Abstract
The genetic polymorphism that has the greatest impact on immune control of human immunodeficiency virus (HIV) infection is expression of HLA-B*57. Understanding of the mechanism for this strong effect remains incomplete. HLA-B*57 alleles and the closely related HLA-B*5801 are often grouped together because of their similar peptide-binding motifs and HIV disease outcome associations. However, we show here that the apparently small differences between HLA-B*57 alleles, termed HLA-B*57 micropolymorphisms, have a significant impact on immune control of HIV. In a study cohort of >2,000 HIV C-clade-infected subjects from southern Africa, HLA-B*5703 is associated with a lower viral-load set point than HLA-B*5702 and HLA-B*5801 (medians, 5,980, 15,190, and 19,000 HIV copies/ml plasma; P = 0.24 and P = 0.0005). In order to better understand these observed differences in HLA-B*57/5801-mediated immune control of HIV, we undertook, in a study of >1,000 C-clade-infected subjects, a comprehensive analysis of the epitopes presented by these 3 alleles and of the selection pressure imposed on HIV by each response. In contrast to previous studies, we show that each of these three HLA alleles is characterized both by unique CD8+ T-cell specificities and by clear-cut differences in selection pressure imposed on the virus by those responses. These studies comprehensively define for the first time the CD8+ T-cell responses and immune selection pressures for which these protective alleles are responsible. These findings are consistent with HLA class I alleles mediating effective immune control of HIV through the number of p24 Gag-specific CD8+ T-cell responses generated that can drive significant selection pressure on the virus.
INTRODUCTION
HLA-B*57 is the HLA allele that has the greatest impact on the viral-load set point and the rate of disease progression in HIV infection (10, 17, 18, 23, 28, 29, 34, 42, 46, 55). However, critically for the development of a vaccine designed to optimize immune control of HIV infection, the mechanism by which HLA-B*57 is linked to relatively successful control of HIV infection remains incompletely understood. In studies of a large South African cohort in Durban, South Africa, the number of Gag-specific CD8+ T-cell responses induced and the number of Gag escape mutants selected correlated with the viral-load set point (30, 40), with HLA-B*5703 associated with the lowest viral-load set point, the highest number of Gag epitopes targeted, and the highest number of Gag escape mutations selected. In addition, HLA-mediated immune control of HIV has been linked to natural killer cell activity, with Bw4-80I alleles, such as HLA-B*57, being ligands for the inhibitory KIR3DL1 receptor (37) and also for the activating KIR3DS1 NK cell receptor (9, 37, 44).
The subtype of HLA-B*57 most prevalent in sub-Saharan Africa, the region worst affected by the global HIV epidemic, is HLA-B*5703, which is the allele associated with the lowest viral loads in the region (11, 27, 29, 31, 34, 41, 54). HLA-B*5703 differs by only a single amino acid from the other HLA-B*57 subtype arising at significant levels in these populations, HLA-B*5702 (Leu at HLA residue 156 in HLA-B*5703 and Arg at residue 156 in HLA-B*5702). HLA-B*5801 differs in the alpha 1 and 2 domains by 7 amino acids from HLA-B*5703 and by 6 residues from HLA-B*5702. However, the published peptide-binding motifs for all three closely related HLA alleles are identical (5, 15): Thr/Ser/Ala at position 2 in the peptide, binding in the B pocket of the HLA molecule, and Trp/Phe/Tyr at the carboxy-terminal position in the peptide, binding in the F pocket of the HLA molecule. Because of these similarities, and in order to increase statistical power, the closely related HLA-B*5702, HLA-B*5703, and HLA-B*5801 alleles have often been analyzed together in studies of HIV infection (19, 23, 35, 38). This has appeared justified, since some of the same CD8+ T-cell epitopes are presented by these three alleles, and in some cases, these responses appear, superficially, to impose the same selection pressure on the virus. An example is the Gag epitope, TSTLQEQIAW (TW10, Gag 240 to 249), presented by all 3 closely related alleles, and within TW10, in each case, the same escape mutant, T242N, is commonly selected. However, closer inspection of this TW10 response, and the other HIV-specific epitopes presented by these related alleles, without exception reveals differences that may be relevant to understanding why HLA-B*5703 is linked to superior immune control of HIV.
In these studies, we focus on the differences between HLA-B*5702, HLA-B*5703, and HLA-B*5801 associated with improved immune control of HIV in C-clade infection in KwaZulu-Natal, South Africa. Although these three alleles are all significantly associated with lower viral-load set points than study subjects not expressing these alleles, HLA-B*5703 is associated with a significantly lower viral-load set point than HLA-B*5801 (P = 0.0005; Mann-Whitney test), and B*5801 is associated with a significantly higher absolute CD4 count than either B*5702 or B*5703 (P = 0.0019 and P = 0.0025; data not shown). To determine whether differences in the viral-load set point associated with these three closely related HLA class I alleles could be related to epitope specificity or to the ability of certain responses to exert selection pressure on the virus, as has been previously hypothesized (40), we undertook for the first time a comprehensive analysis of the CD8+ T-cell responses restricted by this group of alleles in a study of >1,000 adults with chronic C-clade infection. In addition, we identified the epitope-specific cytotoxic-T-lymphocyte (CTL) responses that are capable of exerting selection pressure on the virus. These data were then related to differences in the viral-load set points linked to these protective HLA alleles.
MATERIALS AND METHODS
Study subjects.
We studied a total of 2,093 antiretroviral therapy (ART)-naïve adults with chronic HIV-1 infection recruited from the following four cohorts: (i) Durban, South Africa (C clade; n = 1,263), as previously described (13, 16, 29, 40); (ii) Gaborone, Botswana (C clade; n = 525) via the Mma Bana study; (iii) Bloemfontein, Free State, South Africa (C clade; n = 261), as previously described (27); and (iv) Kimberley, South Africa (n = 44). Data describing viral loads and CD4 T-cell counts were available for 1,869 and 1,849 subjects, respectively, in all of whom HLA typing to four-digit resolution had been determined. CD4+ T-cell counts were determined for 23 HLA-B*5702-, 73 HLA-B*5703-, and 198 HLA-B*5801-positive individuals and for 1,555 subjects expressing none of the 3 alleles. The viral load was measured using the Roche Amplicor version 1.5 assay. Informed consent was obtained from all participating individuals, and institutional review boards at the University of KwaZulu-Natal, Massachusetts General Hospital, and the University of Oxford approved the study.
ELISPOT and optimal peptide mapping.
In 1,010 HIV C-clade-infected subjects, the HIV-specific CD8+ T-cell responses were determined in gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assays. Responses were determined to a set of 410 overlapping peptides (OLPs), whose sequences were based on the 2001 C-clade consensus sequence arranged in a matrix system with 11 or 12 peptides in each pool. Responses to matrix pools were deconvoluted by subsequent testing with the individual 18-mer peptides within each pool, and the identities of the individual 18-mers recognized were thus confirmed, as previously described (29). For epitope mapping of the optimal peptide and recognition of responses to mutated escape peptides, we titrated the individual peptides over a range of 8 log units using 100,000 input cells in each well, as described previously (45).
HLA class I typing.
Four-digit high-resolution typing of HLA-A, HLA-B, and HLA-C alleles was performed using Dynal real-time reverse sequence-specific oligonucleotide (SSO) kits, as previously described (26).
HLA restriction assay and presentation of peptide on HLA.
To determine which HLA allele presented the peptide of interest, we generated a panel of Epstein-Barr virus (EBV)-transformed B lymphoblastoid cell lines (BCL), each matching the effector cells through one HLA allele, as previously described (22). Briefly, BCL cells were incubated with 50 μg/ml of peptide or no peptide for 1 h and washed thoroughly 4 times. We generated effector cells by stimulating peripheral blood mononuclear cells (PBMCs) with peptide (50 μg/ml) for 7 to 10 days, and for further rounds of stimulation, we used irradiated peptide-pulsed HLA-matched BCL and feeder cells in an effector/BCL/feeder ratio of 1:1:1. Effector cells were grown for 2 to 4 weeks prior to their use in assays in order to obtain a higher frequency of peptide-specific CD8+ T cells, as previously described (45). For HLA restriction, CTLs were cocultured with BCL in a 1:2 ratio for 6 h at 37°C, 5% CO2, in the presence of brefeldin A (10 μg/ml) and anti-CD107a (1:25) and subsequently stained in an intracellular-cytokine-staining assay using the surface antibodies CD8 AlexaFluor 750 (eBioscience) and CD3 AlexaFluor 700 (BD) and a LIVE/DEAD violet cell stain kit (Invitrogen); fixed and permeabilized (BD cytofix/cytoperm kit); stained intracellularly with IFN-γ-phycoerythrin (PE)-Cy7 (BD) and MIP1β-fluorescein isothiocyanate (FITC) (R&D); and fixed in 2% paraformaldehyde. Samples were acquired on an LSR II (BD) flow cytometer within 12 h of staining and analyzed using FlowJo version 8.8.6. Cells were hierarchically gated on singlets, lymphocytes, live cells, CD3+ T cells, and CD8+ T cells and analyzed for IFN-γ+ MIP1β+ or CD107a+ MIP1β+ double-positive events.
Tetramer synthesis and tetramer staining.
Tetramers were generated as previously described (32). For staining of PE- or allophycocyanin (APC)-conjugated tetramer-specific cells, PBMCs were thawed in R20 medium; rested for 1 h at 37°C, 5% CO2; stained with HLA-class I tetramer for 30 min at room temperature; washed and surface stained with CD8 AlexaFluor 750 (eBioscience), CD3 AlexaFluor 700 (BD), and a LIVE/DEAD violet cell stain kit (Invitrogen) for 30 min; washed in PBS; and fixed in 2% paraformaldehyde. Samples were acquired on an LSR II (BD) flow cytometer within 12 h of staining and analyzed using FlowJo version 8.8.6. Cells were hierarchically gated on singlets, lymphocytes, live cells, and CD3+ T cells and double gated for a distinct tetramer-specific CD8-positive population.
Sequencing of proviral DNA.
Sequences from Gag, Pol, and Nef were generated by extraction of genomic DNA from PBMCs and amplified by nested PCR using previously published primers (26), and the PCR product was purified as previously described (40). Sequencing was undertaken using the BigDye ready-reaction mix as described previously (16, 33), and in this way, HIV Gag, Pol, and Nef sequences were obtained from 1,209, 446, and 436 study subjects, respectively (40), and Vif, Rev, and Env sequences were obtained from HIV full-length sequencing available for 248 study subjects, as previously described (48).
HLA peptide binding and stability assays.
The measurement of peptide major histocompatibility complex class I (MHC I) stability was determined as recently described (25), and peptide-MHC I affinity interactions were undertaken using AlphaScreen technology as previously described (24).
Statistical method to identify HLA and OLP associations.
We used a decision tree based on Fisher's exact test to identify associations between the recognition of individual 18-mer peptides and the expression of HLA-B*5702, HLA-B*5703, and HLA-B*5801. For each 18-mer peptide, we computed the Fisher exact test P value against all 4-digit HLA alleles observed in the cohort and added the most significant HLA allele to the decision tree. We next removed all individuals who expressed that allele and repeated the process until the most significant HLA allele had a P value of >0.2. False-discovery rates (6) were calculated using a procedure specific to Fisher's exact test that analytically computes the null distribution for all permutations of the data, as previously described (8). For comparison of viral loads and CD4 T-cell counts, we used the Mann-Whitney U test as previously described (30). We used the Mann-Whitney U test to compare immunogenic epitopes to nonimmunogenic epitopes for HLA peptide-binding affinities and HLA-peptide stabilities and the Spearman Rank model to test the correlation between the percentages of individuals responding (IFN-γ ELISPOT) to a particular peptide and the peptide-binding values (50% inhibitory concentration [IC50] Kd [dissociation constant; nM]) and peptide-HLA stability (h) for a particular HLA.
RESULTS
HLA-B*5703 is associated with lower viral-load set points and higher CD4+ T-cell counts than HLA-B*5801.
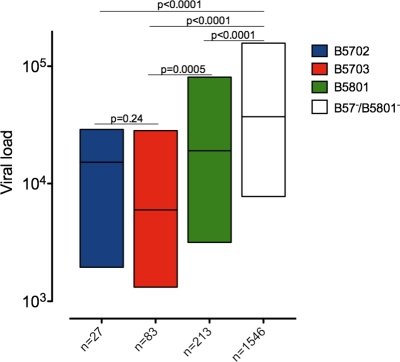
We focused on three HLA alleles associated with control of HIV—HLA-B*5702, HLA-B*5703, and HLA-B*5801—expressed in 1.4%, 4.4%, and 11.9%, respectively, of a large cohort (n = 2,093) of well-characterized chronic HIV C-clade-infected individuals. These closely related alleles are all associated with significantly lower viral-load set points than study subjects not expressing the alleles (median set points, 15,190, 5,980, 19,000, and 37,250 HIV RNA copies/ml plasma, respectively) (Fig. 1). HLA-B*5703 is associated with a significantly lower viral-load set point than HLA-B*5801 (P = 0.0005) and a lower viral-load set point than HLA-B*5702, although the latter difference was not statistically significant (P = 0.2). In this cohort of chronically infected subjects, absolute CD4 T-cell counts were also significantly higher in individuals expressing HLA-B*5702, HLA-B*5703, or HLA-B*5801 than in those not expressing them (706/mm3, 516/mm3, and 412/mm3 versus 336/mm3, respectively; P < 0.0001 in each case) (data not shown).
Fig 1.
Association of HLA-B*57/B*58 allele expression with the steady-state viral load in a cohort size of 1,864 chronically C-clade-infected individuals from South Africa. The y axis shows the association with the viral load (RNA copies/ml) expressed as interquartile ranges, with the number of individuals included in the analysis for each parameter and HLA shown on the x axis. All HLAs were mutually exclusive for coexpression of any of the other HLA-B*57/58 alleles. Significant differences in median viral load between groups are indicated by P values shown above the bars (Mann-Whitney U test).
Distinct p24 Gag epitope targeting and differential selection pressures imposed.
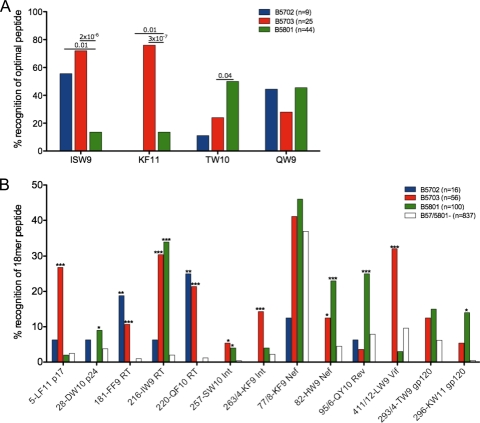
The differences in amino acid residues between HLA-B*5702, HLA-B*5703, and HLA-B*5801 are shown in Table 1. To investigate the consequences of these differences with respect to the epitopes targeted, we initially focused on the four well-characterized p24 Gag epitopes restricted by HLA-B*57/5801, since these responses have been most frequently implicated in studies addressing the association between HLA-B*57 and slow HIV disease progression (40). They are ISPRTLNAW (ISW9; Gag 147 to 155), KAFSPEVIPMF (KF11; Gag 162 to 172), TSTLQEQIAW (TW10; Gag 240 to 249), and QATQDVKNW (QW9; Gag 308 to 316). Three of these p24 Gag epitopes are listed in the Los Alamos Immunology Database “A” list as restricted by HLA-B*5701, namely, ISW9, KF11, and TW10. KF11 is listed as also restricted by HLA-B*5703, and TW10 is listed as also restricted by HLA-B*5801. QATQDVKNW (QW9; Gag 308 to 316) is only listed as HLA-B*5801 restricted. Testing the recognition of these optimal epitopes in ELISPOT assays showed clear differences in recognition according to the HLA type expressed (Fig. 2A). The dominant HLA-B*5703-restricted KF11 epitope was targeted by 76% of subjects expressing HLA-B*5703 compared to 0% and 10% of subjects expressing HLA-B*5702 and HLA-B*5801, respectively (P = 0.01 × 10−7 and P = 3 × 10−7, respectively; Fisher's exact test). ISW9 is targeted significantly less often by subjects expressing HLA-B*5801 than by those expressing HLA-B*5702 or HLA-B*5703 (P = 0.01 = 2 × 10−6 and P = 2 × 10−6, respectively), whereas TW10 is preferentially targeted by HLA-B*5801-positive subjects compared to HLA-B*5703 and HLA-B*5702 (Fig. 2A).
Table 1.
HLA-B*5702, HLA-B*5703, and HLA-B*5801 sequence differences
| Allele | Residue at positiona: |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha 1 domain |
P2b | Alpha 2 domain |
PCb | |||||||||||||||
| 9 | 24 | 45 | 46 | 63 | 66 | 67 | 77 | 94 | 95 | 97 | 99 | 103 | 114 | 116 | 156 | |||
| HLA-B*5702 | Y | A | M | A | E | N | M | N | T/S/A | I | I | V | Y | V | N | Y | R | W/F |
| HLA-B*5703 | − | − | − | − | − | − | − | − | T/S/A | − | − | − | − | − | − | − | L | W/F |
| HLA-B*5801 | − | − | T | E | − | − | − | − | T/S/A | − | − | R | − | L | D | S | − | W/F |
− indicates conservation of the residue shown according to the HLA-B*5702 consensus. Positions 194, 282, 305 and 325, are located outside alpha 1/2 and differ as follows: HLA- B*5702/B*5703, I, V, A, and C; HLA-B*5801, V, I, T, and S, respectively.
Fig 2.
IFN-γ ELISPOT responses to 17 HIV CD8+ T-cell epitopes. (A) Four p24 capsid Gag optimal peptides screened in 9, 25, and 44 HLA-B*5702, HLA-B*5703, and HLA-B*5801 individuals, respectively, expressed as percent recognition of the peptide, ISW9 (ISPRTLNAW), KF11 (KAFSPEVIPMF), TW10 (TSTLQEQIAW), or QW9 (QATQDVKNW), indicated on the x axis. (B) Associations of IFN-γ ELISPOT responses to 18-mer peptides in HLA-B*5702-, HLA-B*5703-, and HLA-B*5801-expressing and HLA-B*57/58-negative individuals expressed as percent responders (y axis) to 13 18-mer peptides grouped according to 4 different HLA-B*57/58 expression levels, with the 18-mer peptide number and epitope abbreviation shown on the x axis. Significant P values between groups are shown above the bars for optimal peptides in panel A and are indicated by asterisks (***, P < 0.001; **, P < 0.001; and *, P < 0.01) for 18-mer peptide recognition compared to HLA-B*57/5801-negative individuals in panel B (Fisher's exact test).
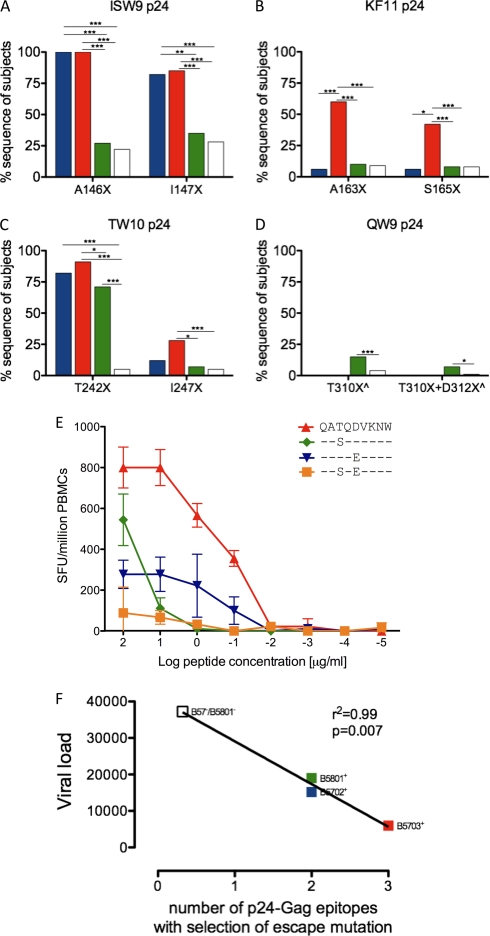
These differences in epitope recognition are, in some cases, at least partially explained by corresponding differences in the selection of escape mutants. Thus, higher recognition of TW10 in subjects with HLA-B*5801 is reflected by lower frequencies of escape mutants at T242 and I247 (Fig. 3). However, it is clear that for ISW9 and KF11, these are epitopes that are not recognized at significant levels by subjects with HLA-B*5801, and there is no escape mutation selected within these epitopes in these subjects; similarly, KF11 is not recognized in HLA-B*5702-positive subjects, nor are escape mutants selected within KF11 in these subjects. With respect to QW9, targeted by a minority of subjects expressing HLA-B*5702, HLA-B*5703, or HLA-B*5801, significant selection for the T310S escape mutant within QW9 (at P3 in the epitope) (Fig. 3D and E) was observed only in subjects expressing HLA-B*5801. However, this selection pressure was relatively weak, and mutants were observed in only 10 to 15% of HLA-B*5801-positive subjects.
Fig 3.
Selection of escape mutations within 4 p24 capsid Gag epitopes. (A to D) Sequence polymorphisms indicated as percent sequences of subjects on the y axis, with the particular polymorphism shown on the x axis for ISW9, KF11, TW10, and QW9 p24 epitopes. HLA expression is color coded as in Fig. 2: B*5702+/B*5703−/B*5801−, n = 17 (blue); B*5703+/B*5702−/B*5801−, n = 54 (red); B*5801+/B*5703−/B*5702−, n = 142 (green); and B*5702−/B*5703−/B*5801−, n = 989 (white). (E) IFN-γ ELISPOT responses for recognition of QW9 QATQDVKNW wild type (WT) and escape peptides, shown as spot-forming units/million PBMCs against log peptide titrations (μg/ml) in donor N067 (HLA-A*0201/0205, B*5101/5801, and Cw*0701/1602). ^ indicates HLA-B*4403 individuals removed from analysis due to selection of B*4403-restricted escape mutations. (F) Correlation of the viral-load set point with the number of p24-Gag epitopes with selection of escape mutations. (A to D) Significant P values are indicated as follows: ***, P < 0.0001; **, P < 0.001; *, P < 0.01 (Fisher's exact test).
Thus, there are clear-cut differences between these three closely related HLA alleles with respect to these four p24 Gag-specific epitopes. From the ELISPOT assays and sequence data shown, HLA-B*5703 restricts three p24 Gag-specific CTL responses that impose strong selection pressure on the virus (ISW9-, KF11-, and TW10-specific responses), whereas HLA-B*5702 and HLA-B*5801 restrict two (ISW9/TW10 and TW10/QW9, respectively). Including the non-B*57/5801 HLA-B alleles in this analysis, which collectively restrict, on average, 0.32 (7/22) p24 Gag epitopes per allele within which escape mutants are selected (40), these data provide a correlation between the viral-load set point and the number of p24 Gag epitopes at which escape mutants are selected (r2 = 0.99; P = 0.007) (Fig. 3F).
All HIV-specific responses restricted by HLA-B*5702/5703/5801 alleles exhibit distinct patterns of targeting and selection pressure.
To determine whether the findings described in relation to the four well-characterized CD8+ T-cell epitopes restricted by HLA-B*5702, HLA-B*5703, and HLA-B*5801 apply more generally to the other HIV-specific epitopes restricted by these three closely related alleles, we first employed a panel of 410 18-mer peptides overlapping by 10 amino acids and spanning the C-clade proteome, as previously described (30), to provide as comprehensive a description as possible of all the epitopes restricted by the alleles. The study cohort of 1,010 adults with chronic C-clade infection studied for responses to these 410 peptides in IFN-γ ELISPOT assays included 14 HLA-B*5702-positive HLA-B*5703/HLA-B*5801-negative subjects, 56 HLA-B5703-positive HLA-B*5702/HLA-B*5801-negative subjects, 100 HLA-B*5801-positive HLA-B*5702/HLA-B*5703-negative subjects, and 837 subjects expressing none of the alleles. One subject coexpressed HLA-B*5703 and HLA-B*5801 and was therefore excluded from analysis. From this evaluation, apart from the 4 p24 Gag responses described above, we identified an additional 4, 11, and 9 epitopes that are presented by HLA-B*5702, HLA-B*5703, and HLA-B*5801, respectively (Fig. 2B and Table 2; see Fig. S1 in the supplemental material). Thus, there are a total of 17 epitopes presented by one or more of these HLA molecules, only 5 of which are listed as optimal epitopes in the Los Alamos Immunology Database “A” list for any of the 3 alleles (Table 2). Despite the apparently identical peptide-binding motifs described previously (5, 15) for HLA-B*5702, HLA-B*5703, and HLA-B*5801, distinct patterns of epitope targeting were observed for all these epitopes (Fig. 2B; see Fig. S1 in the supplemental material). The epitopes within OLP-5-p17, 263/4-Int, and 411/2-Vif were uniquely presented by HLA-B*5703, and 95/6-Rev by HLA-B*5801. The epitopes within OLP-216-RT, 257-Int, 77/8-Nef, 82-Nef, and 296-Env were restricted by both B*5703 and B*5801 alleles. HLA-B*5702 presents only a minority of well-targeted epitopes (FF9-RT and QF10-RT).
Table 2.
Summary of epitopes differentially targeted by HLA-B*5702, B*5703 and B*5801
| Protein | Epitopea |
||
|---|---|---|---|
| B*5702 | B*5703 | B*5801 | |
| p17 | LVWASRELERFb | ||
| ISPRTLNA Wb | ISPRTLNA W | ||
| KAFSPEVIPMF | |||
| p24 | DTINEEAAE Wb | ||
| TSTLQEQIA Wb | TSTLQEQIA W | TSTLQEQIA W | |
| QATQDVKN Wb | QATQDVKN W | QATQDVKN Wb | |
| RT | FSVPLDEG Fb | FSVPLDEG Fb | |
| IAMESIVI Wb | IAMESIVI W | IAMESIVI Wb | |
| QATWIPEWE Fb | QATWIPEWE Fb | ||
| INT | SAAVKAAC Wb | ||
| KTAVQMAV F | KTAVQMAV Fb | ||
| NEF | KAAFDLSF Fb | KAAFDLSF Fb | KAAFDLSF Fb |
| HTQGFFPD W | HTQGFFPD Wb | ||
| Rev | QAVRIIKIL Y | QAVRIIKIL Y | |
| VIF | LGHGVSIE Wb | ||
| Env | TVYYGVPV Wb | TVYYGVPV Wb | |
| KAYEKEVHNVW | KAYEKEVHNVW | ||
Selection of escape mutations is indicated by boldface.
New epitopes not listed in the Los Alamos “A” list database.
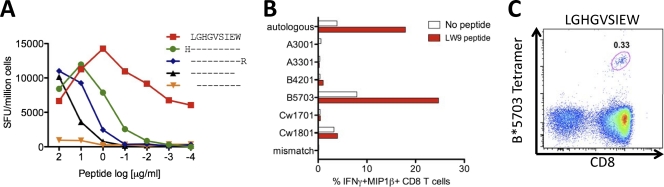
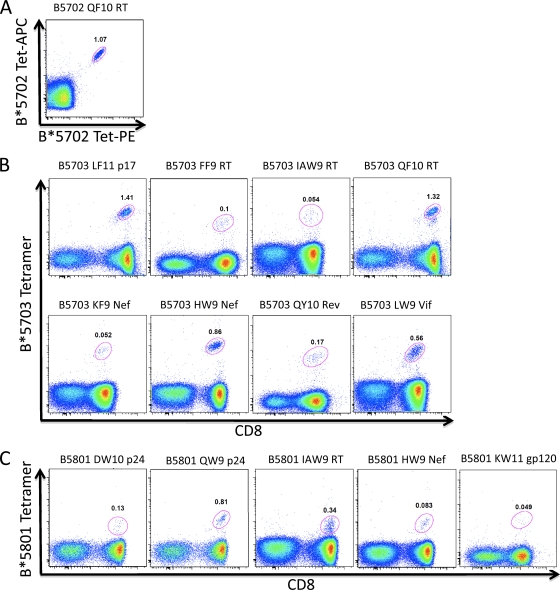
In order to demonstrate the validity of this approach in identifying novel epitopes restricted by these alleles, we proceeded in selected cases to define the optimal epitope and HLA restriction. We first selected the novel overlapping peptide OLP-411/12 Vif response, since targeting this 18-mer OLP had previously been shown to be associated with significantly higher viral loads in HLA-B*5703-positive individuals (30). This association is strengthened in the bigger cohort studied here (median viral-load set point in Vif LW9 responders, 26,492 HIV RNA copies/ml plasma versus 1,705 in Vif LW9 nonresponders; P = 0.005) (data not shown). Peptide truncations demonstrated the optimal epitope to be the 9-mer LGHGVSIEW (LW9; Vif 81 to 89) (Fig. 4A), and a panel of BCL was used to confirm the restriction element as HLA-B*5703 (Fig. 4B). The identity of this optimal epitope was further confirmed when we demonstrated staining of the relevant antigen-specific cells using an HLA-B*5703-LW9 tetramer (Fig. 4C).
Fig 4.
Unequivocal definition of novel HLA-B*57/58-restricted CD8+ T-cell epitopes. (A) HLA restriction of LW9 using peptide-pulsed or unpulsed partial effecter CTL HLA-matched B-cell lines sharing only 1 of the 6 HLA alleles of the R014 CTLs, as indicated on the y axis, in an intracellular cytokine-staining (ICS) assay. (B) Fine mapping of the LW9-Vif epitope using titrated peptide truncations of the predicted epitope in IFN-γ ELISPOT with an LW9-specific CTL line grown out from a chronically infected donor from Zimbabwe, R014 (HLA-A*3001/3301, B*4201/5703, and Cw*1701/1801), and used as effector input cells. (C) HLA-B*5703 tetramer staining of LW9 Vif-specific PBMCs from the same donor (subject R014) used for panels A and B.
We then proceeded to adopt this tetramer-based approach to demonstrate the optimal epitopes and HLA restriction for an additional selection of the 13 novel epitopes identified by the ELISPOT assays (Fig. 5). While only two of these epitopes (KF9 Int and HW9 Nef) have previously appeared in the Los Alamos Immunology Database as HLA-B*5701 restricted, the restriction of the two epitopes by HLA-B*5702, HLA-B*5703, or HLA-B*5801 has not been demonstrated before. In addition, 7 of the epitopes described here (DW10 p24, FF9 RT, QF10 RT, QY10 Rev, LW9 Vif, TW9 Env, and KW9 Env) are entirely novel, whereas 2 epitopes (IW9 RT and SW10 Int) have previously been shown to be presented only by HLA-B*57 (23, 47), and KF9 Nef has been described only for HLA-B*5801 (33).
Fig 5.
Use of HLA-class I tetramers to rapidly define novel HIV-1 CD8+ T-cell epitopes. HLA-B*5703 tetramers were loaded with the indicated optimal peptides and stained against HLA-matched ex vivo donor PBMCs. (A) HLA-B*5702 tetramer loaded with QF10 RT (QATWIPEWEF) (subject, N099). (B) LF11 p17 (LVWASRELERF) (subject, R014), FF9 RT (FSVPLDEGF) (subject, N087), IAW9 RT (IAMESIVIW) (subject, N102), QF10 RT (QATWIPEWEF) (subject, R014), KF9 Nef (KTAVQMAVF) (subject, N102), HW9 Nef (HTQGFFPDW) (subject, R046, B*5701), QY10 Rev (QAVRIIKILY) (subject, R059), and LW9 Vif (LGHGVSIEW) (subject, N102). (C) HLA-B*5801 tetramers loaded with DW10 p24 (DTINEEAAEW) (subject, N067), QW9 p24 (QATQDVKNW) (subject, N067), IAW9 RT (IAMESIVIW) (subject, R007), HW9 Nef (HTQGFFPDW) (subject, N087), and KW11 Env (KAYEKEVHNVW) (subject, R076). The numbers in the gates are the percentages of tetramer-specific cells gated on live cells/CD3+ cells/lymphocytes shown as CD8 versus tetramer (B and C) or gated on CD8+ cells for PE and APC tetramer gating (A).
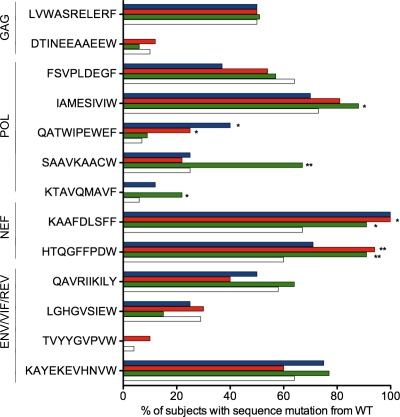
Since the efficacy of a CD8+ T-cell response has been related to its ability to drive selection pressure on HIV, we next sought to characterize the selection of escape mutants within epitopes from the entire HIV proteome for individuals expressing either HLA-B*5702, HLA-B*5703, or HLA-B*5801 and subjects not expressing any of the three alleles (Fig. 6). Among these data, it is also clear that, as with the p24 Gag epitopes, the identical epitope-specific response can have substantially different impacts depending on the particular presenting HLA allele, as identified here for 6 additional epitopes. Of note, no significant selection of escape mutants was observed for the 4 Env/Rev/Vif epitopes.
Fig 6.
Differential selection of HIV sequence polymorphisms among HLA-B*5702, HLA-B*5703, and HLA-B*5801 individuals. The percentages of individuals with sequence variation from wild type are expressed as percent sequence mutation from wild type (x axis) calculated from sequences available from 1,202 (Gag), 426 (Pol), 443 (Nef), and 248 (Env/Vif/Rev) individuals and grouped according to HLA-B*57/58 expression color coded as in Fig. 2. Significant differences (P < 0.05) between each of the three HLA alleles and HLA-B*57/B*5801-negative individuals are indicated as follows: **, P < 0.001; *, P < 0.01 (Fisher's exact test).
Peptide-binding affinities to HLA-B*5702, HLA-B*5703, and HLA-B*5801 and peptide-HLA stability partially explain distinct epitope targeting.
In order to address possible mechanisms by which distinct epitope targeting is observed and differential selection pressure is imposed on HIV by these three closely related HLA alleles, despite the binding motifs for HLA-B*5702, HLA-B*5703, and HLA-B*5801 reportedly being identical (5, 15), we tested the hypothesis that different epitope binding affinities and peptide-HLA stabilities might contribute. Of 15 peptides that were available for testing, differential targeting of 5 epitopes was explained by either peptide-binding affinities, HLA-peptide stabilities, or both (Fig. 7). For example, HLA-B*5801 is the only allele not associated with ISW9p24 targeting or escape, and this allele has almost 10 times lower binding affinity for ISW9 than do HLA-B*5703 and HLA-B*5702 (Kd = 155 nM, 11 nM, and 19 nM, respectively) and 5 times lower peptide-MHC stability (half-life [t1/2] = 1.1 h, 5.2 h, and 5.7 h, respectively). However, for the remainder of the epitopes tested, the HLA peptide-binding affinity or HLA-peptide stability differences did not explain the observed HLA differences. Thus, the distinct targeting of epitopes and selection of escape mutations by HLA-B*5702-, HLA-B*5703-, and HLA-B*5801-expressing individuals could be explained by differential binding affinities and peptide-HLA stabilities for only approximately one-third of these epitopes.
Fig 7.
HLA-peptide affinity and HLA-peptide stability explain differential targeting of epitopes in HLA-B*5702, HLA-B*5703, and HLA-B*5801 individuals. (A and B) ISW9 p24 epitope binding affinity (A) and stability (B) for HLA-B*5702, HLA-B*5703, and HLA-B*5801 molecules. (C to E) Immunogenicity (percent OLP recognition) (C); binding affinity Kd−1 (nM), classified on a log scale and assessed as previously described (24) (D); and peptide-HLA stability (h) of peptides measured as recently described (25) (E) for the indicated three color-coded HLA molecules. The y axis shows the optimal peptide sequences.
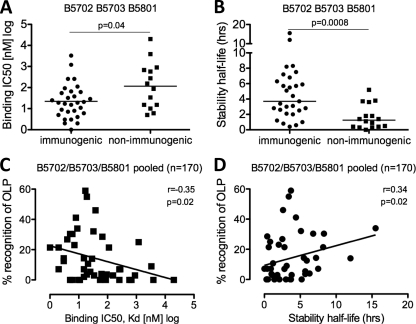
Since recent studies have indicated that peptide-MHC I stability may be a better predictor of immunogenicity than peptide-MHC I binding affinity (M. Harndahl, unpublished data), we compared the contributions of these two measures to peptide immunogenicity for the optimal epitopes shown above in the 170 individuals studied who expressed HLA-B*5702, MHC I B*5703, or MHC I B*5801 (n = 14, n = 56, and n = 100, respectively) (Fig. 8). Although there was a statistically significant difference between immunogenic and nonimmunogenic peptides (Table 2) in peptide-HLA binding affinities (Fig. 8A) and peptide-HLA stabilities (Fig. 8B) (P = 0.04 and P = 0.0008, respectively; Mann Whitney U test), peptide-HLA binding stability did not predict peptide recognition any better than peptide-HLA affinity (Fig. 8C and D; see Fig. S3 in the supplemental material). Furthermore, these data highlight the fact that factors other than peptide-MHC binding affinity and stability also play an important role in determining immunogenicity.
Fig 8.
HLA-peptide affinity and HLA-peptide stability discriminate between immunogenic and nonimmunogenic optimal peptides and correlate with immunogenicity. (A and B) HLA-peptide binding affinities (A) and HLA-peptide stability (B) for immunogenic and nonimmunogenic peptides. Horizontal lines represent median values. (C and D) Binding affinity Kd values (nM) classified on a log scale (C) and peptide-HLA complex stability (h) (D) correlation with the percentage of individuals recognizing the corresponding OLP containing the optimal epitope, shown for groups of HLA-B*5702, HLA-B*5703, and HLA-B*5801 individuals as pooled data. Sloping lines represent Spearman rank correlations. The numbers in brackets refer to the number of individuals used for percent IFN-γ ELISPOT recognition of OLP. A Mann-Whitney U test was used for comparison of median values of immunogenic versus nonimmungenic peptides. P values of <0.05 were considered significant.
Notable absence of previously listed HLA-B*57/5801-restricted epitopes.
The approach we used here to define the epitopes restricted by HLA-B*57/5801 molecules, in addition to identifying them comprehensively and in as unbiased a fashion as possible, also highlighted some notable absences of previously reported epitopes. For those, we tested the abilities of the peptides to bind, and in some cases they proved not to bind. For example, the published HLA-B*5701-restricted epitopes include KAIGTVLV (KV8 Pro), YTPGPGIRY (YY9 Nef), and YFPDWQNYT (YT9 Nef) (14, 20, 21, 51), none of which fit the peptide-binding motif for HLA-B*5701 and none of which bind HLA-B*5701 (Kd > 20,000 nM) (data not shown). These data highlight the value of the approach adopted here to define the epitopes restricted by different HLA alleles and the unequivocal confirmation of optimal epitopes and HLA restriction provided by the use of tetramers, as shown here.
Taken together, this comprehensive characterization of IFN-γ OLP responses and sequence polymorphisms in a large C-clade cohort by the use of MHC class I tetramers and peptide-binding and peptide-HLA stability assays reveals distinct targeting of CD8+ T-cell epitopes within HLA-B*5702, HLA-B*5703, and HLA-B*5801 restriction (Table 2). Importantly, HLA-B*5703 selects escape mutations in 3 of the well-characterized p24 capsid epitopes, ISW9, KF11, and TW10, whereas HLA-B*5702 and HLA-B*5801 select escape in only 2 of these epitopes, which may explain the improved immune control of HIV observed in HLA-B*5703- over HLA-B*5702- and HLA-B*5801-positive individuals.
DISCUSSION
Recent analyses of the STEP trial suggest that T-cell vaccination strategies can be successful, but only if effective CD8+ T-cell responses are induced, and in this case, they were seen only in subjects expressing the already protective alleles HLA-B*27/57/5801 (1, 19). This highlights the importance of fully understanding which specific responses mediate improved immune control of HIV to guide future HIV vaccine design. The study presented here is the first to comprehensively define what epitopes are targeted by the HLA class I alleles most strongly associated with an outcome of immune control in adult chronic C-clade HIV infection. Despite HLA-B*57/5801 alleles having been well studied, it has remained unclear which epitopes are presented and to what extent they are targeted. Here, we report a detailed characterization of all the epitopes within the HIV proteome that are restricted by any of three closely related, protective HLA alleles, HLA-B*5702, HLA-B*5703, and HLA-B*5801, that are significantly targeted in a large cohort (n > 1,000) of chronically infected study subjects. Having undertaken this analysis, we conclude that HLA restriction has a distinct impact on both the immunogenicity and the selection pressure imposed on the virus by each response. In addition, the differences in control of disease progression observed for these closely related HLA alleles are most clearly correlated with the number of p24 Gag-specific responses that drive significant selection pressure on the virus.
The amino acid differences between the 3 closely related HLA-B*57/5801 alleles represent micropolymorphisms, a term previously coined in relation to three closely related HLA-B*44 alleles, HLA-B*4402, HLA-B*4403, and HLA-B*4405, that differ at two positions, 116 and 156 (Table 1) (2). This HLA-B*44 study showed that the amino acid changes do not alter the T-cell receptor (TCR) contact sites on the MHC but alter the confirmation observed for peptide residues 4 to 7 for the three HLA-B*44 alleles. The same principle may apply to peptides presented by HLA-B*5702, HLA-B*5703, and HLA-B*5801, where they bind the same peptides but may present these peptides in very different peptide-MHC landscapes. For example, HLA-B*4402 and HLA-B*4403 differ by only 1 amino acid, and in the same position (Asp and Leu, respectively, at residue 156) as HLA-B*5702 and HLA-B*5703 (Arg and Leu, respectively, at residue 156). The crystal structures of the same peptide presented by HLA-B*4402 and HLA-B*4403 showed a wider peptide-binding cleft for HLA-B*4403 than for HLA-B*4402, and while the majority of peptides presented by HLA-B*4403 are also presented by HLA-B*4402, a broader repertoire of unique peptides is presented by HLA-B*4403 (36). The hydrophobic side chain in residue 156 for HLA-B*5703 (156-Leu) may similarly disrupt water-mediated hydrogen bonds serving to keep the peptide-binding cleft relatively narrow in HLA-B*5702 (156-Arg) compared to HLA-B*5703. This may alter the precise orientation of the same peptide in the groove of B*5702 versus B*5703, as well as increasing the number of possible epitopes presented by HLA-B*5703 compared to HLA-B*5702, as was observed here (Table 2). Other studies of the HLA-B*41 family show how 6 HLA-B*41 subtypes, differing in 6 MHC I residues, present different peptides of various lengths and thereby create structurally distinct peptide-HLA complexes (4). These studies underline the consequences of HLA micropolymorphism changes in residues lining the peptide-binding groove.
In this study, the impact of only 1 residue change between HLA-B*5703 and B*5702 on peptide presentation was highly evident, as these alleles share only 50% of the HIV epitopes, with 7 more epitopes targeted by HLA-B*5703 than by HLA-B*5702. The larger repertoire of unique epitopes presented by HLA-B*5703 is of particular interest in relation to the KF11 Gag epitope, targeting of which has been associated with a lower viral load than HLA-matched nonresponders (30) and in which the accumulation of escape mutations increasingly cripples the virus, thereby facilitating immune control (12, 13). HLA-B*5703 is the only one of these closely related HLA-B*57/5801 molecules capable of a broad p24 Gag-specific response. The differential targeting of these well-studied epitopes in p24 Gag, ISW9, KF11, TW10, and QW9, is related to the viral-load set point in that HLA-B*5703 targets all four, while HLA-B*5702 and B*5801 target three and two, respectively. This result is consistent with our previous findings (40), which showed that the viral-load set point associated with each HLA-B allele is correlated with the number of Gag responses restricted by that HLA-B allele (r = −0.49; P = 0.013). Our findings here also support an additional result of that study, namely, that the ability of a particular HLA-B-restricted Gag response to impose selection pressure on the virus is more strongly correlated with the viral-load set point (r = −0.62; P = 0.0009) (40). Here, we have shown that the only Gag epitopes restricted by the HLA-B*5702/5703/5801 alleles where there is evidence of escape are ISW9, KF11, TW10, and QW9. Furthermore, if one compares the number of p24 Gag epitopes restricted by these 3 HLA-B*57/5801 alleles that show evidence of escape (ISW9 and TW10 for B*5702; TW10 and QW9 for B*5801; and ISW9, KF11, and TW10 for B*5703) with the number of Gag responses driving escape via the other 22 HLA-B alleles (7 responses divided among 22 alleles; mean, 0.32 responses/allele), one arrives at a remarkably strong correlation with the viral-load set point (Fig. 3F). Although it is clear that many other factors contribute to the viral-load set point, in addition to the number of CD8+ T-cell Gag responses that can impose selection pressure on the virus, nonetheless, it is a surprisingly strong correlation, given the small number of data points available. Of note, no such correlation of type was observed when we considered epitopes in Pol, in the accessory/regulatory proteins, or in Env. However, other mechanisms may also be involved, such as the different linkage disequilibrium (LD) to HLA-A and Cw alleles. In particular, the LD of HLA-B*5703 with HLA-A*7401 has recently been shown to have an additive effect for immune control over HLA-A*7401 (34, 39), whereas HLA-B*5801 in LD with HLA-A*0205, for example, may not add any beneficial effect.
From the comprehensive sequence analysis of escape mutants within the 17 epitopes studied here (Fig. 3 and 6), we note that sequence variability from the wild-type consensus is present in >50% of individuals not expressing HLA-B*5702, HLA-B*5703, and HLA-B*5801 for 5 of these epitopes (LF11-p17, FF9-Pol, IAW9-Pol, KF9-Nef, and HW9-Nef). The accumulation of the A83G mutant within KF9-Nef and H119N within HW9-Nef, selected by HLA-B*57/5801, and M377L within IAW9, selected by HLA-B*5801, may represent epitopes being driven toward extinction (33), precipitating new “consensus” sequences. In the cases of LF11-p17 and FF9-Pol, the high sequence variability may not be driven by HLA-B*57/5801 but may result from epitope clustering and selection pressure imposed by other alleles on overlapping epitopes. This finding raises the possibility that protection from HLA-B*5702, HLA-B*5703, and HLA- B*5801 may alter over time. However, whether this protection increases or decreases as a result of such changes is unknown. Taken together, these data support the hypothesis that a broad Gag-specific response is protective against HIV disease progression, that Gag-specific responses capable of driving selection pressure on the virus have the strongest protective effect, and that the outstanding difference between HLA-B*5703 and the other closely related HLA alleles, HLA-B*5702 and HLA-B*5801, is the immunodominant response to KF11.
As described above, we have illustrated an approach to characterizing the significant CD8+ T-cell responses restricted by different HLA class I alleles that is not biased by use of peptide-binding motifs and in which confirmation by a peptide-MHC tetramer demonstrates both the optimal epitope and the restriction element. There are relatively common examples in the literature of epitopes that have proven to be incorrect in sequence and/or by HLA restriction (14, 20, 21, 51).
In this paper, we also took advantage of the opportunity to assess the part played in immunogenicity by peptide-MHC binding avidity and the peptide-MHC stability half-life and found significant differences between immunogenic and nonimmunogenic optimal peptides, which is in agreement with other findings (M. Harndahl, unpublished data). We also found significant correlations between the percentage of chronically infected subjects recognizing the peptide and peptide-MHC binding avidity and peptide-MHC stability. However, the Spearman rank correlation (r) values found here were moderate, which is not unexpected and indicates that other factors also influence immunodominance, as comprehensively reviewed by others (3, 53, 57, 58, 59). Presentation of an MHC class I-restricted epitope to a specific CD8+ T cell is the culmination of a number of individual upstream events, including proteasome cleavage of viral proteins, transportation to the ER lumen by transporters associated with antigen (TAP), and further N-terminal trimming by ERAAP1/2 before peptide loading onto empty MHC class I molecules by the peptide-loading complex (PLC) and subsequent trafficking to the cell surface via the Golgi apparatus for recognition of CD8+ T cells (56, 59). In addition to these processing events, the availability of CD8+ T cells expressing TCRs specific for the processed peptide applies another level of complexity influencing immunodominance, as TCR selection from a vast naïve T-cell repertoire containing millions of unique TCRs is not a stochastic process but is routinely ordered and biased (43). Furthermore, protein abundances differ substantially, in the case of HIV proteins representing an immunogenicity advantage for the highly abundant Gag epitopes (7, 52), and some protein epitopes are presented earlier in the viral life cycle than epitopes from other viral proteins (49, 50). In addition, the presence of viral-sequence escape mutations further affects immunogenicity. Therefore, the measurement of peptide binding and peptide stability of the HLA molecule is unlikely to precisely predict the outcome of this very complex set of individual events that are important for immunogenicity. However, it is evident that peptide-MHC binding is a necessary but not sufficient prerequisite for the initiation of a CD8+ T-cell response (3, 53, 57, 58). This is exemplified by the low LW9-B*5702 stability half-life (<0.5 h) (Fig. 7D), which results in a complete lack of detectable LW9-specific CD8+ T-cell responses in subjects expressing HLA-B*5702 (Fig. 7C).
In conclusion, this study defines the differences between three closely related protective HLA class I alleles in terms of the epitopes presented, which are targeted by a large cohort of HIV-infected study subjects, and in terms of the selection pressure imposed by these responses on the virus. These data support earlier findings (40) that the critical differences distinguishing HLA alleles are the breadth of the Gag-specific CD8+ T-cell responses, in particular the p24 Gag-specific response, and the abilities of those responses to drive selection pressure on the virus.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust (P.G.) and the National Institutes of Health, grant RO1 AI46995.
Contributions of C-clade sequence data made by the Seattle CFAR Computational Biology Core are gratefully acknowledged.
Footnotes
Published ahead of print 16 November 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Altfeld M, Goulder PJ. 2011. The STEP study provides a hint that vaccine induction of the right CD8+ T cell responses can facilitate immune control of HIV. J. Infect. Dis. 203:753–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Archbold JK, et al. 2009. Natural micropolymorphism in human leukocyte antigens provides a basis for genetic control of antigen recognition. J. Exp. Med. 206:209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Assarsson E, et al. 2007. A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J. Immunol. 178:7890–7901 [DOI] [PubMed] [Google Scholar]
- 4. Bade-Doding C, et al. 2011. The impact of human leukocyte antigen (HLA) micropolymorphism on ligand specificity within the HLA-B*41 allotypic family. Haematologica 96:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barber LD, et al. 1997. Polymorphism in the alpha 1 helix of the HLA-B heavy chain can have an overriding influence on peptide-binding specificity. J. Immunol. 158:1660–1669 [PubMed] [Google Scholar]
- 6. Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 57:289–300 [Google Scholar]
- 7. Briggs JA, et al. 2004. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 11:672–675 [DOI] [PubMed] [Google Scholar]
- 8. Carlson J, Heckerman D, Shani G. 2009. Estimating false discovery rates for contingency tables. Microsoft, Mountain View, CA [Google Scholar]
- 9. Carr WH, Pando MJ, Parham P. 2005. KIR3DL1 polymorphisms that affect NK cell inhibition by HLA-Bw4 ligand. J. Immunol. 175:5222–5229 [DOI] [PubMed] [Google Scholar]
- 10. Carrington M, O'Brien SJ. 2003. The influence of HLA genotype on AIDS. Annu. Rev. Med. 54:535–551 [DOI] [PubMed] [Google Scholar]
- 11. Costello C, et al. 1999. HLA-B*5703 independently associated with slower HIV-1 disease progression in Rwandan women. AIDS 13:1990–1991 [DOI] [PubMed] [Google Scholar]
- 12. Crawford H, et al. 2009. Evolution of HLA-B*5703 HIV-1 escape mutations in HLA-B*5703-positive individuals and their transmission recipients. J. Exp. Med. 206:909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crawford H, et al. 2007. Compensatory mutation partially restores fitness and delays reversion of escape mutation within the immunodominant HLA-B*5703-restricted Gag epitope in chronic human immunodeficiency virus type 1 infection. J. Virol. 81:8346–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Culmann B, et al. 1991. Six epitopes reacting with human cytotoxic CD8+ T cells in the central region of the HIV-1 NEF protein. J. Immunol. 146:1560–1565 [PubMed] [Google Scholar]
- 15. Falk K, et al. 1995. Peptide motifs of HLA-B58, B60, B61, and B62 molecules. Immunogenetics 41:165–168 [DOI] [PubMed] [Google Scholar]
- 16. Feeney ME, et al. 2004. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J. Virol. 78:8927–8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fellay J, et al. 2009. Common genetic variation and the control of HIV-1 in humans. PLoS Genet. 5:e1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fellay J, et al. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317:944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fitzgerald DW, et al. 2011. An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: results from a randomized placebo-controlled trial (the Step Study). J. Infect. Dis. 203:765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frahm N, et al. 2005. HLA-B63 presents HLA-B57/B58-restricted cytotoxic T-lymphocyte epitopes and is associated with low human immunodeficiency virus load. J. Virol. 79:10218–10225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frahm N, Baker B, Brander C. 2008. Identification and optimal definition of HIV-derived cytotoxic T-lymphocyte (CTL) epitopes for the study of CTL escape, functional avidity and viral evolution, p 3–24 In Korber BT, et al. (ed), HIV Molecular Immunology. Theoretical Biology and Biophysics, Los Alamos National Laboratory, Los Alamos, NM [Google Scholar]
- 22. Goulder PJ, et al. 2001. Rapid definition of five novel HLA-A*3002-restricted human immunodeficiency virus-specific cytotoxic T-lymphocyte epitopes by ELISPOT and intracellular cytokine staining assays. J. Virol. 75:1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goulder PJ, et al. 1996. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res. Hum. Retroviruses 12:1691–1698 [DOI] [PubMed] [Google Scholar]
- 24. Harndahl M, et al. 2009. Peptide binding to HLA class I molecules: homogenous, high-throughput screening, and affinity assays. J. Biomol. Screen. 14:173–180 [DOI] [PubMed] [Google Scholar]
- 25. Harndahl M, Rasmussen M, Roder G, Buus S. 31 October 2010. Real-time, high-throughput measurements of peptide-MHC-I dissociation using a scintillation proximity assay. J. Immunol. Methods. doi:10.1016/j.jim.2010.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Honeyborne I, et al. 2007. Control of human immunodeficiency virus type 1 is associated with HLA-B*13 and targeting of multiple gag-specific CD8+ T-cell epitopes. J. Virol. 81:3667–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang KH, et al. 2011. Progression to AIDS in South Africa is associated with both reverting and compensatory viral mutations. PLoS One 6:e19018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaslow RA, et al. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405–411 [DOI] [PubMed] [Google Scholar]
- 29. Kiepiela P, et al. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769–775 [DOI] [PubMed] [Google Scholar]
- 30. Kiepiela P, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 [DOI] [PubMed] [Google Scholar]
- 31. Koehler RN, et al. 2010. Class I HLA-A*7401 is associated with protection from HIV-1 acquisition and disease progression in Mbeya, Tanzania. J. Infect. Dis. 202:1562–1566 [DOI] [PubMed] [Google Scholar]
- 32. Leisner C, et al. 2008. One-pot, mix-and-read peptide-MHC tetramers. PLoS One 3:e1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leslie A, et al. 2005. Transmission and accumulation of CTL escape variants drive negative associations between HIV polymorphisms and HLA. J. Exp. Med. 201:891–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leslie A, et al. 2010. Additive contribution of HLA class I alleles in the immune control of HIV-1 infection. J. Virol. 84:9879–9888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leslie AJ, et al. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282–289 [DOI] [PubMed] [Google Scholar]
- 36. Macdonald WA, et al. 2003. A naturally selected dimorphism within the HLA-B44 supertype alters class I structure, peptide repertoire, and T cell recognition. J. Exp. Med. 198:679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin MP, et al. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39:733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martinez-Picado J, et al. 2006. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 80:3617–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matthews PC, et al. 2011. HLA-A*7401-mediated control of HIV viremia is independent of its linkage disequilibrium with HLA-B*5703. J. Immunol. 186:5675–5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matthews PC, et al. 2008. Central role of reverting mutations in HLA associations with human immunodeficiency virus set point. J. Virol. 82:8548–8559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McKinnon LR, et al. 2009. Clade-specific evolution mediated by HLA-B*57/5801 in human immunodeficiency virus type 1 clade A1 p24. J. Virol. 83:12636–12642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Migueles SA, et al. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 97:2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miles JJ, Douek DC, Price DA. 2011. Bias in the alphabeta T-cell repertoire: implications for disease pathogenesis and vaccination. Immunol. Cell Biol. 89:375–387 [DOI] [PubMed] [Google Scholar]
- 44. O'Connor GM, et al. 2007. Functional polymorphism of the KIR3DL1/S1 receptor on human NK cells. J. Immunol. 178:235–241 [DOI] [PubMed] [Google Scholar]
- 45. Payne RP, et al. 2010. Efficacious early antiviral activity of HIV Gag- and Pol-specific HLA-B*2705-restricted CD8+ T cells. J. Virol. 84:10543–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pereyra F, et al. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rodriguez WR, et al. 2004. CD8+ T lymphocyte responses target functionally important regions of protease and integrase in HIV-1 infected subjects. J. Transl. Med. 2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rousseau CM, et al. 2006. Large-scale amplification, cloning and sequencing of near full-length HIV-1 subtype C genomes. J. Virol. Methods 136:118–125 [DOI] [PubMed] [Google Scholar]
- 49. Sacha JB, et al. 2007. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 178:2746–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sacha JB, et al. 2007. Pol-specific CD8+ T cells recognize simian immunodeficiency virus-infected cells prior to Nef-mediated major histocompatibility complex class I downregulation. J. Virol. 81:11703–11712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schellens IM, Kesmir C, Miedema F, van Baarle D, Borghans JA. 2008. An unanticipated lack of consensus cytotoxic T lymphocyte epitopes in HIV-1 databases: the contribution of prediction programs. AIDS 22:33–37 [DOI] [PubMed] [Google Scholar]
- 52. Shehu-Xhilaga M, Crowe SM, Mak J. 2001. Maintenance of the Gag/Gag-Pol ratio is important for human immunodeficiency virus type 1 RNA dimerization and viral infectivity. J. Virol. 75:1834–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sidney J, et al. 2008. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tang J, et al. 2008. Human leukocyte antigen class I genotypes in relation to heterosexual HIV type 1 transmission within discordant couples. J. Immunol. 181:2626–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thomas R, et al. 2009. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat. Genet. 41:1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wearsch PA, Cresswell P. 2008. The quality control of MHC class I peptide loading. Curr. Opin. Cell Biol. 20:624–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yewdell J, et al. 1999. Generating MHC class I ligands from viral gene products. Immunol. Rev. 172:97–108 [DOI] [PubMed] [Google Scholar]
- 58. Yewdell JW, Bennink JR. 2001. Cut and trim: generating MHC class I peptide ligands. Curr. Opin. Immunol. 13:13–18 [DOI] [PubMed] [Google Scholar]
- 59. Yewdell JW, Haeryfar SM. 2005. Understanding presentation of viral antigens to CD8+ T cells in vivo: the key to rational vaccine design. Annu. Rev. Immunol. 23:651–682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.