Abstract
Mammalian orthoreoviruses replicate and assemble in the cytosol of infected cells. A viral nonstructural protein, μNS, forms large inclusion-like structures called viral factories (VFs) in which assembling viral particles can be identified. Here we examined the localization of the cellular chaperone Hsc70 and found that it colocalizes with VFs in infected cells and also with viral factory-like structures (VFLs) formed by ectopically expressed μNS. Small interfering RNA (siRNA)-mediated knockdown of Hsc70 did not affect the formation or maintenance of VFLs. We further showed that dominant negative mutants of Hsc70 were also recruited to VFLs, indicating that Hsc70 recruitment to VFLs is independent of the chaperone function. In support of this finding, μNS was immunoprecipitated with wild-type Hsc70, with a dominant negative mutant of Hsc70, and with the minimal substrate-binding site of Hsc70 (amino acids 395 to 540). We identified a minimal region of μNS between amino acids 222 and 271 that was sufficient for the interaction with Hsc70. This region of μNS has not been assigned any function previously. However, neither point mutants with alterations in this region nor the complete deletion of this domain abrogated the μNS-Hsc70 interaction, indicating that a second portion of μNS also interacts with Hsc70. Taken together, these findings suggest a specific chaperone function for Hsc70 within viral factories, the sites of reovirus replication and assembly in cells.
INTRODUCTION
Mammalian orthoreoviruses have a genome of 10 double-stranded RNA (dsRNA) segments that are encased in a double-layered, nonenveloped capsid. The replication and assembly of reoviruses are thought to take place in distinct cytoplasmic inclusion bodies called viral factories (VFs) (33). The matrix of these structures is formed by the nonstructural viral protein μNS (5). The factories are not static elements but can fuse with other viral factories in the same infected cell (J. S. L. Parker, unpublished findings). During the course of infection, other viral proteins are recruited to the viral factories at distinct times (3, 8, 28). By thin-section electron microscopy, the matrix of viral factories appears to consist of fibrils that have a distinct kink (9, 10, 35). However, no atomic resolution structure of μNS is available. If expressed alone, without other viral proteins, the 80-kDa μNS protein forms viral factory-like structures (VFLs) that resemble VFs in infected cells (5). The carboxyl-terminal (C-terminal) one-third of μNS, comprising amino acids (aa) 471 to 721, is sufficient for VFL formation (2). This minimal factory-forming region has two predicted coiled-coil domains linked by a putative zinc hook and followed by a short C-terminal tail (26). The first one-third of μNS (aa 1 to 221) has been identified as a scaffold for the recruitment of the viral proteins λ1, λ2, μ2, σ2, and σNS; in contrast, the RNA-dependent RNA polymerase (RdRp), λ3, interacts with the C-terminal minimal factory-forming region (5, 27, 28). So far, no function has been elucidated for the middle portion of μNS (aa 222 to 470).
Many viruses are dependent on, or at least aided during their life cycle by, cellular or virally encoded chaperones (24). Cellular chaperones are involved in the folding and refolding of proteins, the disaggregation of protein aggregates, the translocation of proteins across membranes, and the assembly and disassembly of oligomeric protein complexes (reviewed in reference 39). One of the most abundant chaperones in eukaryotic cells is the heat shock cognate protein 70 (Hsc70). In addition, a closely related protein, heat shock protein 70 (Hsp70), is induced during cellular stress. Chaperones are involved in the entry/disassembly of virions, the translocation of the viral genome to the site of replication, replication itself, the packaging of the genome, the folding of capsid proteins, and the assembly of capsids (reviewed in reference 24). During reovirus entry, the outer shell of the double-layered particle is first proteolytically processed to remove the outer capsid protein σ3. The remaining outer capsid protein, μ1, then undergoes autoproteolysis and conformational change that allows the particle to penetrate into the cytosol. Following the entry of this subviral particle into the cytosol of cells, the δ fragment of μ1 remains associated with the viral core particle (reviewed in reference 11). This δ fragment of μ1 is removed from core particles in a process dependent on Hsc70 (19). Furthermore, the folding of the σ1 attachment protein is dependent on Hsp70 and Hsp90 (16, 21).
Here we show that the cellular chaperone Hsc70 specifically interacts with the VF matrix protein μNS.
MATERIALS AND METHODS
Cells and viruses.
CV-1 cells were grown at 37°C under 5% CO2 in Eagle's minimum essential medium (MEM) (CellGro) supplemented with 10% fetal bovine serum (HyClone), 100 U ml−1 of penicillin, 100 μg ml−1 streptomycin, 250 ng ml−1 amphotericin B (antibiotic-antimycotic solution; CellGro), 50 μg ml−1 gentamicin, and nonessential amino acids (CellGro). 293F cells were grown on a shaker (125 rpm) at 37°C under 8% CO2 in FreeStyle 293 expression medium (Gibco) supplemented with 50 U ml−1 penicillin, 50 μg ml−1 streptomycin, and 125 ng ml−1 amphotericin B (antibiotic-antimycotic solution; CellGro). Reoviruses T1L and T3D were laboratory stocks of the isolates previously identified as T1/human/Ohio/Lang/1953 and T3/human/Ohio/Dearing/1955, respectively (17). The superscript N in T3DN differentiates a laboratory stock obtained from the Nibert laboratory from a T3D clone obtained from L. W. Cashdollar (Medical College of Wisconsin), designated T3DC. The T3DC clone differs from the T3DN clone in viral factory morphology and in the nucleotide sequence of its M1 genome segment (32). Viruses were plaque purified and were amplified in murine L929 cells in Joklik's modified minimal essential medium (Gibco) supplemented with 4% fetal bovine serum (HyClone), 2 mM glutamine (CellGro), 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 250 ng ml−1 amphotericin B (antibiotic-antimycotic solution; CellGro), and 50 μg ml−1 gentamicin (Cellgro).
Antibodies and reagents.
The rabbit polyclonal antiserum against μNS has been described previously (4, 36, 37). A rat immunoglobulin G isotype 2a (IgG2a) monoclonal antibody (MAb) against Hsc70 (1B5) was purchased from Stressgen, and a rat IgG1 MAb against hemagglutinin (HA) (3F10) was obtained from Roche Diagnostics (Mannheim, Germany). A rat IgG2a MAb against Trichinella spiralis 18H1.1 (anti-Tsp) was a kind gift from Judith Appleton. A mouse MAb against α-tubulin (DM1A) was purchased from Sigma-Aldrich. A rabbit polyclonal antibody (pAb) against HaloTag7 was purchased from Promega. The secondary antibodies for immunofluorescence (IF) microscopy were goat anti-mouse IgG, goat anti-rabbit IgG, and goat anti-rat IgG conjugated to Alexa 488 or Alexa 594 (Invitrogen). The secondary antibodies for immunoblot detection were donkey anti-rabbit IgG, donkey anti-mouse IgG, and rabbit anti-rat IgG conjugated to horseradish peroxidase (HRP) (Jackson ImmunoResearch). The secondary antibodies used with the Odyssey infrared scanner (Li-Cor) were IRDye 800CW-conjugated goat anti-mouse IgG and IRDye 680-conjugated goat anti-rat IgG (Li-Cor). All antibodies were titrated to optimize signal-to-noise ratios.
Plasmid construction.
Plasmid pCI-M3(T1L) has been described previously (4). The gene encoding bovine Hsc70 was PCR amplified using pEGFP-Hsc70 (courtesy of S. Schmid) as a template. The PCR product was purified, cut with the XhoI and NheI restriction enzymes (New England Biolabs), and then ligated into pCI-neo, which had been cut with the same enzymes. The K71M, D199S, and T204V dominant negative mutants of Hsc70 were cloned similarly from their pEGFP background into pCI-neo. Wild-type (WT) Hsc70 and mutant Hsc70 were tagged at the C terminus with an HA tag by Phusion PCR. The pFN21A-HaloTag7-Flexi and HaloTag control vectors were purchased from Promega. Various truncation and/or point mutants of pCI-Hsc70-HA, pCI-M3(T1L), and pFN21A-HaloTag7-M3(T1L) were constructed using standard mutagenesis. Mutants with the following 20 point mutations of μNS were prepared: H223A, G229A, D232A, E234A, Y236A, N237A, R240A, M242A, F243A, Q245A, H246A, P248A, L249A, Q253A, Y255A, D262A, Y263A, F264A, P268A, and D271A. The R447G point mutation was introduced into pCI-Hsc70-(395-540)-HA. Cloning details and primers are available on request.
Infections and transfections.
CV-1 cells were seeded the day before transfection or infection at a density of 5 × 104 in 12-well plates containing 18-mm-diameter round glass coverslips. Infections were begun by adsorbing virus stocks to cells at a multiplicity of infection (MOI) of 5 PFU/cell, for 1 h at room temperature (RT) in phosphate-buffered saline (PBS) (137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1 mM KH2PO4 [pH 7.5]). Cells were then overlaid with growth medium and were incubated at 37°C for 24 h. CV-1 cells were transfected using the FuGene HD transfection reagent (Roche) according to the manufacturer's instructions. A 7:2 ratio of FuGene HD reagent to DNA was used. Transfected cells were incubated at 37°C for 24 h or as indicated in the figure legends. 293F cells were transfected using 293fectin (Invitrogen) according to the manufacturer's instructions. The day before transfection, 293F cells were set up at a density of 5 × 105 to 7 × 105 per ml in 14 or 28 ml of antibiotic-free FreeStyle 293 expression medium. A 2:1 ratio of 293fectin to DNA was used.
siRNA treatment.
For IF studies, CV-1 cells were seeded the day before transfection at a density of 1.5 × 104 in 12-well plates containing 18-mm-diameter round coverslips. Cells were transfected with 100 nM small interfering RNA (siRNA) J-017609-08 (Dharmacon) by using Dharmafect 1 transfection reagent (Dharmacon) according to the manufacturer's instructions. The complexes were first incubated for 20 min, then added to cells, and finally incubated at 37°C for 48, 72, and 96 h.
To evaluate the knockdown efficiency, CV-1 cells were seeded the day before transfection at a density of 5 × 104 in six-well plates and were transfected as described above with either Hsc70 siRNA or nonspecific control siRNA. Cells were harvested 48 h, 72 h, and 96 h after transfection by trypsinization. Pellets were first lysed in 30 μl of 10% sodium dodecyl sulfate (SDS) and 31.2 mM Tris-HCl (pH 7.4) and were then sonicated with a microtip (power level, 2; duty cycle, 20%; twice, for 10 cycles each time, on ice). The total-protein concentrations of the lysates were determined (DC protein assay; Bio-Rad), and equal amounts were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting. Simultaneous detection of Hsc70 and α-tubulin was performed with the Odyssey infrared scanner (Li-Cor) utilizing rat anti-Hsc70 and mouse anti-α-tubulin as primary antibodies, followed by IRDye 680-conjugated goat anti-rat IgG and IRDye 800CW-conjugated goat anti-mouse IgG as secondary antibodies. The integrated intensity of the selected Hsc70 and α-tubulin bands was measured by the Odyssey program, and the relative amount of Hsc70 was normalized to the amount of α-tubulin. Data from three independent experiments were collected, and differences in the means were evaluated by a paired one-tailed Student t test.
IF microscopy.
Cells on glass coverslips were fixed for 10 min at room temperature in 2% paraformaldehyde in PBS, washed 3 times in PBS, and then permeabilized for 15 min in PBS containing 1% bovine serum albumin (BSA), 0.1% Triton X-100, and 0.05% sodium azide (PBSA-T). Where indicated, cells were fixed with 100% methanol for 20 s at −20°C and were then rehydrated by washing 3 times, for 5 min each time, in PBSA-T. All antibody incubations were carried out for 30 min at room temperature in PBSA-T. Coverslips were washed 3 times in PBS between the primary and secondary antibody incubations. Coverslips were mounted on glass slides with ProLong Gold plus 4′,6-diamidino-2-phenylindole (DAPI) reagent (Molecular Probes). Fluorescence and phase images were obtained with a Nikon TE2000 inverted microscope equipped with fluorescence and phase optics through a 60× (numerical aperture [NA], 1.4) oil objective with 1.5× optical zoom. Images were collected digitally with a Coolsnap HQ charge-coupled device (CCD) camera (Roper) and Openlab software (Improvision) and were then prepared for publication using Photoshop and Illustrator software (Adobe Systems).
Immunoprecipitation (IP) and immunoblotting.
293F cells were harvested by centrifuging the cell suspension for 5 min at 500 × g and 4°C. Cells were washed twice with cold PBS and were lysed in 1 ml lysis buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1% Tween 20, 1 mM phenylmethylsulfonyl fluoride) on ice for 30 min. Lysates were cleared by centrifuging for 15 min at 16,060 × g and 4°C. For each sample, 2.5 μg of anti-Trichinella spiralis (anti-Tsp) or anti-Hsc70 antibody was bound to 30 μl of recombinant protein G agarose (Invitrogen) by rotating for 1 h at room temperature in 500 μl PBS with 0.5 μl of 10% Triton X-100. Afterwards, the antibody-bound agarose was washed 3 times in 1 ml of TBST (Tris-buffered saline–Tween 20) and was incubated 30 min or overnight at 4°C with the precleared cell lysate. Anti-HA antibody 3F10 (and the anti-Tsp control antibody) was added unbound, together with 30 μl of recombinant protein G agarose, to the precleared cell lysate at a concentration of 0.25 μg per sample and was incubated as described above. Samples were washed 4 to 6 times, for 5 min each time, with 1 ml of wash buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 2% Tween 20) on ice. After a final wash with 1 ml PBS, 5 μl of 5× SDS sample buffer was added to each sample, and the mixture was boiled for 5 min at 95°C. Bound proteins were separated by SDS-PAGE. Proteins were transferred from gels to nitrocellulose membranes, which were blocked for 30 min at room temperature in 1× PBS (pH 7.4) containing 0.1% Tween 20, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, and 5% powdered milk. Precipitated proteins were detected with a rabbit anti-μNS antiserum, a rat anti-Hsc70 antibody, and rat anti-HA MAb 3F10 in blocking buffer for 1 h at RT or overnight at 4°C. Membranes were washed 3 times, for 5 min each time, in 1× TBST before incubation with secondary antibodies for 1 h at RT and six final washes of 5 min in 1× TBST. HRP-conjugated donkey anti-rabbit, donkey anti-mouse, and rabbit anti-rat IgG secondary antibodies were used and were detected with SuperSignal West Pico chemiluminescent substrate (Pierce) and fluorography. For consecutive probing with different antibodies, immunoblots were stripped with 10% SDS, 6.25 mM Tris, and 143 mM β-mercaptoethanol, heated to 65°C for 15 min at room temperature, and washed 3 times, for 5 min each time, in 1× TBST.
RESULTS
Hsc70 localizes to VFs in infected cells and colocalizes with ectopically expressed μNS in VFLs.
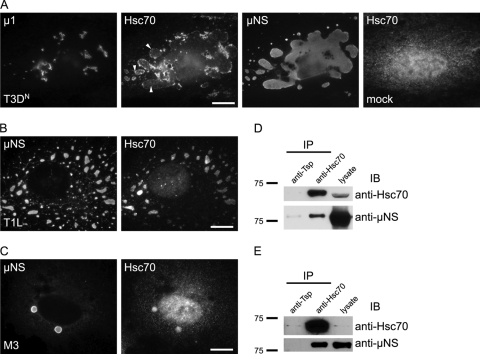
During immunofluorescence experiments to identify cellular proteins that interact with the reovirus outer capsid protein μ1, we noted that the constitutively expressed cellular chaperone Hsc70, in addition to localizing with μ1 on membranous structures, localized to viral factories (Fig. 1A, arrowheads). To confirm this observation, we costained infected cells with antibodies for Hsc70 and μNS. In mock-infected CV-1 cells, Hsc70 was distributed diffusely in the cytosol and nucleus (Fig. 1A, right). However, in cells infected with the T1L, T3DC, or T3DN reovirus, we found that Hsc70 also colocalized with μNS in viral factories (Fig. 1A and B; data not shown for T3DC). Since several viral proteins localize to VFs, we tested whether Hsc70 colocalized with VFLs formed by μNS alone. In CV-1 cells transfected with a plasmid encoding μNS derived from reovirus strain T1L, Hsc70 colocalized with μNS VFLs (Fig. 1C). We conclude from these results that the cellular chaperone Hsc70 colocalizes with VFs and with VFLs formed by μNS expression.
Fig 1.
Hsc70 interacts with the VF matrix protein μNS. (A) (Left and center) T3DN-infected CV-1 cells immunostained for μ1, μNS, and Hsc70. Arrowheads indicate VFs. (Right) Mock-infected CV-1 cells immunostained for Hsc70. (B) T1L-infected CV-1 cells immunostained for μNS and Hsc70. (C) CV-1 cells transfected with a μNS-encoding plasmid [pCI-M3(T1L)] were immunostained for μNS and Hsc70. Bars, 10 μm. (D and E) IP of μNS by Hsc70 from pCI-M3(T1L)-transfected 293F cells (D) or from T3DC-infected L929 cells (E). IB, immunoblot.
μNS coimmunoprecipitates with Hsc70 in infected cells and in cells ectopically expressing μNS.
Having found that Hsc70 and μNS colocalized in VFLs, we determined whether the two proteins interacted by immunoprecipitating endogenous Hsc70 from 293F cells ectopically expressing μNS. Immunoblots probed with an anti-μNS antiserum revealed that μNS coimmunoprecipitated with Hsc70, while a control antibody did not precipitate μNS or Hsc70 (Fig. 1D). To confirm that this interaction also occurred in infected cells, we immunoprecipitated Hsc70 from T3DC-infected cells and similarly found that μNS was coprecipitated with an anti-Hsc70 antibody but not with a control antibody (Fig. 1E). Based on these findings, we conclude that Hsc70 and μNS interact.
The formation and maintenance of VFLs are not impaired by siRNA knockdown of Hsc70.
The matrix of VFs is believed to form by oligomerization of μNS, a process that might require the assistance of chaperones. Given the interaction of Hsc70 and μNS, we tested the possibility that Hsc70 might be required for the formation and/or maintenance of VFLs, since Hsc70 chaperones aid in the oligomerization of some proteins (23). To test this hypothesis, we depleted Hsc70 from CV-1 cells using small interfering RNA (siRNA) and then transfected the cells with a plasmid encoding μNS. We measured the efficiency of Hsc70 depletion by immunoblotting and detection by infrared scanning and found that after treatment with Hsc70 siRNA, 68%, 87%, and 96% of Hsc70 was depleted at 48 h, 72 h, and 96 h posttreatment, respectively (Fig. 2A). To evaluate the role of Hsc70 in the initial formation of VFLs, cells were treated with an Hsc70 or control siRNA and were then transfected with a μNS-encoding plasmid 24 h, 48 h, or 72 h post-siRNA treatment. In this way, μNS was introduced into a cellular environment in which less and less Hsc70 was present. At the last transfection time point, when only 13% of Hsc70 was available, we detected VFLs that were indistinguishable from VFLs formed in control siRNA-treated cells (Fig. 2B).
Fig 2.
Depletion of endogenous Hsc70 does not affect the formation or maintenance of μNS VFLs. (A) siRNA depletion of endogenous Hsc70. (Left) IF images of CV-1 cells stained for Hsc70 at 48, 72, and 96 h after treatment with a control or Hsc70 siRNA. (Right) The efficiency of Hsc70 depletion was quantified by immunoblotting. CV-1 cells were treated with a control (−) or Hsc70 (+) siRNA for 48 h, 72 h, or 96 h. (Top) A representative immunoblot. (Bottom) Quantitation. Bars represent means ± standard deviations for three independent experiments. Asterisks indicate statistically significant differences (by a one-tailed Student t test) between control siRNA- and Hsc70 siRNA-treated cells (*, P < 0.05; **, P < 0.001). (B and C) siRNA depletion of Hsc70 does not affect the formation (B) or maintenance (C) of μNS VFLs. Shown are IF images of CV-1 cells treated with a control (con) or Hsc70 (si) siRNA for 48 h, 72 h, or 96 h and then transfected with pCI-M3(T1L) as diagrammed in the schematics above the IF images. Cells were fixed and immunostained for μNS and Hsc70. Bars, 10 μm.
Viral factories in infected cells are not static in the cytosol but can divide or fuse with other VFs (Parker, unpublished). Hsc70, if dispensable for the initial formation of VFs, could be important for the long-term maintenance or remodeling of VFs. Therefore, we treated cells with Hsc70 siRNA as before and then transfected the cells with a plasmid encoding μNS 24 h later. We then evaluated the morphology of VFLs at 48, 72, and 96 h after siRNA treatment (24, 48, and 72 h after μNS transfection) by immunofluorescence. We found no obvious difference in the size, shape, or number of μNS VFLs between control siRNA-treated and Hsc70 siRNA-treated cells (Fig. 2C). Because the data were evaluated subjectively, it is possible that Hsc70 knockdown had minor effects on VFLs that we did not detect. However, after we carefully examined three independent experiments for the initial formation and long-term maintenance of VFLs after Hsc70 depletion, we concluded that a lack of Hsc70 does not affect the formation and/or maintenance of VFLs.
Dominant negative mutants of Hsc70 still colocalize with μNS.
To further investigate how Hsc70 was recruited by μNS, we examined the capacity of different dominant negative mutants of Hsc70 to be recruited to μNS VFLs. Dominant negative mutants of Hsc70 have a mutation in their nucleotide binding site that affects the ATP-ADP cycle of the chaperone (29). The ATPase rate of Hsc70(K71M) is undetectable, while that of Hsc70(D199S) is reduced 50-fold. These two mutants are predominantly ATP bound, and they bind and release the substrate rapidly (30, 38). A third mutant, Hsc70(T204V), has normal ATP hydrolysis activity but 100-fold-reduced ATP-binding activity (31). Thus, Hsc70(T204V) is predominantly ADP bound and binds and releases the substrate slowly. Because the anti-Hsc70 antibody cannot distinguish between endogenous Hsc70 and mutant Hsc70, the dominant negative mutants were HA tagged. When we coexpressed the dominant negative Hsc70 mutants with μNS, we found that, like wild-type Hsc70, all the dominant negative mutants colocalized with VFLs formed by μNS (Fig. 3). Note that differences in the numbers of VFLs in individual cells were not related to overexpression of dominant negative forms of Hsc70. Such differences are seen in all populations of cells ectopically expressing μNS (S. Kaufer and J. S. L. Parker, unpublished observations). We conclude from these results that the interaction between Hsc70 and μNS likely does not require Hsc70 chaperone activity.
Fig 3.

Dominant negative mutants of Hsc70 colocalize with μNS VFLs. CV-1 cells were cotransfected with plasmids expressing μNS and HA-tagged WT or dominant negative mutant Hsc70. Cells were fixed and were immunostained for μNS and the HA tag 24 h posttransfection. Bars, 10 μm.
Mapping of the minimal region of Hsc70 required for interaction with μNS.
The colocalization of the dominant negative mutants of Hsc70 with μNS VFLs suggested that the interaction of Hsc70 with μNS was independent of the Hsc70 chaperone function. Therefore, to identify the region of Hsc70 that interacted with μNS, we constructed C-terminally HA-tagged truncation mutants of Hsc70 that comprised either the N-terminal ATPase domain (aa 1 to 394), including the interdomain linker (aa 384 to 394), or the C-terminal substrate-binding domain (SBD) (aa 395 to 650) (20) (Fig. 4A). As a control, we also tested an HA-tagged version of the VP1 protein of feline calicivirus, which is not known to interact with μNS. We coexpressed each of the truncated Hsc70 domains with μNS and immunoprecipitated the HA-tagged Hsc70 domains with an anti-HA antibody. Immunoblotting showed that μNS coimmunoprecipitated with the SBD (aa 395 to 650), but not with the ATPase domain, of Hsc70 (Fig. 4B). As expected, μNS did not coimmunoprecipitate with the VP1-HA control protein (data not shown).
Fig 4.

The ATPase domain of Hsc70 is not required for its interaction with μNS. (A) Diagram of Hsc70 subdomains and truncation mutants. SBD, substrate-binding domain. (B) 293F cells were cotransfected with the μNS-encoding plasmid and either the HA-tagged ATPase domain or the HA-tagged SBD of Hsc70. HA-tagged constructs were immunoprecipitated from cell lysates with an anti-HA MAb and were then immunoblotted (IB) and probed for the HA tag or μNS. A control antibody (anti-Tsp) did not immunoprecipitate tagged Hsc70 or μNS. (C) 293F cells were cotransfected with plasmids encoding μNS and the HA-tagged truncation mutants of Hsc70 diagramed in panel A. Immunoprecipitates were prepared and immunoblotted as for panel B. (D) IF images of CV-1 cells ectopically expressing μNS and Hsc70(1-394) (top) or μNS and Hsc70(395-540) (bottom). Cells were fixed with 100% methanol. Bars, 2 μm.
The SBD of Hsc70 can be subdivided into the substrate-binding site, which consists of β-sheets connected by loops, and an α-helical 10-kDa C-terminal tail (Fig. 4A). The C-terminal tail of Hsc70 is not involved in substrate binding but has been found to interact with different cochaperones via distinct interaction motifs (13, 22). The EEVD motif at the very C terminus of Hsc70 is necessary for Hsc70 interaction with Hsp40 cochaperones (14). Another cochaperone interaction domain comprises an α-helical domain and multiple degenerate repeats of the tetrapeptide GGMP (1). To define the interacting region of the SBD of Hsc70 for μNS, we created (i) a mutant of the SBD that was truncated at aa 540 and thus only contained the minimal substrate-binding site, (ii) a truncation mutant comprising aa 395 to 560, which includes the first α-helix of the α-helical domain, (iii) a mutant that lacks the GGXP repeats and the EEVD motif (aa 395 to 614), and (iv) the SBD without the C-terminal EEVD motif (aa 395 to 646) (Fig. 4A). When these mutants were coexpressed with μNS, we found that the 395-540, 395-614, and 395-646 constructs interacted with μNS (Fig. 4C). We confirmed that the minimal region of Hsc70 (aa 395 to 540) localized to VFLs by coexpressing this construct with μNS in CV-1 cells (Fig. 4D, bottom panels). In agreement with our IP findings, the ATPase domain of Hsc70 (aa 1 to 394) did not colocalize with μNS VFLs (Fig. 4D, top panels). In the immunoblot shown in Fig. 4C, the HA-tagged deletion mutant Hsc70(395-560) did not pull down μNS; however, we have observed that this mutant is pulled down at very low levels in some immunoprecipitations. This mutant colocalized with μNS VFLs in IF experiments (data not shown), suggesting that the interaction in vitro may be sterically hindered or that this particular mutant does not always fold properly. Taken together, these results indicate that the GGMP tetrapeptide repeats and the EEVD motif are dispensable for the interaction of Hsc70 with μNS and that μNS interacts with the minimal substrate-binding site (aa 395 to 540) of Hsc70.
Mapping of the minimal necessary μNS interaction domain.
Our knockdown experiments suggested that Hsc70 is dispensable for VFL formation. VFs are the sites of viral replication and assembly. The viral structural proteins (λ1, λ2, λ3, μ1, μ2, σ2, and σ3) and the nonstructural protein σNS are recruited to VFs in infected cells (3, 5, 8, 28, 34). In cells ectopically expressing μNS, the λ1, λ2, λ3, μ2, σ2, and σNS proteins are recruited to VFLs independently of each other (3, 27, 28). Therefore, it is possible that Hsc70 plays a role within VFs in preventing the aggregation of partially folded viral proteins prior to their assembly. Alternatively, Hsc70 may play a role in virion assembly or replication. To identify the minimal region of μNS necessary for its interaction with Hsc70, we prepared different μNS truncation mutants and tested their capacities to coimmunoprecipitate with full-length Hsc70. In contrast to full-length μNS, the minimal region of μNS necessary for the formation of VFLs (aa 471 to 721) (2) did not interact with Hsc70 (Fig. 5A). This result supported our finding that viral factory formation occurred independently of Hsc70 and indicated that the region encompassing the N-terminal 470 amino acids of μNS interacts with Hsc70.
Fig 5.

The region of μNS that interacts with Hsc70 lies between aa 222 and 470. (A) 293F cells were transfected with a plasmid encoding full-length μNS (aa 1 to 721) or the minimal factory-forming region of μNS (aa 471 to 721). Cell lysates were prepared and immunoprecipitated with an anti-Tsp (control) or anti-Hsc70 antibody. Immunoblots were probed for μNS or Hsc70 as indicated. (Bottom left) A longer exposure of the blot probed with anti-μNS. (B) Hsc70 in lysates from 293F cells ectopically expressing full-length μNS or the indicated μNS truncation mutant was immunoprecipitated as described above, and the immunoblots were probed for μNS and Hsc70. (C) Immunoprecipitation of full-length μNS (aa 1 to 721) and truncated μNS (aa 1 to 221) with the anti-Tsp (control) or anti-Hsc70 antibody using four times as much cell lysate for aa 1 to 221 as for aa 1 to 721. Immunoblots were probed for μNS or Hsc70 as indicated. (D) 293F cells were cotransfected with full-length μNS(1-721) or μNS(1-470) together with the HA-tagged SBD (aa 395 to 650) of Hsc70 or the dominant negative T204V mutant of Hsc70 (DN). An anti-HA MAb was used to immunoprecipitate the HA-tagged Hsc70 constructs, and immunoblots were probed for coprecipitation of μNS. The boxed area at the top right of the blot probed with anti-HA shows a longer exposure of the higher-molecular-weight bands.
The first 221 aa of μNS serve as a scaffold for the recruitment of several of the reoviral structural proteins (3, 5, 27). To determine whether Hsc70 interacts with this N-terminal region of μNS, we created two truncation mutants comprising aa 1 to 221 and aa 1 to 470. We found that the longer of these truncation mutants (aa 1 to 470), but not the shorter (aa 1 to 221), coimmunoprecipitated with Hsc70 (Fig. 5B). Because the short fragment (aa 1 to 221) was not expressed at levels similar to those of full-length μNS, we repeated the immunoprecipitation using 4 times the volume of transfected cells. μNS(1-221) still was not coimmunoprecipitated with Hsc70 (Fig. 5C). Since Hsc70 did not interact with aa 471 to 721 of μNS, we inferred that the Hsc70-interacting region of μNS lies between amino acids 222 and 470.
Hsc70 is often recruited to protein aggregates (25). To ensure that the truncation mutants of μNS were not misfolding, we examined immunofluorescence images of the expressed proteins for colocalization with polyubiquitin. The distribution of μNS(1-470) and μNS(1-221) in transfected cells was diffuse throughout the cytosol, and we did not see aggregates that colocalized with polyubiquitin (data not shown). In addition, we found that a coexpressed HA-tagged dominant negative form of Hsc70 (the T204V mutant) and the SBD of Hsc70 immunoprecipitated both μNS(1-470) and WT μNS(1-721) (Fig. 5D). A small amount of full-length μNS was nonspecifically pulled down by the control antibody, but this was not a consistent finding (compare Fig. 5B and D). These results suggest that the interaction of Hsc70 with the μNS fragment was not due to misfolding of the μNS fragment and did not require functional chaperone activity.
To further narrow down the minimal domain of μNS necessary for interaction with Hsc70, we prepared additional truncation mutants of μNS comprising aa 1 to 271, 1 to 301, 1 to 341, 1 to 381, 1 to 396, and 1 to 431. To reduce the risk that the truncated proteins would misfold, the truncations were placed in predicted hydrophilic locations of the primary sequence of μNS. Although we had already established that the N-terminal 221 amino acids of μNS did not interact with Hsc70, we included this portion of the protein in the new mutants in order to maintain proper N-terminal folding. After co-IP using an anti-Hsc70 antibody, we found that all six truncation mutants were able to bind Hsc70, suggesting that the minimal region of μNS necessary for interaction with Hsc70 lies between amino acids 222 and 271 (Fig. 6A).
Fig 6.
Amino acids 222 to 271 of μNS interact with Hsc70. (A) 293F cells were transfected with plasmids encoding the indicated C-terminal truncations of μNS. Cell lysates were prepared 24 h posttransfection, and Hsc70 was immunoprecipitated with anti-Hsc70 antibodies. Immunoblots were then probed for μNS or Hsc70 as indicated. Numbers below the gels indicate the amino acids of μNS in the truncation mutant; 1-721 represents full-length μNS. (B) 293F cells were cotransfected with plasmids encoding the HA-tagged SBD of Hsc70 (aa 395 to 650) and the indicated HaloTagged fragments of μNS. Numbers below the gels indicate the amino acids of μNS in the construct. HA-tagged proteins were immunoprecipitated from cell lysates, immunoblotted, and probed with anti-HaloTag and anti-HA antibodies. (C) 293F cells were cotransfected with plasmids encoding HaloTagged μNS(222-271) or with the HaloTag vector alone (Halo) together with HA-tagged Hsc70(395-540) or Hsc70(395-650) (SBD). HA-tagged proteins were immunoprecipitated from cell lysates, immunoblotted, and probed with anti-HaloTag and anti-HA antibodies. (D) 293F cells were cotransfected with plasmids encoding HaloTagged μNS(222-271) and a HA-tagged point mutant of Hsc70(395-540) carrying the R447G mutation. HA-tagged proteins were immunoprecipitated from cell lysates, immunoblotted, and probed with anti-HaloTag and anti-HA antibodies.
Mapping of the minimal region of μNS sufficient to interact with Hsc70.
To determine if aa 222 to 271 of μNS were sufficient to recruit Hsc70 to the viral factories, we prepared HA-tagged μNS truncation mutants that lacked the N-terminal 221 amino acids, resulting in fragments starting at aa 222 and ending at aa 271, 301, 341, 381, 396, or 431. Unfortunately, none of these constructs was expressed at detectable levels (data not shown). We hypothesized that the loss of the natural N terminus led to misfolding and rapid degradation of the μNS fragments.
Because the HA-tagged μNS fragments were not expressed, we tagged the constructs at their N termini with a larger soluble protein tag, HaloTag, that is derived from bacterial hydrolase in hopes that it might stabilize the μNS fragments and allow their proper folding. We prepared the truncation mutants described above plus an additional truncation mutant comprising aa 222 to 470. All of the constructs bearing the N-terminal HaloTag were expressed at detectable levels. We therefore coexpressed the constructs with the HA-tagged SBD of Hsc70 (aa 395 to 650) and immunoprecipitated using an anti-HA antibody. We found that all of the HaloTagged μNS fragments tested interacted with the SBD of Hsc70. However, the HaloTag protein expressed alone did not interact with the Hsc70 SBD (Fig. 6B). We concluded from this finding that aa 222 to 271 of μNS are sufficient for interaction with the SBD of Hsc70.
We also tested whether the minimal region of μNS that interacted with full-length Hsc70 was capable of interacting with the minimal interaction domain of Hsc70. We coexpressed HaloTagged μNS(222-271) or the HaloTag protein alone together with the HA-tagged substrate-binding site (aa 395 to 540) or SBD (aa 395 to 650) of Hsc70 and immunoprecipitated using an anti-HA antibody. We found that the μNS fragment was coimmunoprecipitated (Fig. 6C), and we thus deduced that the minimal interaction domains identified in μNS and Hsc70 are sufficient for interaction with each other.
Although we had found that Hsc70 did not require its chaperoning function to interact with μNS, we found that μNS bound the minimal substrate-binding site of Hsc70. To investigate whether this interaction was a consequence of the binding of μNS as a substrate, we introduced the R447G point mutation into the minimal substrate-binding site of Hsc70 [Hsc70(395-540)-R447G-HA]. This mutation increases the rate of substrate dissociation from the substrate-binding domain of Hsc70, thus reducing the interaction of the SBD with the substrate to undetectable levels (18). We coexpressed the minimal interaction domains of μNS and Hsc70 carrying the R447G mutation and immunoprecipitated them via the HA-tagged Hsc70 fragment. Although the R447G mutation is known to cause a marked decrease in substrate binding, we detected no difference between the capacities of Hsc70(395-540)-R447G-HA and the wild-type form of this Hsc70 fragment to interact with μNS(222-271) in three independent experiments (Fig. 6D). We conclude that although μNS binds the substrate-binding domain of Hsc70, the μNS-Hsc70 association is not a substrate-chaperone complex.
Point mutants in μNS in the region between amino acids 222 and 271 fail to disrupt the μNS-Hsc70 interaction.
Having identified the region between amino acids 222 and 271 of μNS as sufficient for interaction with Hsc70, we prepared 20 point mutants (see Materials and Methods) with mutations within this region in order to identify individual residues important for the μNS-Hsc70 interaction. We chose these mutations on the basis of the predicted secondary structure of this region of μNS (Jpred, APSSP, CFSSP, NetSurfP) and residue conservation with other homologous reovirus proteins (the Muscovy duck reovirus μC protein, the avian orthoreovirus μNS protein, and the American grass carp reovirus NS73 protein) (the μNS protein is not homologous to any nonreovirus protein). We prepared several mutations within two predicted α-helices spanning aa 235 to 243 and aa 261 to 266, and we used immunofluorescence to analyze the capacities of the mutants to form VFLs. Three mutants, the F243A, H246A, and Y263A mutants, differed from WT μNS by being unable to form large VFLs and instead forming many very small, pinpoint-like structures in transfected cells (data not shown). Costaining for polyubiquitin revealed that these structures colocalized with ubiquitin, indicating that these three point mutants were likely misfolding and aggregating. The other mutants formed VFLs comparable to those formed by wild-type μNS and did not colocalize with ubiquitin [an example, μNS(D271A), is shown in Fig. 7A]. All of the mutants coimmunoprecipitated with Hsc70 (data not shown). From these results we concluded that either single point mutants were insufficient to disrupt the μNS-Hsc70 association or an additional Hsc70-interacting region of μNS existed.
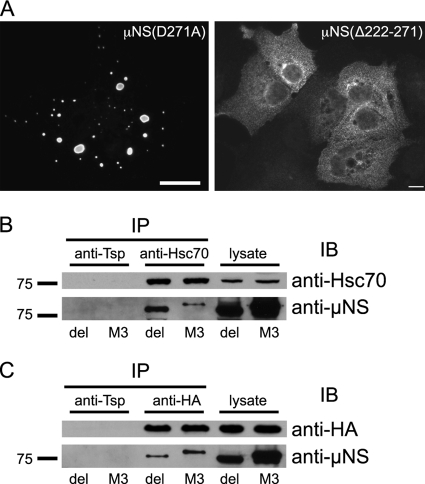
Fig 7.
A deletion mutant of μNS lacking the minimal interaction domain (aa 222 to 271) still interacts with Hsc70. (A) Immunofluorescence images showing the distribution of the μNS(D271A) point mutant (left) and the μNS(Δ222-271) mutant (right). CV-1 cells were fixed and immunostained for μNS at 24 h posttransfection. Bars, 10 μm. (B) 293F cells were transfected with a plasmid encoding μNS(Δ222-271) (del) or full-length μNS (M3). Hsc70 was immunoprecipitated from cell lysates, immunoblotted, and probed with anti-Hsc70 and anti-μNS antibodies. (C) 293F cells were cotransfected with a plasmid encoding μNS(Δ222-271) (del) or full-length μNS (M3) together with the HA-tagged SBD of Hsc70 [Hsc70(395-650)]. HA-tagged proteins were immunoprecipitated from cell lysates, immunoblotted, and probed with anti-HA and anti-μNS antibodies.
Deletion of residues 222 to 271 from μNS does not abrogate its capacity to interact with Hsc70.
To test the hypothesis that an additional region of μNS interacted with Hsc70, we prepared a deletion mutant of μNS lacking aa 222 to 271 [μNS(Δ222-271)]. This deletion mutant was unable to form VFLs at 24 h posttransfection. Instead, it was diffusely distributed in most transfected cells, occasionally forming aggregates (Fig. 7A) that colocalized with polyubiquitin (data not shown). This deletion mutant coimmunoprecipitated with full-length Hsc70 (Fig. 7B). This interaction was not likely due to misfolding of the protein, since we found that it also interacted with the SBD of Hsc70, which lacks a chaperoning function (Fig. 7C). Taken together, these findings suggest that the initially defined minimal interaction domain lying between aa 222 and 271 of μNS is not the only region of μNS that interacts with Hsc70 and that an additional region of μNS between aa 272 and 470 likely interacts with Hsc70.
DISCUSSION
Chaperones are required for the efficient replication of many viruses. The Escherichia coli Hsp70 homolog DnaK was discovered as an essential bacterial factor for the replication of bacteriophage λ (15). Cellular or viral chaperones play roles in virus entry, the translocation of viral proteins to different cellular compartments, genome replication, or the assembly and morphogenesis of progeny virions (reviewed in reference 24). The cellular chaperone Hsc70 is thought to be involved in the uncoating of reovirus particles during virus entry, and Hsp70 is required for posttranslational folding and assembly of the globular portion of the trimeric σ1 attachment protein (16, 19, 21). Our findings in this paper indicate that the constitutively expressed protein Hsc70 is involved in other phases of the reovirus life cycle. Hsc70 was recruited to viral factories via interaction with the factory matrix protein μNS. Although this interaction was mediated by the minimal substrate-binding site of Hsc70, it likely occurs independently of the chaperoning function of Hsc70, since we found that μNS coimmunoprecipitated with dominant negative mutants of Hsc70. Coexpression of these dominant negative mutants of Hsc70 had no detectable effect on VFLs, and they were found to colocalize with the μNS factories. Also, a mutant of the minimal substrate-binding site of Hsc70 (the R447G mutant) with an impaired capacity to bind substrates was able to coimmunoprecipitate μNS as well as did the wild-type form of this fragment. Additional support for the idea that μNS is not a substrate for Hsc70 was provided by our finding that Hsc70 knockdown did not influence the size or morphology of VFLs or their maintenance. Despite these findings, it remains possible that μNS is a substrate for Hsc70. The knockdown of Hsc70 was not complete, and we have found that the inducible form of Hsc70, Hsp70, is also recruited to viral factories (unpublished data). In addition, although Hsc70 (HSPA8) is the major cytosolic chaperone that is constitutively expressed, at least five other HSP70 family members (HSPA1A, HSPA1B, HSPA1L, HSPA2, and HSPA6) are expressed in the cytosol (12). The siRNA used in the experiments described here would not be predicted to reduce the levels of the other five HSP70 chaperones. Thus, we cannot rule out the possibility that μNS is a substrate for Hsc70; however, the co-IP of dominant negative mutants of Hsc70 strongly suggests that μNS serves as a spatial anchor for the recruitment of Hsc70.
The N-terminal one-third (aa 1 to 221) of μNS is responsible for recruiting most of the viral core proteins individually (λ1, λ2, μ2, and σ2), as well as the assembled core particle (3, 5, 27) and nonstructural protein σNS (28), while the C-terminal one-third (aa 471 to 721) contains the minimal factory forming region and has been shown to recruit the λ3 polymerase (27). In contrast, the middle part of μNS (aa 222 to 470) has no assigned function. Our findings here indicate that a region between amino acids 222 and 271 within this portion of μNS serves to recruit the Hsc70 chaperone. Although our data clearly show that μNS(222-271) is sufficient to recruit Hsc70, when these residues were deleted from the full-length μNS protein, we still detected an association with Hsc70 that was not likely due to misfolding of this mutant, suggesting that an additional region of μNS, potentially between aa 272 and 470, also interacts with Hsc70. The presence of a second region of μNS that interacts with Hsc70 may explain our failure to identify point mutants in the μNS(222-271) region that prevented the interaction.
Why is Hsc70 recruited to μNS VFLs and VFs in infected cells? The μNS protein has been hypothesized to act as a scaffold for the recruitment of viral proteins required for the assembly of new viral particles and the replication of the viral genome (3, 27). To this end, it seems possible that chaperones are required to assemble the viral structural proteins to form virions. The reovirus virion is a double-shelled particle that comprises eight structural proteins (λ1, λ2, λ3, μ1, μ2, σ1, σ2, and σ3). The addition of the outer shell of the virion to core particles shuts off transcription, and it seems possible that this process is regulated by chaperones to prevent premature termination of viral transcription. We have found that the μ1 and σ3 heterohexameric complexes are associated with Hsc70 (Kaufer and Parker, unpublished). Chaperones have also been shown to be involved in genome replication for several viruses (6, 7). It is also possible that Hsc70 is involved in the assortment of genome segments for packaging into assembling viral core particles.
Another possible reason for the recruitment of the Hsc70 chaperone is to aid in the oligomerization or deoligomerization of μNS itself. In vitro experiments indicate that viral cores readily associate with purified μNS; however, the presence of μNS on viral cores inhibits the assembly of outer capsid proteins (4). Therefore, another possible function for Hsc70 chaperones would be to remove μNS from core particles in order to allow the assembly of the outer capsid proteins to form infectious virions. We found that Hsc70 is capable of interacting with a deoligomerized form of μNS, namely, a point mutant (the H570Q mutant) that does not form viral factories but is diffusely distributed in cells (unpublished findings).
A mutant of μNS in which the region between aa 222 and 271 was deleted formed VFLs. However, these VFLs were small in comparison to those derived from WT μNS protein. This observation suggests that the region between aa 222 and 271 of μNS is important for the formation of larger VFLs. Whether and how Hsc70 binding to μNS and factory formation are connected remain to be elucidated. Our experiments with siRNA-mediated knockdown of Hsc70 failed to reveal any detectable effect of the absence of Hsc70 on VFL formation. However, Hsp70 is also recruited to VFLs and could compensate for the loss of Hsc70 in these experiments.
In summary, we have defined a region of μNS that specifically interacts with the constitutively expressed cellular chaperone Hsc70. Future experiments will define the function of this chaperone in reovirus replication.
ACKNOWLEDGMENTS
We thank Brian Ingel, Lynne Anguish, and Brenda Werner for excellent technical assistance. We thank Judy Appleton and Sondra Schmid for the kind gift of reagents.
This research was supported by Public Health Service award R01 AI063036 (to J.S.L.P.).
Footnotes
Published ahead of print 16 November 2011
REFERENCES
- 1. Ballinger CA, et al. 1999. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 19:4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Broering TJ, et al. 2005. Carboxyl-proximal regions of reovirus nonstructural protein μNS necessary and sufficient for forming factory-like inclusions. J. Virol. 79:6194–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broering TJ, et al. 2004. Reovirus nonstructural protein μNS recruits viral core surface proteins and entering core particles to factory-like inclusions. J. Virol. 78:1882–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broering TJ, McCutcheon AM, Centonze VE, Nibert ML. 2000. Reovirus nonstructural protein μNS binds to core particles but does not inhibit their transcription and capping activities. J. Virol. 74:5516–5524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broering TJ, Parker JS, Joyce PL, Kim J, Nibert ML. 2002. Mammalian reovirus nonstructural protein μNS forms large inclusions and colocalizes with reovirus microtubule-associated protein μ2 in transfected cells. J. Virol. 76:8285–8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown G, et al. 2005. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology 338:69–80 [DOI] [PubMed] [Google Scholar]
- 7. Chen YJ, et al. 2010. Heat shock protein 72 is associated with the hepatitis C virus replicase complex and enhances viral RNA replication. J. Biol. Chem. 285:28183–28190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coffey CM, et al. 2006. Reovirus outer capsid protein μ1 induces apoptosis and associates with lipid droplets, endoplasmic reticulum, and mitochondria. J. Virol. 80:8422–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dales S. 1963. Association between the spindle apparatus and reovirus. Proc. Natl. Acad. Sci. U. S. A. 50:268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dales S, Gomatos P, Hsu KC. 1965. The uptake and development of reovirus in strain L cells followed with labelled viral ribonucleic acid and ferritin-antibody conjugates. Virology 25:193–211 [DOI] [PubMed] [Google Scholar]
- 11. Danthi P, et al. 2010. From touchdown to transcription: the reovirus cell entry pathway. Curr. Top. Microbiol. Immunol. 343:91–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daugaard M, Rohde M, Jaattela M. 2007. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 581:3702–3710 [DOI] [PubMed] [Google Scholar]
- 13. Demand J, Luders J, Hohfeld J. 1998. The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol. Cell. Biol. 18:2023–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freeman BC, Myers MP, Schumacher R, Morimoto RI. 1995. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 14:2281–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Georgopoulos CP. 1977. A new bacterial gene (groPC) which affects lambda DNA replication. Mol. Gen. Genet. 151:35–39 [DOI] [PubMed] [Google Scholar]
- 16. Gilmore R, Coffey MC, Leone G, McLure K, Lee PW. 1996. Co-translational trimerization of the reovirus cell attachment protein. EMBO J. 15:2651–2658 [PMC free article] [PubMed] [Google Scholar]
- 17. Goral MI, Mochow-Grundy M, Dermody TS. 1996. Sequence diversity within the reovirus S3 gene: reoviruses evolve independently of host species, geographic locale, and date of isolation. Virology 216:265–271 [DOI] [PubMed] [Google Scholar]
- 18. Hu SM, Liang PH, Hsiao CD, Wang C. 2002. Characterization of the L399P and R447G mutants of hsc70: the decrease in refolding activity is correlated with an increase in the rate of substrate dissociation. Arch. Biochem. Biophys. 407:135–141 [DOI] [PubMed] [Google Scholar]
- 19. Ivanovic T, Agosto MA, Chandran K, Nibert ML. 2007. A role for molecular chaperone Hsc70 in reovirus outer capsid disassembly. J. Biol. Chem. 282:12210–12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang J, Prasad K, Lafer EM, Sousa R. 2005. Structural basis of interdomain communication in the Hsc70 chaperone. Mol. Cell 20:513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leone G, et al. 1996. C-terminal trimerization, but not N-terminal trimerization, of the reovirus cell attachment protein is a posttranslational and Hsp70/ATP-dependent process. J. Biol. Chem. 271:8466–8471 [DOI] [PubMed] [Google Scholar]
- 22. Liu FH, Wu SJ, Hu SM, Hsiao CD, Wang C. 1999. Specific interaction of the 70-kDa heat shock cognate protein with the tetratricopeptide repeats. J. Biol. Chem. 274:34425–34432 [DOI] [PubMed] [Google Scholar]
- 23. Mayer MP. 2010. Gymnastics of molecular chaperones. Mol. Cell 39:321–331 [DOI] [PubMed] [Google Scholar]
- 24. Mayer MP. 2005. Recruitment of Hsp70 chaperones: a crucial part of viral survival strategies. Rev. Physiol. Biochem. Pharmacol. 153:1–46 [DOI] [PubMed] [Google Scholar]
- 25. Mayer MP, Bukau B. 2005. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 62:670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCutcheon AM, Broering TJ, Nibert ML. 1999. Mammalian reovirus M3 gene sequences and conservation of coiled-coil motifs near the carboxyl terminus of the μNS protein. Virology 264:16–24 [DOI] [PubMed] [Google Scholar]
- 27. Miller CL, Arnold MM, Broering TJ, Hastings CE, Nibert ML. 2010. Localization of mammalian orthoreovirus proteins to cytoplasmic factory-like structures via nonoverlapping regions of μNS. J. Virol. 84:867–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller CL, Broering TJ, Parker JS, Arnold MM, Nibert ML. 2003. Reovirus σNS protein localizes to inclusions through an association requiring the μNS amino terminus. J. Virol. 77:4566–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newmyer SL, Schmid SL. 2001. Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J. Cell Biol. 152:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Brien MC, Flaherty KM, McKay DB. 1996. Lysine 71 of the chaperone protein Hsc70 is essential for ATP hydrolysis. J. Biol. Chem. 271:15874–15878 [DOI] [PubMed] [Google Scholar]
- 31. O'Brien MC, McKay DB. 1993. Threonine 204 of the chaperone protein Hsc70 influences the structure of the active site, but is not essential for ATP hydrolysis. J. Biol. Chem. 268:24323–24329 [PubMed] [Google Scholar]
- 32. Parker JS, Broering TJ, Kim J, Higgins DE, Nibert ML. 2002. Reovirus core protein μ2 determines the filamentous morphology of viral inclusion bodies by interacting with and stabilizing microtubules. J. Virol. 76:4483–4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rhim JS, Jordan LE, Mayor HD. 1962. Cytochemical, fluorescent-antibody and electron microscopic studies on the growth of reovirus (ECHO 10) in tissue culture. Virology 17:342–355 [DOI] [PubMed] [Google Scholar]
- 34. Schmechel S, Chute M, Skinner P, Anderson R, Schiff L. 1997. Preferential translation of reovirus mRNA by a σ3-dependent mechanism. Virology 232:62–73 [DOI] [PubMed] [Google Scholar]
- 35. Spendlove RS, Lennette EH. 1963. The role of the mitotic apparatus in the intracellular location of reovirus antigen. J. Immunol. 90:554–560 [PubMed] [Google Scholar]
- 36. Tyler KL, Mann MA, Fields BN, Virgin HW., IV 1993. Protective anti-reovirus monoclonal antibodies and their effects on viral pathogenesis. J. Virol. 67:3446–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Virgin HW, IV, Mann MA, Fields BN, Tyler KL. 1991. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J. Virol. 65:6772–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilbanks SM, DeLuca-Flaherty C, McKay DB. 1994. Structural basis of the 70-kilodalton heat shock cognate protein ATP hydrolytic activity. I. Kinetic analyses of active site mutants. J. Biol. Chem. 269:12893–12898 [PubMed] [Google Scholar]
- 39. Young JC, Agashe VR, Siegers K, Hartl FU. 2004. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 5:781–791 [DOI] [PubMed] [Google Scholar]