Indianapolis, IN). Taq DNA polymerase was from Clontech (Palo Alto, CA). Alizarin red, TRAP staining kit, sodium orthovanadate, ascorbic acid, and β-glycerol phosphate were from Sigma-Aldrich (St. Louis, MO). enzyme-linked immunosorbent assay (ELISA) kits for mouse RANKL, osteoprotegerin (OPG), and m-CSF were purchased from R&D Systems (Minneapolis, MN). Recombinant mouse RANKL was from Santa Cruz Biotechnology (Santa Cruz, CA). The bicinchoninic acid (BCA) protein assay kit was from Pierce Biotechnologies (Rockford, IL), and AquaBlock enzyme immunoassay/Western immunoblot (EIA/WIB) solution was from East Coast Biologicals (North Berwick, ME). Immobilon-FL was from Millipore Corporation (Billerico, MA). Antibodies were purchased from the following commercial vendors: anti-Smad1, anti-Akt, anti-phospho-Akt (Ser473), and anti-Akt2, Cell Signaling Technology (Beverly, MA); anti-Runx2, anti-phospho-Smad, Santa Cruz Biotechnology; anti-Akt1 (monoclonal), AbCam (Cambridge, United Kingdom); anti-α-tubulin, Sigma-Aldrich. Goat anti-rabbit IgG-IR800 and goat anti-mouse IgG-IR680 were from Rockland Immunochemical (Gilbertsville, PA). TransIT-LT1 was from Mirus (Madison, WI). Other chemicals and reagents were purchased from commercial suppliers.
Recombinant adenoviruses and lentiviruses.
Recombinant adenoviruses (Ad-βgal, Ad-shAkt1, Ad-shAkt2, Ad-BMP2) were generated and purified by centrifugation through CsCl density gradients, and the titers were determined as described previously (26, 42). Recombinant lentiviruses expressing enhanced green fluorescent protein (EGFP), Akt1, or Akt2 were prepared by transfecting a pWXPL plasmid containing each specific cDNA along with packaging plasmids into Hek293FT cells as described previously (27) and were purified and concentrated by centrifugation of the cell culture supernatant at 20,000 × g at 4°C for 2 h. Lentiviruses were used at the lowest dose resulting in >90% infection efficiency.
Osteoblast differentiation.
Primary bone marrow stromal cells (MSCs) were isolated and grown from 8- to 12-week-old male C57BL/6 mice as described previously (25). C3H10T1/2 cells, mouse embryo fibroblasts (MEFs), and MSCs were incubated in growth medium (DMEM plus 10% fetal calf serum) at 37°C in humidified air with 5% CO2 and were infected with lentiviruses at ∼10% confluent density and/or with adenoviruses at ∼50% confluent density. At confluence, cells were washed and medium was replaced with osteogenic medium (OM) (DMEM, 10% fetal calf serum, 50 mg/liter ascorbic acid, 10 mM β-glycerol phosphate) plus 200 ng/ml BMP2. OM with BMP2 was replaced every 2 days. Staining for alkaline phosphatase and Alizarin red was performed as previously described (26), using cells fixed with 70% ethanol. For measurement of alkaline phosphatase activity, fixed cells were incubated with NBT/BCIP solution for 20 min at 20°C and quantified as described previously (26). For mineralization, Alizarin red stain was added to the fixed cells for 1 min, and after three washes with distilled water, images were captured and analyzed with the LiCoR Odyssey Infrared Imaging System, using software version 3.0 (LiCoR, Lincoln, NE), or by scanning plates on a Canon flatbed scanner (26).
Osteoclast differentiation.
Bone marrow cells were isolated from 8-to-12-week-old C57BL/6 mice and enriched for marrow macrophages by Ficoll gradient centrifugation (1). For osteoclast differentiation, bone marrow macrophages were incubated in an equal mixture of growth medium (α-MEM with 10% fetal calf serum) and conditioned osteoblast medium for 7 days or were cocultured with osteoblasts plated in 0.4 μm transwell tissue culture inserts (37). Osteoclasts were stained for expression of tartrate-resistant acid phosphatase (TRAP) as described previously (1), after fixation with 70% ethanol by incubation with Fast Garnet and tartrate solution for 1 h at 37°C, followed by three washes with distilled water. Counterstaining was performed with hematoxylin dye, and images were captured using a Nikon Eclipse E800 compound microscope with charge-coupled-device (CCD) camera. Image J software (NIH, Bethesda, MD) was used to quantify the TRAP-positive area.
ELISA.
Medium was collected from differentiating osteoblasts at specified days and centrifuged at 3,000 rpm for 10 min to remove cell debris. The supernatant was used for quantitative ELISA in 96-well coated plates for mouse RANKL, mouse OPG, or mouse m-CSF using kits and protein standards from R&D Systems (19). Plates were read at 405 nm.
Analysis of gene expression.
Whole-cell RNA was extracted and reverse transcribed (2 μg) with the Superscript III first-strand cDNA synthesis kit using oligo(dT) primers (26). PCR was performed with 1 μl of osteoblast cDNA and previously published primer pairs for mouse Id1, Dlx5, Runx2, and S17 (26) and with primers for mouse RANKL (top strand, 5′-TGTACTTTCGAGCGCAGATG-3′; bottom strand, 5′-ACATCCAACCATGAGCCTTC-3′), m-CSF (top strand, 5′-ATGGACACCTGAAGGTCCTG-3′; bottom strand, 5′-GCTGGAGAGGAGTCTCATGG-3′), and OPG (top strand, 5′-GTGAAGCAGGAGTGCAAC-3′; bottom strand, 5′-GCAAACTGTGTTTCGCTC-3′). Mouse brain cDNA was from Severyn et al. (33). The following primer pairs were used to amplify mouse Akt mRNAs: Akt1 (top strand, 5′-ATACACATCCTGCCACACGA-3′; bottom strand, 5′-CCCTTCTACAACCAGGACCA-3′), Akt2 (top strand, 5′-CTTGTAATCCATGGCGTCCT-3′; bottom strand, 5′-GAAGACTGAGAGGCCACGAC-3′), Akt3 (top strand, 5′-GCCCAACCTCACAGATTGAT-3′; bottom strand, 5′-CACTTGCCTTCTCTCTCGAACC-3′). Osteoclast cDNA (1 μl) was analyzed by PCR for cathepsin K, calcitonin receptor, and S17 gene expression. Primer pairs included the following: cathepsin K (top strand, 5′-AAGTGGTTCAGAAGATGACGGGAC-3′; bottom strand, 5′-TCTTCAGAGTCAATGCCTCCGTTC-3′) and calcitonin receptor (top strand, 5′-CGGACTTTGACACAGCAGAA-3′; bottom strand, 5′-GTCACCCTCTGGCAGCTAAG-3′). Cycle numbers ranged from 20 to 30, and results were visualized after agarose gel electrophoresis.
Protein extraction and immunoblotting.
Whole-cell protein lysates were prepared as described previously (26) and protein aliquots (15 to 30 μg/lane) resolved by SDS-PAGE. After transfer of samples to Immobilon-FL membranes and blocking with 50% AquaBlock solution, membranes were incubated sequentially with primary and secondary antibodies, as described previously (26). Primary antibodies were used at dilutions ranging from 1:1,000 to 1:15,000, and secondary antibodies were used at 1:5,000. Images were captured using the LiCoR Odyssey and version 3.0 analysis software.
Promoter-reporter gene assays.
C3H10T1/2 cells were transfected using TransIT-LT1 with promoter-reporter plasmids (100 ng) containing the mouse Id1 promoter (39) or six copies of the osteoblast response element (OSE2) from the mouse osteocalcin gene promoter fused to a minimal thymidine kinase promoter (9). Twenty-four hours later, cells were incubated for 48 h in OM plus BMP2, and cell lysates were harvested and used for luciferase assays (26). Results were normalized to total cellular protein concentrations.
Statistical analysis.
Data are presented as mean ± standard deviation (SD). Statistical significance was calculated using a paired Student t test. Results were considered statistically significant at P < 0.05.
RESULTS
Loss of Akt1 enhances BMP2-mediated osteoblast differentiation.
Mesenchymal precursor cells induced to undergo osteoblast differentiation express Akt1 and Akt2, two of three members of the Akt family found in mammals (Fig. 1A). Recent studies from our laboratory showed that targeted deficiency of Akt2 inhibited BMP2-initiated osteoblast differentiation of mouse mesenchymal stem cells, including bone marrow stromal cells in primary culture, but loss of Akt1 did not (27). Osteoblast differentiation could be returned to normal by restoration of Akt2 expression or by forced expression of the osteogenic transcription factor Runx2 but not with Akt1 (27). The focus of current experiments was to define the role of Akt1-mediated signaling in osteogenesis.
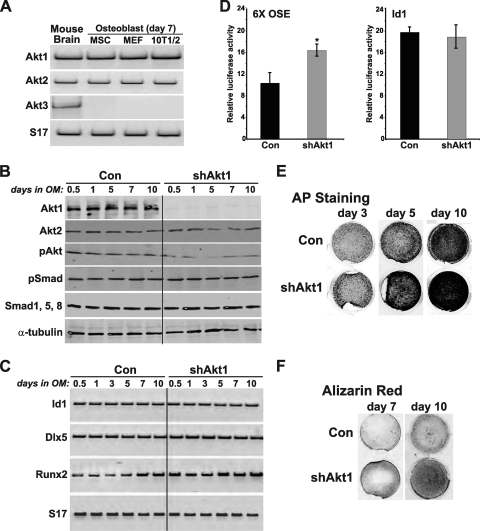
Fig 1.
Akt1 deficiency enhances BMP2-stimulated osteoblast differentiation. (A) Detection by reverse transcription-PCR (RT-PCR) of mRNAs for Akt1, Akt2, Akt3, and S17 in RNA from mouse brain (positive control), and from osteoblasts derived from MSCs, MEFs, and C3H10T1/2 mesenchymal stem cells after 7 days of differentiation. (B to F) C3H10T1/2 cells, infected with Ad-shAkt1 or Ad-βgal (Con), were incubated in osteogenic medium (OM) with BMP2 (200 ng/ml) for up to 15 days. (B) Immunoblots of whole-cell protein lysates for Akt1, Akt2, phospho-Akt (pAkt), pSmad, Smad1, 5, 8, and α-tubulin. (C) Measurement of mRNAs for Id1, Dlx5, Runx2, and S17 by RT-PCR. (D) Results of luciferase activity assays after transient transfection with reporter plasmids containing six copies of a Runx2 responsive element from the osteocalcin gene (OSE) or the BMP2-responsive mouse Id1 promoter and incubation in OM with BMP2 for 2 days (mean ± standard error of results of three experiments, each performed in triplicate; ∗, P < 0.01 versus Con). (E) Alkaline phosphatase (AP) staining on days 3, 5, and 10. F. Alizarin red staining for mineralization on days 7 and 10.
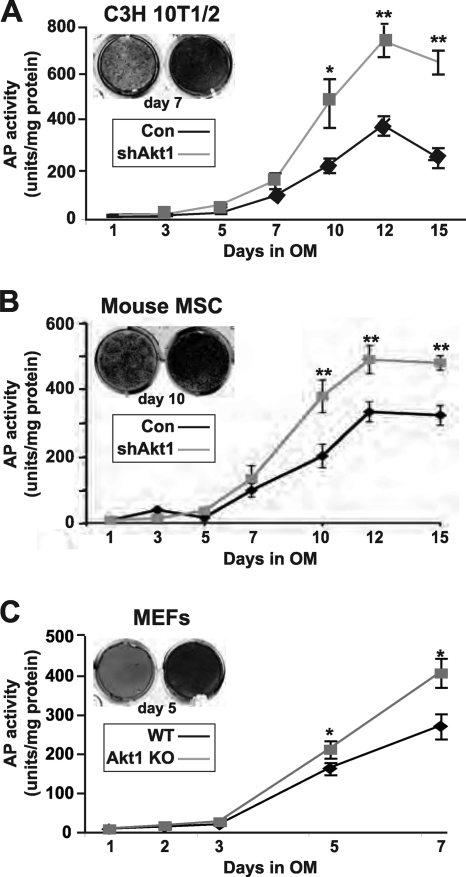
To address this question, we first reduced Akt1 levels by infection with an adenovirus encoding a short hairpin (sh) interfering RNA for mouse Akt1 that targeted the 3′ untranslated region (UTR) of Akt1 mRNA (43). As found previously (27, 43), knockdown of Akt1 by Ad-shAkt1 was effective and specific (>90% reduction in Akt1 protein levels by immunoblotting) and did not lead to compensatory upregulation of Akt2 (Fig. 1B). As a consequence, Akt phosphorylation was reduced by ∼50% (Fig. 1B). Akt1 deficiency did not alter BMP2-stimulated Smad phosphorylation and did not change BMP2- and Smad-activated reporter gene transcription or BMP2- and Smad-induced expression of transcripts for Id1 and Dlx5 (Fig. 1B to D) but did lead to increased expression of Runx2 mRNA by ∼ 2-fold, as well as boosting its transcriptional activity by a similar amount (Fig. 1C and D). As a result of higher levels of Runx2 after knockdown of Akt1, osteoblast differentiation was more rapid and more extensive than in control cells infected with the Ad-βgal, as demonstrated by progressively higher levels of bone-specific alkaline phosphatase (AP) activity and by increased mineralization of extracellular matrix, as measured by Alizarin red staining (Fig. 1E, F). When quantified, osteoblast-associated AP activity was twice as high in C3H10T1/2 cells lacking Akt1 than in controls (Fig. 2A). Analogous observations were seen using MSCs in primary cultures infected with Ad-shAkt1 versus those infected with Ad-βgal (Fig. 2B) and using mouse embryo fibroblasts (MEFs) derived from Akt1-deficient versus Akt1-sufficient control mice (Fig. 2C), with the former results arguing against cell-line specific effects of knockdown of Akt1 and the latter data against nonspecific influences of adenoviral infection.
Fig 2.
Lack of Akt1 upregulates bone-specific alkaline phosphatase activity during osteogenic differentiation. Quantitative assessment of AP activity during BMP2-induced osteoblast differentiation for up to 15 days. (A) C3H10T1/2 cells. (B) Mouse bone marrow stromal cells (MSC). (C) Embryo fibroblasts (MEFs) from wild-type (WT) or Akt1 knockout (KO) mice (mean ± SD of results of three experiments; ∗, P < 0.05; ∗∗, P < 0.01 versus Con or WT).
Akt1 negatively regulates osteoblast differentiation.
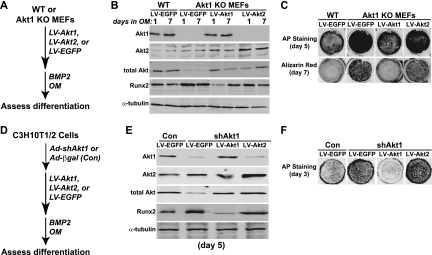
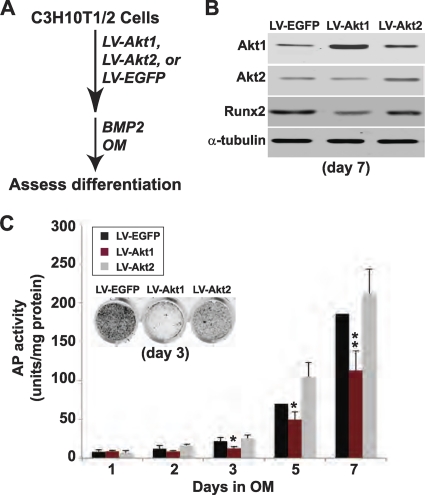
To further test the hypothesis that Atk1 is a negative regulator of osteogenesis, we infected Akt1-null MEFs and C3H10T1/2 mesenchymal stem cells in which Akt1 expression was reduced with recombinant lentiviruses (LV) encoding EGFP (control), mouse Akt1, or mouse Akt2, and we incubated the cells in osteogenic medium plus BMP2 (Fig. 3A and D). The Akt LVs contained only the coding region and thus would be resistant to Ad-shAkt1 (or Ad-shAkt2 [27]), which targets the 3′ UTR of Akt mRNAs (43). LV-Akt1 infection restored Akt1 expression to levels nearly identical to those of control cells, and infection with LV-Akt2 caused a doubling of Akt2 abundance over control cells (Fig. 3B and E). In each case, the amount of Runx2 was decreased from levels seen in cells infected with LV-EGFP, although the reduction was far greater with LV-Akt1 (Fig. 3B and E). Restoration of Akt1 expression also led to declines in AP activity and in extracellular mineralization to levels similar to Akt1-sufficient wild-type (WT) or control (Con) cells (Fig. 3C and F). In contrast, overexpression of Akt2 had a minimal effect on these parameters of osteoblast differentiation (Fig. 3C and F). Moreover, overexpression of Akt1 in C3H10T1/2 cells incubated in osteogenic medium with BMP2 led to reduced accumulation of Runx2 and caused a decline in AP activity compared with those of controls, while by contrast, overexpression of Akt2 had a minimal impact on osteoblast differentiation (Fig. 4). Taken together, the results in Fig. 1 to 4 show that Akt1 is a negative regulator of osteogenic differentiation, and they contrast with our previous observations indicating that Akt2 was required for initiation of osteoblast differentiation in culture (27).
Fig 3.
Restoration of Akt1 expression reduces osteogenic differentiation. (A to C) Experimental scheme: MEFs from WT and Akt1 KO mice were infected with recombinant lentiviruses (LV) encoding mouse Akt1, mouse Akt2, or EGFP and were incubated in OM plus 200 ng/ml BMP2 for up to 7 days. (B) Immunoblots of whole-cell protein lysates for Akt1, Akt2, total Akt, Runx2, and α-tubulin. (C) AP staining on day 5 and Alizarin red on day 7. (D to F) Experimental scheme: C3H10T1/2 cells were infected with Ad-shAkt1 or Ad-βgal (Con), followed by infection with LV-Akt1, LV-Akt2, or LV-EGFP, and incubation in OM plus 200 ng/ml BMP2. (E) Immunoblots of whole-cell protein lysates for Akt1, Akt2, total Akt, Runx2, and α-tubulin on day 5. (F) AP staining on day 3.
Fig 4.
Forced overexpression of Akt1 but not Akt2 decreases BMP2-mediated osteogenic differentiation. (A) Experimental scheme: C3H10T1/2 cells were infected with LV-Akt1, LV-Akt2, or LV-EGFP and were incubated in OM with 200 ng/ml BMP2 for up to 7 days. (B) Immunoblots for Akt1, Akt2, Runx2, and α-tubulin on day 7. (C) Quantitative measurement of AP activity on days 1 to 7 (mean ± SD of results of three experiments; ∗, P < 0.05; ∗∗, P < 0.01 versus cells infected with LV-EGFP). Inset shows AP staining on day 3.
Loss of Akt1 reduces osteoblast-mediated osteoclast differentiation.
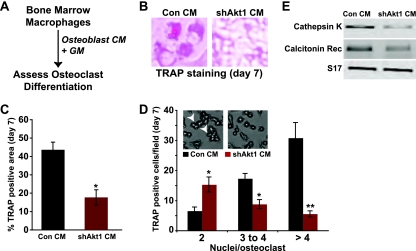
In the adult skeleton, bone remodeling is a highly integrated process in which resorption by osteoclasts is tightly linked to new bone formation by osteoblasts (11, 18, 31, 47). On a cellular and biochemical level, osteoclasts secrete or liberate factors that attract osteoblast precursors to sites of bone resorption, and differentiating osteoblasts produce signaling molecules that promote differentiation of osteoclast progenitors and stimulate the activity of mature osteoclasts (2, 18, 31). Since lack of Akt1 upregulated osteoblast differentiation (Fig. 1 and 2), we hypothesized that it also would lead to an increase in osteoblast-mediated osteoclast differentiation. To test this idea, we incubated osteoclast progenitors (bone marrow macrophages in primary culture) with medium conditioned by MSCs undergoing osteoblast differentiation (see experimental protocol in Fig. 5A). Osteoclast differentiation was measured by staining cells for tartrate-resistant acid phosphatase (TRAP), by counting multinucleated osteoclasts, and by analyzing osteoclast-specific gene expression. Surprisingly, a lack of Akt1 in osteoblasts led to a severe reduction in osteoclast differentiation, as measured by a 60% decline in TRAP activity (Fig. 5B and C), by a halving of multinucleated osteoclasts (and a 80% reduction in osteoclasts with >4 nuclei) (Fig. 5D), and by diminished expression of mRNAs for cathepsin K and the calcitonin receptor (Fig. 5E). A comparable decrease in osteoclastogenesis also was observed when bone marrow macrophages were cocultured with Akt1-deficient osteoblasts (Fig. 6), and additionally was seen when Akt1-null MEFs incubated in osteogenic medium plus BMP2 was the source of conditioned medium for osteoclast differentiation (Fig. 7).
Fig 5.
Conditioned medium from Akt1-deficient osteoblasts reduces osteoclast formation. (A) Experimental scheme to assess osteoclast differentiation in mouse bone marrow macrophages incubated in a combination of 50% osteoblast conditioned medium (CM, collected at day 7 from control or shAkt1 osteoblasts) and 50% growth medium (GM). (B) Example of TRAP staining to assess formation of multinucleated osteoclasts on day 7. (C) Percentage of TRAP-positive cells expressed as area per microscopic field (×200 magnification, mean ± S.D. of 6 fields, ∗, P < 0.01 versus Con CM). (D) Quantitative assessment of the percentage of multinucleated TRAP-positive osteoclasts on day 7 per microscopic field (×200 magnification; mean ± SD of results of six fields; ∗, P < 0.01; ∗∗, P < 0.001 versus Con CM). Inset depicts representative examples (white arrowheads indicate osteoclasts with >4 nuclei). (E) Expression of mRNAs for cathepsin K, the calcitonin receptor (Rec), and S17 by RT-PCR on day 7.
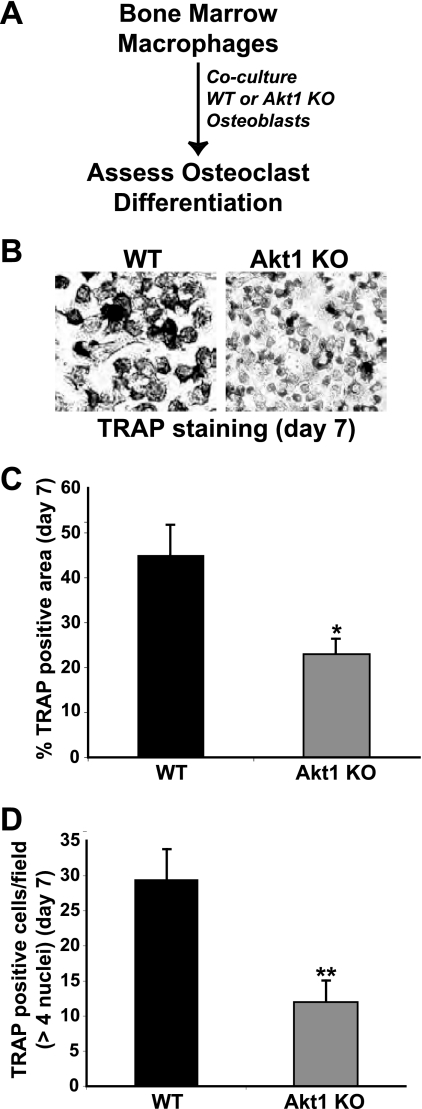
Fig 6.
Coculture of bone marrow macrophages with Akt1-deficient osteoblasts reduces osteoclast formation. (A) Mouse bone marrow macrophages were incubated with MEFs from wild type (WT) or Akt1 knockout (KO) mice in osteogenic medium plus BMP2 for 7 days. (B) TRAP staining to assess formation of multinucleated osteoclasts at day 7. (C) Percentage of TRAP-positive cells at day 7 expressed as area per microscopic field (×200 magnification; mean ± SD of results of six fields; ∗, P < 0.005 versus WT). (D) Comparison of the number of TRAP-positive osteoclasts per microscopic field with >4 nuclei on day 7 (×200 magnification; mean ± SD of results of six fields; ∗∗, P < 0.001 versus WT).
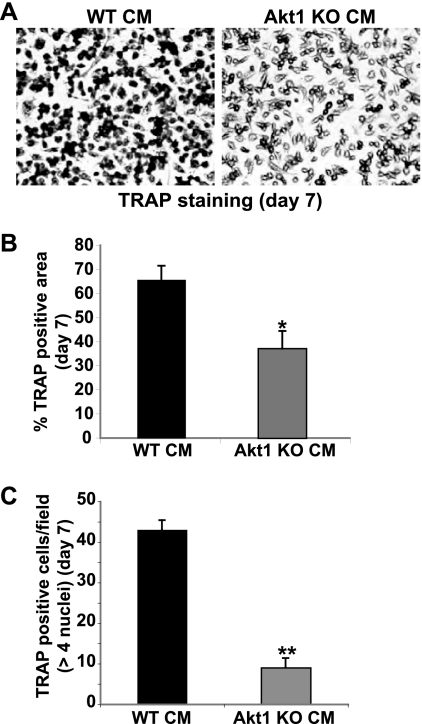
Fig 7.
Conditioned medium from Akt1-knockout osteoblasts reduces osteoclast formation. Conditioned medium (CM) was collected from wild type (WT) or Akt1-deficient MEFs after incubation in OM plus BMP2 for 7 days and added to mouse bone marrow macrophages as described in the legend to Fig. 5. (A) Image of TRAP staining to assess formation of multinucleated osteoclasts on day 7 (×100 magnification). (B) Percentage of TRAP-positive cells expressed as area per microscopic field (mean ± SD of results of six fields; ∗, P < 0.01 versus WT CM). (C) Quantitative assessment of the percentage of multinucleated TRAP-positive osteoclasts on day 7 per microscopic field (n > 4 nuclei; ×200 magnification; mean SD of results of three experiments; ∗∗, P < 0.001 versus WT CM).
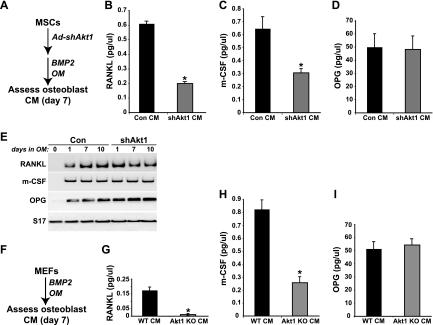
Differentiating osteoblasts produce RANKL and m-CSF, two critical signaling molecules for osteoclastogenesis that are key components of osteoblast-osteoclast coupling during bone remodeling (11, 14). Osteoblasts also secrete osteoprotegerin (OPG), a soluble inhibitor of osteoclast differentiation that acts by binding to RANKL and sequestering it from the receptor RANK on the surface of osteoclast progenitors (14, 23). We measured the accumulation of each of these proteins in medium conditioned by differentiating osteoblasts to determine if any alteration in their expression could explain the diminished osteoblast-osteoclast coupling observed with osteoblasts lacking Akt1. The amount of RANKL was reduced by nearly 70% and the abundance of m-CSF by 55% compared with controls in medium obtained from Akt1-deficient mouse MSCs collected from days 5 to 7 of osteoblast differentiation, while levels of OPG were nearly identical (Fig. 8B to D). Despite the substantial decline in concentrations of RANKL and m-CSF, there was a minimal decrease in the abundance of their mRNAs in differentiating Akt1-deficient osteoblasts compared with controls, although there was a slight increase in OPG transcripts (Fig. 8E). Comparably large reductions in RANKL and m-CSF were detected in conditioned medium from Akt1-null MEFs at day 7 of osteoblast differentiation, except that baseline RANKL levels were 75% lower in control MEFs than in MSCs and were reduced in Akt1-deficient MEFs by ∼90% versus those in controls (Fig. 8F to I).
Fig 8.
Reduced secretion of osteoclast coupling factors RANKL and m-CSF by Akt1-deficient osteoblasts. (A) Experimental scheme for assessment of secreted factors found in conditioned medium (CM) from MSCs induced to undergo osteoblast differentiation for 7 days. (B to D) Measurement by ELISA for RANKL (B), m-CSF (C), and OPG (D) (mean ± SD of results of three experiments; ∗, P < 0.01 versus Con CM). (E) Steady-state levels of RANKL, m-CSF, OPG, and S17 mRNAs assessed by RT-PCR after 0 to 10 days of osteoblast differentiation in control and Akt1-deficient MSCs. (F) Experimental scheme for assessment of secreted factors found in CM from MEFs induced to undergo osteoblast differentiation for 7 days. (G to I) Measurement by ELISA for RANKL (G), m-CSF (H), and OPG (I) (mean ± SD of results of three experiments; ∗, P < 0.01 Akt1 KO versus WT CM).
Akt1 promotes osteoblast-mediated osteoclast differentiation.
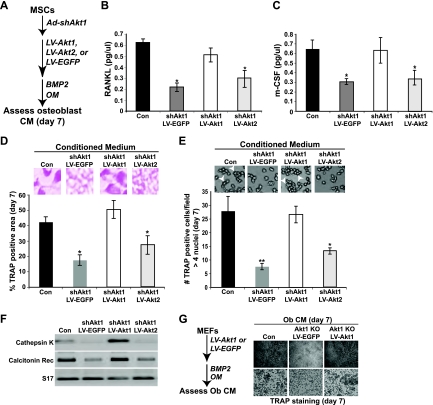
We next tested the hypothesis that restoration of Akt1 expression in osteoblasts could stimulate coupled osteoclastogenesis, even while reducing osteoblast differentiation. Mouse MSCs in primary culture were sequentially infected with Ad-shAkt1, plus LV-EGFP (control), LV-Akt1, or LV-Akt2, and incubated in osteogenic medium plus BMP2, and conditioned medium was collected from days 5 to 7 (Fig. 9A). Reexpression of Akt1 caused an increase in levels of RANKL and m-CSF that were equal to those in medium from intact MSCs (Fig. 9B and C) and restored osteoclastogenesis of bone marrow macrophages to levels observed under control conditions, as measured by TRAP activity, formation of multinucleated osteoclasts, and expression of mRNAs encoding osteoclast-specific genes, cathepsin K and the calcitonin receptor (Fig. 9D to F). In contrast, substitution with Akt2 did not reverse the defects in osteoclastogenesis seen in the absence of Akt1. Equivalent results were seen using conditioned medium collected from Akt1-null MEFs in which Akt1 was reintroduced (Fig. 9G). Thus, osteoblast Akt1 acts as a positive regulator of coupled osteoclast differentiation and function.
Fig 9.
Expression of Akt1 but not Akt2 by differentiating osteoblasts restores production of RANKL and m-CSF and promotes coupled osteoclast differentiation. (A) Experimental scheme: MSCs in which Akt1 was knocked down were infected with LV-Akt1, LV-Akt2, or LV-EGFP and were incubated in OM plus BMP2 for up to 7 days. (B and C) Detection of RANKL (B) and m-CSF (C) by ELISA (mean ± SD of results of three experiments; ∗, P < 0.01 versus Con CM). (D) Percentage of TRAP-positive osteoclasts expressed as area per microscopic field (×200 magnification; mean ± SD of results of six fields; ∗, P < 0.05 versus Con CM). Inset depicts representative example of TRAP-stained cells. (E) Quantitative measurement of the percentage of multinucleated TRAP-positive osteoclasts on day 7 per microscopic field (×200 magnification; mean ± SD of results of six fields; ∗, P < 0.01; ∗∗, P < 0.001 versus Con CM). Inset illustrates representative examples (white arrowheads indicate osteoclasts with >4 nuclei). (F) Expression of mRNAs for cathepsin K, the calcitonin receptor (Rec), and S17 by RT-PCR on day 7. (G) Left: experimental scheme. MEFs lacking Akt1 were infected with LV-EGFP or LV-Akt1 and incubated in OM plus BMP2 for 7 days, and conditioned medium (CM) was collected. Right: images are shown of TRAP staining to assess formation of multinucleated osteoclasts on day 7 (top row, ×40 magnification; bottom row, ×100 magnification).
RANKL promotes osteoblast-mediated osteoclast differentiation in the absence of Akt1.
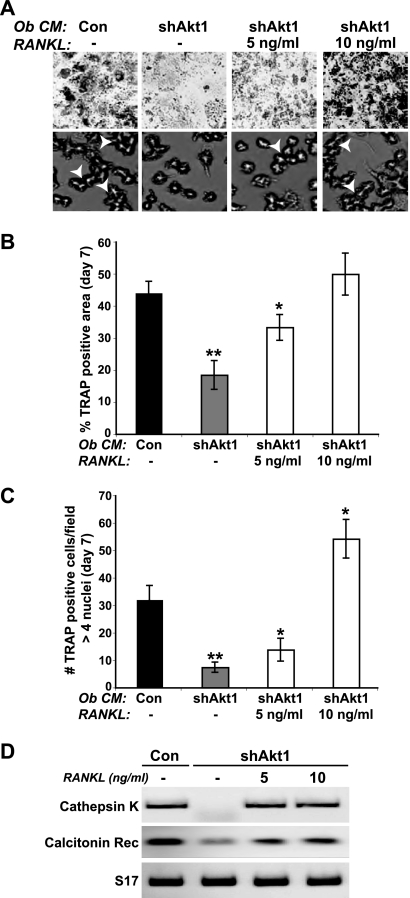
We next asked if recombinant RANKL could restore normal osteoclastogenesis when added to conditioned medium from Akt1-deficient osteoblasts. C3H10T1/2 mesenchymal stem cells were infected with Ad-shAkt1 and incubated in osteogenic medium plus BMP2, and conditioned medium was collected from days 5 to 7. RANKL addition caused a dose-dependent rise in osteoclast differentiation of bone marrow macrophages to levels seen with control-conditioned medium, as evidenced by increases in TRAP activity and formation of multinucleated osteoclasts and by boosts in expression of mRNAs for cathepsin K and the calcitonin receptor (Fig. 10A to D). These results support the idea that Akt1 deficiency in osteoblasts does not lead to production of a soluble inhibitor of osteoclastogenesis but rather impairs accumulation of the osteoclast differentiation factors RANKL and m-CSF.
Fig 10.
RANKL restores osteoclast differentiation when added to conditioned medium from osteoblasts lacking Akt1. Conditioned medium (Ob CM) was collected from control (Con) C3H10T1/2 cells or cells infected with Ad-shAkt1 after incubation in osteoblast differentiation medium plus BMP2 for 7 days and was added to mouse bone marrow macrophages (as described in the legend to Fig. 5) without or with recombinant mouse RANKL (5 or 10 ng/ml). (A) Images of TRAP staining to assess formation of multinucleated osteoclasts on day 7 (top row, ×40 magnification; bottom row, ×200 magnification; white arrowheads indicate osteoclasts with >4 nuclei). (B) Percentage of TRAP-positive osteoclasts on day 7 expressed as area per microscopic field (×200 magnification; mean ± SD of results of six fields; ∗, P = 0.01; ∗∗, P < 0.002 versus Con CM). (C) Percentage of multinucleated TRAP-positive osteoclasts on day 7 per microscopic field (n > 4 nuclei; ×200 magnification; mean ± SD of results of three fields; ∗, P = 0.02; ∗∗, P = 0.002 versus Con CM). (D) Expression by RT-PCR of mRNAs for cathepsin K, the calcitonin receptor (Rec), and S17 on day 7.
DISCUSSION
Multiple local and systemic factors control bone remodeling in the adult skeleton (18, 31, 47), and are responsible for the tightly coupled temporal and spatial interplay between bone-resorbing osteoclasts and bone-forming osteoblasts that normally maintains skeletal mass and integrity (11, 18, 31, 47). Here we have identified two distinct roles for Akt1 in osteoblasts that critically modify the relationship between these two skeletal cell types. We found first that Akt1 uniquely functions as a negative modulator of osteoblast differentiation, in direct contrast to the positive role of Akt2 as an essential gatekeeper for initiating and sustaining osteoblast differentiation in culture (27). The loss of Akt1 in osteoblast progenitors led to their accelerated and enhanced differentiation, as measured by increases in the rate and extent of both AP activity and matrix mineralization, which were reduced to normal by reexpression of Akt1 by lentiviral delivery but not by substitution with Akt2 and which were diminished further by overexpression of Akt1 (Fig. 1 to 4). The biochemical mechanism by which Akt1 deficiency promoted osteogenesis appears to be secondary to modest upregulation of expression of the critical osteoblast differentiation-promoting transcription factor Runx2 (Fig. 1 and 3), which proportionately increased its transcriptional activity (Fig. 1).
Second, we surprisingly discovered that Akt1 is a key facilitator of osteoblast-osteoclast coupling. Akt1 deficiency in osteoblasts impaired linked osteoclastogenesis, as measured by reductions in expression of osteoclast-specific mRNAs, including cathepsin K and the calcitonin receptor, by markedly decreased production of TRAP, and by diminished numbers of multinucleated osteoclasts (Fig. 5 to 8). The mechanism appears to be decreased accumulation of RANKL and m-CSF, the critical secreted factors that stimulate osteoclast differentiation (11, 14, 15). Readdition of Akt1 to Akt1-null osteoblasts, but not substitution with Akt2, boosted osteoblast-mediated osteoclastogenesis back to normal, coincident with increases in RANKL and m-CSF to normal levels (Fig. 9), and addition of recombinant RANKL to conditioned medium from Akt1-deficient osteoblasts also could restore osteoclastogenesis (Fig. 10). Taken together, our results demonstrate that Akt1 occupies a unique signaling niche during osteoblast differentiation and that its activity is necessary to ensure adequate coupled osteoclast differentiation.
Distinct actions of Akt1 in other cell types.
Despite similarities in overall protein structure and kinase activity of the three mammalian Akts (3, 22, 44), a growing literature has defined a range of selective functions for each of these proteins, although in most instances to date the biochemical mechanisms have not been defined. For Akt1 the most compelling examples relate to its potential role in restraining the migration and the propensity for metastasis of breast cancer cells (16, 20, 46). In several model cell culture systems, where tumorigenesis was driven by overexpression of the IGF1 receptor (16, 46), Akt1 deficiency led to changes in cell morphology, stimulation of migration, and upregulation of markers of epithelial-mesenchymal transition. A loss of Akt2, by contrast, either had no effect or caused alterations that were the opposite of those seen with Akt1 deficiency (16, 46). Moreover, forced expression of a constitutively active Akt1 could stimulate invasiveness of breast cancer cells after implantation into nude mice (20). In more recent studies, the actin-associated protein palladin has been identified as a potential mediator of the inhibitory effects of Akt1 on cell migration (5).
In spite of clear evidence linking a lack of Akt1 to a more migratory phenotype in breast cancer cells, distinctly different observations have been made with MEFS and with endothelial cells from Akt1 knockout mice (35, 48). In both cases, Akt1 deficiency led to reduced cell migration (35, 48), and in MEFs it was the isolated loss of Akt2 rather than lack of Akt1 that promoted both migratory and invasive phenotypes (48). Taken together, these results and others (4, 8, 38) suggest that there are many nuances in the mechanisms of action of each Akt that need to be identified and that in most cases the precise signaling steps that uniquely distinguish the biological effects of one Akt from those of another have not been characterized beyond a rudimentary level. It addition, it is clear that putative cell-type-specific modifiers that might explain the distinct biological effects of a single Akt within a background of otherwise shared signaling pathways have yet not been identified.
Akt actions and bone biology.
The biological effects of individual Akts in bone cells remain an underexplored area of investigation. Although osteoclastogenesis appears to require the actions of both Akt1 and Akt2, as targeted knockdown of either molecule in mouse bone marrow macrophages in primary culture caused reduced osteoclast differentiation without altering cell viability or proliferation (36), the biochemical mechanisms have not been elucidated. The adipokine adiponectin also is able to block osteoclastogenesis by selectively interfering with Akt1 in osteoclast progenitors, and adiponectin knockout mice have diminished bone growth and reduced bone mass because of enhanced osteoclast differentiation and activity (40). In addition, osteoclast precursors from Akt1-deficient mice showed reduced differentiation in the presence of m-CSF and RANKL compared with controls (17, 41). Thus, intact Akt-mediated signaling in progenitors is important for normal osteoclastogenesis.
Only a few publications have assessed the impact of Akt1 deficiency in osteoblast differentiation and bone development (17, 41), and no studies have examined the role of Akt2 in bone in vivo. Using calvarial osteoblasts from globally Akt1-deficient mice, Kawamura et al. found that these cells were more susceptible to apoptotic death than controls and also differentiated more poorly, although the response to BMP2 appeared to be relatively equivalent to that of control cells (17). In contrast, we observe enhanced osteogenic differentiation in the absence of Akt1 in cell lines and in primary MSCs with Akt1 deficiency, and we find in preliminary experiments that MSCs from Akt1 knockout mice also differentiated more robustly than did controls (data not shown). Taken together, our data and the results of Kawamura et al. (17) raise the possibility that there may be intrinsic differences between osteoblast progenitors that undergo intramembranous (calvariae) versus endochondral (long bones) osteogenesis in terms of the role of Akt1 in regulating their differentiation.
Modifying osteoblast-osteoclast coupling.
The most remarkable aspect of our observations is the key role that Akt1 appears to play in mediating coupling between osteoblast and osteoclast precursors. Despite the enhanced differentiation seen in Akt1-deficient osteoblasts, linked osteoclastogenesis was dramatically diminished. To date, no other signaling intermediate shares this property, although an extracellular molecule, the long-chain fatty acid-amide oleoyl serine, appears able to stimulate bone formation and to reduce bone resorption and osteoclast numbers in ovariectomized mice but not in normal controls (34). Even though several other signaling proteins can interfere with osteoblast-mediated osteoclastogenesis, they do so in predictable and expected ways. For example, targeted loss of the MAP kinases, Erks 1 and 2, in chondro-osteoprogenitors in mice led to reduced osteogenesis but enhanced chondrogenesis (24), and to diminished linked osteoclast differentiation secondary to a decline in RANKL production (24). In addition, knockdown of components of the p38α and β MAP kinase pathway in osteoblast precursors reduced their differentiation through loss of activating phosphorylation of Runx2 (13). Thus, with the exception of Akt1, signaling molecules that impaired osteoblast differentiation also reduced coupled osteoclast differentiation.
In summary, our studies have identified unique roles for Akt1 as both a negative regulator of osteoblast differentiation and a robust positive mediator of osteoblast-coupled osteoclastogenesis. Since treatments for osteoporosis have not been able to dissociate coupling between osteoblasts and osteoclasts, potential targeting of Akt1 or its downstream signaling pathways may lead to new therapeutic opportunities for enhancing osteoblast differentiation and bone formation while limiting osteoblast-coupled osteoclast development and bone resorption.
ACKNOWLEDGMENTS
We thank Morris Birnbaum of the University of Pennsylvania for wild-type and Akt-deficient MEFs. We also thank our OHSU colleagues, Robert Klein and Emily Larson, for reagents and Ronen Schweitzer for use of his microscope and imaging system.
These studies were supported in part by National Institutes of Health grant R01 DK42748 (to P.R.).
Footnotes
Published ahead of print 7 November 2011
REFERENCES
- 1. Abu-Amer Y, et al. 2000. Tumor necrosis factor receptors types 1 and 2 differentially regulate osteoclastogenesis. J. Biol. Chem. 275: 27307–27310 [DOI] [PubMed] [Google Scholar]
- 2. Blair HC, Schlesinger PH, Huang CL, Zaidi M. 2007. Calcium signalling and calcium transport in bone disease. Subcell. Biochem. 45: 539–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brazil DP, Yang ZZ, Hemmings BA. 2004. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 29: 233–242 [DOI] [PubMed] [Google Scholar]
- 4. Buzzi F, et al. 2010. Differential effects of protein kinase B/Akt isoforms on glucose homeostasis and islet mass. Mol. Cell. Biol. 30: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chin YR, Toker A. 2010. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol. Cell 38: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho H, et al. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731 [DOI] [PubMed] [Google Scholar]
- 7. Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. 2001. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276: 38349–38352 [DOI] [PubMed] [Google Scholar]
- 8. DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. 2006. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J. Biol. Chem. 281: 32841–32851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ducy P, Karsenty G. 1995. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 15: 1858–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Easton RM, et al. 2005. Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol. Cell. Biol. 25: 1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng X, McDonald JM. 2011. Disorders of bone remodeling. Annu. Rev. Pathol. 6: 121–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghosh-Choudhury N, et al. 2002. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J. Biol. Chem. 277: 33361–33368 [DOI] [PubMed] [Google Scholar]
- 13. Greenblatt MB, et al. 2010. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J. Clin. Invest. 120: 2457–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanada R, Hanada T, Sigl V, Schramek D, Penninger JM. 2011. RANKL/RANK-beyond bones. J. Mol. Med. (Berl.) 89: 647–656 [DOI] [PubMed] [Google Scholar]
- 15. Henriksen K, Bollerslev J, Everts V, Karsdal MA. 2011. Osteoclast activity and subtypes as a function of physiology and pathology—implications for future treatments of osteoporosis. Endocr. Rev. 32: 31–63 [DOI] [PubMed] [Google Scholar]
- 16. Irie HY, et al. 2005. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J. Cell Biol. 171: 1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawamura N, et al. 2007. Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS One 2: e1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khosla S, Westendorf JJ, Oursler MJ. 2008. Building bone to reverse osteoporosis and repair fractures. J. Clin. Invest. 118: 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwak HB, et al. 2008. Reciprocal cross-talk between RANKL and interferon-gamma-inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis Rheum. 58: 1332–1342 [DOI] [PubMed] [Google Scholar]
- 20. Liu H, et al. 2006. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc. Natl. Acad. Sci. U. S. A. 103: 4134–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu X, et al. 2007. Lifelong accumulation of bone in mice lacking Pten in osteoblasts. Proc. Natl. Acad. Sci. U. S. A. 104: 2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manning BD, Cantley LC. 2007. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsuo K, Irie N. 2008. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 473: 201–209 [DOI] [PubMed] [Google Scholar]
- 24. Matsushita T, et al. 2009. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol. Cell. Biol. 29: 5843–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mukherjee A, Rotwein P. 2009. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J. Cell Sci. 122: 716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mukherjee A, Rotwein P. 2008. Insulin-like growth factor-binding protein-5 inhibits osteoblast differentiation and skeletal growth by blocking insulin-like growth factor actions. Mol. Endocrinol. 22: 1238–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mukherjee A, Wilson EM, Rotwein P. 2010. Selective signaling by Akt2 promotes bone morphogenetic protein 2-mediated osteoblast differentiation. Mol. Cell. Biol. 30: 1018–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Novack DV, Teitelbaum SL. 2008. The osteoclast: friend or foe? Annu. Rev. Pathol. 3: 457–484 [DOI] [PubMed] [Google Scholar]
- 29. Osyczka AM, Leboy PS. 2005. Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology 146: 3428–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peng XD, et al. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17: 1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raggatt LJ, Partridge NC. 2010. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 285: 25103–25108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raucci A, Bellosta P, Grassi R, Basilico C, Mansukhani A. 2008. Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J. Cell. Physiol. 215: 442–451 [DOI] [PubMed] [Google Scholar]
- 33. Severyn CJ, Rotwein P. 2010. Conserved proximal promoter elements control repulsive guidance molecule c/hemojuvelin (Hfe2) gene transcription in skeletal muscle. Genomics 96: 342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smoum R, et al. 2010. Oleoyl serine, an endogenous N-acyl amide, modulates bone remodeling and mass. Proc. Natl. Acad. Sci. U. S. A. 107: 17710–17715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Somanath PR, Kandel ES, Hay N, Byzova TV. 2007. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. J. Biol. Chem. 282: 22964–22976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sugatani T, Hruska KA. 2005. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J. Biol. Chem. 280: 3583–3589 [DOI] [PubMed] [Google Scholar]
- 37. Teti A, et al. 1991. Osteoblast-osteoclast relationships in bone resorption: osteoblasts enhance osteoclast activity in a serum-free co-culture system. Biochem. Biophys. Res. Commun. 179: 634–640 [DOI] [PubMed] [Google Scholar]
- 38. Thrash BR, Menges CW, Pierce RH, McCance DJ. 2006. AKT1 provides an essential survival signal required for differentiation and stratification of primary human keratinocytes. J. Biol. Chem. 281: 12155–12162 [DOI] [PubMed] [Google Scholar]
- 39. Tournay O, Benezra R. 1996. Transcription of the dominant-negative helix-loop-helix protein Id1 is regulated by a protein complex containing the immediate-early response gene Egr-1. Mol. Cell. Biol. 16: 2418–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tu Q, et al. 2011. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J. Biol. Chem. 286: 12542–12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vandoorne K, et al. 2010. Bone vascularization and trabecular bone formation are mediated by PKB alpha/Akt1 in a gene-dosage-dependent manner: in vivo and ex vivo MRI. Magn. Reson. Med. 64: 54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson EM, Hsieh MM, Rotwein P. 2003. Autocrine growth factor signaling by insulin-like growth factor-II mediates MyoD-stimulated myocyte maturation. J. Biol. Chem. 278: 41109–41113 [DOI] [PubMed] [Google Scholar]
- 43. Wilson EM, Rotwein P. 2007. Selective control of skeletal muscle differentiation by Akt1. J. Biol. Chem. 282: 5106–5110 [DOI] [PubMed] [Google Scholar]
- 44. Woodgett JR. 2005. Recent advances in the protein kinase B signaling pathway. Curr. Opin. Cell Biol. 17: 150–157 [DOI] [PubMed] [Google Scholar]
- 45. Wu X, et al. 2010. Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell 7: 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yoeli-Lerner M, et al. 2005. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell 20: 539–550 [DOI] [PubMed] [Google Scholar]
- 47. Zaidi M. 2007. Skeletal remodeling in health and disease. Nat. Med. 13: 791–801 [DOI] [PubMed] [Google Scholar]
- 48. Zhou GL, et al. 2006. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J. Biol. Chem. 281: 36443–36453 [DOI] [PubMed] [Google Scholar]