Abstract
Intercellular tight junctions (TJs) exhibit a complex molecular architecture involving the regulated cointeraction of cytoplasmic adaptor proteins (e.g., zonula occludens) and integral membrane linker proteins (e.g., occludin and claudins). They provide structural integrity to epithelial and endothelial tissues and create highly polarized barriers essential to homeostatic maintenance within vertebrate physiological systems, while their dysregulation is an established pathophysiological hallmark of many diseases (e.g., cancer, stroke, and inflammatory lung disease). The junctional complex itself is a highly dynamic signaling entity wherein participant proteins constantly undergo a blend of regulatory modifications in response to diverse physiological and pathological cues, ultimately diversifying the overall adhesive properties of the TJ. Occludin, a 65-kDa tetraspan integral membrane protein, contributes to TJ stabilization and optimal barrier function. This paper reviews our current knowledge of how tissue occludin is specifically modified at the posttranscriptional and posttranslational levels in diverse circumstances, with associated consequences for TJ dynamics and epithelial/endothelial homeostasis. Mechanistic concepts such as splice variance and alternate promoter usage, proteolysis, phosphorylation, dimerization, and ubiquitination are comprehensively examined, and possible avenues for future investigation highlighted.
TIGHT JUNCTIONS AND OCCLUDIN
Tight junctions.
The tight junction (TJ), originally identified in the early 1960s by Farquhar and Palade (34), is an intramembrane multiprotein complex that provides apical intercelluar connections between adjacent cells in both epithelial and endothelial monolayers. Working in concert with other structurally distinct intercellular junctions (adherens junctions, gap junctions, and desmosomes), TJs provide structural integrity to tissues and create highly polarized barriers with selective paracellular permeability to water, solutes, larger molecules, and other cells, an essential feature of homeostatic maintenance within vertebrate physiological systems. Over the years, additional roles for TJs in cellular differentiation, proliferation, migration, signal transduction, and gene expression have also emerged, highlighting their functional diversity (for reviews, see references 10, 73, and 106), while TJ dysregulation has been associated with the pathogenesis of various diseases, including cancers, stroke, diabetic retinopathy, pulmonary disorders, and inflammatory bowel disease (38).
The molecular architecture of the TJ exhibits a complex arrangement of cointeracting cytoplasmic adaptor proteins (e.g., zonula occludens [ZO-1, ZO-2, and ZO-3], as well as 7H6, AF6, vinculin, and cingulin), which mediate the cytoskeletal tethering and cell-cell partnering of transmembrane linker proteins (e.g., occludin, claudins, and junctional adhesion molecules 1, 2, and 3) (1). The overall junctional complex is therefore a highly dynamic signaling entity in which the individual protein components are frequently subjected to an array of regulatory modifications that may introduce changes in protein size and structural conformation, modify protein-protein interactions and localization, and either enhance or subdue protein function, ultimately modulating the adhesive properties of the TJ. In an effort to illustrate this concept, this minireview examines how the prototypical TJ protein occludin is subjected to various modifications at the posttranscriptional and posttranslational levels, with specific consequences for TJ assembly and barrier function in diverse circumstances. Relevant concepts such as splice variance and alternate promoter usage, proteolysis, phosphorylation, dimerization, and ubiquitination are considered, and the functional consequences (if known) of occludin variants are highlighted. Moreover, regular comparison of findings from both epithelial and endothelial barrier studies are included within the general scope of the paper.
Occludin structure.
Originally discovered in avian tissues by Furuse et al. (41) using anti-chick occludin antisera prepared in rats, occludin (∼65 kDa) was found localizing to epithelial and endothelial TJs and was subsequently confirmed as the first integral membrane TJ protein to be identified. Later cloning studies revealed up to 90% homology among mammalian occludins, with both avian and mammalian forms exhibiting a similar multidomain tetraspan structure (Fig. 1) (3) and with individual occludin domains exhibiting distinct functions and regulatory features (for a review, see reference 36). The extended C terminus, for example, has been shown to be essential for occludin interactions with ZO-1, subsequently mediating its intracellular trafficking to the plasma membrane TJ site. The C terminus also has essential signaling functions and mediates occludin dimerization (21, 40, 63, 122, 123). Investigations have also confirmed roles for the extracellular loops and at least one transmembrane domain in occludin localization and TJ stability (8), while evidence also points to possible copolymerization of occludin with a claudin(s) for proper stabilization of TJ strands (39). Occludin also exhibits a MARVEL (MAL and related proteins for vesicle trafficking and membrane link) domain, a four-transmembrane structural motif common among junctional proteins involved in membrane apposition and fusion events (Fig. 2). Other proteins within the occludin family displaying this domain include MARVEL-D3 and MARVEL-D2 (tricellulin). The former is an alternately spliced protein that colocalizes with occludin at TJs in both epithelial and endothelial cells (107), while the latter is expressed in regions where three cells make contact (89). Notwithstanding their collective importance to barrier maintenance, this review will focus exclusively on occludin.
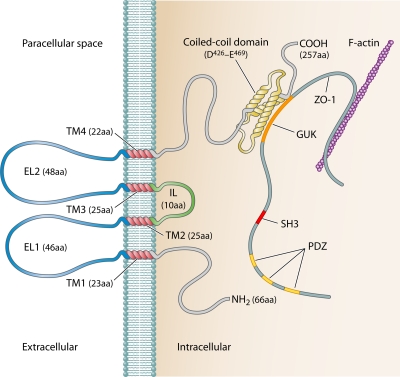
Fig 1.
Plasma membrane-associated human occludin. Individual occludin domains (and amino acid lengths) are indicated. C-terminal occludin interaction with the ZO-1 GUK domain is also shown (Note that ZO-1 PDZ domains indicated are believed to mediate ZO-1 interaction with claudins.) ZO-1 is not to scale (∼25% of actual protein size). EL1/2, extracellular loops 1 and 2; GUK, guanylate kinase domain; IL, intracellular loop; SH3, Src homology 3 domain; TM1 to -4, transmembrane domains 1 to 4.
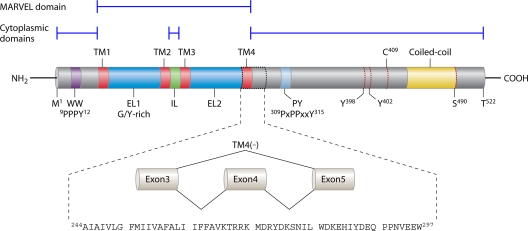
Fig 2.
Structural organization of the TM4(+) and TM4(−) variants of human occludin protein. Canonical/TM4(+) occludin is pictured. Also pictured is the 54-aa deletion corresponding to the TM4(−) variant resulting from skipping of exon 4. C409, cysteine-409; EL1/2, extracellular loops 1 and 2 (EL1 is glycine/tyrosine rich); IL, intracellular loop; M1, methionine-1; PY, Nedd4-2-binding domain; T522, threonine-522; TM1 to -4, transmembrane domains 1 to 4; Y398/402, tyrosine-398/402; WW, Itch-binding domain.
Occludin function.
The importance of occludin to proper TJ function has been conclusively demonstrated through numerous investigations. Overexpression of occludin in Madin-Darby canine kidney (MDCK) cells, for example, enhances transepithelial electrical resistance (75), while synthetic peptides mimicking the extracellular loop structure of occludin have been found to increase permeability in A6 epithelial cells by disrupting normal loop-loop interactions (61, 126). In knockout mice (occludin−/−), TJs were seen to be morphologically intact, although the investigators noted complex histological phenotypes characterized by chronic inflammation and poor TJ integrity in several epithelial tissues (95), pointing to a more likely role for occludin in TJ stability and barrier function as opposed to TJ assembly. The indispensability of occludin for cortical actin organization and barrier integrity in various endothelial cell models has also been robustly demonstrated (60). Research has shown, for example, that downregulation of occludin via either proteolysis or treatment with permeabilizing factors such as vascular endothelial growth factor (VEGF) or phospholipase D2 (PLD2) is directly linked to elevated endothelial permeability (78, 120, 129).
POSTTRANSCRIPTIONAL MODIFICATIONS
Splice variance and alternate promoter usage. (i) Tight junctions and gene splicing.
Gene splicing is a eukaryotic posttranscriptional mechanism that extends the coding capacity of a single gene by enabling it to encode multiple protein isoforms that are structurally and functionally distinct from one another. In this way, the variety and frequency of reactive sites/domains within a protein can be altered, thereby enhancing its functional diversity. The emergence of gene splicing as a regulatory force in TJ assembly stretches back almost 2 decades with the discovery by Willott et al. (125) of two different ZO-1 isoforms. Sequence analysis of ZO-1 cDNA by this group revealed an additional 240-bp sequence in only some of the ZO-1 cDNAs studied. This coded for an 80-amino-acid in-frame insertion in ZO-1 to yield the ZO-1 alpha(+) isoform, an observation made in normal rat and human tissues as well as a variety of epithelial cell lines (Caco-2, T84, and Hep G2). Investigators subsequently deduced that the ZO-1 alpha(+) variant reduces TJ lability and enhances tightness, while the ZO-1 alpha(−) variant is restricted to more structurally dynamic junctions that exhibit a broader range of paracellular flux rates and/or are readily opened by physiological signals (e.g., such as those found in endothelial cells, Sertoli cells, and renal glomeruli) (9, 59, 72, 86, 102).
(ii) Occludin N-terminal extension.
In mapping the human occludin gene to its chromosomal band (5q13.1) in epithelial cells, Saitou and coworkers identified several occludin mRNA bands by Northern blotting, suggesting that, like that of ZO-1, occludin expression was also subject to gene splicing events (96). Using MDCK cells, Muresan et al. (79) went on to identify occludin 1B, an alternatively spliced transcript containing a 193-bp insertion that encoded an occludin isoform with an extended N-terminal sequence of 56 amino acids. Although the functional significance of this insertion was not investigated (the subcellular distribution of occludin 1B was identical to that of normal occludin), the authors speculated that the N-terminal extension may be shielding an SH3 consensus motif (XPXXPPP*XP, where * is any hydrophobic residue and X is any amino acid), thereby potentially blocking binding interactions between occludin 1B and SH3 adaptor proteins.
(iii) Occludin TM4 deletion.
More recently, an alternatively spliced occludin isoform generated by skipping exon 4 has been identified by Ghassemifar and coworkers in human tissues, embryos, and a range of cell types (both epithelial and endothelial). This variant comprises a 162-bp deletion encoding the entire fourth transmembrane domain (TM4) and proximal C-terminal region and was subsequently designated TM4(−), as opposed to canonical occludin or TM4(+) (42, 43) (Fig. 2). Both TM4(−) and TM4(+) isoforms were also identified in primate epithelial cells but not in murine or canine cells, suggesting that this splicing event has a more recent evolutionary origin. A related study conducted by Mankertz et al. (70) further demonstrated the presence of four differentially spliced occludin mRNA transcripts in human epithelial tissues (placenta and colon) and colonic epithelial cells (HT29/B6). These isoforms were designated types I (522 amino acids [aa]), II (468 aa), III (290 aa), and IV (504 aa), with type II being identical to the TM4(−) isoform identified previously (42). Unlike TM4(+) occludin, the TM4(−) isoform exhibits an externalized C-terminal domain, presumably altering its ability to interact with ZO-1 and properly localize to the tight junction. In this respect, Mankertz et al. (70) reported that the type II/TM4(−) isoform in fact does not colocalize with ZO-1, reinforcing the importance of both TM4 and C-terminal domains to the correct intracellular trafficking of occludin. By employing “live” cell immunofluorescent staining using a C-terminally directed antioccludin IgG, Ghassemifar et al. (42) also demonstrated that TM4(−) immunoreactivity was to be found only at the periphery of subconfluent cell islands or wounded cell monolayers, leading one to speculate that low-level expression of this isoform occurs only under conditions of tissue remodelling that involve TJ “loosening” (a feature of both normal physiological regulation and pathologies such as cancer). These aforementioned investigations, however, provide only limited insight into the regulation and putative function(s) of the TM4(−) isoform, with firmer conclusions necessitating further research.
(iv) Other occludin variants (exons 1 and 9).
In other studies, Mankertz and coworkers (70) also demonstrated the existence of a second occludin promoter and transcription start site (exon 1a) displaying enhanced sensitivity to proinflammatory tumor necrosis factor alpha (TNF-α) signaling, leading the authors to speculate that this could be a pathological mechanism to reduce occludin expression, causing colonic epithelial inflammation characteristic of ulcerative colitis. Finally, a more recent study by Gu et al. (46) demonstrated the existence of an exon 9-deleted occludin splice variant (OccΔE9) in human liver cells (Chang, Huh7, and Hep3B) acting via an alternative (P2) promoter site. Interestingly, OccΔE9 localized in the cytoplasm and, unlike wild-type occludin, did not induce mitochondrion-mediated apoptosis or reduce cell invasiveness. As previous publications have demonstrated the antitumorigenic properties of occludin (71, 84), these findings have led investigators to speculate on a potential role for the OccΔE9 variant in cancer progression. Again, further investigations will be necessary to confirm such a role.
POSTTRANSLATIONAL MODIFICATION OF OCCLUDIN
Proteolytic degradation. (i) MMP-dependent proteolysis.
The proteolytic degradation of both adherens and tight junction proteins constitutes an important regulatory feature of physiological tissue remodeling (e.g., barrier regulation, migration, proliferation, and angiogenesis) and pathology (e.g., cancer, blood-brain barrier [BBB] inflammation, and diapedesis) (54, 55, 67, 80). Within the endothelium, proteolytic cleavage of occludin to inactive fragments (presumably destined for cellular recycling) invariably leads to barrier disruption and is frequently mediated by matrix metalloproteinases (MMPs). Indeed, various papers report that occludin serves as a substrate for gelatinases (MMP-2/9) (44, 65) and, to a lesser extent, stromelysins (MMP-3) (47, 93; for a review, see reference 2). While the endothelial cell itself is undoubtedly a principal source of MMPs, basolaterally located astrocytes and pericytes within microvascular beds, as well as circulating cells, are also important MMP sources. These MMPs contribute in tandem to the degradation of basal lamina proteins and TJ-associated occludin, the former providing structural support for the capillary wall as well as some degree of proteolytic protection for intercellular TJ proteins (115, 118, 128). By way of illustration, a report by Ishihara et al. (55) demonstrates how glioma-derived transforming growth factor β2 (TGF-β2) can induce endothelial MMP expression, leading to occludin reduction and TJ dysregulation during glioma-mediated BBB impairment. In contrast, the degradation of occludin (and ZO-1/claudin 5) by leukemic cell-derived MMP-2 and -9, leading to BBB dysfunction and leukemic cell invasion into the central nervous system (CNS) during leukemia, has also been reported (37).
(ii) In vivo endothelial MMP studies.
For the endothelium, much in vivo evidence exists to support a role for MMP-mediated occludin proteolysis, particularly during blood-brain barrier (BBB) pathologies. Rodent models of cerebral ischemic injury, for example, demonstrate elevated levels of MMP-2/9 leading to occludin fragmentation in brain microvessels, with resultant elevated leakage and brain edema (13, 20, 64, 114, 128). Yang et al. (128) went on to describe the temporal dynamics of these events, confirming that constitutively active MMP-2 is responsible for occludin cleavage and TJ “loosening” during the early transient phase of the ischemia (3 h), while MMP-9 and other products of extended neuroinflammation and reperfusion injury (e.g., reactive oxygen species) cause further occludin degradation and more long-term (24-h) alterations to BBB integrity. In other models, the depletion of occludin by MMP-9, leading to macrophage infiltration, has been reported during cerebral aneurysm formation in rats (111). Higashida et al. (51) also recently confirmed a role for MMP-9 in the degradation of both laminin and occludin, leading to BBB disruption and brain edema following traumatic brain injury in rats, a mechanism also involving hypoxia-inducible factor 1α (HIF-1α) and aquaporin 4 (AQP-4). Degradation of occludin by MMP-2/9 is also a feature of blood-brain and blood-retinal barrier disruption in various rat models of type II diabetes and diabetic retinopathy (44, 50, 62). In other studies, elevated levels of MMP-9 observed in the medial thalamus and brain microvascular endothelium of thiamine-deficient mouse brain (an established model of Wernicke's encephalopathy, which is characterized by petechial hemorrhagic lesions and BBB compromise) have been shown to correlate with reductions of occludin (and ZO-1/2) protein levels and membrane immunolocalization pattern (14). Degradation of occludin (and claudin 5) arising from elevated MMP-9 levels in mouse brain endothelium following azoxymethane-induced acute liver failure (ALF) has also been reported, further corroborating the role of MMP-9 in the vasogenic mechanism of brain edema associated with this disease (19). Finally, a slightly earlier study by Caron et al. (16) has demonstrated that elevated levels of occludin proteolysis correlate with increases in ProMMP-2/9, MMP-9, and TIMP-2 in rat kidney endothelium during ischemic injury.
(iii) In vitro endothelial MMP studies.
In line with in vivo studies, brain-derived microvascular endothelial cell (BMEC) models have also robustly demonstrated the involvement of MMP-dependent occludin processing in the regulation of cell-cell contacts and monocyte diapedesis (66, 92), as well as during pathological disruption of the BBB via oxidative stress (65) and infection (e.g., for N. meningitidis, HIV-infected monocytes, and West Nile virus-infected cortical astrocytes) (53, 100, 118). Moreover, a recent study by Blecharz et al. (15) has shown that incubation of the murine brain endothelial cell line cEND with sera from multiple sclerosis (MS) patients (in either active phase or remission) leads to downregulation of occludin (and claudin 5) mRNA/protein in parallel with upregulation of MMP-9 levels, with the authors also reporting minor BBB reconstitution following glucocorticoid treatment of cEND monolayers. Finally, in vitro coculture of BMECs with glioblastoma cells (and growth factors, e.g., TGF-β2) reduced expression of occludin (and JAM-A/ZO-1) in an MMP-dependent manner in parallel with enhanced endothelial permeability (55).
(iv) Epithelial MMP studies.
MMP-dependent proteolysis of occludin in epithelial cells, while far less common, has also been reported. A recent study by Casas et al. (17), for example, has demonstrated reduced barrier resistance and increased basolateral accumulation of occludin C-terminal fragments (i.e., 41 and 48 kDa) following methyl-beta-cyclodextrin-induced cholesterol depletion in MDCK cells. More importantly, these authors demonstrate that the occludin hydrolysis in this instance results from the action of a GM6001-sensitive MMP. Moreover, in a slightly earlier study by Gorodeski (45), elevated activation and release of MMP-7 following estrogen treatment of normal human vaginal epithelial cells led to increased occludin proteolysis, prompting the author to suggest this as a potential mechanism whereby estrogens may regulate epithelial paracellular permeability during the human estrous cycle.
(v) MMP-independent proteolysis.
Considerable evidence exists, however, for MMP-independent proteolytic processing of occludin in epithelial cells. Studies by Wan et al. (124) and Runswick et al. (94) confirmed roles for both serine and cysteine peptidases (e.g., Der p 1 from house dust mite allergen) in the cleavage of occludin with attendant elevation in epithelial permeability, and they went on to propose this as a pathological mechanism for allergen delivery across lung and nasal epithelial barriers in asthma and allergic rhinitis sufferers. Calpains (Ca2+-dependent cysteine proteases) have also been demonstrated to cleave occludin in epithelial models of bacterial infection, thereby accommodating paracellular translocation of either pathogens (e.g., group A Streptococcus pyogenes) or polymorphonuclear leukocytes (24, 108).
Phosphorylation. (i) Occludin phosphorylation.
Reversible phosphorylation of occludin and other TJ proteins is now established as a vital aspect of barrier regulation. In an early barrier experiment using MDCK I cells, for example, Sakakibara et al. (97) demonstrated that epithelial barrier restoration following a switch from low to normal calcium levels induced elevated levels of NP-40-insoluble occludin coincident with increased phosphoserine (pSer) and phosphothreonine (pThr) occludin levels. This led the authors to conclude that highly phosphorylated occludin selectively localized to intact epithelial TJs as a detergent-insoluble form. Later studies confirmed the necessity of occludin phosphorylation on key serine, threonine, and tyrosine residues to the TJ assembly process in MDCK cells (35, 117), while occludin phosphorylation on serine and threonine residues in epithelial junctions of Xenopus laevis embryos has also been reported (27, 28). The regulation of endothelial TJs through occludin phosphorylation has also received extensive attention, with many studies focusing on how the phosphorylation status of occludin correlates to vessel barrier dysfunction (6, 23, 29, 30, 77, 85). In line with this mechanism of regulatory covalent modification, occludin has repeatedly been shown to serve as a substrate for a wide range of cellular kinases and phosphatases in various experimental/pathophysiological contexts (Table 1).
Table 1.
Phosphatase- and kinase-dependent modification of occludina
| Phosphatase/kinase | Experimental model | Phosphosite(s) | Barrier effectb | Reference(s) |
|---|---|---|---|---|
| CK1 | HUVEC, GST-OccC (human) | Thr, Ser | 76 | |
| GST-OccC (human), GST-OccN (human) | Thr, Ser | 31 | ||
| CK2 | Crude brain extract (porcine) | Thr, Ser | 105 | |
| GST-OccC (human) | Thr-404, Ser-408 | 31 | ||
| Occludin domain E (Xenopus laevis) | Thr-375, Ser-379 | 28 | ||
| Embryo (Xenopus laevis) | Thr, Ser | 27 | ||
| c-Src | Isolated brain capillary (rat) | Tyr | − | 56, 113 |
| Rat-1, MDCK | Tyr-398, Tyr-402 | − | 33 | |
| MDCK | Tyr-473 | 32 | ||
| GST-OccC (chicken) | Tyr | 57 | ||
| c-Yes | MDCK | Tyr | + | 22 |
| DEP-1 (PTPη) | MCF10A, MDCK II, A431 | Tyr | + | 98 |
| p-ERK | Caco-2 | Tyr, Thr | + | 11 |
| PI3K | Caco-2, GST-OccC (chicken) | Tyr | + | 103 |
| PKC | CaSki | Thr | + | 130 |
| MDCK | Ser-338 | + | 5 | |
| PKCβII | REC (bovine) | Thr, Ser | − | 49 |
| PKCη | Caco-2, MDCK | Thr-403, Thr-404 | + | 110 |
| PP1 | Caco-2, GST-OccC (chicken) | Ser | − | 101 |
| PP2A | MECs (murine) | Thr, Ser | − | 48 |
| HPAF-II | Thr, Ser | − | 88 | |
| Caco-2, GST-OccC (chicken) | Thr | − | 101 | |
| MDCK | Thr, Ser | − | 81 | |
| RhoK | BMEC (murine) | Thr-382, Ser-507 | − | 127 |
| ECV304 | Thr, Ser | − | 52 |
BMEC, brain microvascular endothelial cell; Caco-2, human colorectal epithelial adenocarcinoma; CaSki, HPV16/18+ cervical epithelial carcinoma; CK1/2, casein kinase 1/2; c-Src, tyrosine kinase; c-Yes, non-receptor protein tyrosine kinase; DEP1, density-enhanced phosphatase 1; p-ERK, phospho-extracellular signal-regulated kinase; ECV304, transformed HUVEC; GST, glutathione S-transferase; HUVEC, human umbilical vein endothelial cell; MCF10A, human mammary epithelial cell; MDCK, Madin-Darby canine kidney; PI3K, phosphoinositide-3 kinase; PKC, protein kinase C; PP, protein phosphatase; REC, retinal endothelial cell; RhoK, Rho-associated protein kinase.
+, increased barrier integrity; −, decreased barrier integrity.
(ii) pTyr occludin. (a) Barrier reduction.
The highly dynamic nature of the occludin phosphorylation state has since dictated the focus of numerous TJ studies, yielding functionally distinct modification patterns. Elevated occludin phosphotyrosine (pTyr) levels, for example, have been strongly linked to barrier dysfunction during oxidative stress in Caco-2 cells (91, 103), acetaldehyde treatment in human colonic mucosa (12), ATP treatment in human cervical epithelial CaSki cells (130), mainstream cigarette smoke exposure in Calu-3 lung epithelium cells (83), and cholesterol depletion in MDCK II cells (69), with the last study also demonstrating how occludin association with detergent-insoluble membrane fractions (consistent with intact TJs) is partly cholesterol dependent. As an explanation of these events, groundbreaking studies by Kale et al. (57) and Elias et al. (33) support the hypothesis that barrier-disrupting stimuli enhance tyrosine phosphorylation of occludin within a highly conserved C-terminal motif (YETDYTT, corresponding to Tyr-398 and Tyr-402 in human occludin) (Fig. 2). This attenuates its interaction with ZO-1 (and ZO-2/3, but not F-actin) and leads to occludin delocalization from the membrane with attendant barrier disruption (see reference 90 for a more detailed review). Other epithelial barrier studies directly and indirectly support this notion (91, 103).
In endothelial studies, both focal cerebral ischemia (56, 113) and glutamate treatment of BMEC cultures (4), which are in vivo and in vitro models of BBB dysfunction, respectively, also manifest elevated pTyr occludin levels. This is fully consistent with the reduced endothelial pTyr occludin levels that appear to accompany the barrier-strengthening impact of blood flow-associated hemodynamic forces such as cyclic strain and shear stress on bovine aortic endothelial cells (BAECs) and BMECs, respectively (26, 121). In this regard, the recent in vitro shear study by Walsh et al. (121) is particularly noteworthy. In that paper, the authors describe an intriguing mechanism in BMECs whereby mechanosensory VE-cadherin can, through activation of a Tiam1/Rac1 signaling pathway, transmit physiological shear signals to TJs, in part via tyrosine phosphatase-dependent reduction of pTyr occludin levels. Importantly, this study highlights how molecular cross talk between endothelial adherens and tight junctions can adapt TJ adhesiveness through the covalent modification of occludin (121).
(b) Barrier enhancement.
Paradoxically, a reduced pTyr occludin level has also been linked to barrier dysfunction following either Ca2+ or ATP depletion in MDCK cell monolayers (22, 117) and following poly-l-arginine treatment in rabbit nasal epithelium (82), highlighting the potential diversity in occludin pTyr sites and cell-specific kinase/phosphatase effectors that likely affect the regulation of occludin trafficking and protein-protein interactions.
(iii) pSer/pThr occludin. (a) Barrier enhancement.
Another common feature of intact TJs appears to be hyperphosphorylation of occludin at serine and threonine residues, as evidenced through several early MDCK studies (5, 35, 69, 81, 97). Consistent with this, occludin Ser/Thr dephosphorylation has been shown to increase paracellular permeability in both intestinal epithelium and Caco-2 cells following infection by either enteropathogenic Escherichia coli or Pseudomonas aeruginosa, respectively (104, 119). Other recent studies, also with Caco-2 monolayers, confirm the importance of threonine phosphorylation within the occludin C-terminal tail for mediating its ability to localize to the TJ (101, 110), while reduced pSer/pThr occludin levels have been shown to accompany barrier disruption following septic insult and glutamate treatment in microvascular endothelial cell cultures (4, 48).
(b) Barrier reduction.
Contrasting studies, however, again highlight the potential multiplicity of occludin pSer and pThr sites and related cell-specific signaling effectors that mediate the functional diversity of this protein. In this respect, elevated pSer/pThr occludin levels have been correlated to increased epithelial permeability (58, 88, 130) as well as to BBB dysfunction in encephalitic mouse and human brain (127). Collins et al. (26) also showed a reduction in pSer/pThr occludin levels in BAECs in response to physiological cyclic strain (i.e., a barrier-strengthening influence). An early study by Antonetti et al. (6) also deserves mention, as it was one of the first papers to relate occludin phosphorylation changes with vascular endothelial permeability. In that study, VEGF-induced barrier disruption in bovine retinal endothelial cells (BRECs), an underlying pathological mechanism in diabetic retinopathy, was shown to involve enhanced occludin phosphorylation. Subsequent studies by the same group attributed these events to a protein kinase Cβ (PKCβ)-dependent increase in occludin pSer levels (49). By combining mass spectrometry data analysis with bioinformatics, Sundstrom and coworkers subsequently mapped the VEGF-induced occludin phosphorylation sites to include Thr-168, Thr-404, Ser-408, Ser-471, and Ser-490 (numbering is for human occludin), with further investigations specifically identifying Ser-490 as a pivotal amino acid mediating occludin interaction with its other TJ binding partners (e.g., ZO-1) (109), in addition to its subsequent ubiquitination and degradation after VEGF treatment (discussed below) (78).
(iv) Occludin dimerization.
Finally, in addition to regulating occludin cointeraction with other TJ binding partners (33, 57), phosphorylation may also putatively regulate the ability of occludin to cointeract with itself to form homodimers, a phenomenon first noted by Chen et al. (21) while studying TJ assembly in early Xenopus embryos. Recent studies of the BBB endothelium have demonstrated that the C-terminal coiled-coil domain in occludin contributes to its redox-dependent dimerization via disulfide bridge formation (Cys-409 in human occludin), a critical step in the generation of tightly packed multiprotein TJ assemblies (74, 122, 123). Similar studies using HEK293 cells have also reported that redox-dependent occludin homodimerization is essential to the recruitment of ZO-1 to the plasma membrane (18). Interestingly, the close proximity of Cys-409 to Tyr-398/402 (Fig. 2) (these tyrosine phosphosites are known to mediate cointeraction of occludin/ZO-1 [33, 57]) suggests functional overlap between these specific phosphorylation and dimerization sites. Indeed, one might speculate that the reversible phosphorylation of occludin at Tyr-398/402 may induce a localized conformational change within the coiled-coil domain to regulate steric masking/unmasking of the nearby sulfhydryl side chain on Cys-409 and consequently disulfide bridge formation. Future investigations will likely shed further light on this possible mechanism.
Ubiquitination. (i) Occludin ubiquitination.
Ubiquitin, a 76-amino-acid heat stable protein found in all eukaryotic cells, can be coupled as either a monomer or a polymer to lysine residues in an ATP-dependent manner, thereby directing proteins to the proteasomal degradation pathway (99). Ubiquitination is therefore an important mechanism for regulating a target protein's function through modification of its intracellular trafficking and degradation and, in this respect, is now emerging as a significant regulatory mechanism within both epithelial and endothelial TJs. Early work by Traweger et al. (116) reported ubiquitination of occludin in vivo using HEK293 cells, an event that was sensitive to proteasomal inhibition with MG-132. The same authors also employed yeast two-hybrid screening to identify a novel interaction between the N terminus of occludin (Pro9-Pro10-Pro11-Tyr12) and the WW domain of the E3 ubiquitin ligase, Itch (Fig. 2). A slightly later study by Lui and Lee (68) went on to report that occludin coassociation with Itch and UBC-4 (a ubiquitin-conjugating enzyme) was involved in regulating TJ integrity in Sertoli cells of the blood-testis barrier. More recently, Coëffier et al. (25) presented evidence that proteasome-dependent degradation of occludin within the colonic mucosa contributes to the pathophysiology of irritable bowel syndrome (IBS). Moreover, coimmunoprecipitation of occludin with another E3 ubiquitin ligase family member, Nedd4-2, has also been reported (87). In that study, occludin was found to interact with Nedd4-2 via a conserved C-terminal PY motif (Fig. 2), which when mutated almost doubled the half-life of transfected occludin in HEK293 cells. In a parallel series of experiments, these authors also went on to show that small interfering RNA (siRNA)-mediated knockdown of Nedd4-2 increased occludin expression and reduced paracellular permeability in mpkCCD(c14) cells (a collecting duct epithelial cell line), with Nedd4-2 overexpression having the opposite effects. On a related note, a recent study by Takahashi et al. (112) has confirmed a role for another E3 ubiquitin ligase, Ligand of Numb protein X1 p80 isoform (LNX1 p80), in regulating claudin 1 ubiquitination (and subsequent endocytosis and degradation) in MDCK cells, while the contribution of the ubiquitin-proteasome system to claudin 1 membrane trafficking in EA hy.926 cells has also recently been described (7).
(ii) Ubiquitination and Ser-490.
More recently, Murakami et al. (78) have demonstrated the contribution of occludin ubiquitination to endothelial TJ regulation using a BREC model of VEGF-induced permeability. In a comprehensive series of investigations, the authors convincingly demonstrate that Ser-490 phosphorylation of occludin is an essential prerequisite for its ubiquitination following VEGF treatment of BRECs. This occludin ubiquitination event subsequently induced the endocytosis of coassociated TJ proteins, claudin 5 and ZO-1, ultimately leading to TJ disruption. The authors go on to speculate that phosphorylation of Ser-490 possibly decreases occludin interactions with other TJ proteins, while also exposing its N-terminal WW-binding motif for enhanced interaction with Itch. Moreover, they also report that ubiquitinated occludin interacts more readily with modulators of intracellular trafficking and endocytosis such as epsin 1 and epidermal growth factor receptor substrate 15 (Eps15).
FINAL COMMENTS
Intercellular TJs are integral to the regulated maintenance of barrier function in vertebrate physiological systems. In response to diverse physiological and pathological cues, individual TJ proteins may undergo an array of regulatory modifications at posttranscriptional and posttranslational levels, thereby enhancing the functional plasticity of these highly dynamic signaling complexes. To illustrate this concept, this minireview has focused on occludin, a prototypical TJ protein well known to regulate junctional organization and the ensuing restriction of the paracellular transport pathway. In this regard, published findings clearly point to a highly processed biomolecule subject to size alterations (e.g., splice variance and proteolysis) and extensive covalent modification (e.g., phosphorylation and ubiquitination). Moreover, despite the various biochemical and morphological differences that distinguish epithelial and endothelial TJs, considerable overlap in their occludin modification characteristics is evidenced from the literature.
Notwithstanding the wealth of information already generated within this field, several avenues for future investigation still remain. The identification of new phosphosites and other regulatory domains (including binding partners), characterization of tissue- and cell-specific occludin modifications, and deciphering the functional rapport between different modification events (e.g., phosphorylation, ubiquitination, proteolysis, and dimerization), will likely typify future studies in this field. This will ultimately yield a fuller understanding of how modifications to occludin affect TJ characteristics and will help to unlock the therapeutic potential of the TJ by identifying new cellular targets for intervention in diseases characterized by barrier dysregulation.
ACKNOWLEDGMENTS
I acknowledge financial support provided through Science Foundation Ireland (04/BR/B0421, BICF708), the Irish Research Council for Science, Engineering & Technology (IRCSET ID 493, October 2006), and the Irish Higher Education Authority Programme for Research in Third Level Institutes (HEA-PRTLI Cycle 4: T3 Targeted Therapeutics & Theranostics).
Footnotes
Published ahead of print 14 November 2011
REFERENCES
- 1. Aijaz S, Balda MS, Matter K. 2006. Tight junctions: molecular architecture and function. Int. Rev. Cytol. 248:261–298 [DOI] [PubMed] [Google Scholar]
- 2. Alexander JS, Elrod JW. 2002. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J. Anat. 200:561–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ando-Akatsuka Y, et al. 1996. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J. Cell Biol. 133:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. András IE, et al. 2007. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J. Cereb. Blood Flow Metab. 27:1431–1443 [DOI] [PubMed] [Google Scholar]
- 5. Andreeva AY, Krause E, Müller EC, Blasig IE, Utepbergenov DI. 2001. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J. Biol. Chem. 276:38480–38486 [DOI] [PubMed] [Google Scholar]
- 6. Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. 1999. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 274:23463–23467 [DOI] [PubMed] [Google Scholar]
- 7. Asaka M, Hirase T, Hashimoto-Komatsu A, Node K. 2011. Rab5a-mediated localization of claudin-1 is regulated by proteasomes in endothelial cells. Am. J. Physiol. Cell Physiol. 300:C87–C96 [DOI] [PubMed] [Google Scholar]
- 8. Balda MS, Flores-Maldonado C, Cereijido M, Matter K. 2000. Multiple domains of occludin are involved in the regulation of paracellular permeability. J. Cell. Biochem. 78:85–96 [PubMed] [Google Scholar]
- 9. Balda MS, Anderson JM. 1993. Two classes of tight junctions are revealed by ZO-1 isoforms. Am. J. Physiol. 264:C918–C924 [DOI] [PubMed] [Google Scholar]
- 10. Balda MS, Matter K. 2009. Tight junctions and the regulation of gene expression. Biochim. Biophys. Acta 1788:761–767 [DOI] [PubMed] [Google Scholar]
- 11. Basuroy S, Seth A, Elias B, Naren AP, Rao R. 2006. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem. J. 393:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basuroy S, Sheth P, Mansbach CM, Rao RK. 2005. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am. J. Physiol. Gastrointest. Liver Physiol. 289:G367–G375 [DOI] [PubMed] [Google Scholar]
- 13. Bauer AT, Bürgers HF, Rabie T, Marti HH. 2010. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J. Cereb. Blood Flow Metab. 30:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beauchesne E, Desjardins P, Hazell AS, Butterworth RF. 2009. Altered expression of tight junction proteins and matrix metalloproteinases in thiamine-deficient mouse brain. Neurochem. Int. 55:275–281 [DOI] [PubMed] [Google Scholar]
- 15. Blecharz KG, et al. 2010. Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cEND incubated with sera from patients with multiple sclerosis. Mult. Scler. 16:293–302 [DOI] [PubMed] [Google Scholar]
- 16. Caron A, Desrosiers RR, Langlois S, Béliveau R. 2005. Ischemia-reperfusion injury stimulates gelatinase expression and activity in kidney glomeruli. Can. J. Physiol. Pharmacol. 83:287–300 [DOI] [PubMed] [Google Scholar]
- 17. Casas E, et al. 2010. Cholesterol efflux stimulates metalloproteinase-mediated cleavage of occludin and release of extracellular membrane particles containing its C-terminal fragments. Exp. Cell Res. 316:353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castro V, et al. 2010. The membrane recruitment of ZO-1 and its interplay with occludin is redox sensitive and relies on the disulfide bridge-mediated homodimerization of occludin, abstr P-5. Abstr. 13th Int. Symp. Signal Transduction Blood-Brain University Hospital, Zurich, Switzerland [Google Scholar]
- 19. Chen F, Ohashi N, Li W, Eckman C, Nguyen JH. 2009. Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 50:1914–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen W, et al. 2009. Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J. Neurochem. 111:726–736 [DOI] [PubMed] [Google Scholar]
- 21. Chen Y, Merzdorf C, Paul DL, Goodenough DA. 1997. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J. Cell Biol. 138:891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen YH, Lu Q, Goodenough DA, Jeansonne B. 2002. Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol. Biol. Cell 13:1227–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen YS, et al. 2007. Propofol-induced vascular permeability change is related to the nitric oxide signaling pathway and occludin phosphorylation. J. Biomed. Sci. 14:629–636 [DOI] [PubMed] [Google Scholar]
- 24. Chun J, Prince A. 2009. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe 5:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coëffier M, et al. 2010. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am. J. Gastroenterol. 105:1181–1188 [DOI] [PubMed] [Google Scholar]
- 26. Collins NT, et al. 2006. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler. Thromb. Vasc. Biol. 26:62–68 [DOI] [PubMed] [Google Scholar]
- 27. Cordenonsi M, et al. 1997. Occludin dephosphorylation in early development of Xenopus laevis. J. Cell Sci. 110:3131–3139 [DOI] [PubMed] [Google Scholar]
- 28. Cordenonsi M, et al. 1999. Xenopus laevis occludin. Identification of in vitro phosphorylation sites by protein kinase CK2 and association with cingulin. Eur. J. Biochem. 264:374–384 [DOI] [PubMed] [Google Scholar]
- 29. DeMaio L, et al. 2006. Oxidized phospholipids mediate occludin expression and phosphorylation in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 290:H674–H683 [DOI] [PubMed] [Google Scholar]
- 30. DeMaio L, Chang YS, Gardner TW, Tarbell JM, Antonetti DA. 2001. Shear stress regulates occludin content and phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 281:H105–H113 [DOI] [PubMed] [Google Scholar]
- 31. Dörfel MJ, Westphal JK, Huber O. 2009. Differential phosphorylation of occludin and tricellulin by CK2 and CK1. Ann. N. Y. Acad. Sci. 1165:69–73 [DOI] [PubMed] [Google Scholar]
- 32. Du D, et al. 2010. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev. Cell 18:52–63 [DOI] [PubMed] [Google Scholar]
- 33. Elias BC, et al. 2009. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J. Biol. Chem. 284:1559–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farquhar MG, Palade GE. 1963. Junctional complexes in various epithelia. J. Cell Biol. 17:375–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farshori P, Kachar B. 1999. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J. Membr. Biol. 170:147–156 [DOI] [PubMed] [Google Scholar]
- 36. Feldman GJ, Mullin JM, Ryan MP. 2005. Occludin: structure, function and regulation. Adv. Drug Deliv. Rev. 57:883–917 [DOI] [PubMed] [Google Scholar]
- 37. Feng S, et al. 2011. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One 6:e20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Förster C. 2008. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 130:55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Furuse M, Sasaki H, Fujimoto K, Tsukita S. 1998. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 143:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Furuse M, et al. 1994. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 127:1617–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Furuse M, et al. 1993. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123:1777–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghassemifar MR, et al. 2002. Occludin TM4(−): an isoform of the tight junction protein present in primates lacking the fourth trans-membrane domain. J. Cell Sci. 115:3171–3180 [DOI] [PubMed] [Google Scholar]
- 43. Ghassemifar MR, et al. 2003. Gene expression regulating epithelial intercellular junction biogenesis during human blastocyst development in vitro. Mol. Hum. Reprod. 9:245–252 [DOI] [PubMed] [Google Scholar]
- 44. Giebel SJ, Menicucci G, McGuire PG, Das A. 2005. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 85:597–607 [DOI] [PubMed] [Google Scholar]
- 45. Gorodeski GI. 2007. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodeling of occludin. Endocrinology 148:218–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gu JM, Lim SO, Park YM, Jung G. 2008. A novel splice variant of occludin deleted in exon 9 and its role in cell apoptosis and invasion. FEBS J. 275:3145–3156 [DOI] [PubMed] [Google Scholar]
- 47. Gurney KJ, Estrada EY, Rosenberg GA. 2006. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol. Dis. 23:87–96 [DOI] [PubMed] [Google Scholar]
- 48. Han M, et al. 2010. Ascorbate protects endothelial barrier function during septic insult: role of protein phosphatase type 2A. Free Radic. Biol. Med. 48:128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harhaj NS, et al. 2006. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest. Ophthalmol. Vis. Sci. 47:5106–5115 [DOI] [PubMed] [Google Scholar]
- 50. Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. 2007. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia 50:202–211 [DOI] [PubMed] [Google Scholar]
- 51. Higashida T, et al. 2011. The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J. Neurosurg. 114:92–101 [DOI] [PubMed] [Google Scholar]
- 52. Hirase T, et al. 2001. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J. Biol. Chem. 276:10423–10431 [DOI] [PubMed] [Google Scholar]
- 53. Huang W, Eum SY, András IE, Hennig B, Toborek M. 2009. PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB J. 23:1596–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ichikawa Y, et al. 2006. Matrilysin (MMP-7) degrades VE-cadherin and accelerates accumulation of beta-catenin in the nucleus of human umbilical vein endothelial cells. Oncol. Rep. 15:311–315 [PubMed] [Google Scholar]
- 55. Ishihara H, et al. 2008. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J. Neuropathol. Exp. Neurol. 67:435–448 [DOI] [PubMed] [Google Scholar]
- 56. Kago T, et al. 2006. Cerebral ischemia enhances tyrosine phosphorylation of occludin in brain capillaries. Biochem. Biophys. Res. Commun. 339:1197–1203 [DOI] [PubMed] [Google Scholar]
- 57. Kale G, Naren AP, Sheth P, Rao RK. 2003. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem. Biophys. Res. Commun. 302:324–329 [DOI] [PubMed] [Google Scholar]
- 58. Kawedia JD, et al. 2008. The protein kinase A pathway contributes to Hg2+-induced alterations in phosphorylation and subcellular distribution of occludin associated with increased tight junction permeability of salivary epithelial cell monolayers. J. Pharmacol. Exp. Ther. 326:829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kurihara H, Anderson JM, Farquhar MG. 1992. Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc. Natl. Acad. Sci. U. S. A. 89:7075–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kuwabara H, et al. 2001. Occludin regulates actin cytoskeleton in endothelial cells. Cell Struct. Funct. 26:109–116 [DOI] [PubMed] [Google Scholar]
- 61. Lacaz-Vieira F, Jaeger MM, Farshori P, Kachar B. 1999. Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J. Membr. Biol. 168:289–297 [DOI] [PubMed] [Google Scholar]
- 62. Li W, et al. 2010. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes 59:228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li Y, Fanning AS, Anderson JM, Lavie A. 2005. Structure of the conserved cytoplasmic C-terminal domain of occludin: identification of the ZO-1 binding surface. J. Mol. Biol. 352:151–164 [DOI] [PubMed] [Google Scholar]
- 64. Lischper M, Beuck S, Thanabalasundaram G, Pieper C, Galla HJ. 2010. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 1326:114–127 [DOI] [PubMed] [Google Scholar]
- 65. Liu W, Hendren J, Qin XJ, Shen J, Liu KJ. 2009. Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J. Neurochem. 108:811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lohmann C, Krischke M, Wegener J, Galla HJ. 2004. Tyrosine phosphatase inhibition induces loss of blood-brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res. 995:184–196 [DOI] [PubMed] [Google Scholar]
- 67. Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS. 2010. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J. Neuropathol. Exp. Neurol. 69:801–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lui WY, Lee WM. 2005. cAMP perturbs inter-Sertoli tight junction permeability barrier in vitro via its effect on proteasome-sensitive ubiquitination of occludin. J. Cell. Physiol. 203:564–572 [DOI] [PubMed] [Google Scholar]
- 69. Lynch RD, et al. 2007. Cholesterol depletion alters detergent-specific solubility profiles of selected tight junction proteins and the phosphorylation of occludin. Exp. Cell Res. 313:2597–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mankertz J, et al. 2002. Gene expression of the tight junction protein occludin includes differential splicing and alternative promoter usage. Biochem. Biophys. Res. Commun. 298:657–666 [DOI] [PubMed] [Google Scholar]
- 71. Martin TA, Mansel RE, Jiang WG. 2010. Loss of occludin leads to the progression of human breast cancer. Int. J. Mol. Med. 26:723–734 [DOI] [PubMed] [Google Scholar]
- 72. Martínez-Contreras R, Galindo JM, Aguilar-Rojas A, Valdés J. 2003. Two exonic elements in the flanking constitutive exons control the alternative splicing of the alpha exon of the ZO-1 pre-mRNA. Biochim. Biophys. Acta 1630:71–83 [DOI] [PubMed] [Google Scholar]
- 73. Matter K, Aijaz S, Tsapara S, Balda MS. 2005. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr. Opin. Cell Biol. 17:453–458 [DOI] [PubMed] [Google Scholar]
- 74. McCaffrey G, et al. 2008. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J. Neurochem. 106:2395–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McCarthy KM, et al. 1996. Occludin is a functional component of the tight junction. J. Cell Sci. 109:2287–2298 [DOI] [PubMed] [Google Scholar]
- 76. McKenzie JA, Riento K, Ridley AJ. 2006. Casein kinase I epsilon associates with and phosphorylates the tight junction protein occludin. FEBS Lett. 580:2388–2394 [DOI] [PubMed] [Google Scholar]
- 77. Morgan L, et al. 2007. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience 147:664–673 [DOI] [PubMed] [Google Scholar]
- 78. Murakami T, Felinski EA, Antonetti DA. 2009. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem. 284:21036–21046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Muresan Z, Paul DL, Goodenough DA. 2000. Occludin 1B, a variant of the tight jnction protein occludin. Mol. Cell. Biol. 11:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Navaratna D, McGuire PG, Menicucci G, Das A. 2007. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes 56:2380–2387 [DOI] [PubMed] [Google Scholar]
- 81. Nunbhakdi-Craig V, et al. 2002. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 158:967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ohtake K, et al. 2003. Poly-l-arginine enhances paracellular permeability via serine/threonine phosphorylation of ZO-1 and tyrosine dephosphorylation of occludin in rabbit nasal epithelium. Pharm. Res. 20:1838–1845 [DOI] [PubMed] [Google Scholar]
- 83. Olivera D, Knall C, Boggs S, Seagrave J. 2010. Cytoskeletal modulation and tyrosine phosphorylation of tight junction proteins are associated with mainstream cigarette smoke-induced permeability of airway epithelium. Exp. Toxicol. Pathol. 62:133–143 [DOI] [PubMed] [Google Scholar]
- 84. Osanai M, et al. 2006. Epigenetic silencing of occludin promotes tumorigenic and metastatic properties of cancer cells via modulations of unique sets of apoptosis-associated genes. Cancer Res. 66:9125–9133 [DOI] [PubMed] [Google Scholar]
- 85. Pang Z, Antonetti DA, Tarbell JM. 2005. Shear stress regulates HUVEC hydraulic conductivity by occludin phosphorylation. Ann. Biomed. Eng. 33:1536–1545 [DOI] [PubMed] [Google Scholar]
- 86. Pelletier RM, Okawara Y, Vitale ML, Anderson JM. 1997. Differential distribution of the tight-junction-associated protein ZO-1 isoforms alpha+ and alpha− in guinea pig Sertoli cells: a possible association with F-actin and G-actin. Biol. Reprod. 57:367–376 [DOI] [PubMed] [Google Scholar]
- 87. Raikwar NS, Vandewalle A, Thomas CP. 2010. Nedd4-2 interacts with occludin to inhibit tight junction formation and enhance paracellular conductance in collecting duct epithelia. Am. J. Physiol. Renal Physiol. 299:F436–F444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rajasekaran SA, et al. 2007. Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G124–133 [DOI] [PubMed] [Google Scholar]
- 89. Raleigh DR, et al. 2010. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol. Biol. Cell 21:1200–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rao R. 2009. Occludin phosphorylation in regulation of epithelial tight junctions. Ann. N. Y. Acad. Sci. 1165:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. 2002. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 368:471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reijerkerk A, et al. 2006. Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB J. 20:2550–2552 [DOI] [PubMed] [Google Scholar]
- 93. Rosenberg GA, Yang Y. 2007. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg. Focus 22:E4. [DOI] [PubMed] [Google Scholar]
- 94. Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. 2007. Pollen proteolytic enzymes degrade tight junctions. Respirology 12:834–842 [DOI] [PubMed] [Google Scholar]
- 95. Saitou M, et al. 2000. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11:4131–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Saitou M, et al. 1997. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur. J. Cell Biol. 73:222–231 [PubMed] [Google Scholar]
- 97. Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. 1997. Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 137:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sallee JL, Burridge K. 2009. Density-enhanced phosphatase 1 regulates phosphorylation of tight junction proteins and enhances barrier function of epithelial cells. J. Biol. Chem. 284:14997–15006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Salomons FA, Acs K, Dantuma NP. 2010. Illuminating the ubiquitin/proteasome system. Exp. Cell Res. 316:1289–1295 [DOI] [PubMed] [Google Scholar]
- 100. Schubert-Unkmeir A, et al. 2010. Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP-8. PLoS Pathog. 6:e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Seth A, Sheth P, Elias BC, Rao R. 2007. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J. Biol. Chem. 282:11487–11498 [DOI] [PubMed] [Google Scholar]
- 102. Sheth B, et al. 1997. Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha+ isoform. Development 124:2027–2037 [DOI] [PubMed] [Google Scholar]
- 103. Sheth P, Basuroy S, Li C, Naren AP, Rao RK. 2003. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J. Biol. Chem. 278:49239–49245 [DOI] [PubMed] [Google Scholar]
- 104. Simonovic I, Rosenberg J, Koutsouris A, Hecht G. 2000. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell. Microbiol. 2:305–315 [DOI] [PubMed] [Google Scholar]
- 105. Smales C, et al. 2003. Occludin phosphorylation: identification of an occludin kinase in brain and cell extracts as CK2. FEBS Lett. 545:161–166 [DOI] [PubMed] [Google Scholar]
- 106. Steed E, Balda MS, Matter K. 2010. Dynamics and functions of tight junctions. Trends Cell Biol. 20:142–149 [DOI] [PubMed] [Google Scholar]
- 107. Steed E, Rodrigues NT, Balda MS, Matter K. 2009. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell. Biol. 10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sumitomo T, et al. 2011. Streptolysin S contributes to group A streptococcal translocation across an epithelial barrier. J. Biol. Chem. 286:2750–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sundstrom JM, et al. 2009. Identification and analysis of occludin phosphosites: a combined mass spectrometry and bioinformatics approach. J. Proteome Res. 8:808–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Suzuki T, et al. 2009. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc. Natl. Acad. Sci. U. S. A. 106:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tada Y, et al. 2010. Reduction of endothelial tight junction proteins is related to cerebral aneurysm formation in rats. J. Hypertens. 28:1883–1891 [DOI] [PubMed] [Google Scholar]
- 112. Takahashi S, et al. 2009. The E3 ubiquitin ligase LNX1p80 promotes the removal of claudins from tight junctions in MDCK cells. J. Cell Sci. 122:985–994 [DOI] [PubMed] [Google Scholar]
- 113. Takenaga Y, Takagi N, Murotomi K, Tanonaka K, Takeo S. 2009. Inhibition of Src activity decreases tyrosine phosphorylation of occludin in brain capillaries and attenuates increase in permeability of the blood-brain barrier after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 29:1099–1108 [DOI] [PubMed] [Google Scholar]
- 114. Tang C, Xue H, Bai C, Fu R, Wu A. 2010. The effects of Tanshinone IIA on blood-brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomedicine 17:1145–1149 [DOI] [PubMed] [Google Scholar]
- 115. Thanabalasundaram G, Pieper C, Lischper M, Galla HJ. 2010. Regulation of the blood-brain barrier integrity by pericytes via matrix metalloproteinases mediated activation of vascular endothelial growth factor in vitro. Brain Res. 1347:1–10 [DOI] [PubMed] [Google Scholar]
- 116. Traweger A, et al. 2002. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch. J. Biol. Chem. 277:10201–10208 [DOI] [PubMed] [Google Scholar]
- 117. Tsukamoto T, Nigam SK. 1999. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am. J. Physiol. 276:F737–F750 [DOI] [PubMed] [Google Scholar]
- 118. Verma S, Kumar M, Gurjav U, Lum S, Nerurkar VR. 2010. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 397:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vikström E, Bui L, Konradsson P, Magnusson KE. 2009. The junctional integrity of epithelial cells is modulated by Pseudomonas aeruginosa quorum sensing molecule through phosphorylation-dependent mechanisms. Exp. Cell Res. 315:313–326 [DOI] [PubMed] [Google Scholar]
- 120. Wachtel M, et al. 1999. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J. Cell Sci. 112:4347–4356 [DOI] [PubMed] [Google Scholar]
- 121. Walsh TG, et al. 2011. Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. J. Cell. Physiol. 226:3053–3063 [DOI] [PubMed] [Google Scholar]
- 122. Walter JK, et al. 2009. The oligomerization of the coiled coil-domain of occludin is redox sensitive. Ann. N. Y. Acad. Sci. 1165:19–27 [DOI] [PubMed] [Google Scholar]
- 123. Walter JK, et al. 2009. Redox-sensitivity of the dimerization of occludin. Cell. Mol. Life Sci. 66:3655–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wan H, et al. 2000. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin. Exp. Allergy 30:685–698 [DOI] [PubMed] [Google Scholar]
- 125. Willott E, Balda MS, Heintzelman M, Jameson B, Anderson JM. 1992. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am. J. Physiol. 262:C1119–C1124 [DOI] [PubMed] [Google Scholar]
- 126. Wong V, Gumbiner BM. 1997. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J. Cell Biol. 136:399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yamamoto M, et al. 2008. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am. J. Pathol. 172:521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. 2007. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 27:697–709 [DOI] [PubMed] [Google Scholar]
- 129. Zeiller C, et al. 2009. Phospholipase D2 regulates endothelial permeability through cytoskeleton reorganization and occludin downregulation. Biochim. Biophys. Acta 1793:1236–1249 [DOI] [PubMed] [Google Scholar]
- 130. Zhu L, Li X, Zeng R, Gorodeski GI. 2006. Changes in tight junctional resistance of the cervical epithelium are associated with modulation of content and phosphorylation of occludin 65-kilodalton and 50-kilodalton forms. Endocrinology 147:977–989 [DOI] [PMC free article] [PubMed] [Google Scholar]