Abstract
Virion uncoating is an essential early event in reovirus infection. In natural enteric infections, rapid proteolytic uncoating of virions is mediated by pancreatic serine proteases. The proteases that promote reovirus disassembly and cell entry in the respiratory tract remain unknown. In this report, we show that endogenous respiratory and inflammatory proteases can promote reovirus infection in vitro and that preexisting inflammation augments in vivo infection in the murine respiratory tract.
TEXT
Mammalian orthoreovirus (reovirus) entry into cells is characterized by the stepwise disassembly of virions into two functional subvirion particles, a membrane penetration-competent infectious subvirion particle (ISVP) and a transcriptionally active core (19). In cell culture, entry is initiated by attachment of viral protein σ1 (44) to a cell surface carbohydrate (6, 20, 32) and junctional adhesion molecule A (7). Virions are then internalized by β1 integrin-mediated endocytosis (15, 29, 47, 48). Within the endocytic compartment, host proteases remove the outer capsid protein σ3, exposing μ1, the membrane penetration protein (4, 25, 58). In murine fibroblasts, virion disassembly is mediated by the acid-dependent cysteine proteases, cathepsins (Cats) L and B (4, 5, 28). In immune cells, acid-independent proteases, including Cat S and neutrophil elastase, can promote virion uncoating (34, 36).
After oral inoculation of newborn mice, reovirus virions are rapidly uncoated in the enteric tract by the secreted pancreatic serine proteases chymotrypsin (CHT) and trypsin (8, 13). Extracellular uncoating is required for reovirus adherence to Peyer's patch M cells and subsequent entry into intestinal tissue (3). In humans and animals, reoviruses are also associated with pulmonary infections (10, 22, 46, 51), but little is known about the proteases that mediate virion uncoating when infection is initiated by this route. In this study, we investigated the role of inflammatory and endogenous respiratory proteases in reovirus infection.
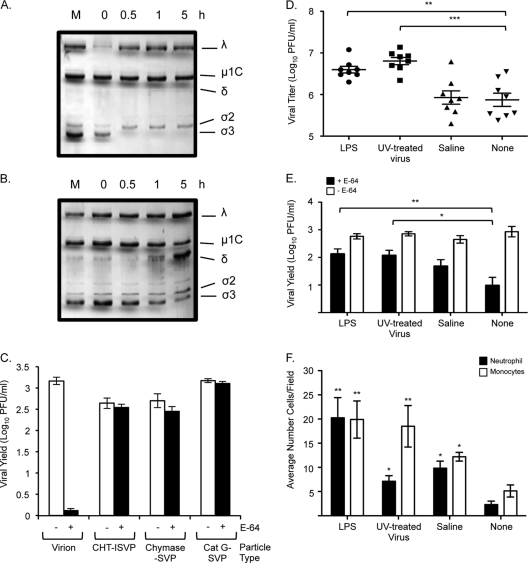
Type II transmembrane serine proteases (TTSPs) expressed in the human airway have been shown to activate membrane fusion and promote cell entry of a number of respiratory pathogens, including influenza virus, human metapneumovirus, and severe acute respiratory syndrome (SARS) coronavirus (11, 12, 16, 17, 18, 33, 55–57). To assess the capacity of a respiratory TTSP to promote reovirus uncoating, we incubated purified reovirus type 1 Lang virions with human airway trypsin-like protease (HAT) and analyzed the products by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1A). After 6 h of treatment, only 2% of the σ3 outer capsid protein remained associated with virions, as determined by NIH Image J quantitation. The particles resembled ISVPs generated by pancreatic serine proteases (14), although, unlike treatment with CHT, HAT treatment did not lead to significant cleavage of the underlying capsid protein μ1C. To assess the infectivity of the HAT-treated subviral particles (HAT-SVPs) and determine whether they require additional intracellular proteolysis, we measured viral yields in cultures of L929 mouse fibroblasts that were left untreated or treated with the broad-spectrum cysteine protease inhibitor E-64 for 3 h prior to infection. E-64 inhibits Cats L and B, which mediate virion disassembly in L929 cells. As expected, E-64 treatment inhibited virion infection in L929 cells, whereas CHT-generated ISVPs (CHT-ISVPs), which lack the σ3 capsid protein, replicated to high yields (Fig. 1B). Like CHT-ISVPs, HAT-generated subviral particles replicated to high yields in the presence and absence of the protease inhibitor. These results indicate that the HAT respiratory protease can productively uncoat reovirus virions.
Fig 1.
Effect of type II transmembrane serine proteases on reovirus virions and infectivity. (A) Reovirus strain Lang virions (5 × 1010) in virion dialysis buffer (150 mM NaCl, 10 mM MgCl2, 10 mM Tris [pH 7.5]) were incubated with purified HAT (R&D Systems) (21 μg/ml) for the indicated times. Reactions were stopped with 5 mM benzamidine. The mock sample (M) consisted of virions held in reaction buffer in the absence of protease for 23 h. Treated particles were analyzed using SDS–12% polyacrylamide gels, and proteins were visualized by Coomassie staining. The positions of reovirus capsid proteins are labeled. (B) L929 cells were left untreated (white bars) or pretreated for 3 h with 300 μM E-64 (black bars) and infected with Lang virions, CHT-ISVPs, or HAT-SVPs at a multiplicity of infection (MOI) of 5 PFU/cell. Infections were carried out in the presence or absence of E-64 and terminated at 1 day postinfection. Viral yields were determined by plaque assay on L929 cells. Values represent the means (± standard errors [SE]) of the results of experiments performed with triplicate samples. (C) Vero cell cultures were transfected with HAT, TMPRSS2, or HSV ICP22 expression plasmids 30 h prior to infection with reovirus strain Lang virions or CHT-ISVPs (MOI, 10 PFU/cell). At 18 h postinfection, cells were fixed and permeabilized with 4% paraformaldehyde. Indirect immunofluorescence was used to detect expression of the reovirus nonstructural protein σNS and the presence of the FLAG-tagged protease or control protein. Cells were counterstained with Hoechst stain to facilitate quantification. Reovirus-infected and transfected cells were quantified by counting fluorescent cells in a minimum of three fields in each of 3 independent experiments. Between 85 and 150 cells were counted per field. Data are presented as the percentage of cells expressing σNS relative to the number of FLAG-positive cells in the field. Error bars indicate SEM. *, P < 0.05 (Mann-Whitney test).
We used an additional approach to test whether HAT and a second TTSP, TMPRSS2, could promote reovirus infection. Vero cells, which are permissive for reovirus infection but do not support efficient virion disassembly (35), were transfected with FLAG-tagged protease expression plasmids (16) or a similarly tagged control plasmid (9). At 30 h after transfection, the cultures were infected with reovirus virions. At 18 h postinfection, cells were fixed and analyzed for evidence of viral replication and expression of the FLAG antigen (Fig. 1C). In the transfected cultures, expression of either HAT or TMPRSS2 led to a significant increase in the number of reovirus-infected cells, whereas expression of FLAG-tagged herpes simplex virus protein ICP22 from the control plasmid did not. Levels of infection in protease gene-transfected cultures were comparable to those in mock-transfected cultures that had been infected with CHT-ISVPs (Fig. 1C). When we compared infection by CHT-ISVPs in cultures expressing TTSPs and in control-transfected cultures, we found no significant difference in σNS expression in these samples (data not shown). Together, our results reveal that endogenous respiratory TTSPs are capable of productively uncoating reovirus virions in vitro and of promoting infection in cell culture.
In vivo, HAT and TMPRSS2 are expressed on the membrane of bronchiolar epithelial cells (12, 21, 27, 49, 59) and have murine homologues, namely, MAT and epitheliasin (37, 38). However, the activity of these and other respiratory proteases is balanced by the presence of locally produced protease inhibitors (31). To assess the efficiency of reovirus uncoating in the respiratory tract, we used a murine respiratory model (46) to compare viral loads after infection with virions or ISVPs. Four-week-old CBA/J mice were inoculated intranasally (10, 51) with type 1 Lang virions or ISVPs. Organs were harvested at various times postinoculation, and viral titers were determined by plaque assay on L929 cells. We observed equivalent lung titers at early times (12 h) following infection with virions or ISVPs (Fig. 2A), likely reflecting the inoculum. However, by 24 h postinoculation (hpi), we recovered significantly higher titers in the lungs of ISVP-infected animals. Titers from the lungs of virion-infected animals increased more slowly. Peak lung titers in ISVP-infected animals were over 1 log greater than peak lung titers in virion-infected animals. Immunohistochemistry of lung samples taken at 24 hpi from both virion- and ISVP-infected animals revealed type 1 alveolar pneumocytes and monocytes expressing the μNS reovirus nonstructural protein (evidence of viral replication), although we observed many more μNS-positive cells in the lungs of ISVP-infected animals (Fig. 2B). These data suggest that, while endogenous respiratory proteases can productively uncoat reovirus virions in vitro, they do not do so efficiently in the murine respiratory tract.
Fig 2.
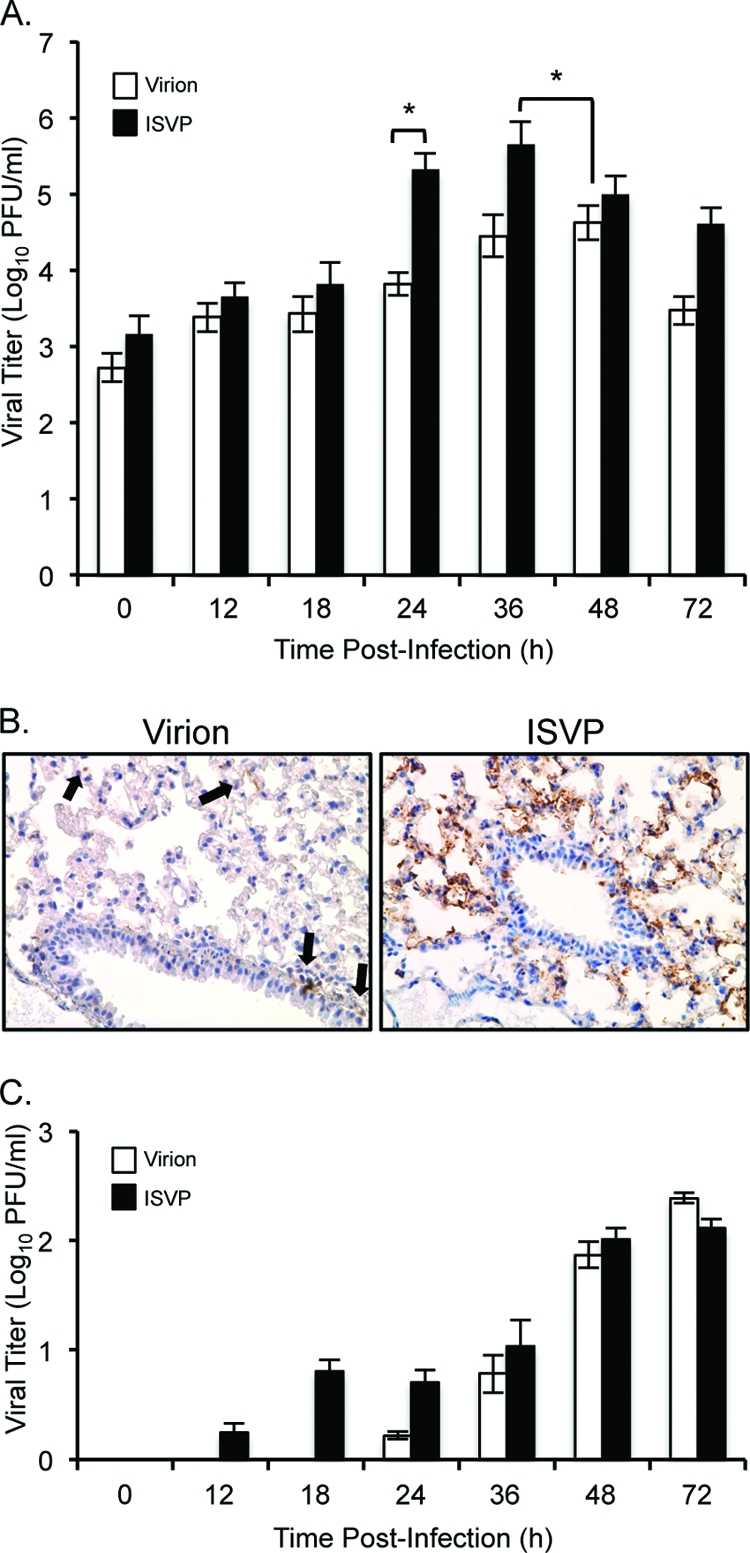
Comparative analysis of reovirus infection in the respiratory tract after intranasal inoculation with virions or ISVPs. CBA/J mice (4 weeks old) were inoculated intranasally with 1 × 107 PFU Lang virions or CHT-ISVPs. Organs were harvested at the indicated times postinoculation, and viral titers in the lungs (A) and spleen (C) were determined by plaque assay on L929 cells. Results are expressed as the means of viral titers for 6 to 12 mice per time point. Error bars indicate standard errors of the means (SEM). *, P < 0.05 (as determined by the Mann Whitney test). (B) At 24 hpi, lungs were isolated, sectioned, and stained with antiserum directed against the reovirus μNS nonstructural protein. Viral antigen was detected using a horseradish peroxidase-linked secondary antibody and diaminobenzidine, and slides were counterstained with hematoxylin. Representative sections are shown, and arrows point to some antigen-positive cells. Specificity of the staining was determined from control slides, which included infected lung sections incubated with preimmune serum and uninfected lung sections incubated with μNS-specific antiserum (data not shown).
To determine how the kinetics of replication in the respiratory tract impact dissemination, we analyzed viral titers in spleen tissue after intranasal infection with virions or ISVPs (Fig. 2C). Virus was detected as early as 12 hpi in spleen samples from the ISVP-infected animals. At 24 hpi, virion-infected animals began to show evidence of viral spread; by 36 hpi, there was no significant difference between the samples. We considered the possibility that early spread in the ISVP-infected animals reflected quicker drainage of the inoculum, but the virus recovered in the spleen samples was E-64 sensitive, arguing that it represented progeny virions (data not shown). Because mice infected intranasally can swallow some of the inoculum, we measured virus in the intestines at early time points. Recovered titers ranged broadly between animals (data not shown). While this virus might have contributed to the observed spread in our intranasally infected animals, Kauffman and colleagues detected less than half a log of spread to the spleen 24 h after direct intragastric inoculation of 108 Lang virions into mice that were similar in age (40). Together, these data suggest that efficient replication of ISVPs in the lung leads to quicker dissemination.
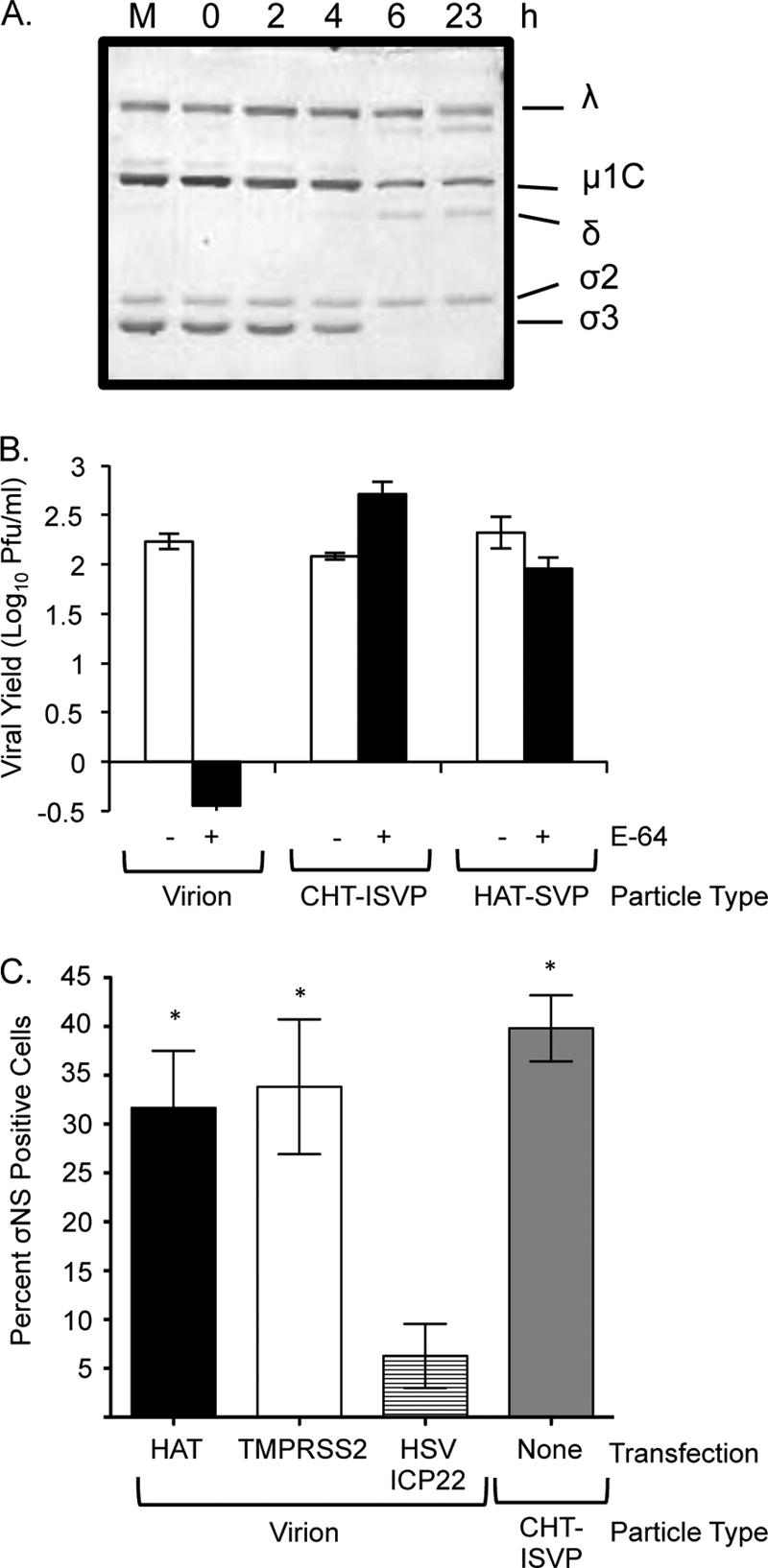
While studies in the murine enteric tract have shown that reovirus virions are converted to ISVPs within minutes after oral inoculation (13), our data suggest that the endogenous respiratory proteases in the lung do not rapidly convert virions to ISVPs. However, the balance of proteases and protease inhibitors in the respiratory tract can be impacted by inflammation (2, 24, 50, 52, 53, 61). Inflammatory cells, recruited to sites of infection or pathology, release a variety of microbicidal products (23, 26, 45). Serine proteases are expressed as components of this response (31), and we have previously shown that one such protease, neutrophil elastase, can promote reovirus infection in cell culture (36). Using the in vitro uncoating assay, we investigated the capacity of two other inflammatory proteases, Cat G (expressed by neutrophils) and mast cell chymase, to uncoat reovirus virions. We found that chymase mediated rapid virion disassembly, with almost all σ3 disappearing after 0.5 h (Fig. 3A). The kinetics of σ3 removal from virions treated with Cat G (Fig. 3B) was not as rapid, but by 5 h, particles had lost 70% of σ3, and the underlying μ1C protein had been cleaved to the characteristic δ fragment. When we used the chymase- and Cat G-SVPs in single-cycle growth experiments in E-64-treated L929 cells, we found that they were infectious in the absence of cysteine protease activity (Fig. 3C). The finding that HAT- and chymase-SVPs with uncleaved μ1C replicate to high yields in E-64-treated cells supports other published work suggesting that cleavage of μ1C to yield the δ and φ fragments is not essential for reovirus infection in cell culture (19).
Fig 3.
Analysis of the effects of inflammatory proteases and inflammation on reovirus virions and pathogenesis in the murine respiratory model. (A, B, and C) Reovirus strain Lang virions (5 × 1010) were incubated with purified chymase (Calbiochem) (50 μg/ml) (A) or purified Cat G (Calbiochem) (25 μg/ml) (B) in virion dialysis buffer for the indicated times. Reactions were stopped with 5 mM benzamidine. The mock sample (M) consisted of virions held in reaction buffer in the absence of protease for 5 h. Treated particles were analyzed using SDS–12% polyacrylamide gels, and proteins were visualized by Coomassie staining. The positions of reovirus capsid proteins are labeled. (C) L929 cells were left untreated (white bars) or pretreated for 3 h with 300 μM E-64 (black bars) and infected with Lang virions, CHT-ISVPs, Cat G-SVPs, or chymase-SVPs at an MOI of 5 PFU/cell. Infections were carried out in the presence or absence of E-64 and terminated at 1 day postinfection. Viral yields were determined by plaque assay on L929 cells. Values represent the means (± SE) of the results of experiments performed with triplicate samples. (D) Four-week-old CBA/J mice were left untreated or treated intranasally with 1.5 μg of LPS, 1.7 × 109 UV-inactivated T3D virions, or saline solution 24 h prior to intranasal infection with 1 × 107 PFU of reovirus strain Lang virions. At day 1 postinfection, lungs were harvested and viral titers determined by plaque assay on L929 cells. (E and F) Four-week-old CBA/J mice were left untreated or treated and infected as described for panel D. At 8 hpi, BALF was harvested from infected mice. (E) Virus was released from half of the BALF sample by three cycles of freezing and thawing. The presence of uncoated particles and the relative levels of virus were assessed by using the BALF in single-cycle growth experiments. L929 cells were left untreated or pretreated with E-64 for 3 h and then infected with 100 μl of BALF. Viral yields were determined by plaque assay on L929 cells 1 day postinfection. (F) Neutrophils and monocytes in the BALF were quantified by Cytospin analysis and hematoxylin and eosin staining. Error bars indicate SEM. *, P < 0.05; **, P < 0.01 (as determined by the Mann Whitney test).
Since our results demonstrated that the inflammatory proteases elastase (36), Cat G, and chymase mediated productive reovirus uncoating, we hypothesized that lung injury or induction of inflammation would potentiate reovirus infection in the lungs by promoting virion uncoating. To test this, we pretreated mice intranasally with lipopolysaccharide (LPS) or UV-inactivated reovirus type 3 Dearing for 24 h to induce inflammation (30, 42, 43, 54). Treated and control mice were then inoculated with type 1 Lang reovirus virions, and virus was quantified from lung tissue 24 h after infection. We recovered significantly more virus at 24 hpi from animals pretreated with UV-inactivated virus and LPS than we did from untreated animals (Fig. 3D). To determine whether preexisting inflammation promoted extracellular conversion of virions to ISVPs, we harvested bronchoalveolar lavage fluid (BALF) samples from untreated or pretreated and infected animals and used these as inocula in single-cycle-yield experiments in E64-treated and untreated L929 cells. In mice that had been pretreated with LPS or UV-inactivated virus, the BALF samples contained significantly more E-64-resistant (uncoated) virus (Fig. 3E), consistent with our hypothesis. To determine the extent to which the pretreatments caused infiltration of inflammatory cells that might also serve as target cells, we quantified the influx of neutrophils and monocytes by the use of Cytospin preparations of the BALF collected 8 h after treatment (Fig. 3F). Pretreatment with LPS resulted in the greatest increase in neutrophil numbers; significant numbers of monocytes were recruited to the lungs after treatment with either LPS or UV-inactivated virus. It was notable that the control treatment (saline solution) also resulted in an influx of inflammatory cells relative to untreated animals; however, levels were significantly lower than in mice pretreated with LPS or UV-treated virus, and they did not correlate with a significant increase in ISVPs in the BALF. Together, our findings reveal that preexisting inflammation in the lungs promotes reovirus uncoating in the respiratory lumen and results in the infiltration of potential target cells. These factors likely contribute to the increases in viral load in mice with preexisting inflammation.
In this report, we have shown that TTSPs can uncoat reovirus virions and promote infection in cell culture. Whether reovirus uncoating in HAT- or TMPRSS2-transfected cells occurs extracellularly, at the cell membrane, or intracellularly remains unknown. Böttcher-Friebertshäuser et al. demonstrated that influenza HA cleavage occurs at the cell membrane in HAT-expressing MDCK cells and in intracellular compartments in TMPRSS2-expressing cells (17). Interestingly, TMPRSS2 overexpression has been associated with prostate cancer (60). Since protease-mediated uncoating is a major determinant of reovirus oncolysis (1), our results suggest that reovirus might selectively target prostate tumors that overexpress and secrete TMPRSS2.
In enteric reovirus infections, pancreatic proteases in the intestinal lumen rapidly convert virions to ISVPs (8). In contrast, in healthy lungs, there are few active proteases in the extracellular environment of the respiratory tract and this may impact reovirus infection. Consistent with this, we found that inoculation with uncoated particles resulted in quicker viral replication in the lungs and spread from the respiratory tract. We explored the capacity of inflammatory serine proteases to promote infection and found that, in addition to neutrophil elastase (36), Cat G and chymase were capable of mediating productive reovirus disassembly in vitro.
To probe the role of specific proteases in vivo, we attempted to inhibit protease activity in the respiratory tract by prior intranasal inoculation of protease inhibitors. These preliminary studies suggested that the pretreatment protocol itself induced some gross inflammation and slightly increased viral titers from the lung (data not shown). When we modified the model of respiratory infection to specifically induce inflammation prior to reovirus infection (by inoculation with LPS or UV-treated virus), we recovered a higher percentage of ISVPs in the BALF, as well as a significant infiltration of monocytes. These animals also had significantly higher viral yields in the lungs. In contrast, prior inflammation did not promote infection when animals were inoculated with ISVPs (data not shown). These results are consistent with a model in which inflammation promotes reovirus infection through multiple mechanisms, including increased access to target cells and the presence of inflammatory proteases. Our findings may have broader implications for chronic pulmonary disease. Inflammation induced under a variety of chronic conditions, including asthma, emphysema, chronic obstructive pulmonary disease, and acute respiratory distress syndrome (39, 41), may further compromise human health by providing an environment that is conducive to viral infection. Further work is needed to address the possibility that other respiratory viruses (including influenza virus and SARS coronavirus) can also be activated by inflammatory proteases.
ACKNOWLEDGMENTS
We thank Linse Lahti, Megan Ahl Fischer, Jesse Bahe, and Lindsey Foran for excellent technical support and Stephen Schmechel for analysis of histopathology. We also thank Max Nibert and Terry Dermody for their generous gifts of μNS and σNS antiserum. We are also grateful to Mikhail Matrosovich for providing the HAT and TMPRSS2 plasmids and Stephen Rice for providing the pcDNA22 plasmid. Stephen Rice, Linse Lahti, and Wade Schulz provided helpful feedback on the manuscript.
This work was supported by Public Health Service grant AI-045990 from the National Institutes of Health.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Alain T, et al. 2007. Proteolytic disassembly is a critical determinant for reovirus oncolysis. Mol. Ther. 15:1512–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Altiok O, et al. 2006. Imbalance between cysteine proteases and inhibitors in a baboon model of bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 173:318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amerongen HM, Wilson GA, Fields BN, Neutra MR. 1994. Proteolytic processing of reovirus is required for adherence to intestinal M cells. J. Virol. 68:8428–8432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baer GS, Dermody TS. 1997. Mutations in reovirus outer-capsid protein sigma3 selected during persistent infections of L cells confer resistance to protease inhibitor E64. J. Virol. 71:4921–4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baer GS, Ebert DH, Chung CJ, Erickson AH, Dermody TS. 1999. Mutant cells selected during persistent reovirus infection do not express mature cathepsin L and do not support reovirus disassembly. J. Virol. 73:9532–9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barton ES, Connolly JL, Forrest JC, Chappell JD, Dermody TS. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200–2211 [DOI] [PubMed] [Google Scholar]
- 7. Barton ES, et al. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441–451 [DOI] [PubMed] [Google Scholar]
- 8. Bass DM, et al. 1990. Intraluminal proteolytic activation plays an important role in replication of type 1 reovirus in the intestines of neonatal mice. J. Virol. 64:1830–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bastian TW, Rice SA. 2009. Identification of sequences in herpes simplex virus type 1 ICP22 that influence RNA polymerase II modification and viral late gene expression. J. Virol. 83:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellum SC, et al. 1997. Respiratory reovirus 1/L induction of intraluminal fibrosis: a model for the study of bronchiolitis obliterans organizing pneumonia. Am. J. Pathol. 150:2243–2254 [PMC free article] [PubMed] [Google Scholar]
- 11. Bertram S, et al. 2010. TMPRSS2 and TMPRSS4 facilitate trypsin-independent influenza virus spread in Caco-2 cells. J. Virol. 84:10016–10025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bertram S, et al. 12 October 2011. Cleavage and activation of the SARS-coronavirus spike-protein by human airway trypsin-like protease (HAT). J. Virol. doi:10.1128/JVI.05300-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bodkin DK, Nibert ML, Fields BN. 1989. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J. Virol. 63:4676–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borsa J, Copps TP, Sargent MD, Long DG, Chapman JD. 1973. New intermediate subviral particles in the in vitro uncoating of reovirus virions by chymotrypsin. J. Virol. 11:552–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borsa J, et al. 1979. Two modes of entry of reovirus particles into L cells. J. Gen. Virol. 45:161–170 [DOI] [PubMed] [Google Scholar]
- 16. Böttcher E, et al. 2006. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 80:9896–9898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Böttcher-Friebertshäuser E, et al. 2010. Cleavage of influenza virus hemagglutinin by airway proteases TMPRSS2 and HAT differs in subcellular localization and susceptibility to protease inhibitors. J. Virol. 84:5605–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaipan C, et al. 2009. Proteolytic activation of the 1918 influenza virus hemagglutinin. J. Virol. 83:3200–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chandran K, Parker JSL, Ehrlich M, Kirchhausen T, Nibert ML. 2003. The δ region of outer-capsid protein μ1 undergoes conformational change and release from reovirus particles during cell entry. J. Virol. 77:13361–13375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chappell JD, Duong JL, Wright BW, Dermody TS. 2000. Identification of carbohydrate-binding domains in the attachment proteins of type 1 and type 3 reoviruses. J. Virol. 74:8472–8479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chokki M, et al. 2004. Human airway trypsin-like protease increases mucin gene expression in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 30:470–478 [DOI] [PubMed] [Google Scholar]
- 22. Chua KB, et al. 2007. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc. Natl. Acad. Sci. U. S. A. 104:11424–11429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crosby LM, Waters CM. 2010. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 298:715–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davies PL, et al. 2010. Relationship of proteinases and proteinase inhibitors with microbial presence in chronic lung disease of prematurity. Thorax 65:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dermody TS, Nibert ML, Wetzel JD, Tong X, Fields BN. 1993. Cells and viruses with mutations affecting viral entry are selected during persistent infections of L cells with mammalian reoviruses. J. Virol. 67:2055–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doerschuk CM. 2000. Leukocyte trafficking in alveoli and airway passages. Respir. Res. 1:136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Donaldson SH, et al. 2002. Regulation of the epithelial sodium channel by serine proteases in human airways. J. Biol. Chem. 277:8338–8345 [DOI] [PubMed] [Google Scholar]
- 28. Ebert DH, Deussing J, Peters C, Dermody TS. 2002. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277:24609–24617 [DOI] [PubMed] [Google Scholar]
- 29. Ehrlich M, et al. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118:591–605 [DOI] [PubMed] [Google Scholar]
- 30. Farone AL, et al. 2006. Reovirus strain-dependent inflammatory cytokine responses and replication patterns in a human monocyte cell line. Viral Immunol. 19:546–557 [DOI] [PubMed] [Google Scholar]
- 31. Garcia-Verdugo I, Descamps D, Chignard M, Touqui L, Sallenave J-M. 2010. Lung protease/anti-protease network and modulation of mucus production and surfactant activity. Biochimie 92:1608–1617 [DOI] [PubMed] [Google Scholar]
- 32. Gentsch JR, Pacitti AF. 1985. Effect of neuraminidase treatment of cells and effect of soluble glycoproteins on type 3 reovirus attachment to murine L cells. J. Virol. 56:356–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glowacka I, et al. 2011. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 85:4122–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Golden JW, Bahe JA, Lucas WT, Nibert ML, Schiff LA. 2004. Cathepsin S supports acid-independent infection by some reoviruses. J. Biol. Chem. 279:8547–8557 [DOI] [PubMed] [Google Scholar]
- 35. Golden JW, Linke J, Schmechel S, Thoemke K, Schiff LA. 2002. Addition of exogenous protease facilitates reovirus infection in many restrictive cells. J. Virol. 76:7430–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Golden JW, Schiff LA. 2005. Neutrophil elastase, an acid-independent serine protease, facilitates reovirus uncoating and infection in U937 promonocyte cells. Virol. J. 2:48 http://www.virologyj.com/content/2/1/48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hansen IA, et al. 2004. The adrenal secretory serine protease AsP is a short secretory isoform of the transmembrane airway trypsin-like protease. Endocrinology 145:1898–1905 [DOI] [PubMed] [Google Scholar]
- 38. Jacquinet ER, Rao NVGV, Hoidal JR. 2000. Cloning, genomic organization, chromosomal assignment and expression of a novel mosaic serine proteinase: epitheliasin. FEBS Lett. 468:93–100 [DOI] [PubMed] [Google Scholar]
- 39. Jeffery PK. 2001. Remodeling in asthma and chronic obstructive lung disease. Am. J. Respir. Crit. Care Med. 164(Pt. 2):S28–S38 [DOI] [PubMed] [Google Scholar]
- 40. Kauffman RS, Wolf JL, Finberg R, Trier JS, Fields BN. 1983. The sigma 1 protein determines the extent of spread of reovirus from the gastrointestinal tract of mice. Virology 124:403–410 [DOI] [PubMed] [Google Scholar]
- 41. Kim EY, Battaile JT, Patel AC, You Y, Agapov E. 2008. Persistent activation of an innate immune axis translates respiratory viral infection into chronic lung disease. Nat. Med. 14:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kinniry P, et al. 2006. KL4-surfactant prevents hyperoxic and LPS-induced lung injury in mice. Pediatr. Pulmonol. 41:916–928 [DOI] [PubMed] [Google Scholar]
- 43. Knapp S, Florquin S, Golenbock DT, van der Poll T. 2006. Pulmonary lipopolysaccharide (LPS)-binding protein inhibits the LPS-induced lung inflammation in vivo. J. Immunol. 176:3189–3195 [DOI] [PubMed] [Google Scholar]
- 44. Lee PW, Hayes EC, Joklik WK. 1981. Protein sigma 1 is the reovirus cell attachment protein. Virology 108:156–163 [DOI] [PubMed] [Google Scholar]
- 45. Ley K. 2004. Weird and weirder: how circulating chemokines coax neutrophils to the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L463–L464 [DOI] [PubMed] [Google Scholar]
- 46. London L, Majeski EI, Paintlia MK, Harley RA, London SD. 2002. Respiratory reovirus 1/L induction of diffuse alveolar damage: a model of acute respiratory distress syndrome. Exp. Mol. Pathol. 72:24–36 [DOI] [PubMed] [Google Scholar]
- 47. Maginnis MS, et al. 2006. Beta1 integrin mediates internalization of mammalian reovirus. J. Virol. 80:2760–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maginnis MS, et al. 2008. NPXY motifs in the beta1 integrin cytoplasmic tail are required for functional reovirus entry. J. Virol. 82:3181–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matsushima R, et al. 2006. Human airway trypsin-like protease stimulates human bronchial fibroblast proliferation in a protease-activated receptor-2-dependent pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L385–L395 [DOI] [PubMed] [Google Scholar]
- 50. Moraes TJ, Chow CW, Downey GP. 2003. Proteases and lung injury. Crit. Care Med. 31:S189–S194 [DOI] [PubMed] [Google Scholar]
- 51. Morin MJ, Warner A, Fields BN. 1996. Reovirus infection in rat lungs as a model to study the pathogenesis of viral pneumonia. J. Virol. 70:541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parks WC, Shapiro SD. 2001. Matrix metalloproteinases in lung biology. Respir. Res. 2:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reid PT, Sallenave J-M. 2001. Neutrophil-derived elastases and their inhibitors: potential role in the pathogenesis of lung disease. Curr. Opin. Investig. Drugs 2:59–67 [PubMed] [Google Scholar]
- 54. Sajjan U, et al. 2009. Elastase- and LPS-exposed mice display altered responses to rhinovirus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 297:L931–L944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shirogane Y, et al. 2008. Efficient multiplication of human metapneumovirus in Vero cells expressing the transmembrane serine protease TMPRSS2. J. Virol. 82:8942–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shulla A, et al. 2011. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 85:873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simmons G, et al. 2011. Different host cell proteases activate the SARS-coronavirus spike-protein for cell-cell and virus-cell fusion. Virology 413:265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sturzenbecker LJ, Nibert ML, Furlong D, Fields BN. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 61:2351–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takahashi M, et al. 2001. Localization of human airway trypsin-like protease in the airway: an immunohistochemical study. Histochem. Cell Biol. 115:181–187 [DOI] [PubMed] [Google Scholar]
- 60. Tomlins SA, et al. 2005. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648 [DOI] [PubMed] [Google Scholar]
- 61. Wolters PJ, Chapman HA. 2000. Importance of lysosomal cysteine proteases in lung disease. Respir. Res. 1:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]