Abstract
Cytotoxic T lymphocyte (CTL) responses play a central role in viral suppression in human immunodeficiency virus (HIV) infections. Prophylactic vaccination resulting in effective CTL responses after viral exposure would contribute to HIV control. It is important to know how CTL memory induction by vaccination affects postexposure CTL responses. We previously showed vaccine-based control of a simian immunodeficiency virus (SIV) challenge in a group of Burmese rhesus macaques sharing a major histocompatibility complex class I haplotype. Gag206-216 and Gag241-249 epitope-specific CTL responses were responsible for this control. In the present study, we show the impact of individual epitope-specific CTL induction by prophylactic vaccination on postexposure CTL responses. In the acute phase after SIV challenge, dominant Gag206-216-specific CTL responses with delayed, naive-derived Gag241-249-specific CTL induction were observed in Gag206-216 epitope-vaccinated animals with prophylactic induction of single Gag206-216 epitope-specific CTL memory, and vice versa in Gag241-249 epitope-vaccinated animals with single Gag241-249 epitope-specific CTL induction. Animals with Gag206-216-specific CTL induction by vaccination selected for a Gag206-216-specific CTL escape mutation by week 5 and showed significantly less decline of plasma viral loads from week 3 to week 5 than in Gag241-249 epitope-vaccinated animals without escape mutations. Our results present evidence indicating significant influence of prophylactic vaccination on postexposure CTL immunodominance and cooperation of vaccine antigen-specific and non-vaccine antigen-specific CTL responses, which affects virus control. These findings provide great insights into antigen design for CTL-inducing AIDS vaccines.
INTRODUCTION
Human immunodeficiency virus (HIV) infection induces chronic, persistent viral replication leading to AIDS onset in humans. Virus-specific cytotoxic T lymphocyte (CTL) responses play a central role in the resolution of acute peak viremia (3, 4, 13, 22, 28) but mostly fail to contain viral replication in the natural course of HIV infection. Vaccination resulting in more effective CTL responses after viral exposure than in natural HIV infections would contribute to HIV control (30, 33). CTL memory induction by prophylactic vaccination may lead to efficient secondary CTL responses, but naive-derived primary CTL responses specific for viral nonvaccine antigens can also be induced after viral exposure. It is important to know how CTL memory induction by vaccination affects these postexposure CTL responses.
Cumulative studies on HIV-infected individuals have shown association of HLA genotypes with rapid or delayed AIDS progression (5, 14, 31, 34). For instance, most of the HIV-infected individuals possessing HLA-B*57 have been indicated to show a better prognosis with lower viral loads, implicating HLA-B*57-restricted epitope-specific CTL responses in this viral control (1, 8, 23, 24). Indian rhesus macaques possessing certain major histocompatibility complex class I (MHC-I) alleles, such as Mamu-A*01, Mamu-B*08, and Mamu-B*17, tend to show simian immunodeficiency virus (SIV) control (19, 25, 36). This implies possible HIV control by induction of particular effective CTL responses (2, 7, 12, 16, 27).
Recent trials of prophylactic T-cell-based vaccines in macaque AIDS models have indicated the possibility of reduction in postchallenge viral loads (6, 15, 17, 21, 35). We previously developed a prophylactic AIDS vaccine consisting of a DNA prime and a boost with a Sendai virus (SeV) vector expressing SIVmac239 Gag (SeV-Gag) (20). Our trial showed vaccine-based control of an SIVmac239 challenge in a group of Burmese rhesus macaques sharing the MHC-I haplotype 90-120-Ia (21). Animals possessing 90-120-Ia dominantly elicited Mamu-A1*043:01 (GenBank accession number AB444869)-restricted Gag206-216 (IINEEAADWDL) epitope-specific and Mamu-A1*065:01 (AB444921)-restricted Gag241-249 (SSVDEQIQW) epitope-specific CTL responses after SIV challenge and selected for viral gag mutations, GagL216S (leading to a leucine [L]-to-serine [S] substitution at amino acid [aa] 216 in Gag) and GagD244E (aspartic acid [D]-to-glutamic acid [E] at aa 244), resulting in escape from CTL recognition with viral fitness costs in the chronic phase (9, 26). Vaccinees possessing 90-120-Ia failed to control a challenge with a mutant SIV carrying these two CTL escape mutations, indicating that Gag206-216-specific and Gag241-249-specific CTL responses play a crucial role in the vaccine-based control of wild-type SIVmac239 replication (10). Furthermore, in an SIVmac239 challenge experiment with 90-120-Ia-positive rhesus macaques that received a prophylactic vaccine expressing the Gag241-249 epitope fused with enhanced green fluorescent protein (EGFP), this single-epitope vaccination resulted in control of SIVmac239 replication with dominant induction of Gag241-249-specific CTL responses in the acute phase postchallenge (32).
Thus, it is hypothesized that induction of single Gag206-216 or Gag241-249 epitope-specific CTL responses by vaccination may result in different patterns of CTL immunodominance and viral replication after SIV challenge. In the present study, we analyzed the impact of prophylactic vaccination inducing single Gag206-216 epitope-specific CTL responses on SIV control in 90-120-Ia-positive macaques and compared the results with those of vaccination inducing single Gag241-249 epitope-specific CTL responses. This analysis revealed differences in CTL responses and patterns of viral control after SIV challenge between these vaccinated groups, indicating significant effects of prophylactic vaccination on postexposure CTL immunodominance and cooperation of vaccine antigen-specific and non-vaccine antigen-specific CTL responses.
MATERIALS AND METHODS
Animal experiments.
Animal experiments were conducted through the Cooperative Research Program at Tsukuba Primate Research Center, National Institute of Biomedical Innovation, with the help of the Corporation for Production and Research of Laboratory Primates. Blood collection, vaccination, and virus challenge were performed under ketamine anesthesia. All animals were maintained in accordance with the Guideline for Laboratory Animals of the National Institute of Infectious Diseases.
Five Burmese rhesus macaques (Macaca mulatta) possessing the MHC-I haplotype 90-120-Ia (26) (group IV) received a DNA-prime/SeV-boost vaccine eliciting Gag206-216-specific CTL responses followed by an SIVmac239 challenge and were compared with three groups (I, II, and III) of 90-120-Ia-positive animals reported previously (10, 32) (Table 1). Group I animals (n = 6) received no vaccination, while group II animals (n = 5) received a DNA-prime/SeV-boost vaccine eliciting Gag-specific CTL responses. The DNA, CMV-SHIVdEN, used for the vaccination was constructed from a simian/human immunodeficiency virus (SHIVMD14YE) molecular clone DNA with env and nef deleted (29) and has the genes encoding SIVmac239 Gag, Pol, Vif, and Vpx; SIVmac239–HIV-1 chimeric Vpr; and HIV-1 Tat and Rev (21). In group II animals, CTL responses were undetectable after DNA prime but Gag-specific CTL responses became detectable after SeV-Gag boost. Group III animals (n = 6) received a DNA-prime/SeV-boost vaccine eliciting Gag241-249-specific CTL responses. A pGag236-250-EGFP-N1 DNA and an SeV-Gag236-250-EGFP vector, both expressing an SIVmac239 Gag236-250 (IAGTTSSVDEQIQWM)-EGFP fusion protein, were used for the group III vaccination. After the SeV-Gag236-250-EGFP boost, group III animals induced Gag241-249-specific CTL responses; the animals showed no Gag236-250-specific CD4+ T-cell responses but elicited SeV/EGFP-specific CD4+ T-cell responses (32). For the group IV vaccination, A pGag202-216-EGFP-N1 DNA and an SeV-Gag202-216-EGFP vector, both expressing an SIVmac239 Gag202-216 (IIRDIINEEAADWDL)-EGFP fusion protein, were used (Fig. 1). Approximately 3 months after the boost, all animals were challenged intravenously with 1,000 50% tissue culture infective doses of SIVmac239 (11). In our previous study (32), the unvaccinated and the control-vaccinated animals receiving a DNA and an SeV expressing EGFP showed no significant differences in viral loads after SIV challenge.
Table 1.
Animals analyzed in this study
| Group | No. of animals | Vaccinationa | SIV-specific CTL response postboost |
|---|---|---|---|
| I | 6 | None | None |
| II | 5 | Gag (pCMV-SHIVdEN DNA prime, SeV-Gag boost) | Gag-specific CTL |
| III | 6 | Gag241-249-specific (pGag236-250-EGFP-N1 DNA prime, SeV-Gag236-250-EGFP boost) | Gag241-249-specific CTL |
| IV | 5 | Gag206-216-specific (pGag202-216-EGFP-N1 DNA prime, SeV-Gag202-216-EGFP boost) | Gag206-216-specific CTL |
All animals were challenged with SIVmac239.
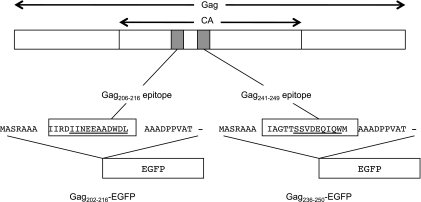
Fig 1.
Schema of the cDNA constructs encoding Gag202-216-EGFP and Gag236-250-EGFP fusion proteins. A DNA fragment that encodes a 31-mer peptide (boxes) including the Gag202-216 or Gag236-250 sequence (underlining) was introduced into the 5′ end of the EGFP cDNA.
Analysis of antigen-specific CTL responses.
We measured virus-specific CD8+ T-cell levels by flow cytometric analysis of gamma interferon (IFN-γ) induction after specific stimulation, as described previously (21). Peripheral blood mononuclear cells (PBMCs) were cocultured with autologous herpesvirus papioimmortalized B-lymphoblastoid cell lines pulsed with 1 μM SIVmac239 Gag206-216 (IINEEAADWDL), Gag241-249 (SSVDEQIQW), or Gag367-381 (ALKEALAPVPIPFAA) peptide for Gag206-216-specific, Gag241-249-specific, or Gag367-381-specific stimulation. Intracellular IFN-γ staining was performed with a CytofixCytoperm kit (BD, Tokyo, Japan) and fluorescein isothiocyanate-conjugated anti-human CD4 (BD), peridinin chlorophyll protein-conjugated anti-human CD8 (BD), allophycocyanin (APC)-Cy7-conjugated anti-human CD3 (BD), and phycoerythrin (PE)-conjugated anti-human IFN-γ (Biolegend, San Diego, CA) monoclonal antibodies. Specific T-cell levels were calculated by subtracting nonspecific IFN-γ T-cell frequencies from those after peptide-specific stimulation. Specific T-cell levels lower than 100 per million PBMCs were considered negative.
Sequencing of the viral genome.
Plasma RNA was extracted using the High Pure viral RNA kit (Roche Diagnostics, Tokyo, Japan). Fragments corresponding to nucleotides from 1231 to 2958 (containing the entire gag region) in the SIVmac239 genome (GenBank accession number M33262) were amplified by nested reverse transcription (RT)-PCR. The PCR products were sequenced using dye terminator chemistry and an automated DNA sequencer (Applied Biosystems, Tokyo, Japan).
Statistical analysis.
Statistical analyses were performed using R software (R Development Core Team). Differences in geometric means of plasma viral loads were examined by one-way analysis of variance (ANOVA) and Tukey-Kramer's multiple-comparison test. Plasma viral loads at week 3 were examined for differences between group III and groups II and IV by analysis of covariance (ANCOVA) with week 5 viral loads as a covariate.
RESULTS
CTL responses after prophylactic vaccination.
We previously reported the efficacy of vaccination eliciting whole Gag-specific or single Gag241-249 epitope-specific CTL memory against SIVmac239 challenge (10, 32). In the present study, we examined the efficacy of prophylactic induction of single Gag206-216 epitope-specific CTL memory against SIVmac239 challenge and compared the results with those of the previous experiments.
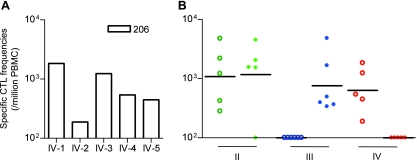
Five Burmese rhesus macaques possessing MHC-I haplotype 90-120-Ia received a DNA-prime/SeV-boost vaccine eliciting single Gag206-216 epitope-specific CTL responses. A plasmid DNA (pGag202-216-EGFP-N1) and an SeV (SeV-Gag202-216-EGFP) vector, both expressing an SIVmac239 Gag202-216-EGFP fusion protein, were used for the vaccination (Fig. 1). We confirmed Gag206-216-specific CTL responses 1 week after SeV-Gag202-216-EGFP boost in all five animals (Fig. 2A). As expected, no Gag241-249-specific CTL responses were detected in these animals. No Gag202-216-specific CD4+ T-cell responses were detected in the animals except for one (IV-5) showing marginal levels of responses (data not shown).
Fig 2.
Gag206-216-specific and Gag241-249-specific CTL responses after prophylactic vaccination. (A) Gag206-216-specific CD8+ T-cell frequencies 1 week after SeV-Gag202-216–EGFP boost in group IV macaques (open boxes). (B) Gag206-216-specific (open circles) and Gag241-249-specific (closed circles) CD8+ T-cell frequencies 1 week after boost in group II (green), III (blue), and IV (red) macaques. The bars indicate the geometric mean of each group. No animal showed detectable Gag-specific CTL responses before the boost.
Plasma viral loads after SIV challenge.
We compared these five animals (referred to as group IV) with other groups (I, II, and III) of 90-120-Ia-positive macaques reported previously (Table 1). Group I animals (n = 6) received no vaccination, group II (n = 5) received a DNA-prime/SeV-boost vaccine eliciting whole Gag-specific CTL responses, and group III (n = 6) received a DNA-prime/SeV-boost vaccine eliciting single Gag241-249 epitope-specific CTL responses. Both Gag206-216-specific and Gag241-249-specific CTL responses were detectable after SeV-Gag boost in four of five group II animals except for one animal (II-3), in which Gag206-216-specific, but not Gag241-249-specific, CTL responses were detected. In all group III animals, Gag241-249-specific CTL responses were confirmed, while no Gag206-216-specific CTL responses were detected after SeV-Gag236-250-EGFP boost (Fig. 2B).
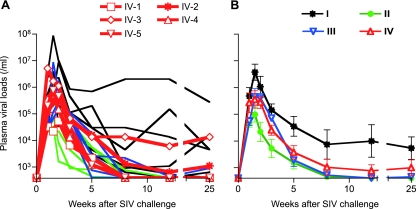
After SIVmac239 challenge, all animals were infected and showed plasma viremia during the acute phase. Plasma viremia was maintained in five of six unvaccinated animals in group I but became undetectable in one animal (I-2) at week 12. In contrast, all animals in groups II and III contained SIV replication with significantly reduced plasma viral loads compared to group I at the set point. In group IV, however, vaccine efficacy was not so clear; while three out of five animals contained SIV replication, the remaining two (IV-2 and IV-3) failed to control viral replication with persistent plasma viremia (Fig. 3).
Fig 3.
Plasma viral loads after SIVmac239 challenge. The plasma viral loads in group I, group II, group III, and group IV animals were determined as described previously (21). The lower limit of detection was approximately 4 × 102 copies/ml. (A) Changes in plasma viral loads (SIV gag RNA copies/ml plasma) after challenge. (B) Changes in geometric means of plasma viral loads after challenge. Groups II and III (but not group IV) showed significantly lower set point viral loads than group I (P = 0.0390 between groups I and II, P = 0.0404 between groups I and III, and P > 0.05 between groups I and IV at week 25 by one-way ANOVA and Tukey-Kramer's multiple-comparison test).
Gag-specific CTL responses after SIV challenge.
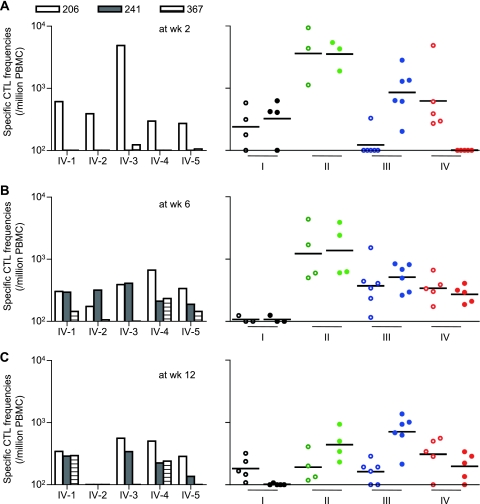
We then measured Gag206-216-specific and Gag241-249-specific CTL responses after SIVmac239 challenge by detection of peptide-specific IFN-γ induction. At week 2 (Fig. 4A), most animals in groups I and II elicited both Gag206-216-specific and Gag241-249-specific CTL responses, whereas group III animals induced Gag241-249-specific CTL responses dominantly. Remarkably, all animals in group IV showed efficient Gag206-216-specific CTL responses without detectable Gag241-249-specific CTL responses at week 2. These results indicate dominant Gag206-216-specific CTL responses with delayed induction of Gag241-249-specific CTL responses postchallenge in group IV animals with prophylactic Gag206-216-specific CTL induction, and vice versa in group III animals.
Fig 4.
Gag206-216-specific and Gag241-249-specific CTL responses after SIVmac239 challenge. CTL responses at week 2 (A), week 6 (B), and week 12 (C) are shown. In the graphs on the left, Gag206-216-specific (open boxes), Gag241-249-specific (closed boxes), and Gag367-381-specific (striped boxes) CD8+ T-cell frequencies in group IV macaques are shown. On the right, Gag206-216-specific (open circles) and Gag241-249-specific (closed circles) CD8+ T-cell frequencies in group I (black), II (green), III (blue), and IV (red) macaques are shown. The bars indicate the geometric mean of each group. Samples from macaques I-1, I-6, II-1, and II-3 at week 2; macaques I-1, I-2, I-6, and II-5 at week 6; and macaques I-1 and II-5 at week 12 were unavailable for this analysis. Statistical analyses among four groups at week 12 revealed significant differences in Gag241-249-specific CTL levels (I and III, P < 0.0001; I and II, and III and IV, P < 0.01; I and IV, II and III, and II and IV, P > 0.05 by one-way ANOVA and Tukey-Kramer's multiple-comparison test) but not in Gag206-216-specific CTL levels (P > 0.05 by one-way ANOVA).
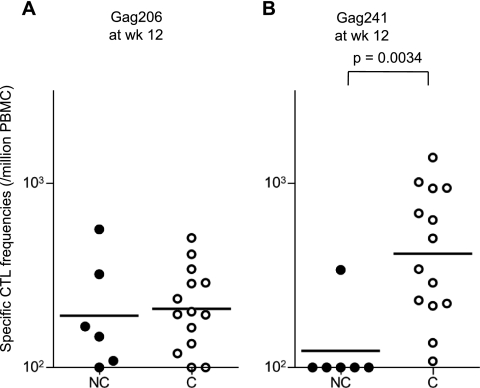
At week 6 (Fig. 4B), efficient Gag206-216-specific and Gag241-249-specific CTL responses were observed in all vaccinated animals in groups II, III, and IV, but not in group I. Gag206-216-specific and Gag241-249-specific CTL responses were induced equivalently even in groups III and IV. We also examined subdominant Gag367-381 epitope-specific CTL responses, which were undetectable at week 2 but became detectable at week 6 in most group IV animals (Fig. 4, graphs on left). At week 12 (Fig. 4C), however, different CTL immunodominance patterns were observed among the groups. Gag241-249-specific CTL levels were higher than Gag206-216-specific levels in groups II and III but were reduced in groups I and IV. Interestingly, comparison between the animals with persistent viremia (referred to as noncontrollers) and those controlling SIV replication (referred to as controllers) revealed significant differences in Gag241-249-specific CTL levels, but not in Gag206-216-specific levels, at week 12 (P = 0.0034 by Mann-Whitney test) (Fig. 5).
Fig 5.
Comparison of Gag206-216-specific or Gag241-249-specific CTL responses in noncontrollers and controllers at week 12. (A) Gag206-216-specific CD8+ T-cell frequencies in noncontrollers (NC; closed circles) and controllers (C; open circles). (B) Gag241-249-specific CD8+ T-cell frequencies in noncontrollers and controllers. Gag241-249-specific CTL levels in controllers were significantly higher than those in noncontrollers (P = 0.0034 by Mann-Whitney test). The bars indicate the geometric mean of each group. Data on a noncontroller (I-1) and a controller (II-5) were unavailable.
Selection of a CTL escape mutation.
Next, we examined viral genome gag sequences at weeks 5 and 12 after challenge to determine whether CTL escape mutations were selected in these animals (Table 2). At week 5, a mutation leading to an L-to-S substitution at the 216th residue in Gag (L216S) was selected in all the group II animals. This GagL216S change results in escape from Gag206-216-specific CTL recognition, as described previously (21). All the group IV animals with Gag206-216-specific CTL induction also showed rapid selection of this CTL escape mutation at week 5. Analysis at week 3 found the GagL216S mutation dominant in two (II-2 and II-5) group II and two (IV-1 and IV-3) group IV animals (data not shown). However, animals in group III showed no gag mutations at week 5, except for one animal (III-5) selecting a mutation leading to an L-to-F substitution at the 216th residue. Later, at week 12, the Gag206-216-specific CTL escape mutation, GagL216S, was selected even in group III animals. No animals showed mutations around the Gag241-249 epitope-coding region even at week 12. These results indicate that selection of this Gag206-216-specific CTL escape mutation may be accelerated by prophylactic vaccination inducing Gag206-216-specific CTL responses. On the other hand, in group III animals with single Gag241-249 epitope-specific CTL induction, selection of a Gag206-216-specific CTL escape mutation was delayed but was observed before selection of a Gag241-249-specific CTL escape mutation, suggesting strong selective pressure by delayed Gag206-216-specific CTL responses after SIV challenge.
Table 2.
Selection of a CTL escape mutation
| Group | Macaque ID | Amino acid change for Gag residuesb: |
|||
|---|---|---|---|---|---|
| 206–216 | 241–249 | ||||
| Wk 5 | Wk 12 | Wk 5 | Wk 12 | ||
| I | I-1 | None | ND | None | ND |
| I-2a | None | L216S | None | None | |
| I-3 | None | L216S | None | None | |
| I-4 | None | None | None | None | |
| I-5 | None | None | None | None | |
| I-6 | None | None | None | None | |
| II | II-1a | L216S | ND | None | ND |
| II-2a | L216S | ND | None | ND | |
| II-3a | L216S | ND | None | ND | |
| II-4a | L216S | ND | None | ND | |
| II-5a | L216S | ND | None | ND | |
| III | III-1a | None | L216S | None | None |
| III-2a | None | L216S | None | None | |
| III-3a | None | NA | None | NA | |
| III-4a | None | NA | None | NA | |
| III-5a | L216F | L216S | None | None | |
| III-6a | None | L216S | None | None | |
| IV | IV-1a | L216S | L216S | None | None |
| IV-2 | L216S | L216S | None | None | |
| IV-3 | L216S | L216S | None | None | |
| IV-4a | L216S | L216S | None | None | |
| IV-5a | L216S | NA | None | NA | |
Animals that controlled SIV replication at week 12 (controllers).
Plasma viral gag genome mutations were examined at weeks 5 and 12. Amino acid substitutions in Gag206-216 and Gag241-249 epitope regions are shown. L216S results in viral escape from Gag206-216-specific CTL recognition. It remains undetermined whether L216F results in CTL escape. ND, not determined; NA, not determined because Gag fragments were unable to be amplified from plasma RNA.
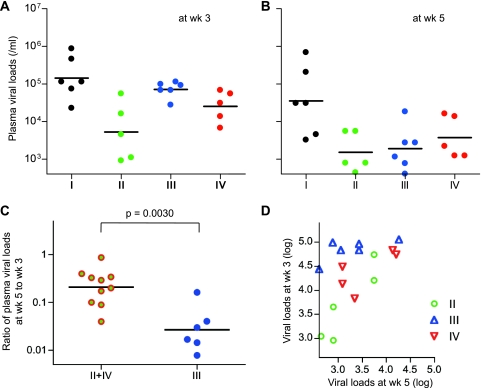
In order to see the effect of rapid selection of the Gag206-216-specific CTL escape mutation on SIV control, we compared plasma viral loads at weeks 3 and 5 between groups II and IV (referred to as group II+IV) with rapid selection of the GagL216S mutation and group III without the mutation at week 5 (Fig. 6). Ratios of plasma viral loads at week 5 to week 3 in group III were significantly lower than those in group II+IV (P = 0.0030 by Mann-Whitney test) (Fig. 6C). To confirm this result, we examined the difference in week 3 viral loads between groups III and II+IV by ANCOVA, with week 5 viral loads as a covariate. This analysis revealed that week 3 viral loads controlled for by week 5 viral loads were significantly higher in group III than those in group II+IV (Fig. 6D and Table 3); i.e., the decline in viral loads from week 3 to week 5 was significantly sharper in group III than in group II+IV, possibly reflecting viral escape from suppressive pressure by Gag206-216-specific CTL responses in the latter group during this period (from week 3 to week 5).
Fig 6.
Comparison of plasma viral loads at weeks 3 and 5 among four groups. (A) Plasma viral loads at week 3 in group I, II, III, and IV animals. (B) Plasma viral loads at week 5 in group I, II, III, and IV animals. (C) Comparison of ratios of plasma viral loads at week 5 to week 3 in group II+IV animals and group III animals. The ratios in group III were significantly lower than those in group II+IV (P = 0.0030 by Mann-Whitney test). The bars indicate the geometric mean of each group. (D) Scatter plots between plasma viral loads at weeks 3 and 5 in group II, III, and IV animals.
Table 3.
ANCOVA on week 3 viral loads with week 5 viral loads as a covariate between groups III and II+IV
| ANOVA | Parameter | SSa | dfb | MSc | F | P value |
|---|---|---|---|---|---|---|
| Homogeneity of slopes of regression | Group × slope | 0.304 | 1 | 0.304 | 2.099 | 0.173 |
| Residual | 1.735 | 12 | 0.145 | |||
| Total | 2.038 | 13 | 0.157 | |||
| Difference in week 3 viral loads with week 5 viral loads as a covariate between groups III and II+IV | Effect and group | 1.106 | 1 | 1.106 | 7.052 | 0.020 |
| Residual | 2.038 | 13 | 0.157 | |||
| Total | 3.144 | 14 | 0.225 |
SS, sum of squares.
df, degrees of freedom.
MS, mean squares.
DISCUSSION
In the present study, we analyzed the impact of vaccination inducing single Gag206-216 epitope-specific CTL memory on postchallenge CTL responses and SIV control in 90-120-Ia-positive macaques and then compared the results with those of vaccination inducing single Gag241-249 epitope-specific CTL responses. Our results indicate that these prophylactic vaccinations result in different patterns of Gag206-216-specific and Gag241-249-specific CTL immunodominance and cooperation after SIVmac239 challenge.
Unvaccinated 90-120-Ia-positive macaques (group I) showed both Gag206-216-specific and Gag241-249-specific CTL responses after SIV challenge. In group IV animals with prophylactic induction of single Gag206-216 epitope-specific CTL responses, Gag206-216-specific CTL responses were induced dominantly but Gag241-249-specific CTL responses were undetectable at week 2. In contrast, Gag241-249-specific CTL responses were induced dominantly at week 2 in group III. Both groups showed Gag206-216-specific and Gag241-249-specific CTL responses equivalently at week 6. It may be difficult to compare these results with those in group II animals inducing whole Gag antigen-specific CTL and CD4+ T-cell responses before challenge; the group II animals elicited Gag206-216-specific and Gag241-249-specific CTL responses equivalently at week 2. Our results indicate that prophylactic vaccination results in dominant induction of vaccine antigen-specific CTL responses and may delay CTL responses specific for viral antigens other than vaccine antigens (referred to as nonvaccine antigens) after viral exposure.
A significant difference between groups III and IV is the pattern of selection of CTL escape mutation. All group IV animals showed rapid selection of a Gag206-216-specific CTL escape mutation, while most group III animals showed no gag mutation at week 5 but selection of the Gag206-216-specific CTL escape mutation later, at week 12. Thus, prophylactic vaccination may affect the patterns of viral genome diversification, possibly accelerating selection of CTL escape mutations. Interestingly, Gag241-249-specific CTL mutations were not detected even at week 12 in group III animals, although a previous study observed not only the Gag206-216-specific CTL escape mutation (GagL216S), but also a Gag241-249-specific CTL escape mutation (GagD244E) in the chronic phase of SIV infection in 90-120-Ia-positive macaques (9). These results indicate that delayed, naive-derived Gag206-216-specific CTL responses, as well as preceding Gag241-249-specific CTL responses, exert strong suppressive pressure on SIV replication in group III animals, implying cooperation between vaccine antigen-specific and non-vaccine antigen-specific CTL responses for virus control.
Rapid selection of the Gag206-216-specific CTL escape mutation (GagL216S) in group II and delayed selection of this muta-tion without a detectable Gag241-249-specific CTL escape mutation (GagD244E) in group III suggest that the virus with GagL216S (SIVmac239Gag216S) replicates more efficiently than the virus with GagD244E (SIVmac239Gag244E) under both Gag206-216-specific and Gag241-249-specific CTL responses. Our previous competition assay did not find a significant difference in viral fitness between these mutant viruses. Possibly, escape of SIVmac239Gag216S from Gag206-216-specific CTL pressure may be more efficient than that of SIVmac239Gag244E from Gag241-249-specific CTL pressure.
Our analysis revealed that the decline of plasma viral loads from week 3 to week 5 in group II+IV with rapid selection of the GagL216S mutation was significantly less than that in group III without the mutation at week 5, possibly reflecting viral escape from suppressive pressure by Gag206-216-specific CTL responses in the former groups around weeks 3 to 5. Even the comparison between groups II and III, both showing dominant Gag241-249-specific CTL responses at week 2, revealed a significantly sharper decline in the latter (P = 0.0087). Thus, our results suggest three patterns of Gag206-216-specific and Gag241-249-specific CTL cooperation for virus control after SIVmac239 challenge. First, as observed in group II, dominantly induced Gag206-216-specific and Gag241-249-specific CTL responses both work against wild-type SIV replication around week 2, but then a mutant virus escaping from the former CTL responses is selected, and the responses work against this mutant virus replication. Second, as observed in group III, dominantly induced Gag241-249-specific CTL responses work against wild-type SIV replication around week 2 and then contribute to virus control, together with delayed, naive-derived Gag206-216-specific CTL responses. Third, as observed in group IV, dominantly induced Gag206-216-specific CTL responses work against wild-type SIV replication around week 2, but then a mutant virus escaping from Gag206-216-specific CTL responses is selected, and delayed, naive-derived Gag241-249-specific CTL responses instead work against this mutant virus replication. Viral loads at week 3 in group III looked higher than those in group IV, implying that Gag206-216-specific CTL responses may exert a stronger suppressive effect on SIV replication in the acute phase than Gag241-249-specific CTL responses. However, viral loads at week 5 in group III looked lower than those in group IV, and the comparison between the two groups showed significantly less decline in the latter (P = 0.0303). It is speculated that the third pattern observed in group IV is prone to failure in virus control. Indeed, two of five animals in group IV failed to control SIV replication. Even if vaccines are designed to express multiple antigens, of the vaccine-induced CTLs generated, only several epitope-specific cells may recognize the incoming HIV because of viral diversity and host MHC polymorphisms (18), and cooperation of these vaccine antigen-specific and non-vaccine antigen-specific CTL responses would be required for viral control. Thus, our results may imply a rationale of inducing escape-resistant, epitope-specific CTL memory by prophylactic AIDS vaccines.
In summary, this study showed dominant induction of vaccine antigen-specific CTL responses and delay in non-vaccine antigen-specific CTL responses in the acute phase of SIV infection, clearly describing the impact of prophylactic vaccination on CTL immunodominance and cooperation after virus exposure. Our results indicate that the patterns of cooperation of vaccine antigen-specific and non-vaccine antigen-specific CTL responses affect virus control and selection of CTL escape mutations. These findings provide great insights into antigen design in the development of a CTL-inducing AIDS vaccine.
ACKNOWLEDGMENTS
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology; grants-in-aid from the Ministry of Health, Labor, and Welfare; and a grant from the Takeda Science Foundation, Japan.
We thank F. Ono, K. Oto, K. Komatsuzaki, M. Hamano, Y. Emoto, H. Akari, and Y. Yasutomi for their assistance in animal experiments.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Altfeld M, et al. 2003. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 17:2581–2591 [DOI] [PubMed] [Google Scholar]
- 2. Berger CT, et al. 2011. High functional avidity CTL responses to HLA-B-restricted Gag-derived epitopes associate with relative HIV control. J. Virol. 85:9334–9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. 1994. Virus-specific CD8 cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103–6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goulder PJ, Watkins DI. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4:630–640 [DOI] [PubMed] [Google Scholar]
- 5. Goulder PJ, Watkins DI. 2008. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansen SG, et al. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Julg B, et al. 2010. Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. J. Virol. 84:5540–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaslow RA, et al. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405–411 [DOI] [PubMed] [Google Scholar]
- 9. Kawada M, et al. 2006. Involvement of multiple epitope-specific cytotoxic T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J. Virol. 80:1949–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawada M, et al. 2008. Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J. Virol. 82:10199–10206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kestler HW, III, et al. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651–662 [DOI] [PubMed] [Google Scholar]
- 12. Kiepiela P, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 [DOI] [PubMed] [Google Scholar]
- 13. Koup RA, et al. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leslie A, et al. 2010. Additive contribution of HLA class I alleles in the immune control of HIV-1 infection. J. Virol. 84:9879–9888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Letvin NL, et al. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li F, et al. 2011. Mapping HIV-1 vaccine induced T-cell responses: bias towards less-conserved regions and potential impact on vaccine efficacy in the Step study. PLoS One 6:e20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, et al. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, McNevin JP, Holte S, McElrath MJ, Mullins JI. 2011. Dynamics of viral evolution and CTL responses in HIV-1 infection. PLoS One 6:e15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loffredo JT, et al. 2008. Patterns of CD8 immunodominance may influence the ability of Mamu-B*08-positive macaques to naturally control simian immunodeficiency virus SIVmac239 replication. J. Virol. 82:1723–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matano T, Kano M, Nakamura H, Takeda A, Nagai Y. 2001. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA prime/Sendai virus vector boost regimen. J. Virol. 75:11891–11896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matano T, et al. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 199:1709–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matano T, et al. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Migueles SA, et al. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 97:2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miura T, et al. 2009. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. J. Virol. 83:2743–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mothé BR, et al. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77:2736–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naruse TK, et al. 2010. Diversity of MHC class I genes in Burmese-origin rhesus macaques. Immunogenetics 62:601–611 [DOI] [PubMed] [Google Scholar]
- 27. Ndhlovu ZM, et al. 2011. Mosaic HIV-1 Gag antigens can be processed and presented to human HIV-specific CD8+ T cells. J. Immunol. 186:6914–6924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmitz JE, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860 [DOI] [PubMed] [Google Scholar]
- 29. Shibata R, et al. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362–373 [DOI] [PubMed] [Google Scholar]
- 30. Streeck H, et al. 2009. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J. Virol. 83:7641–7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang J, et al. 2002. Favorable and unfavorable HLA class I alleles and haplotypes in Zambians predominantly infected with clade C human immunodeficiency virus type 1. J. Virol. 76:8276–8284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsukamoto T, et al. 2009. Impact of cytotoxic-T-lymphocyte memory induction without virus-specific CD4+ T-cell help on control of a simian immunodeficiency virus challenge in rhesus macaques. J. Virol. 83:9339–9346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turnbull EL, et al. 2009. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J. Immunol. 182:7131–7145 [DOI] [PubMed] [Google Scholar]
- 34. Wang YE, et al. 2009. Protective HLA class I alleles that restrict acute-phase CD8+ T-cell responses are associated with viral escape mutations located in highly conserved regions of human immunodeficiency virus type 1. J. Virol. 83:1845–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilson NA, et al. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80:5875–5885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yant LJ, et al. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80:5074–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]