Abstract
In order to characterize simian foamy retroviruses (SFVs) in wild-born nonhuman primates (NHPs) in Gabon and to investigate cross-species transmission to humans, we obtained 497 NHP samples, composed of 286 blood and 211 tissue (bush meat) samples. Anti-SFV antibodies were found in 31 of 286 plasma samples (10.5%). The integrase gene sequence was found in 38/497 samples, including both blood and tissue samples, with novel SFVs in several Cercopithecus species. Of the 78 humans, mostly hunters, who had been bitten or scratched by NHPs, 19 were SFV seropositive, with 15 cases confirmed by PCR. All but one were infected with ape SFV. We thus found novel SFV strains in NHPs in Gabon and high cross-species transmission of SFVs from gorilla bites.
TEXT
Foamy viruses belong to the Spumaretrovirus subfamily of the Retroviridae family. They are highly prevalent in several animal species, particularly in nonhuman primates (NHPs), in which they establish persistent infections (19), with the documented level of infection being 75 to 100% of captive adult NHPs. Most of the captive animals examined have been macaques and baboons (3, 7). Recently, it was shown that colonies of NHPs living in the wild, including various species of monkeys and apes in Africa (mandrills, gorillas, chimpanzees) and Asia (subspecies of macaques), are also infected with simian foamy virus (SFV) (6, 13, 17). The oral mucosa has been shown to be the main site of SFV replication in vivo, and saliva appears to be the principal reservoir of SFV (20). In NHPs, SFV is presumed to be transmitted mainly through severe bites, thus involving contact between infected saliva and blood (3, 7, 19). Other factors could play an important role in SFV transmission: for example, Leendertz et al. demonstrated that SFV can be transmitted to chimpanzees after the consumption of smaller NHPs, such as colobus monkeys (16).
Zoonotic transmission of SFV from various NHPs to occupationally exposed people, such as zookeepers, veterinarians, and personnel of animal care facilities, is well documented (9, 14), and bites from adult NHPs are presumed to be the major risk factor for viral acquisition. Acquired SFV infection has also been reported in hunters in the rainforest area of southern Cameroon and in people living in close contact with macaques in various Asian countries (5, 12, 27). In Cameroon, 24% of people who were bitten while hunting apes were infected with SFV (5). Neither signs of infection-associated disease in humans nor human-to-human transmission of SFV has, however, been documented (2, 5, 9, 14).
Few data are available on SFV infection in Gabon (18). In this central African country, a wide diversity of NHPs live deep in the rainforest, and hunting monkeys and apes is still frequent, with wide circulation of “bush meat.” The aims of the study reported here were to detect and characterize SFVs in wild-born NHPs in Gabon, to investigate interspecies transmission of SFVs from NHPs to humans, and to identify risk factors for such zoonotic infection.
Blood and tissue (bush meat) were collected from 497 monkeys and apes, representing 13 species of NHPs (Table 1) from various regions in rural Gabon (see the supplemental material). We obtained 273 blood samples from animals kept as pets after their parents were killed by hunters in the forest and 13 other blood samples from mandrills from Lopé National Park after they received anesthetics through arrows. Killed animals are sold in stalls in villages; we sampled 211 NHPs sold as bush meat (Table 1). All samples were stored at −20°C before they were transferred to the Centre International de Recherches Médicales de Franceville (CIRMF) in Gabon for analysis. Samples were collected in accordance with the rules of the animal care committee in Gabon (22).
Table 1.
Species of monkeys and apes caught in the wild in Gabon, central Africa, and results of SFV serology and PCR
| Species | Common name | No. of animals positive/no. tested (%) by: |
||
|---|---|---|---|---|
| Serological tests of blood samplesa | PCR |
|||
| Buffy coatsb | Bush meat (tissues)c | |||
| Cercopithecus solatus | Sun-tailed monkey | 8/16 (50) | 6/16 (37.5) | |
| Cercopithecus pogonias | Crowned guenon | 0/6 | 0/6 | |
| Cercopithecus nictitans | Greater white-nosed monkey | 0/30 | 0/30 | 3/29 (10.3) |
| Cercopithecus neglectus | De Brazza guenon | 0/3 | 0/3 | 1/3 (33.3) |
| Cercopithecus cephus | Red-eared guenon | 0/67 | 0/67 | 2/46 (4.3) |
| Cercocebus torquatus | Red-capped mangabey | 1/13 (7.7) | 1/13 (7.7) | 0/13 |
| Mandrillus sphinx | Mandrill | 12/77 (15.6) | 12/77 (15.6) | 4/78 (5.1) |
| Pan troglodytes troglodytes | Central African chimpanzee | 9/49 (18.4) | 8/49 (18.6) | 0/34 |
| Gorilla gorilla | Gorilla | 0/3 | 0/3 | 0/8 |
| Miopithecus ogouensis | Gabon talapoin | 0/2 | 0/2 | |
| Miopithecus talapoin | Talapoin monkey | 0/8 | 0/8 | |
| Colobus guereza | Mantled guereza | 0/2 | 0/2 | |
| Lophocebus albigena | Gray-cheeked mangabey | 1/10 (10) | 1/10 (10) | |
| Total | 31/286 (10.8) | 28/286 (9.8) | 10/211 (4.7) | |
Serological tests were performed by Western blotting using sera obtained from the monkeys.
PCR was performed using buffy coats obtained from monkeys and apes tested serologically with WB.
PCR was performed using only tissue samples obtained from bush meat (lymph nodes, muscles, lung, and heart) collected from dead monkeys.
Blood was also collected from 78 people (10 women, 59 men, and 9 children) who had received severe bites or scratches from NHPs while hunting or playing with pets (see Table S1 in the supplemental material). All received detailed information and gave consent during personal interviews. Ethical approval was received from the Ministry of Health and from the Ethical Committee of the CIRMF.
Plasma from NHPs and humans was screened by Western blotting (WB) for the presence of SFV antibodies as described previously (7, 10, 18, 26). We used the same antigens of SFV (chimpanzee foamy virus antigens) that were used in the study reported by Calattini et al. (5). Cell lysates without SFV were used as negative antigen controls. WB seropositivity was defined as the presence of reactivity to the Gag doublet of 70 kDa and 74 kDa, as previously described (5, 18). Samples with no reactivity to either Gag protein were considered seronegative, and those with reactivity to a single band in the 70- to 74-kDa molecular mass range were considered indeterminate. The WB positive control was serum from an SFV-positive chimpanzee, obtained from Calattini et al. (5); the negative control was obtained from a human who had never been in contact with NHPs.
DNA was extracted from buffy coats or tissue (bush meat conserved in RNAlater) with the Qiagen kit (QIAamp blood minikit; Courtaboeuf, France). The quality of the extracted DNA was verified by amplifying an albumin gene fragment, as described previously (18). The set of primers used in the round of PCR has been described previously (7, 24). These primers can amplify the integrase gene fragment of SFV from humans and apes, as well as from several Cercopithecus monkeys, and also some divergent SFV sequences from Asian monkeys. Positive PCR products were directly sequenced with an automatic sequencing system (Macrogen, Republic of Korea).
The SFV integrase gene sequences obtained were aligned with the ClustalW (1.81) program and then analyzed with Bioedit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Phylogenetic trees were constructed by the Bayesian method, as implemented in MrBayes version 3.1 software (23), and maximum likelihood was estimated by running the general time-reversible model of evolution (GTR model) with a gamma distribution of rates across sites for 1,000,000 generations with a burn-in of 25%. Parameters were examined with the Tracer program (http://tree.bio.ed.ac.uk/software/tracer/), and all estimated sample sizes were greater than 545, as previously described (18). The trees were visualized using the FigTree program (http://tree.bio.ed.ac.uk/software/figtree/).
SFV infection in wild-born nonhuman primates.
Antibodies to SFV were found in 31 (10.8%) of the 286 NHP plasma samples obtained from wild-born NHPs. The samples showed clear Gag doublet reactivity and were thus considered SFV seropositive (Table 1). Eleven samples were indeterminate, and the other 244 were seronegative.
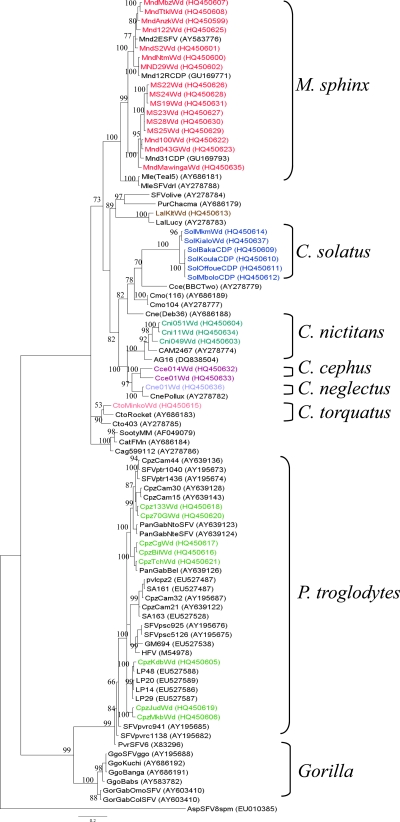
PCR was performed on all 497 NHP DNA samples obtained from 286 buffy coats and 211 tissues (bush meat), including lymph nodes, muscles, lung, and heart. The SFV integrase gene fragment was detected and sequenced in a total of 38 samples (22 from pets, 6 from mandrills in the national park, and 10 from bush meat), including 16 Mandrillus sphinx samples, 8 Pan troglodytes troglodytes samples, 6 Cercopithecus solatus samples, 3 Cercopithecus nictitans samples, 2 Cercopithecus cephus samples, 1 Cercopithecus neglectus sample, 1 Cercocebus torquatus sample, and 1 Lophocebus albigena sample (Table 1). Phylogenetic analyses (Fig. 1) showed that the new sequences obtained from these mandrills and chimpanzees clearly clustered within their respective clades (containing prototypic sequences). Three new clades, supported by high bootstrap values, were identified: the first corresponded to five new sequences of C. solatus, the second to the new sequences of C. cephus, and the third to three new C. nictitans sequences. The three sequences from L. albigena (LalKltWd; see the legend to Fig. 1 for sequence designations), C. neglectus (Cne01Wd), and Cercocebus torquatus (CtoMinkoWd) also clustered with their respective species clades.
Fig 1.
Phylogenetic relationships of integrase gene sequences (425 bp) obtained from 38 wild-born monkeys and apes in Gabon. Phylogenetic tree of new sequences isolated from 16 Mandrillus sphinx samples (red), one Lophocebus albigena sample (Lal; brown), one C. neglectus sample (Cne; turquoise), one Cercocebus torquatus sample (Cto; red), six C. solatus samples (blue), three C. nictitans samples (Cni; green), and two C. cephus samples (Cce; purple) and eight new sequences isolated from Pan troglodytes troglodytes (Cpz for chimpanzee; green). NHPs are indicated by the name of the species (e.g., Mnd for mandrill) and the number of the sample (e.g., 122), followed by Wd (wild) for the origin.
Cross-species transmission of SFV to humans.
Antibodies against SFV were detected in 19 of 78 plasma samples (24%) from people who recalled having been bitten, injured, or scratched by monkeys or apes (see Table S1 in the supplemental material). The SFV integrase gene fragment was detected by PCR in 15 DNA samples from the 19 seropositive persons and then sequenced (see Table S1 in the supplemental material). Twelve of the DNA samples were from people who had been severely bitten by gorillas, two were from people bitten by chimpanzees, and one was from a person who had been bitten by an unspecified Cercopithecus monkey. In all cases, there was a perfect match between the history of the contact with a given species and the simian viral sequence found in the infected person. The sequences from people bitten by gorillas belonged to the large clade of gorilla foamy viruses (Fig. 2), while the two people (sequences H3Gab56 and H4Gab59) who had been bitten by the same chimpanzee were infected with the same chimpanzee (P. troglodytes troglodytes) foamy virus. The sequence obtained from the person who had been bitten by an unknown Cercopithecus species clustered with the CneDeb36 SFV strain, obtained from a De Brazza monkey. A clinical examination conducted in the field by a physician showed that all the SFV-infected humans were apparently healthy, even several decades after the bites.
Fig 2.
Phylogenetic relationships of integrase gene sequences (425 bp) obtained from 15 persons bitten by an NHP. Asterisks indicate SFV sequences obtained from humans. The sequence isolated from a man bitten by a Cercopithecus species is shown in blue, the 12 sequences from people bitten by gorillas are in red, and the 2 sequences from the people bitten by the same P. t. troglodytes monkey are in green. Sequence Asp8SFVsp (from a spider monkey) was included as an outgroup. Values above the branches are bootstrap values. Human sequences are indicated by H, an order number, Gab (for Gabon), and a number of the person.
In univariate analysis (Table 2), the presence of foamy viral infection was clearly associated with the sex of the infected person (24% male versus 0% female, P = 0.035), the circumstances of contact (26% hunting versus 0% pets, P = 0.011), and the type of NHP (33% apes versus 3% monkeys, P = 0.001). Most of the contacts that resulted in an SFV infection were due to a bite by a gorilla, accounting for 42% (16/38) of infections, while only two of the six people bitten by a chimpanzee (33%) were infected.
Table 2.
Risk factors for infection with SFVa
| Characteristic | No. of humans positive for SFV | Total no. of humans tested | No. positive/no. tested (%) | P value |
|---|---|---|---|---|
| Age at contact (yr) | 0.533 | |||
| ≤45 | 10 | 57 | 17.5 | |
| >45 | 5 | 21 | 23.8 | |
| Sex | 0.035 | |||
| Male | 15 | 63 | 23.8 | |
| Female | 0 | 15 | 0 | |
| Circumstance of contact | 0.011 | |||
| Pet | 0 | 20 | 0 | |
| Hunting | 15 | 58 | 25.9 | |
| Type of nonhuman primate | 0.001 | |||
| Monkey | 1 | 36 | 2.8 | |
| Ape | 14 | 42 | 33.3 | |
| Type of wound | 0.316 | |||
| Scratch | 0 | 4 | 0 | |
| Bite | 15 | 74 | 20.3 | |
| Location of wound | 0.860 | |||
| Upper body | 8 | 40 | 20.0 | |
| Lower body | 7 | 38 | 18.4 | |
| Presence of scars | 0.018 | |||
| No | 0 | 18 | 0 | |
| Yes | 15 | 60 | 25.0 |
Univariate analyses were performed by STATA software with χ2 tests and Fisher's exact tests, with a critical P value of 0.05.
In the present study, we found that SFVs naturally infect three species of monkeys (C. solatus, C. nictitans, and C. cephus), and we found a high rate of SFV infection in humans who had been bitten, mainly by apes, indicating a high level of interspecies transmission from infected NHPs in Gabon.
A low prevalence of SFV was found in blood obtained from wild monkeys and in various tissues collected as bush meat. It has been reported previously that 50 to 100% of monkeys are infected with SFV; however, the majority of these studies were done with monkeys born in the wild but living in semifree conditions, such as zoos, national parks, and breeding colonies. Last year, we reported a large study on free-living mandrills and showed a prevalence of SFV of 50 to 100%, with a significant increase in SFV infection prevalence with increased age; the lowest prevalence was found in juvenile monkeys (18). A seroprevalence of 89.5% was found in a small macaque population (mostly adults) living in a temple in Bali, Indonesia, with a higher prevalence in adults than in juveniles (11). Thus, young monkeys are less frequently infected than older ones. In the present study, the majority of blood samples were obtained from pets, which were monkeys generally captured as juveniles after their mothers had been killed by hunters. This may explain, in part, the low prevalence in our blood samples. The mandrills are typical of this situation. The mandrills consisted of two groups: those collected as pets in various villages and those collected from adult groups living in Lopé National Park (n = 13 mandrills). A prevalence of 47% was found in the latter group, compared with 9% in pets in various villages.
In our study, a lower prevalence of SFV was also found in various tissues collected as bush meat. To our knowledge, the SFV prevalence in bush meat has not been reported previously. The tissues were collected for simian immunodeficiency virus (SIV) studies and kept for several years in RNAlater reagent. We used the same collection for our present studies. We cannot exclude the possibility that conservation of tissue samples in RNAlater affected the presence of SFV; however, the quality of the extracted DNA was verified in all samples by amplifying an albumin gene fragment. It has been reported that oral tissues are the major reservoir of SFV replication in monkeys (8, 20, 21). Unfortunately, none of the bush meat tissues contained oral tissue, such as salivary glands. The conservation of these tissues for many years and the origin of the samples might explain the low prevalence of SFV in our tissue collection. Our PCR methods were able to detect three new species of monkey (C. solatus, C. nictitans, and C. cephus) infected with species-specific SFVs which had not been shown previously to be infected with SFV.
C. solatus monkeys, which are found specifically in Gabon, are a species of guenon. The six new sequences obtained from this species were closely related and clustered, forming a clade supported by a 100% bootstrap value. The three new sequences obtained from C. nictitans were closely related and formed a strongly supported clade with two other sequences (CAM2467 and AG16) from two people living in southern Cameroon (5, 27). AG16 was from a hunter who had been bitten by an unspecified small monkey (5); our data strongly suggest that the monkey was a C. nictitans monkey. This species, which is widely distributed in central Africa, including Gabon and southern Cameroon, is one of the most frequently hunted in these areas. We also showed that wild-born C. cephus monkeys are naturally infected with SFV. The two new sequences clustered and were found to be related to but different from two other sequences obtained from De Brazza guenons (Cne01Wd and CnePollux).
The newly described sequences from 8 chimpanzees and 16 mandrills clustered in the two large groups of known strains, P. t. troglodytes for the chimpanzees and M. sphinx for the mandrills. By adding new sequences of known geographical origin, we have confirmed that two groups of strains naturally infect M. sphinx, as shown recently by our group (18). Further studies are necessary to better understand the apparent presence of subclades of P. t. troglodytes and have already been initiated by others (17, 25).
In this study, we found 19 SFV-seropositive people among the 78 who had been bitten or scratched by an NHP, and an integrase gene fragment was detected by PCR in peripheral blood DNA from 15 of them. The majority of individuals were infected with gorilla SFV. Although the number of samples obtained from gorillas was low (only three blood samples and eight tissue samples), none of these samples was positive for SFV by serology or PCR. This could be due to the fact that these samples were obtained from pets, a group in which a low prevalence of SFV was found. Our results for SFV in humans are similar to those observed by Calattini et al. (5) in rural Cameroon. They showed a prevalence of SFV of 24.1% of people who had had contact with apes (gorillas or chimpanzees). This is not surprising, as the two cohorts are similar and from similar geographical areas of central Africa. It was also shown previously by Calattini et al. (5) that transmission of SFV from NHPs to humans is much more likely after a deep bite, specifically a bite by an ape. In our study, 36 of the 78 individuals had been bitten by monkeys other than apes, and only one was positive for SFV. Furthermore, we collected 154 blood samples from people living in Gabon who reported hunting NHPs but had never been bitten. None of these samples was found to be SFV positive by serology or by PCR (A. Mouinga and M. Kazanji, unpublished data).
We found that the major risk factor for humans for acquiring SFV infection after being bitten by an NHP is the species and history of the NHP. Free-living apes confer a greater risk than pets and small monkeys. This confirms the findings of a recent study of a comparable series in southern Cameroon (5, 9, 27). It may be that most pets, including wild-caught gorillas, are not infected, as they are very young when captured. Furthermore, most wounds due to pet bites are not serious, with mainly superficial tissue damage. This contrasts with the situation for free-living apes, where most of the animals, mainly gorillas, are adults and give bites that lead to major tissue damage, resulting in contact between the ape's saliva and human muscles, bones, and blood, thus favoring cross-species viral infection.
Some authors showed rare cases of transmission of SFV without a bite after contact with NHPs (25, 27). In previous studies, few women were found to be SFV positive. In Africa, women do not hunt, but they generally cut up the bush meat and are also exposed to the blood of animals after injury and are thus exposed to SFV. Calattini et al. (4) found a woman who was infected with SFV after handling bush meat in Cameroon. We cannot exclude the possibility that other factors, such as the proviral load of SFV in the animals and the type of contact with humans, play an indirect role in SFV transmission. Furthermore, transmission of SFV between monkeys could occur by routes other than bites. In our previous study on a mandrill colony, 50% of the animals were SFV positive at the age of 1 year, perhaps due to the exchange of saliva with their mothers during feeding. It was reported recently that mandrills have a prominent muzzle-muzzle behavior, usually between young, naive monkeys and older individuals (15). Further studies are needed to understand the factors influencing the transmission of SFV.
No virus could be detected by PCR in the blood samples of four individuals who were seropositive for SFV. The lack of SFV sequences in WB-seropositive people was previously reported in hunters in southern Cameroon (5, 9, 27). Although the presence of divergent SFVs, exposure of the NHPs and humans, or abortive infections might explain this discrepancy, a low viral load in the blood is more likely. Our PCR can amplify a large variety of SFVs, including several divergent Asian variants (7).
In the present study, we showed a strong similarity (93 to 97%) between SFV sequences obtained from gorillas and those obtained from humans. We showed previously that SFV shows extremely low genetic drift in both monkeys and humans (18). In our previous study, the genetic variability of SFV was evaluated in a mandrill identified to be SFV positive and after transmission to a person severely bitten by the same mandrill at the Primatology Center in Gabon. Comparative sequence analysis also showed strong nucleotide sequence similarity (99.7%) between SFV sequences obtained from the human and those obtained from the mandrill.
In Africa, SFV zoonosis was previously reported in Zaire and most likely occurred in Kenya when human foamy virus (HFV),which is now documented to be of chimpanzee origin, was described (1); also, in southern Cameroon, 16 cases have been demonstrated serologically and molecularly (9, 5). Our series of 15 well-documented cases expands such findings to Gabon and doubles the number of known SFV-infected people in this area of the world. Our findings suggest that cross-species transmission of SFV is widespread in central Africa, especially in villages and settlements in lowland forest regions of Equatorial Guinea, Congo, and the Democratic Republic of the Congo. Hunting has increased in these countries due to a combination of urban demand for bush meat and easier access to NHP habitats via logging roads, and this has increased the frequency of human exposure to NHP retroviruses.
Further examination of humans infected by SFV is ongoing in both central Africa and Southeast Asia to investigate interhuman transmission in familial studies and the morbidity and mortality that might be associated with this zoonotic infection, especially in people infected with human immunodeficiency virus (HIV).
Nucleotide sequence accession numbers.
SFV sequences from NHPs obtained in this study have been submitted to GenBank under accession numbers HQ450599 to HQ450623 and HQ450625 to HQ450637. SFV sequences from humans obtained in this study have been submitted to GenBank under accession numbers HQ450584 to HQ450598.
Supplementary Material
ACKNOWLEDGMENTS
We thank the CIRMF, which is funded by the Gabonese Government, Total-Gabon, and the French Foreign Ministry. The sources of funding had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
We thank Benjamin Ollomo, Bertrand Mpiga-Mickoto, and Delphine Verrier for technical help.
Footnotes
Published ahead of print 9 November 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Achong BG, Mansell PW, Epstein MA. 1971. A new human virus in cultures from a nasopharyngeal carcinoma. J. Pathol. 103:P18. [PubMed] [Google Scholar]
- 2. Boneva RS, et al. 2007. Clinical and virological characterization of persistent human infection with simian foamy viruses. AIDS Res. Hum. Retroviruses 23:1330–1337 [DOI] [PubMed] [Google Scholar]
- 3. Broussard SR, et al. 1997. Characterization of new simian foamy viruses from African nonhuman primates. Virology 237:349–359 [DOI] [PubMed] [Google Scholar]
- 4. Calattini S, et al. 2011. Multiple retroviral infection by HTLV type 1, 2, 3 and simian foamy virus in a family of Pygmies from Cameroon. Virology 410:48–55 [DOI] [PubMed] [Google Scholar]
- 5. Calattini S, et al. 2007. Simian foamy virus transmission from apes to humans, rural Cameroon. Emerg. Infect. Dis. 13:1314–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calattini S, et al. 2004. Natural simian foamy virus infection in wild-caught gorillas, mandrills and drills from Cameroon and Gabon. J. Gen. Virol. 85:3313–3317 [DOI] [PubMed] [Google Scholar]
- 7. Calattini S, et al. 2006. Modes of transmission and genetic diversity of foamy viruses in a Macaca tonkeana colony. Retrovirology 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falcone V, et al. 1999. Sites of simian foamy virus persistence in naturally infected African green monkeys: latent provirus is ubiquitous, whereas viral replication is restricted to the oral mucosa. Virology 257:7–14 [DOI] [PubMed] [Google Scholar]
- 9. Gessain A, Calattini S. 2008. Emergence of simian foamy viruses in humans: facts and unanswered questions. Future Virol. 3:71–81 [Google Scholar]
- 10. Hussain AI, et al. 2003. Screening for simian foamy virus infection by using a combined antigen Western blot assay: evidence for a wide distribution among Old World primates and identification of four new divergent viruses. Virology 309:248–257 [DOI] [PubMed] [Google Scholar]
- 11. Jones-Engel L, et al. 2005. Primate-to-human retroviral transmission in Asia. Emerg. Infect. Dis. 11:1028–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones-Engel L, et al. 2008. Diverse contexts of zoonotic transmission of simian foamy viruses in Asia. Emerg. Infect. Dis. 14:1200–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones-Engel L, et al. 2007. Sensitive assays for simian foamy viruses reveal a high prevalence of infection in commensal, free-ranging Asian monkeys. J. Virol. 81:7330–7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan AS. 2009. Simian foamy virus infection in humans: prevalence and management. Expert Rev. Anti Infect. Ther. 7:569–580 [DOI] [PubMed] [Google Scholar]
- 15. Laidre ME. 2009. Informative breath: olfactory cues sought during social foraging among Old World monkeys (Mandrillus sphinx, M. Leucophaeus, and Papio anubis). J. Comp. Psychol. 123:34–44 [DOI] [PubMed] [Google Scholar]
- 16. Leendertz FH, et al. 2008. Interspecies transmission of simian foamy virus in a natural predator-prey system. J. Virol. 82:7741–7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu W, et al. 2008. Molecular ecology and natural history of simian foamy virus infection in wild-living chimpanzees. PLoS Pathog. 4:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mouinga-Ondeme A, et al. 2010. Two distinct variants of simian foamy virus in naturally infected mandrills (Mandrillus sphinx) and cross-species transmission to humans. Retrovirology 7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murray SM, Linial LM. 2006. Foamy virus infection in primates. J. Med. Primatol 35:225–235 [DOI] [PubMed] [Google Scholar]
- 20. Murray SM, et al. 2008. Replication in a superficial epithelial cell niche explains the lack of pathogenicity of primate foamy virus infections. J. Virol. 82:5981–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray SM, Picker LJ, Axthelm MK, Linial ML. 2006. Expanded tissue targets for foamy virus replication with simian immunodeficiency virus-induced immunosuppression. J. Virol. 80:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ollomo B, et al. 2009. A new malaria agent in African hominids. PLoS Pathog. 5:e1000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- 24. Schweizer M, et al. 1995. Markers of foamy virus infections in monkeys, apes, and accidentally infected humans: appropriate testing fails to confirm suspected foamy virus prevalence in humans. AIDS Res. Hum. Retroviruses 11:161–170 [DOI] [PubMed] [Google Scholar]
- 25. Switzer WM, et al. 2004. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J. Virol. 78:2780–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tobaly-Tapiero J, et al. 2000. Isolation and characterization of an equine foamy virus. J. Virol. 74:4064–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolfe ND, et al. 2004. Naturally acquired simian retrovirus infections in central African hunters. Lancet 363:932–937 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.