Abstract
Despite the worldwide distribution, most of the known Seoul viruses (SEOV) are closely related to each other. In this study, the M and the S segment sequences of SEOV were recovered from 130 lung tissue samples (mostly of Norway rats) and from six patient serum samples by reverse transcription-PCR. Genetic analysis revealed that all sequences belong to SEOV and represent 136 novel strains. Phylogenetic analysis of all available M and S segment sequences of SEOV, including 136 novel Chinese strains, revealed four distinct groups. All non-Chinese SEOV strains and most of the Chinese variants fell into the phylogroup A, while the Chinese strains originating from mountainous areas clustered into three other distinct groups (B, C, and D). We estimated that phylogroup A viruses may have arisen only within the last several centuries. All non-Chinese variants appeared to be directly originated from China. Thus, phylogroup A viruses distributed worldwide may share a recent ancestor, whereas SEOV seems to be as diversified genetically as other hantaviruses. In addition, all available mitochondrial DNA (mtDNA) sequences of Norway rats, including our 44 newly recovered mtDNA sequences, were divided into two phylogenetic groups. The first group, which is associated with the group A SEOV variants, included most of rats from China and also all non-Chinese rats, while the second group consisted of a few rats originating only from mountain areas in China. We hypothesize that an ancestor of phylogroup A SEOV variants was first exported from China to Europe and then spread through the New World following the migration of Norway rats.
INTRODUCTION
Emerging or reemerging infectious diseases cause extensive damage and present a threat to human and/or wildlife health, agricultural production, and public security, and they will remain a considerable challenge in the foreseeable future (3, 34). The emergence of these infectious diseases results from rapid evolution of infectious agents, as well as changes in the environment and hosts' behavior that provide such agents with new ecological niches (10, 34, 35). Furthermore, the impact of these diseases on human populations is affected by the rate and degree to which they spread across geographical areas following the movement of humans, natural hosts, and vectors (34). Numerous data indicate that the translocation or trafficking of domestic and wild animals plays an important role in the rapid spread of many zoonotic pathogens (4, 9, 10).
Unlike the other four genera of viruses in the family Bunyaviridae, the viruses of Hantavirus genus are hosted and transmitted by rodents and insectivores. Hantaviruses can cause two human diseases: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) (45). Currently, the Hantavirus genus includes 23 distinct species and at least as many provisional species (38). Among the known hantaviruses, Hantaan virus (HTNV), Seoul virus (SEOV), Dobrava-Belgrade virus, Saaremaa virus, and Puumala virus (PUUV) have been found to cause HFRS in Asia and Europe, whereas Sin Nombre virus, Andes virus, and related viruses are the etiological agents of HPS in North and South Americas (20, 38, 45). Most recently, there was a rapid increase in the number of novel hantaviruses found in insectivores of families Crocidurinae, Soricinae, and Talpinae (38, 53). It remains to be seen whether any of these hantaviruses is human pathogens. As important (re)emerging zoonotic pathogens, hantaviruses have been gaining more and more attention worldwide during the past decades (20, 45, 47, 68).
Hantaviruses show a specific and close association with their reservoir hosts, rodents and insectivores (37, 38, 53), and a high degree of genetic diversity, even within a small region (for examples, see other studies of HTNV and PUUV [51, 69]). In addition, hantavirus genetic variants show geographical clustering. Thus, the distribution of rodent hosts determines the spread of hantaviruses, as well as the distribution of HFRS and HPS cases (20, 33, 64). Recently, Ramsden et al. reported that hantaviruses exhibit short-term evolutionary rates equivalent to those seen for rapidly evolving RNA viruses (41, 42). They proposed that there might not be codivergence between hantaviruses and their hosts, and the present geographic distribution of hantaviruses might be not determined longer than previously thought (20, 41, 42). Further analyses are needed to see if this is the case indeed.
Unlike other hantaviruses, SEOV has been found across a worldwide geographic range, from Asia to Africa, Europe, and both Americas (7, 8, 15, 16, 21–23, 28, 32, 37, 39, 43, 48, 50, 57–59, 61). Remarkably, most of the SEOV variants identified thus far, including the majority of Chinese strains and also all known non-Chinese strains, are genetically homogeneous and closely related to each other, with up to 95% nucleotide sequence identity (37, 39, 59, 66).
In China, HFRS remains serious public health problem even after 3 decades of comprehensive prevention, including vaccination (68). To date, only HTNV and SEOV have been found to cause HFRS in China (6, 59, 68). HFRS caused by SEOV was first identified in humans in 1981 in the neighboring regions of Henan and Shanxi provinces along the Yellow River (13). Subsequently, the virus was isolated from Norway rats (Rattus norvegicus) in regions of Henan in 1982 where the virus is endemic (52). Since then, hantavirus-positive rats and SEOV have been found in all HFRS areas of endemicity in China (5, 6, 30, 59, 66, 68). Recently, HFRS caused by SEOV occurred in the areas where HFRS had never been reported before, suggesting that both the virus and the disease are spreading further (67).
Information about the geographic distribution, genetic diversity, and phylogeny of SEOV in China is limited. Little is known about the evolutionary relationship between SEOV genetic variants in and outside China (37, 68). In order to fill this gap, the M and S segment sequences of SEOV were amplified from 130 hantavirus antigen-positive animals and from six patient serum samples. Cytochrome b (Cyt-b) gene sequences were also recovered from rats collected in major HFRS areas of endemicity during the last 7 years and subjected to phylogenetic and phylogeographic analyses.
MATERIALS AND METHODS
Samples.
Rodents were captured with snap-traps or cage-traps in residential habitats of major HFRS areas of endemicity of China from the spring of 2002 through the autumn of 2008 (Table 1). Lung tissues were collected from captured rodents, placed immediately at −196°C, and then transported to the laboratory for screening. Hantavirus antigen-positive lung tissues were also obtained from one cat in Anhui province. Serum samples from six patients with acute HFRS were included in the present study as well.
Table 1.
Prevalence of hantavirus in rodents by species and location in China from 2002 to 2008
| Species | No. of positive for hantavirus antigen/no. of rodents captured |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beijing | Hebei | Henan | Hubei | Hunan | Guangdong | Inner Mongolia | Jiangsu | Jilin | Liaoning | Shandong | Zhejiang | Total | |
| Rattus norvegicus | 1/30 | 25/635 | 17/481 | 4/105 | 5/96 | 6/197 | 2/44 | 3/106 | 32/1,421 | 7/188 | 5/173 | 4/149 | 111/3,625 |
| Rattus flavipectus | 0/0 | 0/0 | 3/176 | 0/32 | 0/10 | 0/41 | 0/0 | 0/16 | 0/0 | 0/0 | 0/0 | 1/47 | 4/322 |
| Rattus rattus | 0/0 | 0/0 | 1/29 | 0/3 | 0/0 | 0/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/33 |
| Mus musculus | 0/0 | 2/48 | 1/27 | 1/26 | 0/51 | 0/33 | 0/2 | 2/27 | 0/35 | 0/16 | 1/18 | 0/0 | 7/283 |
| Cricetulus barabensis | 0/0 | 1/34 | 0/21 | 0/0 | 0/0 | 0/0 | 0/0 | 1/32 | 0/0 | 0/3 | 0/12 | 0/0 | 2/102 |
| Cricetulus triton | 0/0 | 2/59 | 0/17 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2/36 | 0/0 | 4/112 |
| Total | 1/30 | 30/776 | 22/751 | 5/166 | 5/157 | 6/272 | 2/46 | 6/181 | 32/1,456 | 7/207 | 8/239 | 5/196 | 129/4,477 |
Serological assays.
Hantavirus-specific antigen in animal lungs and IgM antibodies in human sera were detected by indirect immunofluorescence assay (IFA) as described previously (65). Scattered, granular fluorescence in the cytoplasm was considered a positive reaction.
Amplification of viral genome and rodent mtDNA and sequencing.
Total RNA was extracted from the animal lung tissue and human serum samples with the TRIzol reagent (Invitrogen, Beijing, China) according to the manufacturer's instructions. In order to amplify M and S segment sequences, primer P14 (46) was used for cDNA syntheses with avian myeloblastosis virus reverse transcriptase (Promega, Beijing, China). The partial M and S segment sequences of SEOV were obtained by nested reverse transcription-PCR as described previously (66, 67). The complete M and S segments were amplified according to the method of Zou et al. (70). Mitochondrial DNA (mtDNA) was extracted from rat lung tissues using a genomic DNA extraction kit (SBS, Beijing, China) according to the manufacturer's protocol. The primer pair CB1/CB2 was used to amplify the sequences of the rat Cyt-b gene (29).
The PCR products were gel purified and subjected to sequencing with an ABI Prism dye termination sequencing kit and an ABI 373-A genetic analyzer.
Detection of recombination.
Detection of potential recombinant sequences, identification of likely parental sequences, and localization of possible recombination breakpoints were carried out by using the RDP, GENECONV, BOOTSCAN, MAXIMUM CHI SQUARE, CHIMAERA, SISCAN, and 3SEQ recombination detection methods as implemented in RDP3 (31). The analysis was carried out with default settings for the different detection methods and a Bonferroni corrected P value cutoff of 0.05. Only events found with two or more methods coupled with significant phylogenetic support were considered credible evidence of recombination. The recombinant sequences were not included in the present study for phylogenetic analyses and estimation of rates of nucleotide substitution.
Phylogenetic analyses.
Hantaviral S and M segment sequences and animal Cyt-b sequences were aligned using the CLUSTAL W program (version 1.83). Their nucleotide (nt) and amino acid (aa) identities were calculated using the DNAStar program. The best-fit phylogenetic model was determined by Modeltest v3.7. General time reversible (GTR), with a gamma distribution model of site rate heterogeneity, and a proportion of invariable sites (GTR+Γ+I) was found to be the best model for the partial or complete S segment and Cyt-b gene of the host, GTR+Γ, for the partial or complete M segment.
The MCMC method in MrBayes v3.1.2 was also used to perform phylogenetic analyses (17). The analysis used three hot chains and one cold chain and ran until the average standard deviation of split frequencies was <0.01, with 25% burn-in. The convergence of parameters was tested by calculating the effective sample size (ESS) using TRACER v1.4, and all ESS were well over 200. Consensus trees were supported by posterior-node probabilities. Maximum-likelihood (ML) trees were reconstructed using RAxML Blackbox web server (54). The tree files were visualized using Dendroscope program (2.4). Tree-Map software (2.0b) was used to analyze the degree of concordance between rat and SEOV cladograms (19). The trees of the virus and host were constructed by the Bayesian method. The optimal virus tree was mapped onto the host tree. Hantavirus sequences recovered in the present study are shown in Table S1 in the supplemental material, and those retrieved from GenBank are given in Table S2 in the supplemental material. The GenBank accession numbers for the animal Cyt-b sequences obtained in the present study are GU592954 to GU592997; those retrieved from GenBank are shown in Table S3 in the supplemental material.
Estimating rates of nucleotide substitution.
The rates of nucleotide substitution and divergence times (the most recent common ancestors [TMRCA]) for the S and the M segment sequences of SEOV were estimated using the Bayesian MCMC approach available in the BEAST v1.6.0 package (11), with uncertainty in all estimates reflected in the 95% high-probability density (HPD) intervals. The data sets were based on the complete S and M sequences for which the year-of-sampling was known (dates ranged from 1982 to 2008).
BEAUTi v1.6.0 was used to generate BEAST XML input files, specifying a strict molecular clock model and an uncorrelated lognormal-distribution relaxed molecular clock model (12). When analyzing separate partitions of the codon position sites, we used GTR+Γ+I for the open reading frame (ORF) sequence of the S segment and GTR+Γ for the ORF sequence of the M segment determined by Modeltest 3.7 and both the constant and the extended Bayesian Skyline trees for all analyses. For the analysis of all three codon positions, the SRD06 substitution model was used (49). For each data set with sampling every 1,000 generations, we performed a minimum of two independent runs. Each run was performed until the effective sample size of all parameters was larger than 200. Tracer v1.5 (http://tree.bio.ed.ac.uk/software/tracer) was used to summarize, analyze, and visualize the resulting posterior sample. We used TreeAnnotator program (v1.6.0) to generate a maximum clade credibility (MCC) tree with a burn-in of 10% of the sampled trees to summarize the sample of trees produced by each BEAST run. Model tests in a Bayesian framework were performed by calculating the Bayes Factor (BF) (55) with Tracer v1.5. According to the BF analysis results of clock model (see Table S4 in the supplemental material), an uncorrelated lognormal-distribution relaxed molecular clock model and extended Bayesian Skyline tree prior fit both ORF sequences of S and M segments (see Table S4 in the supplemental material). Because temporal structure was essential to the accurate estimation of substitution rates, we also assessed the strength of the temporal signal in these data. Under the best model of clock and tree prior model, BEAST analyses described above were repeated on data sets in which sampling times were randomized such that they lack any temporal structure. Runs for randomized data were repeated 10 times. The mean rates and 95% HPD estimates from the randomized data were then compared to those from the real data. If these samples contain clear temporal structure, the mean rates and 95% HPDs from the real data have major differences compared to those from the resampled data.
We calculated posterior summaries for the nodes in the MCC tree that had a posterior probability greater than 0.5 and used FigTree v1.3.1 (Rambaut, http://tree.bio.ed.ac.uk) to visualize the tree.
Spatial pattern analysis.
In order to elucidate phylodynamic spread in time and space, a recently developed Bayesian statistical inference framework was used (25). In this framework, spatial diffusion on time-measured genealogies as a continuous-time Markov chain (CTMC) process over discrete sampling locations was performed. This spatial process model was matched simultaneously with well-established models of sequence evolution in a Bayesian genealogical approach by using the software package BEAST.
The spatial dynamics of SEOV variants in real timescales were inferred by simultaneous integration of the CTMC model for discretized diffusion in BEAST, which is centered on time-scaled phylogenies. The Bayesian stochastic search variable selection procedure, which allows the CTMC rates to become zero with some positive and prior probability, was applied to achieve statistical efficiency. By using this procedure, the inference at a parsimonious set of spatial diffusion rates, which could explain appropriately the spatial process, was obtained.
The MCC tree was constructed according to the method described above. An uncorrelated lognormal-distribution relaxed molecular clock model (12), GTR+Γ, an extended Bayesian Skyline tree prior, and the SRD06 substitution model were used (49) for analysis. Only the MCC tree, having a posterior probability greater than 0 in the node, was used to perform spatial pattern analysis. To provide a spatial projection, the tree was converted into a keyhole markup language (KML) file suitable for viewing with Google Earth (http://earth.google.com). The temporal information on the marked-up tree using the TimeSpan KML function was used to animate viral dispersal over the time.
RESULTS
Samples and identification.
In the past 7 years (2002 to 2008), a total of 4,477 rodents belonging to six species (Table 1) were captured in the residential habitats of 12 provinces (major HFRS areas of endemicity) in China (68). Most of these rodents (3,625 [80.97%]) were Norway rats. A total of 129 rodents (111 Norway rats, 4 Rattus flavipectus, 1 Rattus rattus, 7 Mus musculus, 2 Cricetulus barabensis, and 4 Cricetulus triton) were found to be positive for hantavirus antigens by IFA. Interestingly, hantavirus antigen was also identified in one cat collected from Anhui province. IgM antibodies against SEOV were detected by indirect IFA in all six serum samples from acutely ill HFRS patients. The year of collection, species, origin, and geographical location for each animal and human sample (positive for hantavirus antigens or antibodies) are presented in detail in Table S1 in the supplemental material.
Genetic diversity of the Chinese SEOV variants and their phylogenetic relationship with those from around the world.
Partial M segment sequences (nucleotides [nt] 2001 to 2301) were successfully recovered from 129 hantavirus antigen-positive animal lung tissues and 6 human serum samples (see Table S1 in the supplemental material). Partial S segment sequences (nt 620 to 999) were successfully recovered from 108 hantavirus antigen-positive animal samples and 4 human serum samples. Comparison of these partial M and S sequences revealed nucleotide and amino acid divergences up to 18.5 and 14.6% (nucleotides) and 5.9 and 3.2% (amino acids), respectively. The nucleotide divergences between phylogroups (see below) were over 14% for the M segment or 10% for the S segment and >10% for the M segment or >5% between clusters within the second phylogroup. In addition, all non-Chinese SEOV strains were found to be closely related to the Chinese phylogroup A strains, with <5% nucleotide divergence. A total of 64 complete M sequences and 69 complete S sequences were recovered from these samples. The nucleotide divergences of the complete S and M sequences between phylogroups and between clusters within phylogroups were similar to those of the partial S and M sequences.
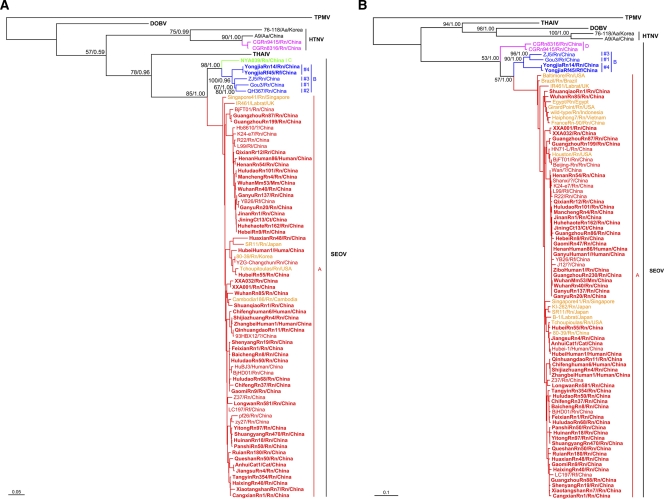
To investigate the evolutionary relationships between SEOV variants from both inside and outside China, we calculated phylogenetic trees using partial or complete S sequences recovered in the present study as well as those reported previously (retrieved from the GenBank) using ML and Bayesian methods. As shown in Fig. 1A, nearly all known Chinese strains (excluding the two reassortants [70]) belong to SEOV, regardless the host species (R. norvegicus, R. rattus, R. flavipectus, M. musculus, C. barabensis, C. triton, cat, or human) from which they were derived, suggesting that cross-species transmission (spillover) of SEOV from the main natural host R. norvegicus to other species has occurred. On the phylogenetic trees, these viruses formed phylogroups A, B, and C. Notably, most of the strains were grouped into the well-supported phylogroup A. Phylogroup B contained two strains (YongjiaRn14 and YongjiaRf45) identified in the present study and those identified previously (Gou3 isolated from R. rattus [56], QH367 [DQ081717], and ZJ5 [FJ753400] detected in R. norvegicus) and could be divided into four distinct clusters (1 to 4). The NYA039 strain, which was identified in R. norvegicus from Hunan province, formed phylogroup C. Phylogroup C viruses are closely related to phylogroup B strains, but are more distantly related to phylogroup A strains. Although phylogroup A viruses could be divided into four clusters in the present study (data not shown) or more in previous studies by using the neighbor-joining method (39, 50, 59), similar clustering patterns could not be obtained by using both ML and Bayesian methods.
Fig 1.
Phylogenetic relationships between Seoul viruses (SEOV) detected or isolated in and outside China. ML/Bayesian trees were based on the S segment sequences (A), and ML/Bayesian trees were based on the M segment sequences (B). Numbers (>50%/>0.5) above or below branches indicate bootstrap values or posterior node probabilities. Thottapalayam virus (TPMV) was used as the outgroup. The SEOV strains in groups A, B, C, and D are shown in red, blue, green, and purple, respectively. The SEOV strains of group A outside China are shown in yellow. Sequences obtained in the present study are indicated in boldface. The scale bar represents the genetic distance. Aa, Apodemus agrarius; Rn, Rattus norvegicus; Rr, Rattus rattus; Rf, Rattus flavipectus; Cb, Cricetulus barabensis; Ct, Cricetulus tyiton; Mm, Mus musculus. These abbreviations represent the same species of host in Table S1 in the supplemental material.
On the trees based on partial or complete M sequences (Fig. 1B), all Chinese SEOV variants formed phylogroups A, B, and D. The clustering patterns of phylogroups A and B were similar to those on the S tree described above. However, within phylogroup B, the M segment sequences of the two strains (YongjiaRn14 and YongjiaRf45) identified in here were distinct from the other two strains, with >10% nucleotide divergence. Two M segment sequences from HTNV-SEOV reassortants isolated from R. norvegicus in Guizhou province (70) formed phylogroup D. Notably, these two strains are quite distinct from all other SEOV strains. Unfortunately, attempts to amplify either complete or even partial M segment sequence from strain NYA039 (phylogroup C of Fig. 1A) were not successful.
Among the non-Chinese strains, the strain Houston from the United States formed a small branch (previously called cluster 2) in phylogroup A with strains from Beijing and Guangdong of China (Fig. 1B). The strains that originated from Cambodia, Egypt, France, Indonesia, Japan, Singapore, South Korea, the United States, and Vietnam could be grouped into phylogroup A. The strains from Brazil, the United States, and the United Kingdom were closely related to each other and were grouped into phylogroup A (called previously cluster 6 [50]) as well. These non-Chinese variants were identified not only in wild rats but also in laboratory rats (e.g., IR461 and B1) (50).
Geographic distribution of SEOV variants recovered in and outside of China.
Most of the phylogroup A viruses identified thus far, including the earliest isolate R22 (59) and variants described in the present and previous studies (26, 27, 56, 59, 60, 63, 71), are distributed throughout most of the major HFRS areas of endemicity of China (Fig. 2). In China, SEOV strains from phylogroup A have been found in the most of the provinces except for Chongqing, Gansu, Ningxia, Qinghai, Shannxi, Tibet, and Xinjiang.
Fig 2.
Geographic distribution of SEOV variants found in China. The phylogroups and clusters of SEOV are consistent with those in Fig. 1. Sequences recovered in the present study, retrieved from GenBank (the accession numbers are given in Tables S1 and S2 in the supplemental material), and from previous studies (26, 27, 56, 60, 63, 71) were used for the map.
To date, all five strains of phylogroup B (one from R. rattus, three from R. norvegicus, and one from R. flavipectus) were identified in Zhejiang province, and one strain NYA039 in the phylogroup C had been found in R. norvegicus in Hunan province, while two HTNV-SEOV reassortants in the phylogroup D were isolated from R. norvegicus in Guizhou province. Notably, all of these variants were identified in rats trapped in the mountainous regions.
Thus far, SEOV has been found in at least 22 countries outside China (see Fig. S1 in the supplemental material), from Asia to Africa, Europe, and the Americas. Interestingly, almost all of these countries have extended coastal regions.
Phylogeographic analysis.
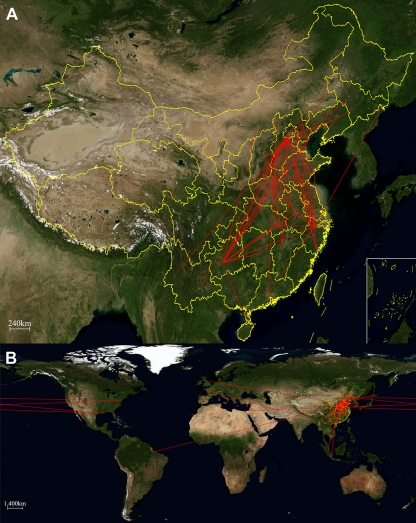
The model of sequence evolution in the Bayesian genealogical approach was used to infer the epidemiological connections between SEOV variants in and outside China sampled through time and across space. Since the available M segment sequences from outside China are more numerous than S and L segment sequences, the spatial pattern of SEOV variants was performed using the partial M segment sequences. The MCC trees of SEOV in or outside China with CTMC spatial reconstruction are shown in Fig. S2 and S3 in the supplemental material, respectively. As shown in Fig. 3A, the early evolution of SEOV might have occurred in the neighboring areas of Henan and Shanxi along the Yellow River in China. An ancestor could then spread to other regions, such as Zhejiang, Hunan, and Guizhou provinces, and diversify into the present phylogroups A, B, C, and D. Interestingly, the strains within the phylogroup A seemed to be frequently dispersed through different locations over the last decades.
Fig 3.
Inferred migration graph for SEOV in China (A) and around the world (B) and its reflection of the vents reconstructed from the MCC tree. The maps are based on satellite pictures made available in Google Earth (http://earth.google.com; doi:10.1371/journal.pcbi.1000520.g007).
As shown on Fig. 3B, some SEOV strains from outside China (e.g., the Houston strain from the United States) might have been directly originated from China, while others (e.g., the IR461 strain from the United Kingdom, the Baltimore strain from the United States, and the strain from Brazil) might have evolved from the ancestor in China. Since the knowledge on the genetic diversity and geographic distribution of SEOV outside China is limited, further studies are needed to clarify what routes SEOV variants spread around the world.
Rates of nucleotide substitution in SEOV.
We performed the Bayesian Markov chain Monte Carlo analysis on the S and M segment sequences with a known year-of-sampling. The parameter values estimated from the S and M segment sequence data set deviated substantially from those of the random data set with no overlapping 95% HPD, suggesting that there is a significant temporal signal in these data. The mean substitution rates calculated using an uncorrelated lognormal-distribution relaxed molecular clock model and an extended Bayesian Skyline model for the S and M segments of the SEOVs were 2.15 × 10−4 and 2.30 × 10−4 substitutions/site/year, respectively, with a 95% HPD that ranged from 5.21 × 10−5 to 4.58 × 10−4 and from 9.10 × 10−5 to 4.08 × 10−4 substitutions/site/year (Table 2; see also Fig. S4 in the supplemental material), findings in agreement with the data reported by Ramsden et al. (41, 42). As shown in Table 2 and in Fig. S4 in the supplemental material, the mean values of TMRCA estimated for all SEOVs based on the currently sampled genetic diversity were between 959 and 1,211 years before present (ybp) (95% HPD, 186 to 2,040 ybp). The similarly mean values of recent timescales estimated for the divergences of the phylogroup A viruses were between 223 and 281 ybp (95% HPD, 74 to 381 ybp).
Table 2.
Summary of evolution rates in the complete S and M segments
| Summary statistic | Mean | SEM | GMa | 95% HPD |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| S segment | |||||
| TMRCA(SEOV) | 959 | 64.96 | 605 | 186 | 1,345 |
| TMRCA(A) | 281 | 21.09 | 189 | 74 | 381 |
| TMRCA(B and C) | 605 | 32.79 | 370 | 117 | 847 |
| Mean rate | 2.15E-4 | 1.25E-5 | 1.89E-4 | 5.21E-5 | 4.58E-4 |
| M segment | |||||
| TMRCA(SEOV) | 1211 | 80.30 | 1132 | 479 | 2040 |
| TMRCA(A) | 223 | 10.32 | 209 | 95 | 362 |
| TMRCA(B) | 478 | 26.95 | 440 | 166 | 849 |
| TMRCA(D) | 115 | 5.98 | 105 | 40 | 211 |
| Mean rate | 2.30E-04 | 1.38E-05 | 2.15E-04 | 9.10E-05 | 4.08E-04 |
GM, geometric mean.
Phylogeny of SEOV host Norway rats and its relationship to that of the SEOV variants.
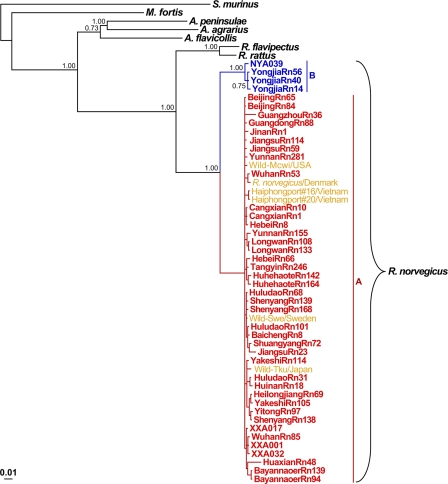
The current distribution of hantaviruses is the result of rodent migrations and continuing adaptation of viruses to their hosts (37). To clarify the evolutionary relationships between SEOV variants and their hosts, Norway rats (R. norvegicus), we performed phylogenetic analysis of the 1,143-nt fragment of the Cyt-b gene sequences recovered from Norway rats captured in China and around the world. A total of 44 mtDNA sequences recovered from hantavirus antigen-positive Norway rats captured from diverse HFRS areas of endemicity in China were included in this analysis. The results showed that most rats from China were closely related to each other, with overall Cyt-b sequence identities of 97.5 to 100%. Remarkably, these Norway rats were genetically closely related to those from Japan, Vietnam, Sweden, and the United States, with a <1% nucleotide difference. As shown in Fig. 4, the animals clustered together and formed the well-supported phylogroup A (100%). Interestingly, the rats, which were grouped into phylogroup A, completely coincide with the first phylogroup of SEOV variants. In contrast, the Norway rats captured in the mountainous areas of Ningyuan in Hunan province and Yongjia in Zhejiang province, which carried distinct SEOV strains from phylogroups B and C, were distant from the phylogroup A rats, with a >5% nucleotide difference, and formed a distinct well-supported phylogroup B (100%). The tree constructed using the ML method had the same topology as the tree constructed using the Bayesian method (data not shown).
Fig 4.
Phylogenetic relationships between Norway rats captured in and outside China. The Bayesian tree was constructed with mtDNA sequences of cytochrome b. The sequences of Suncus murinus were used as the outgroup. The groups A and B are shown in red and blue, respectively. The GenBank accession numbers of the rat mtDNA sequences recovered outside China are shown in yellow. The scale bar represents the genetic distance.
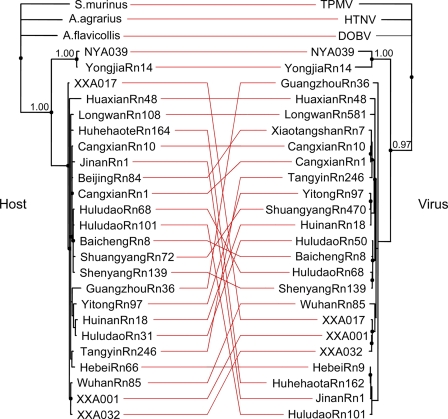
In order to infer phylogenetic relationships between SEOV variants and their hosts, the phylogenies of rats and viruses were reconstructed by TreeMap 2.0b. As shown in Fig. 5, similar topologies revealed a high degree of concordance between phylogroup A to C SEOV variants and their respective rat carriers. However, with few exceptions, the strains within phylogroup A were not concordant with their rat carriers, suggesting a free transmission of viruses between Norwegian rats with slightly different genetic backgrounds.
Fig 5.
Tanglegram constructed with the TreeMap program, showing SEOV variants and their rat carriers in China. The host tree on the left was based on cytochrome b sequences, and the hantavirus tree on the right was based on the complete S segment sequences. MrBayes v3.1.2 was used to construct the phylogenetic trees using the Bayesian method. The two trees were rooted with Suncus muinus and TPMV as outgroups, respectively.
DISCUSSION
The long-term surveillance carried out since 1984 and epidemiologic investigations have indicated that Norway rats (R. norvegicus) are the predominant carriers for hantaviruses (>50%) in the residential habitats of the most HFRS areas of endemicity in China (5, 6, 30, 59, 66–68). SEOV-positive rats have been found in all provinces of China except Qinghai and Tibet (68). The present study confirms that R. norvegicus is the predominant host of hantavirus in these habitats and that SEOV is distributed widely in China. SEOV has been found in almost all major HFRS areas of endemicity since its discovery in 1981 (68). After decades of fast socioeconomic development and extensive changes in agricultural production in rural areas of China, SEOV has become the most important hantavirus possessing threat to public heath in the country.
Earlier molecular epidemiological investigations based on partial (nt 2001 to 2301) or complete M segment sequences suggested that SEOV strains could be divided into five or more phylogenetic lineages (39, 50, 59). Except strains of the Gou3 variants (which, at the moment, is considered a provisional distinct hantavirus species), all known Chinese and non-Chinese SEOV strains are genetically homogeneous and do not display geographical clustering (39, 50, 59, 66). In line with these results, most of the SEOV strains characterized here are genetically homogeneous and widely distributed. However, two reassortants, CGRn8316 and CGRn9415, isolated from R. norvegicus from Guizhou (70), the virus NYA039 identified in R. norvegicus from Hunan, and the strains YongjiaRn14 and YongjiaRf45 identified in R. norvegicus and R. flavipectus from Zhejiang, showed ca. 15% nucleotide divergence from the majority of SEOV (besides Gou3). Moreover, they formed distinct phylogroups on both the M and the S trees in accordance with their geographic origins. Particularly, phylogroup B variants show high genetic diversity within a small region of Zhejiang. Our results revealed that the genetic diversity of SEOV is comparable to that of other hantaviruses. There are at least four genetically distinct phylogroups of SEOV circulating in rats in China. We propose to designate these phylogroups A to D (Fig. 1). Since all SEOV strains from phylogroups B to D were identified only in rats inhabiting mountainous areas (Fig. 4), our results also suggested that still unknown phylogroups (subtypes) of SEOV may be present in rat species from the mountainous regions. These rats are indigenous and have not been sharing habitats with rats from other places for a long time. Moreover, the strains from phylogroups B, C and D show geographical clustering typical to other hantaviruses, such as, e.g., HTNV and PUUV (51, 69). Since the viruses from phylogroups B to D, which have been identified only in China, occupy more ancestral positions on the M and S trees (Fig. 1) and since Norway rats from phylogroup A are closely related to each other, the present study strongly supports our earlier proposal that the phylogroup A variants of SEOV share a common ancestor (66, 68). Moreover, our results point to the direction that all of these variants of SEOV might be originated from China and then radiated to other parts of the world.
In the 1980s and 1990s, mild HFRS cases were reported in many regions of China after the first identification of mild HFRS cases caused by SEOV in 1981 (5, 6, 30, 68). Interestingly, the increase in mild HFRS coincided with the fast socioeconomic development beginning in 1978. The surveillance and epidemiological investigations revealed that R. norvegicus had not been found until the 1970s in Xinjiang, and hantavirus antigens or hantavirus antibodies were only detected in rats collected around railway stations in the 1980s, in which HFRS had not been reported thus far, suggesting that SEOV spread along railway routes, along with its hosts (5, 30, 66). Other studies also reported that seaports might be a source of hantaviruses (21, 62). Recently, we found that the HFRS emerged in the Bayannaoer district of Inner Mongolia might have been caused by SEOV imported from the neighboring HFRS areas of endemicity (67). The present study indicated that the SEOV variants of phylogroup A spread widely through China over the last decades (Fig. 2) and even around the world (see Fig. S1 in the supplemental material). Especially, all identified non-Chinese SEOV strains have been found in countries with coastal lines (see Fig. S1 in the supplemental material). In addition, phylogeographic analysis also revealed that all non-Chinese variants appeared to be directly originated from China. Together, these results suggest that the present geographic distribution of phylogroup A variants of SEOV in China and even around the world might have resulted from the widespread of rats which followed human activities.
Hantaviruses show a host specificity and their phylogeny also exhibit a high degree of concordance with the phylogeny of their hosts (with a few exceptions that probably originated from host-switching). Therefore, it is a widely shared opinion that hantaviruses present an example of a long-term codivergence of a virus and its host (18–20, 37, 40, 53). Recently, however, the evolutionary rate of hantaviruses was estimated to be similar to the rate seen for rapidly evolving RNA viruses (41, 42). Consequently, it was suggested that the association between hantaviruses and their mammalian hosts might be the result of a more recent history of preferential host switching and local adaptation. In the present study, the evolutionary rates of hantaviruses based on both the S and the M segment sequences are comparable to those reported by Ramsden et al. (41, 42), suggesting that hantaviruses evolved orders of magnitude more recently than expected under codivergence.
The distribution of hantaviruses today is the result of rodent migrations and a history of hantaviruses adaptation to their hosts (37). The species R. norvegicus probably originated from northern China (Asia) (2, 16) and the rats migrated to Europe in the 18th century (2). They may have entered Europe after an earthquake in 1727 by swimming the Volga River. They reached North America at around the year 1755 on the ships of the new settlers (1, 13, 24). The worldwide spread of Norway rats can be directly attributed to its relationship with humans (44). All these data suggest that the current worldwide distribution of the Norway rat following human activities might have taken place within the last few centuries. In the present study, phylogenetic analysis of the mtDNA sequences indicates that most of the Chinese sequences are not only closely related to each other but also closely related to those from other countries in Asia, Europe, and the United States (Fig. 4), suggesting that most rats from China and all known rats outside China share an ancestor. Remarkably, the rats trapped from mountainous areas of China, which carry distinct SEOV variants of phylogroups B and C, are quite distinct from the phylogroup A variants with which they form a sister taxa. Furthermore, the phylogenetic positions for most of the viruses within the phylogroup A are not in concordance with their rat carriers, and the current genetic diversity in phylogroup A from diverse geographical locations in and outside China may have been generated in a few recent centuries. Phylogeographic analysis also reveals the frequent dispersion of the viruses within the phylogroup A over the last decades in and outside China. To summarize, our data support the notion that Norway rats originated in China and then migrated to Europe and subsequently to the Americas in the 18th century (1, 2). These analyses also suggest that the migration might have caused the worldwide distribution of SEOV.
Supplementary Material
ACKNOWLEDGMENTS
We thank Zhen F. Fu at the Department of Pathology of the College of Veterinary Medicine, University of Georgia, for help with this study. We also thank Lin-Hong Li of the Henan Center for Disease Control and Prevention, Qi Li of the Hebei Center for Disease Control and Prevention, Jian-Bo Wang of the Yakeshi Center for Disease Control and Prevention, Guang-Wei Hu and Lai-Shun Yao of the Jilin Center for Disease Control and Prevention, Zhi-Qiang Wang of the Shandong Center for Disease Control and Prevention, and Guo-Qing Yang of the Huludao Center for Disease Control and Prevention for collecting samples. We are grateful to Li Sun, Hong-Xia Wang, and Yu Wang of the National Institute of Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, for their help as well.
This study was supported by grants 2001DIA40037, 2002DIB40095, and 2003BA712A08-02 from the Chinese Ministry of Science and Technology and by the State Key Laboratory for Infectious Disease Prevention and Control (2011SKLID101).
Footnotes
Published ahead of print 16 November 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Anonymous. 2004. History of the Norway rat (Rattus norvegicus). http://www.ratbehavior.org/images/history.htm Accessed 18 November 2011
- 2. Barnett SA. 2002. The story of rats: their impact on us, and our impact on them, p 17–18 Allen and Unwin, Crows Nest, Australia [Google Scholar]
- 3. Bell DM, et al. 2009. Pandemic influenza as 21st century urban public health crisis. Emerg. Infect. Dis. 5:1963–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen H, et al. 2005. Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature 436:191–192 [DOI] [PubMed] [Google Scholar]
- 5. Chen HX, Luo CW. 2001. Hemorrhagic fever with renal syndrome: studies and the surveillance and application of vaccine. Hong Kong Medical Publisher, Hong Kong [Google Scholar]
- 6. Chen HX, et al. 1986. Epidemiological studies on hemorrhagic fever with renal syndrome in China. J. Infect. Dis. 154:394–398 [DOI] [PubMed] [Google Scholar]
- 7. Childs JE, Korch GW, Glass GE, LeDuc JW, Shah KV. 1987. Epizootiology of hantavirus infections in Baltimore: isolation of a virus from Norway rats, and characteristics of infected rat populations. Am. J. Epidemiol. 126:55–68 [DOI] [PubMed] [Google Scholar]
- 8. Cueto GR, Cavia R, Bellomo C, Padula PJ, Suárez OV. 2008. Prevalence of hantavirus infection in wild Rattus norvegicus and R. rattus populations of Buenos Aires City, Argentina. Trop. Med. Int. Health 13:46–51 [DOI] [PubMed] [Google Scholar]
- 9. Cutler SJ, Fooks AR, van der Poel WH. 2010. Public health threat of new, reemerging, and neglected zoonoses in the industrialized world. Emerg. Infect. Dis. 16:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287:443–449 [DOI] [PubMed] [Google Scholar]
- 11. Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drummond AJ, Ho SY, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol. 4:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grzimek B. 1968. Animal life encyclopedia, p 579 Van Nostrand Reinhold Co, New York, NY [Google Scholar]
- 14. Hang CS, et al. 1982. Investigation of the agent causing a mild type of hemorrhagic fever. Zhonghua Liu Xing Bing Xue Za Zhi 3:204–205 [PubMed] [Google Scholar]
- 15. Heyman P, et al. 2009. Serological and genetic evidence for the presence of Seoul hantavirus in Rattus norvegicus in Flanders, Belgium. Scand. J. Infect. Dis. 41:51–56 [DOI] [PubMed] [Google Scholar]
- 16. Heyman P, et al. 2004. Seoul hantavirus in Europe: first demonstration of the virus genome in wild Rattus norvegicus captured in France. Eur. J. Clin. Microbiol. Infect. Dis. 23:711–717 [DOI] [PubMed] [Google Scholar]
- 17. Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755 [DOI] [PubMed] [Google Scholar]
- 18. Hughes AL, Friedman R. 2000. Evolutionary diversification of protein-coding genes of hantaviruses. Mol. Biol. Evol. 7:1558–1568 [DOI] [PubMed] [Google Scholar]
- 19. Jackson AP, Charleston MA. 2004. A cophylogenetic perspective of RNA-virus evolution. Mol. Biol. Evol. 21:45–57 [DOI] [PubMed] [Google Scholar]
- 20. Jonsson CB, Figueiredo LT, Vapalahti O. 2010. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 23:412–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kariwa H, Yoshimatsu K, Arikawa J. 2007. Hantavirus infection in East Asia. Comp. Immunol. Microbiol. Infect. Dis. 30:341–356 [DOI] [PubMed] [Google Scholar]
- 22. Korch GW, Childs JE, Glass GE, Rossi CA, LeDuc JW. 1989. Serologic evidence of hantaviral infections within small mammal communities of Baltimore, Maryland: spatial and temporal patterns and host range. Am. J. Trop. Med. Hyg. 41:230–240 [DOI] [PubMed] [Google Scholar]
- 23. Lam SK, et al. 2001. Serological evidence of hantavirus infections in Malaysia. Southeast. Asian. J. Trop. Med. Public. Health 32:809–813 [PubMed] [Google Scholar]
- 24. Lantz DE. 1909. U.S. Department of Agriculture report. Biol. Surv. Bull. 33:9–54 [Google Scholar]
- 25. Lemey P, Rambaut A, Drummond AJ, Suchard MA. 2009. Bayesian phylogeography finds its roots. PLoS Comput. Biol. 9:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Zhao ZT, Wang ZQ, Liu YX, Hu MH. 2007. Nucleotide sequence characterization and phylogenetic analysis of hantaviruses isolated in Shandong Province, China. Chin. Med. J. (Engl.) 120:825–830 [PubMed] [Google Scholar]
- 27. Liu JJ, et al. 2008. Study on the molecular characteristic of natural infection of rodents with Hantaviruses in Shenzhen city. Zhonghua Yu Fang Yi Xue Za Zhi 42:324–328 [PubMed] [Google Scholar]
- 28. Lledó L, Gegúndez MI, Saz JV, Alves MJ, Beltrán M. 2002. Serological study of hantavirus in man in the Autonomous Community of Madrid, Spain. J. Med. Microbiol. 51:861–865 [DOI] [PubMed] [Google Scholar]
- 29. Luo J, et al. 2004. Molecular phylogeny and biogeography of Oriental voles: genus Eothenomys (Muridae, Mammalia). Mol. Phylogenet. Evol. 33:349–362 [DOI] [PubMed] [Google Scholar]
- 30. Luo ZZ, Liu GZ. 1990. Geographically epidemiological investigation of epidemiological fever in China, p 1–38 Anhui Press Bureau, Hefei, China [Google Scholar]
- 31. Martin DP, et al. 2010. RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26:2462–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCaughey C, et al. 1996. Evidence of hantavirus in wild rodents in Northern Ireland. Epidemiol. Infect. 117:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mills JN, et al. 1997. Patterns of association with host and habitat: antibody reactive with Sin Nombre virus in small mammals in the major biotic communities of the southwestern United States. Am. J. Trop. Med. Hyg. 56:273–284 [DOI] [PubMed] [Google Scholar]
- 34. Morens DM, Folkers GK, Fauci AS. 2004. The challenge of emerging and re-emerging infectious diseases. Nature 430:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neumann G, Noda T, Kawaoka Y. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pacsa AS, Elbishbishi EA, Chaturvedi UC, Chu KY, Mustafa AS. 2002. Hantavirus-specific antibodies in rodents and humans living in Kuwait. FEMS Immunol. Med. Microbiol. 33:139–142 [DOI] [PubMed] [Google Scholar]
- 37. Plyusnin A, Morzunov SP. 2001. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. Curr. Top. Microbiol. Immunol. 256:47–75 [DOI] [PubMed] [Google Scholar]
- 38. Plyusnin A, et al. 2011. Bunyaviridae, p 693–709 In King AM, et al. (ed), Virus taxonomy: 9th Report of the International Committee on Taxonomy of Viruses Elsevier, San Diego, CA [Google Scholar]
- 39. Plyusnina A, et al. 2004. Identification of Seoul hantavirus in Rattus norvegicus in Indonesia. Scand. J. Infect. Dis. 36:356–359 [DOI] [PubMed] [Google Scholar]
- 40. Plyusnin A, Vapalahti O, Vaheri A. 1996. Hantaviruses: genome structure, expression and evolution. J. Gen. Virol. 77:2677–2687 [DOI] [PubMed] [Google Scholar]
- 41. Ramsden C, Holmes EC, Charleston MA. 2009. Hantavirus evolution in relation to its rodent and insectivore hosts: no evidence for codivergence. Mol. Biol. Evol. 26:143–153 [DOI] [PubMed] [Google Scholar]
- 42. Ramsden C, et al. 2008. High rates of molecular evolution in hantaviruses. Mol. Biol. Evol. 25:1488–1492 [DOI] [PubMed] [Google Scholar]
- 43. Reynes JM, et al. 2003. Evidence of the presence of Seoul virus in Cambodia. Microb. Infect. 5:769–773 [DOI] [PubMed] [Google Scholar]
- 44. Robinson R. 1965. Genetics of the Norway rat. Pergamon, Oxford, United Kingdom [Google Scholar]
- 45. Schmaljohn CS, Hjelle B. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmaljohn CS, Jennings GB, Hay J, Dalrymple JM. 1986. Coding strategy of the S genome segment of Hantaan virus. Virology 155:633–643 [DOI] [PubMed] [Google Scholar]
- 47. Schönrich G, et al. 2008. Hantavirus-induced immunity in rodent reservoirs and humans. Immunol. Rev. 225:163–189 [DOI] [PubMed] [Google Scholar]
- 48. Seijo A, et al. 2003. Study of Hantavirus Seoul in a human and rodent population from a marginal area in Buenos Aires City. Medicina (Buenos Aires) 63:193–196 [PubMed] [Google Scholar]
- 49. Shapiro BA, Rambaut A, Drummond AJ. 2006. Choosing appropriate substitution models for the phylogenetic analysis of protein-coding sequences. Mol. Biol. Evol. 23:7–9 [DOI] [PubMed] [Google Scholar]
- 50. Shi X, McCaughey C, Elliott RM. 2003. Genetic characterisation of a Hantavirus isolated from a laboratory-acquired infection. J. Med. Virol. 71:105–109 [DOI] [PubMed] [Google Scholar]
- 51. Sironen T, Vaheri A, Plyusnin A. 2001. Molecular evolution of Puumala hantavirus. J. Virol. 75:11803–11810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Song G, et al. 1982. Isolation of EHF-related agent from Rattus norvegicus captured from patients' home in endemic areas of the mild type of hemorrhagic fever. Acta Microbiol. Sin. 22:373–376 [Google Scholar]
- 53. Song JW, et al. 2009. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura). J. Virol. 83:6184–6191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for RAxML web-servers. Syst. Biol. 57:758–771 [DOI] [PubMed] [Google Scholar]
- 55. Suchard MA, Weiss RE, Sinsheimer JS. 2001. Bayesian selection of continuous-time Markov chain evolutionary models. Mol. Biol. Evol. 18:1001–1013 [DOI] [PubMed] [Google Scholar]
- 56. Sun CQ, et al. 2000. Molecular biological evidence of Seoul virus firstly isolated in the northeast China (Heilongjiang province): complete nucleotide sequence analysis of the S segment of hantavirus Pf26 strain. Chin. J. Dis. Control Prevention 4:313–316 [Google Scholar]
- 57. Tantivanich S, Ayuthaya PI, Usawattanakul W, Imphand P. 1992. Hantaanvirus among urban rats from a slum area in Bangkok. Southeast Asian J. Trop. Med. Public Health 23:504–509 [PubMed] [Google Scholar]
- 58. Truong TT, et al. 2009. Molecular epidemiological and serological studies of hantavirus infection in northern Vietnam. J. Vet. Med. Sci. 71:1357–1363 [DOI] [PubMed] [Google Scholar]
- 59. Wang H, et al. 2000. Genetic diversity of hantaviruses isolated in china and characterization of novel hantaviruses isolated from Niviventer confucianus and Rattus rattus. Virology 278:332–345 [DOI] [PubMed] [Google Scholar]
- 60. Wang SW, Hang CS, Wang H, Xie YX, Ma BJ. 2002. Genotype and clade distribution of hantaviruses in China. Bing Du Xue Bao 18:211–216 [Google Scholar]
- 61. Weissenbacher MC, Cura E, Segura EL, Hortal MLJ, Baek Chu YK, Lee HW. 1996. Serological evidence of human hantavirus infection in Argentina, Bolivia, and Uruguay. Medicina (Buenos Aires) 56:17–22 [PubMed] [Google Scholar]
- 62. Wu YW, et al. 2007. Seaport as a source of hantavirus: a study on isolated isles. Int. J. Environ. Health Res. 17:25–32 [DOI] [PubMed] [Google Scholar]
- 63. Yang F, et al. 2006. Surveillance on natural infection of rodents with hantavirus in Shenzhen city and identification of a hantavirus strain SZ2083. Zhonghua Liu Xing Bing Xue Za Zhi 27:981–984 [PubMed] [Google Scholar]
- 64. Zeier M, et al. 2005. New ecological aspects of hantavirus infection: a change of a paradigm and a challenge of prevention: a review. Virus Genes 30:157–180 [DOI] [PubMed] [Google Scholar]
- 65. Zhang YZ, et al. 2009. Seoul virus and hantavirus disease, Shenyang, People's Republic China. Emerg. Infect. Dis. 15:200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang YZ, et al. 2010. Hantaviruses in small mammals and humans in the coastal region of Zhejiang Province, China. J. Med. Virol. 82:987–995 [DOI] [PubMed] [Google Scholar]
- 67. Zhang YZ, et al. 2009. Hantavirus in rodents and humans, Inner Mongolia Autonomous Region, China. Emerg. Infect. Dis. 15:885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang YZ, Zou Y, Fu ZF, Plyusnin AA. 2010. Hantavirus infection in humans and animals, China. Emerg. Infect. Dis. 16:1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zou Y, et al. 2008. Molecular diversity and phylogeny of Hantaan virus in Guizhou, China: evidence for Guizhou as a radiation center of the present Hantaan virus. J. Gen. Virol. 89:1987–1997 [DOI] [PubMed] [Google Scholar]
- 70. Zou Y, et al. 2008. Genetic characterization of hantaviruses isolated from Guizhou, China: evidence for spillover and reassortment in nature. J. Med. Virol. 80:1033–1041 [DOI] [PubMed] [Google Scholar]
- 71. Zou Y, et al. 2006. The epidemiological investigation and characterization of hantaviruses carried by Rattus in Yunnan. Chin. J. Vector Biol. Control 17:399–403 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.