Abstract
Objective
Ethnic differences in body fat mass and distribution may develop in childhood and contribute to the elevated obesity-related disease risk among Asians. We evaluated adiposity measures of adult women and their adolescent daughters of predominantly Japanese and Caucasian ethnicity using Dual Energy X-ray Absorptiometry (DXA).
Methods
We obtained DXA whole body scans for 101 mothers aged ≥30 years and 112 daughters aged 10–16 years who were classified as Asian, Part-Asian, Mixed-Other, and Caucasian. As a measure of central adiposity, we calculated the trunk/periphery fat ratio (TPFR). General linear models were applied to evaluate differences in adiposity measures by ethnic category.
Results
In mothers, TPFR was significantly higher (ptrend=0.01) in Asians and Part-Asians (1.38±0.42 for and 1.32±0.51) than in Mixed-Others and Caucasians (1.18±0.27 and 1.09±0.21). Daughters showed a similar trend (ptrend =0.001) with respective values of 1.09±0.18, 0.97±0.17, 0.99±0.16, and 0.87±0.11. Among mothers, gynoid fat mass and periphery fat mass were significantly lower in Asians than Caucasians, whereas none of the regional DXA adiposity measures in girls differed by ethnicity.
Conclusions
These results confirm previous reports of greater central adiposity in women of Asian ancestry and indicate that ethnic differences in adiposity are already present in adolescence.
Keywords: DXA, trunk/periphery fat ratio, central adiposity, ethnicity
Introduction
While obesity is a global health concern, associated disease risks differ by ethnicity. Asians experience a higher risk for hypertension, diabetes, and cardiovascular disease at lower levels of body mass index (BMI) than Caucasians.1–3 This paradoxical finding, which led to the evaluation of lower BMI cutoffs for Asians,4 also conveyed the need for additional adiposity measures to supplement BMI in assessing metabolic disease risk. In particular, investigations in Japanese have shown differences in body fat mass and distribution as compared to Caucasians that are not fully captured by BMI alone.5,6 The relatively higher adiposity of Asians compared to Caucasians is associated with lower birth weight, smaller body frame, and shorter leg to trunk length and, therefore, reflects less skeletal and muscle mass and more body and trunk fat for the same BMI, which may be present since childhood.7–10 These characteristics may also relate to the higher proportions of abdominal adiposity, in particular visceral fat, observed in Asian populations.5,11 Dual Energy X-ray Absorptiometry (DXA) is used to assess abdominal fat and compares well to computed tomography (CT) and magnetic resonance imaging (MRI) in adults.12,13 In girls of different ethnic backgrounds, DXA-based ratios between trunk and peripheral fat have performed well to describe ethnic differences.14,15 In the current study, we evaluated ethnic differences in trunk to periphery fat ratio (TPFR) and other DXA adiposity measures among adult women and their adolescent daughters of predominantly Japanese and Caucasian ethnicity in Hawaii to assess whether ethnic differences in body fat mass and distribution develop in early life.
Methods
Study design and procedure
The current analysis was conducted as part of a mother-daughter study that measured breast density and body-fat composition in adult women and adolescent girls using DXA.16–18 The project was approved by the Committee on Human Studies at the University of Hawaii and the Institutional Review Board of Kaiser Permanente (KP) Hawaii, a large health maintenance organization where study participants were recruited. As described in detail elsewhere, 16–18 we mailed 3,915 invitation letters to women aged 30 years and older who had daughters aged 10–16 years; 102 pairs plus 12 additional sisters participated in the study. DXA body scan images were obtained from 101 mothers and 112 daughters for the current analysis.
Prior to DXA scans, mothers signed informed consent and daughters informed assent, answered a questionnaire about demographic and reproductive factors, and completed duplicate height and weight measurements performed by trained research personnel. BMI was classified as overweight (25–<30 kg/m2) and obese (≥30 kg/m2) in mothers. For girls, BMI-for-age percentiles were calculated according to CDC reference data19 and classified as overweight (85–<95th percentile) and obese (≥95th percentile). Tanner stages of breast development were assessed by the same staff member throughout the study.20 In the questionnaire, mothers and daughters reported the percentages of all ethnic backgrounds that applied to their parents. Based on this information, each participant was classified as (1) all Asian; (2) Mixed of partly-Asian descent (Part-Asian), (3) Mixed of non-Asian descent or Other, i.e., one African American, one Hispanic, and one Pacific Islander mother and one African American daughter (Mixed-Other), or (4) all Caucasian.
DXA data collection
At the exam, a urine test excluded pregnancy in all participants. Whole body scans were performed using GE Lunar Prodigy Bone Densitometer (GE Healthcare, software version 10.1) to determine body composition, lean soft tissue mass (kg) and fat mass (kg) of standard body regions including arms, legs, trunk, and total body. A single DXA operator conducted all DXA scans. The percentage of total body fat was calculated as total fat mass divided by total lean soft tissue mass and total fat mass (all in kg). Scans were reanalyzed for android and gynoid fat mass, standardized regions provided by GE.21 Fat mass from arms and legs was first summed to estimate periphery fat mass, and TPFR was calculated by dividing trunk fat mass (kg) by periphery fat mass (kg). Similarly, the android to gynoid fat ratio was calculated as android fat mass (kg) divided by gynoid fat mass (kg).
Statistical Analysis
All statistical analyses were performed using the SAS software package version 9.2 (SAS Institute, Inc., Cary, NC). Anthropometric and DXA adiposity measures across ethnic categories were reported for mothers and daughters as means ± standard deviations. Spearman rank order correlation coefficients were calculated to assess the strength of association between DXA adiposity measures and BMI in both mothers and daughters. General linear models (Proc GLM) were used to evaluate ethnic differences (categorical variable) using log-transformed anthropometric and adiposity data to normalize the distribution; an α level of 0.05 was considered significant. In addition, we converted the ethnic categories into a continuous variable in the order of all Caucasian, Mixed of non-Asian descent, Mixed of partly-Asian, and all Asian to calculate p value for trend. We also conducted separate analyses including only one of the two daughters at a time.
Results
Of the 101 participating mothers (Table 1), 34 were Asian (17 Japanese, 7 Filipino, 5 Chinese, 5 Other), 22 Part-Asian, 23 Mixed-Other, and 22 Caucasian. Among the 112 daughters (Table 2), the respective numbers were 18 (11 Japanese and 7 Other), 47, 30, and 17. Mean ages were 47.0±4.4 years for mothers and 13.9±1.7 years for daughters. Mean BMI was 27.5±6.0 kg/m2 for mothers and 21.7±4.9 kg/m2 for daughters; age and the proportions of overweight and obese individuals did not differ by ethnic category in either mothers or daughters, nor did Tanner stage in girls. Percent total DXA body fat was higher in mothers than daughters (39.8±7.8 vs. 30.2±9.1%). In mothers and daughters, the association between DXA adiposity measures and BMI was strong (Table 3). The correlation coefficients for mothers were 0.94 for android fat mass, 0.81 for gynoid fat mass, 0.95 for trunk fat mass, and 0.83 for periphery fat mass (p<0.0001 for all). The corresponding correlation coefficients for daughters were 0.88, 0.87, 0.86, and 0.82 (p<0.0001 for all). The correlations between the android/gynoid fat ratio and TPFR with BMI were weaker than for the fat mass variables: 0.69 and 0.48 for mothers and 0.68 and 0.49 for daughters (p<0.0001 for all). The TPFR values between mothers and daughters were significantly correlated (r=0.29; p=0.002).
Table 1.
Characteristics of mothers by ethnic category
| Ethnic category | All | Asian | Part-Asian | Mixed-Othera | Caucasian | p valueb |
|---|---|---|---|---|---|---|
| N | 101 | 34 | 22 | 23 | 22 | --- |
| Age (years) | 47.0±4.4 | 48.8±5.7 | 46.3±4.0 | 47.2±4.1 | 47.9±4.3 | 0.27 |
| Postmenopausal status (N)c | 27 | 9 | 4 | 6 | 8 | 0.53 |
| Menopausal hormone use (N) | 22 | 11 | 4 | 2 | 5 | 0.19 |
| Weight (kg) | 71.0±17.0 | 62.8±13.8 | 72.8±16.6 | 80.5±17.5 | 72.2±16.6 | <0.001 |
| Height (cm) | 160.4±7.3 | 155.5±5.4 | 161.1±7.5 | 163.4±6.1 | 164.2±6.8 | <0.0001 |
| BMI (kg/m2) | 27.5±6.0 | 26.0±5.7 | 27.9±5.4 | 30.1±6.3 | 26.9±6.1 | 0.06 |
| Overweight/obese (N)d | 32/28 | 14/4 | 5/8 | 7/10 | 6/6 | 0.17 |
| DXA body fat measures | ||||||
| Total body fat (%) | 39.8±7.8 | 38.1±7.2 | 39.0±7.3 | 42.8±8.0 | 40.0±8.7 | 0.23 |
| Android fat (kg) | 2.6±1.44 | 2.3±1.3 | 2.6±1.3 | 3.2±1.6 | 2.5±1.5 | 0.17 |
| Gynoid fat (kg) | 5.3±1.92 | 4.4±1.4 | 5.2±1.6 | 6.4±1.9 | 5.7±2.3 | 0.0001 |
| Android/gynoid fat ratio | 0.48±0.16 | 0.52±0.18 | 0.50±0.17 | 0.47±0.14 | 0.41±0.11 | 0.08 |
| Trunk fat (kg) | 15.0±13.4 | 13.1±6.0 | 15.3±6.9 | 17.9±7.8 | 14.6±7.2 | 0.11 |
| Periphery (arm and leg) fat (kg) | 12.8±10.8 | 9.5±3.5 | 11.8±4.4 | 14.9±4.7 | 13.1±5.0 | <0.0001 |
| Trunk/periphery fat ratio | 1.26±0.39 | 1.38±0.42 | 1.32±0.51 | 1.18±0.27 | 1.09±0.21 | 0.02 |
Mixed-Other includes 1 African American, 1 Hispanic and 1 Pacific Islander.
P values for anthropometric and DXA measures are based on log-transformed data and reflect the overall difference across ethnicity as categorical variable.
Postmenopausal status is defined as no menstrual period ≥ 365.25 days; one woman with missing data in the Caucasian category.
Based on body mass index: overweight: 25 to <30; obese: ≥ 30.
Table 2.
Characteristics of daughters by ethnic category
| Ethnic category | All | Asian | Part-Asian | Mixed-Othera | Caucasian | p valueb |
|---|---|---|---|---|---|---|
| N | 112 | 18 | 47 | 30 | 17 | --- |
| Age (years) | 13.9±1.7 | 14.4±1.6 | 13.5±1.9 | 14.3±1.6 | 13.8±1.5 | 0.14 |
| Tanner breast stage | ||||||
| 1–2 | 17 | 1 | 10 | 4 | 2 | 0.33 |
| 3 | 38 | 10 | 15 | 7 | 6 | |
| 4–5 | 57 | 7 | 22 | 19 | 9 | |
| Weight (kg) | 53.9±14.5 | 50.7±12.1 | 55.1±16.8 | 54.8±13.3 | 52.6±11.8 | 0.75 |
| Height (cm) | 157.4±8.4 | 154.9±6.7 | 156.3±9.6 | 159.2±7.3 | 159.9±7.9 | 0.12 |
| BMI (kg/m2) | 21.7±5.0 | 21.4±4.1 | 22.3±5.5 | 21.5±4.5 | 20.7±5.4 | 0.62 |
| BMI z-score | 0.40±1.02 | 0.28±1.07 | 0.57±1.07 | 0.36±0.86 | 0.10±1.10 | 0.39 |
| Overweight/obese (N)c | 15/15 | 3/2 | 8/8 | 3/3 | 0/2 | 0.53 |
| DXA body fat measures | ||||||
| Total body fat (%) | 30.2±9.1 | 28.1±8.4 | 30.4±9.4 | 31.0±9.4 | 30.2±8.8 | 0.75 |
| Android fat (kg) | 1.3±1.0 | 1.1±0.6 | 1.3±1.1 | 1.3±1.0 | 1.1±0.7 | 0.91 |
| Gynoid fat (kg) | 3.5±1.5 | 3.1±1.2 | 3.5±1.7 | 3.6±1.5 | 3.3±1.3 | 0.81 |
| Android/gynoid fat ratio | 0.33±0.10 | 0.35±0.09 | 0.34±0.11 | 0.33±0.11 | 0.30±0.09 | 0.51 |
| Trunk fat (kg) | 7.9±5.0 | 7.1±3.9 | 8.2±5.6 | 8.3±5.1 | 7.1±4.2 | 0.81 |
| Periphery (arm and leg) fat (kg) | 7.9±4.3 | 6.5±3.4 | 8.2±4.9 | 8.1±4.2 | 7.9±3.6 | 0.43 |
| Trunk/periphery fat ratio | 0.98±0.17 | 1.09±0.18 | 0.97±0.17 | 0.99±0.16 | 0.87±0.11 | 0.001 |
Mixed-Other includes 1 African American
P values for anthropometric and DXA measures are based on log-transformed data and reflect the overall difference across ethnicity as categorical variable.
Based on BMI-for-age percentile: overweight = 85 to <95; obese ≥95.
Table 3.
Correlation of DXA adiposity measures with BMI in mothers and daughtersa
| % Total body fat | Android fat (kg) | Gynoid fat (kg) | Android: gynoid fat ratio | Trunk fat (kg) | Periphery (arm and leg) fat (kg) | Trunk: periphery fat ratio | ||
|---|---|---|---|---|---|---|---|---|
| Mothers (N=101) | r | 0.85 | 0.94 | 0.81 | 0.69 | 0.95 | 0.83 | 0.48 |
| p value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
| Daughters (N=112) | r | 0.79 | 0.88 | 0.87 | 0.68 | 0.86 | 0.82 | 0.49 |
| p value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
Spearman rank order correlation coefficients
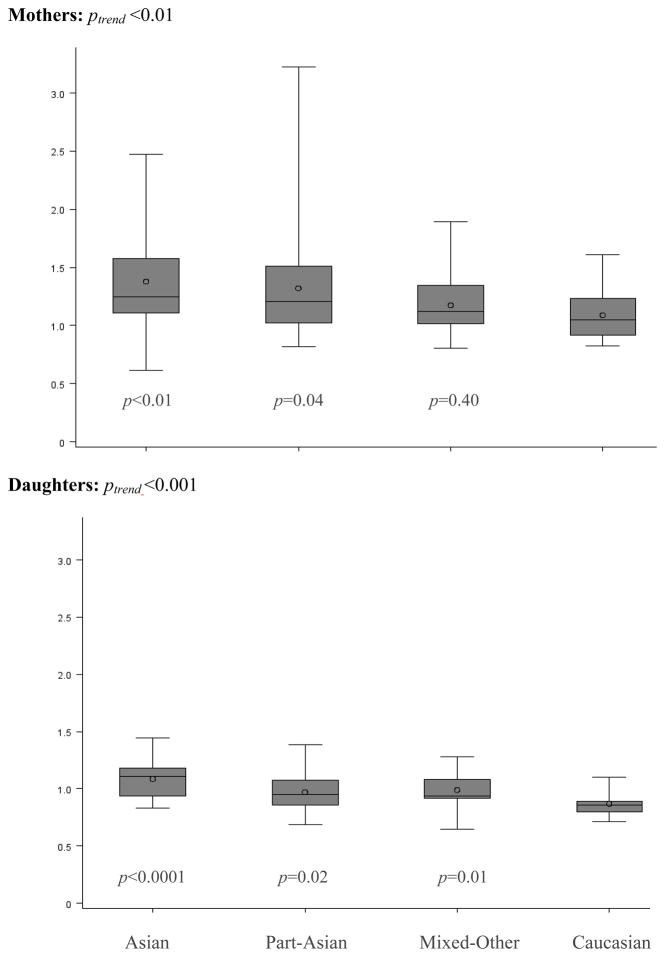
While the android fat mass, android/gynoid fat ratio, and trunk fat mass of mothers did not differ by ethnicity, gynoid fat mass and periphery fat mass were lower in the Asian than non-Asian groups (p =0.001 and p<0.001; Table 1). As the result of the lower periphery fat mass, TPFR was significantly higher in Asian than non-Asian groups (Figure 1): 1.38±0.42 for Asian and 1.32±0.51 for Part-Asian vs. 1.18±0.27 for Mixed-Other and 1.09±0.21 for Caucasian with a significant trend test (ptrend <0.01). When limited to 100% Japanese ancestry (n=17), the mean TPFR was very similar to that of all Asians (1.38±0.50). In daughters, TPFR was the only DXA body fat measure that differed by ethnicity (Table 2); the linear declining trend with decreasing degree of Asian admixture (ptrend <0.001) was similar to the one seen in mothers (Figure 1). The respective mean TPFR values were 1.09±0.18, 0.97±0.17, 0.99±0.16, and 0.87±0.11. Mean TPFR of 100% Japanese daughters (N=10) was 1.08±0.17. All results were similar in analyses that included only one of the two siblings for the 12 participants with sisters. After stratification by weight status, mean TPFR was 1.09±0.28 for normal-weight and 1.37±0.41 for overweight/obese mothers (p<0.0001); the respective values for daughters were 0.95±0.16 vs. 1.07±0.15 (p<0.001).
Figure 1.
Distributions of TPFR in mothers (top) and daughters (bottom) by ethnic category; p values are based on log-transformed data with Caucasian as reference; ptrend values are generated using ethnicity as continuous variable in the order of Caucasian, Mixed-Other, Part-Asian, and Asian. The horizontal lines from the bottom to the top correspond to the 10th, 25th, median, 75th, and 90th percentiles, and the O represents the arithmetic mean.
Discussion
The current analysis observed ethnic differences in DXA adiposity measures among women and girls of predominantly Asian and Caucasian ethnicity in Hawaii. Mothers and adolescent daughters showed a similar trend of higher TPFR for Asian groups as compared to non-Asians strongly indicating that Asian ethnicity is associated with higher central adiposity as previously observed by other studies and measures6,22,23 and that the ethnic differences originate early in life as shown among Indian infants.24 While overall high correlations of DXA adiposity measures with BMI confirm the validity of BMI as a universal obesity measure, lower correlations with TPFR and android/gynoid fat ratio convey that % body fat and adipose tissue distribution are not precisely predicted by BMI. Thus, central adiposity measures are important in evaluating obesity-related disease risks across populations with varying adiposity patterns.11 According to our results, TPFR may be useful in comparing central adiposity across ethnically-diverse populations.
These results agree with previous comparisons in girls14 and support the hypothesis that ethnic differences in adipose tissue distribution arise in childhood, possibly due to ethnic variations in skeletal dimensions14,21 or perinatal events.10,24 TPFR appears to reflect the ethnic variations in skeletal dimensions more closely than the android/gynoid fat ratio; it may be a more precise estimate of central adiposity and provide more information than the android/gynoid fat ratio among younger populations. A recent study demonstrated a DXA measure of trunk to extremity fat ratio similar to TPFR to be a strong indicator of visceral adiposity in normal-weight and obese girls when compared to MRI.15 Ethnic differences in the trunk-to-limb fat mass ratio exist between Caucasian, African-American, and Hispanic adults25,26 and reference values are available from the National Health and Nutrition Examination Survey (NHANES)26 but not for Asians and youths. Similarly, NHANES provides lean and fat mass and other body composition data for children and adolescent among non-Asian ethnic groups.27,28
A major strength of the present study is the inclusion of adult women and adolescent girls, in particular including Asian and mixed-Asian groups that need more data, and the detailed assessment of ethnicity. A number of limitations also need to be noted. Due to its small sample size and the low response rate, the observed ethnic differences need to be confirmed in a larger study. Furthermore, stratified analyses of normal-weight and overweight/obese women should be included in future investigations, as well as analyses among men who have different body compositions and larger abdominal diameters than women.26 An important limitation of DXA is the inability to distinguish subcutaneous from visceral fat mass as achieved by CT and MRI.12 Since visceral adiposity has been found to have stronger associations with diabetes and other chronic conditions than subcutaneous fat,29,30 MRI and CT have been applied in some research studies.23,31 However, these methods are not feasible for use in population-based studies or screening settings due to their high cost and the relatively high radiation exposure associated with CT. In contrast, DXA is more practical due to low cost, minimal radiation exposure, and availability in clinical settings. Given the relatively weak correlation with BMI and the difference in TPFR by BMI status in mothers, this measure appears to provide additional information about body fat distribution that BMI does not provide. Nevertheless, as indicated by the low participation rate, the DXA method is primarily a research instrument to understand fat distribution across ethnic groups and not a clinical assessment tool. Additional evaluations of TPFR and other DXA adiposity measures are needed to consider their use as screening tools. Enhanced DXA methods to distinguish subcutaneous and visceral fat are under development.
This innovative study confirmed the higher prevalence of trunk adiposity among women and girls of primarily Japanese ancestry shown in other populations6,23 and provides evidence that these differences can be observed during adolescence making a genetic, epigenetic, or growth-related origin likely. Given the increase in obesity-related, metabolic disease risks among Japanese and other Asians,1–3 a better understanding of ethnic differences in body fat composition and distribution may provide critical information for developing ethnic-specific, preventive measures for these conditions.
Acknowledgments
The current project was supported by grant BC060615 from the Breast Cancer Research program of the Department of Defense and by a Research Centers in Minority Institutions award (P20 RR11091) from the National Center for Research Resources. SMC was supported by a postdoctoral fellowship on grant R25 CA 90956. The authors declare no conflict of interest. We thank all women and their daughters who participated in this study; Yihe Daida, Aleli Vinoya and Kathryn Mau at KP Hawaii; and Jane Yakuma at the University of Hawaii Clinical Research Center.
References
- 1.Stommel M, Schoenborn CA. Variations in BMI and prevalence of health risks in diverse racial and ethnic populations. Obesity (Silver Spring) 2010;18:1821–1826. doi: 10.1038/oby.2009.472. [DOI] [PubMed] [Google Scholar]
- 2.Maskarinec G, Erber E, Grandinetti A, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes. 2009;58:1732–1738. doi: 10.2337/db08-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huxley R, James WP, Barzi F, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9 (Suppl 1):53–61. doi: 10.1111/j.1467-789X.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 4.WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–1275. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki T, Sekikawa A, Murata K, et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond) 2006;30:1163–1165. doi: 10.1038/sj.ijo.0803248. [DOI] [PubMed] [Google Scholar]
- 7.Deurenberg P, Durenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 8.Deurenberg P, Deurenberg YM, Wang J, Lin FP, Schmidt G. The impact of body build on the relationship between body mass index and percent body fat. Int J Obes Relat Metab Disord. 1999;23:537–542. doi: 10.1038/sj.ijo.0800868. [DOI] [PubMed] [Google Scholar]
- 9.Deurenberg P, Deurenberg-Yap M, Foo LF, Schmidt G, Wang J. Differences in body composition between Singapore Chinese, Beijing Chinese and Dutch children. Eur J Clin Nutr. 2003;57:405–409. doi: 10.1038/sj.ejcn.1601569. [DOI] [PubMed] [Google Scholar]
- 10.Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr. 2004;134:205–210. doi: 10.1093/jn/134.1.205. [DOI] [PubMed] [Google Scholar]
- 11.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) Am J Clin Nutr. 2007;86:353–359. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 12.Snijder MB, Visser M, Dekker JM, et al. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord. 2002;26:984–993. doi: 10.1038/sj.ijo.0801968. [DOI] [PubMed] [Google Scholar]
- 13.Kamel EG, McNeill G, Han TS, et al. Measurement of abdominal fat by magnetic resonance imaging, dual-energy X-ray absorptiometry and anthropometry in non-obese men and women. Int J Obes Relat Metab Disord. 1999;23:686–692. doi: 10.1038/sj.ijo.0800904. [DOI] [PubMed] [Google Scholar]
- 14.Novotny R, Daida YG, Grove JS, Le ML, Vijayadeva V. Asian adolescents have a higher trunk:peripheral fat ratio than Whites. J Nutr. 2006;136:642–647. doi: 10.1093/jn/136.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savgan-Gurol E, Bredella M, Russell M, Mendes N, Klibanski A, Misra M. Waist to hip ratio and trunk to extremity fat (DXA) are better surrogates for IMCL and for visceral fat respectively than for subcutaneous fat in adolescent girls. Nutr Metab (Lond) 2010;7:86. doi: 10.1186/1743-7075-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maskarinec G, Morimoto Y, Daida Y, et al. Comparison of breast density measured by dual energy X-ray absorptiometry with mammographic density among adult women in Hawaii. Cancer Epidemiol. 2011;35:188–193. doi: 10.1016/j.canep.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maskarinec G, Morimoto Y, Daida Y, Shepherd J, Novotny R. A comparison of breast density measures between mothers and adolescent daughters. BMC Cancer. 2011;11:330. doi: 10.1186/1471-2407-11-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novotny R, Daida Y, Morimoto Y, Shepherd J, Maskarinec G. Puberty, body fat, and breast density in girls of several ethnic groups. Am J Hum Biol. 2011;23:359–365. doi: 10.1002/ajhb.21145. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention/National Center for Health Statistics. [Accessed June 22, 2010.];CDC Growth Charts. 2009 August 4; [ www.cdc.gov/growthcharts]
- 20.Tanner JM. Growth at adolescence, with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. 2. Oxford: Blackwell Scientific Publisher; 1962. [Google Scholar]
- 21.Novotny R, Going S, Teegarden D, et al. Hispanic and Asian pubertal girls have higher android/gynoid fat ratio than whites. Obesity (Silver Spring) 2007;15:1565–1570. doi: 10.1038/oby.2007.185. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol. 2003;40 (Suppl 1):S302–S304. doi: 10.1007/s00592-003-0093-z. [DOI] [PubMed] [Google Scholar]
- 23.Lim U, Ernst T, Buchthal SD, et al. Asian women have greater abdominal and visceral adiposity than white women with similar body mass index. Nutrition & Diabetes. 2011 doi: 10.1038/nutd.2011.2. Provisionally accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yajnik CS, Lubree HG, Rege SS, et al. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab. 2002;87:5575–5580. doi: 10.1210/jc.2002-020434. [DOI] [PubMed] [Google Scholar]
- 25.Rahman M, Temple JR, Breitkopf CR, Berenson AB. Racial differences in body fat distribution among reproductive-aged women. Metabolism. 2009;58:1329–1337. doi: 10.1016/j.metabol.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borrud LG, Flegal KM, Looker AC, Everhart JE, Harris TB, Shepherd JA. Body composition data for individuals 8 years of age and older: U.S. population, 1999–2004. Vital Health Stat. 2010;11:1–87. [PMC free article] [PubMed] [Google Scholar]
- 28.Flegal KM, Ogden CL, Yanovski JA, et al. High adiposity and high body mass index-forage in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91:1020–1026. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]