Abstract
HIV-2 has a lower pathogenicity and transmission rate than HIV-1. Neutralizing antibodies could be contributing to these observations. Here we explored side by side the potency and breadth of intratype and intertype neutralizing activity (NAc) in plasma of 20 HIV-1-, 20 HIV-2-, and 11 dually HIV-1/2 (HIV-D)-seropositive individuals from Guinea-Bissau, West Africa. Panels of primary isolates, five HIV-1 and five HIV-2 isolates, were tested in a plaque reduction assay using U87.CD4-CCR5 cells as targets. Intratype NAc in HIV-2 plasma was found to be considerably more potent and also broader than intratype NAc in HIV-1 plasma. This indicates that HIV-2-infected individuals display potent type-specific neutralizing antibodies, whereas such strong type-specific antibodies are absent in HIV-1 infection. Furthermore, the potency of intratype NAc was positively associated with the viral load of HIV-1 but not HIV-2, suggesting that NAc in HIV-1 infection is more antigen stimulation dependent than in HIV-2 infection, where plasma viral loads typically are at least 10-fold lower than in HIV-1 infection. Intertype NAc of both HIV-1 and HIV-2 infections was, instead, of low potency. HIV-D subjects had NAc to HIV-2 with similar high potency as singly HIV-2-infected individuals, whereas neutralization of HIV-1 remained poor, indicating that the difference in NAc between HIV-1 and HIV-2 infections depends on the virus itself. We suggest that immunogenicity and/or antigenicity, meaning the neutralization phenotype, of HIV-2 is distinct from that of HIV-1 and that HIV-2 may display structures that favor triggering of potent neutralizing antibody responses.
INTRODUCTION
Whereas human immunodeficiency virus type 1 (HIV-1) is spread globally, HIV-2 is mainly confined to West Africa, with the highest prevalence being in the general population of Guinea-Bissau (15, 37). It has been proposed that the spread of HIV-2 in this region may have been facilitated by the colonial wars in the 1960s and 1970s (28). Today, the HIV-2 epidemic seems to be contained or even decreasing, while HIV-1 is spreading rapidly (15, 29).
HIV-2 is known to be less virulent and less pathogenic than HIV-1, and the majority of HIV-2-infected individuals remain asymptomatic much longer than HIV-1-infected individuals (20, 22, 30). Plasma viral load is approximately 1 log unit lower in HIV-2 than HIV-1 infection when matched for CD4+ T cell count (4), whereas the proviral load does not appear to differ between the two infections (7, 36). The underlying mechanism for this difference has yet to be clarified. However, factors within the host immune response, including neutralizing antibodies, could play a part. HIV-1 is notoriously known to evade the immune response of the infected host by constantly producing variant viruses that are resistant to neutralizing antibodies (1, 6, 40, 50, 53). In contrast, as shown in earlier work, all HIV-2-infected individuals had sera with contemporaneous autologous neutralizing capacity (11), rarely encountered in HIV-1 infections. Similarly, we noted in another study comprising sera and HIV-2 isolates sequentially collected over a 10-year period, that unlike HIV-1 infection, emergence of neutralization-resistant HIV-2 variants could not be demonstrated in the HIV-2-infected patients (47). In the same study, we also noted that serum IgG of HIV-2-infected individuals displayed a marked ability to cross-neutralize heterologous HIV-2 isolates of different subtypes. Instead, the development of antibodies with the capacity to neutralize a broad range of heterologous viruses has been shown in only a fraction of HIV-1-infected individuals (48). Moreover, the emergence of broadly neutralizing antibodies in HIV-1 infection is usually not detected until several years after infection (32), and it has been suggested that the potency and breadth of these responses are driven by either virus divergence (13, 39) or elevated virus burden (16, 34, 43). In contrast, the presence of autologous neutralizing activity against contemporaneous HIV-2 strains (11), as well as heterologous strains (47), in HIV-2 infections may suggest that HIV-2 infection represents a suitable model for the study of immune responses that may control an HIV infection.
Despite intense research and enormous amounts of effort invested in the search for an HIV vaccine, no effective vaccine has yet been found. In addition, it is still unclear what type of immune response is necessary for control of an HIV infection. Development of a vaccine able to induce both broadly neutralizing antibodies and a T lymphocyte response against the virus would most likely represent the best strategy to pursue (9). So far, no vaccine candidate has managed to induce broadly neutralizing antibodies, leaving many open questions to be answered.
The current study was set up to compare, side by side, the breadth and potency of type-specific, i.e., intratype, and cross-neutralizing, i.e., intertype, neutralizing activity (NAc) in plasma of HIV-1- and HIV-2-infected subjects from the same geographic area, Guinea-Bissau. For the first time, this study also allowed analysis of NAc in plasma of dually HIV-1/HIV-2 (HIV-D)-infected individuals. NAc in plasma was tested against a panel of HIV-1 and HIV-2 primary isolates, including isolates of the CRF02_AG subtype, which is currently the most common HIV-1 subtype in this area (5, 17). The results show that the potency of intratype neutralization differed between HIV-1 and HIV-2 infection, where high-titer NAc characterized the plasma of HIV-2-infected individuals. The fact that in HIV-D infection NAc was present against HIV-2 but not HIV-1 suggests differences in immunogenicity and/or antigenicity of the two viruses.
MATERIALS AND METHODS
Study population.
The study participants resided in three adjacent suburban districts, Bandim 1, Bandim 2, and Belem, in Bissau, Guinea Bissau. They were part of general population cohorts of adult individuals that have been followed for retroviral infections since 1987 within the framework of the Bandim Health project (15, 26, 37, 38). The last serosurvey of HIV infection took place from 2004 to 2006 (15). It comprised 2,548 individuals in 384 randomly selected houses; the number of houses represented a 10% sample of the total number of houses in the study area. From this screening, all individuals with HIV-1/HIV-2 dual infection (HIV-D) were identified. Singly HIV-1- or HIV-2-infected individuals were randomly selected from the same cohort in a case-control manner. In the present study, plasma from 20 HIV-1-infected individuals (of whom 5 were also positive for human T cell leukemia virus type 1 [HTLV-1]), 20 HIV-2-infected individuals (of whom 9 also were positive for HTLV-1), and 7 HIV-D-infected individuals was investigated (Table 1). In addition, since a low number of HIV-D infections were identified, plasma from four HIV-D-infected individuals of a professional cohort of police officers in Guinea Bissau (29) was also included. These samples were collected within the same time frame, i.e., during 2004 and 2005, and handled in the same way. The study participants were characterized by assessment of CD4+ cell count, plasma viral load, and IgG levels (Table 1).
Table 1.
Characteristics of study participants
| Characteristic | HIV-1-positive plasma (n = 20) | HIV-2-positive plasma (n = 20) | HIV-1/2-positive plasma (n = 11) | P valuea |
|---|---|---|---|---|
| Median (IQRb) age (yr) | 35.1 (29.3–49.0) | 61.6 (54.8–68.0) | 47.0 (33.1–55.6) | <0.001 |
| Gender (% women) | 75 | 70 | 73 | 0.939 |
| HTLV status (% positive) | 25 | 45 | 0 | 0.026 |
| % with viral load (no. of RNA copies/ml) ofc: | ||||
| <1,001 | 22 | 72 | 14 | |
| 1,001–10,000 | 22 | 22 | 14 | |
| >10,000 | 56 (data missing for 2) | 6 (data missing for 2) | 72 (data missing for 4) | 0.003 |
| Median (IQR) CD4+ T cell count/μld | 227 (176–341) | 422 (261–500) | 376 (69–489) | 0.198 |
| % with CD4+ T cell count/μld of: | ||||
| >499 | 16 | 28 | 20 | |
| 200–499 | 53 | 67 | 50 | |
| <200 | 31 (data missing for 1) | 5 (data missing for 2) | 30 (data missing for 1) | 0.346 |
| Median (IQR) total IgG level ( mg/dl)e | 22.6 (15.3–25.7) (data missing for 4) | 13.0 (10.3–15.6) (data missing for 2) | 17.8 (10.8–27.1) (data missing for 5) | 0.041 |
P values calculated using the Kruskal-Wallis test, comparing means over the columns, the chi-square test, or Fisher's exact test when appropriate.
IQR, interquartile range.
The plasma HIV-1 and HIV-2 loads were analyzed by measuring RT activity using the Cavidi ExaVir Load kit (Cavidi Tech AB, Uppsala, Sweden) according to the manufacturer's instructions. Both HIV-1 and HIV-2 were detected with this method, and results are presented as number of RNA copy equivalents/ml.
The percentage and absolute CD4+ T cell counts were determined either by flow cytometry on a FACStrak instrument (Becton Dickinson, San Jose, CA) using three two-color immunofluorescence reagents (CD45/CD14, CD3/CD4, and CD3/CD8; Simultest; Becton Dickinson, San Jose, CA) in combination with leukocyte counts or by flow cytometry on a CyFlow instrument (Partec GmbH, Münster, Germany) using a CD4% antibody kit (CyTecs GmbH, Görlitz, Germany) according to the manufacturer's instructions.
IgG levels were measured by an in-house ELISA (47) with reagents from Jackson Immunotech (Marseille, France). In brief, plates were coated overnight with AffiniPure goat anti-human IgG (20 μg/ml), alkaline phosphatase-conjugated anti-human IgG (diluted to 1:5,000) was used as a detection antibody, and purified human IgG was used as a standard.
Blood sampling and HIV-1, HIV-2, and HTLV-1 status determinations.
Venous blood samples were drawn and collected in CPT tubes (BD Biosciences, San Jose, CA) with sodium citrate as anticoagulant, and plasma was separated according to the manufacturer's instructions. Plasma was then kept frozen until use in neutralization assays, virus isolation, viral load determinations, and other laboratory analyses. Serological testing for HIV antibodies was done using the Behring Enzygnost HIV-1/HIV-2 enzyme-linked immunosorbent assay (ELISA; Behring, Marburg, Germany). Confirmation and HIV-1/HIV-2 diagnosis were performed using Capillus HIV-1/HIV-2 (Cambridge Biotech Limited, Galway, Ireland) and Immunocomb II HIV-1 and HIV-2 BiSpot RST (Origenics, Yavne, Israel) test kits. Testing for HTLV antibodies was performed using an HTLV enzyme immunoassay (Murex, Dartford, United Kingdom), and confirmation was done using the line assay INNO-LIA (Innogenetics, Ghent, Belgium). T lymphocyte subsets were determined by conventional flow cytometry (FACStrak; Becton Dickinson, San Jose, CA).
Primary HIV-1 and HIV-2 isolates.
For the analysis of neutralizing activity of HIV-positive plasma, five HIV-1 and five HIV-2 primary isolates were used. The HIV-1 panel included one subtype B isolate from Sweden (B Sw); two subtype C isolates, one from Brazil (C Br) and one from India (C IN); and two HIV-1 CRF02_AG isolates (AG GB/30 and AG GB/48) originating from the same Guinea-Bissau study population from which the analyzed plasma was obtained (Table 2) (23, 42, 52). The HIV-2 panel contained five subtype A HIV-2 isolates, where two isolates originated from Guinea-Bissau (GB/1682 and GB/1812) and three isolates originated from West Africa but from locations other than Guinea-Bissau (A WA/1, A WA/2, and A WA/3) (3, 47). Eight viruses were isolated from peripheral blood mononuclear cells (PBMCs) (1, 2), while HIV-1 AG GB/30 and AG GB/48 were isolated from plasma (14). In brief, 2 days prior to the start of the isolation, PBMCs were separated from buffy coats of healthy blood donors and depleted of CD8+ T cells using a magnetic cell sorting system (MACS; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's instructions. CD8+ T cell-depleted PBMCs were then stimulated by the addition of phytohemagglutinin (PHA) to a final concentration of 2.5 μg/ml in complete medium, i.e., RPMI 1640 GlutaMAX medium supplemented with 10% fetal calf serum (FCS), 0.1 μg/ml streptomycin (Invitrogen, Carlsbad, CA), and 0.1 U/ml penicillin (Invitrogen, Carlsbad, CA). Two days later, 1 ml plasma from the HIV-infected individuals was centrifuged at 18,000 rpm for 1.5 h and the sediment was resuspended in 500 μl infection medium, i.e., complete medium supplemented with 2 μg/ml of the cationic polymer Polybrene and 10 U/ml interleukin 2 (Sigma-Aldrich, St. Louis, MO). The resuspended virus pellet was then added to 500 μl of infection medium containing 10 × 106 PHA-activated CD8+ T cell-depleted PBMCs from two different donors, and next, the mixture was incubated for 2 h at 37°C in a 5% CO2 atmosphere. Subsequently, medium was added to a volume of 10 ml and the incubation was continued. Cultures were kept for 4 weeks with weekly harvest of supernatants and the addition of 3 × 106 fresh PHA-activated CD8+ T cell-depleted PBMCs. Cell-free culture supernatants were analyzed for reverse transcriptase (RT) activity using a Cavidi HS Lenti RT kit (Cavidi Tech AB, Uppsala, Sweden). The viruses included in the HIV-1 and HIV-2 panels were tested for coreceptor use on U87.CD4 indicator cell lines as previously described (12, 33, 47). All isolates were found to use CCR5 for cell entry, which was the rationale for the use of U87.CD4-CCR5 cells as targets in the neutralization assays.
Table 2.
Origins and characteristics of HIV isolates
| Isolate | Original name | Yr | Country of origin | Isolate source | Disease state | ARTa | HIV load | CD4+ T cell count | Reference or source |
|---|---|---|---|---|---|---|---|---|---|
| HIV-1 B Sw | 435:3415b | 1990 | Sweden | PBMCs | Asymptomatic | No | NAc | 290 | 23 |
| HIV-1 C Br | 92Br025d | 1992 | Brazil | PBMCs | Asymptomatic | No | NA | NA | WHO Network (42) |
| HIV-1 C IN | 98IN007d | 1998 | India | PBMCs | Asymptomatic | No | NA | NA | WHO Network |
| HIV-1 AG GB/30 | CC30 | 2005 | Guinea-Bissau | Plasma | AIDS | No | 118,300 | 100 | 52 |
| HIV-1 AG GB/48 | CC48 | 2005 | Guinea-Bissau | Plasma | AIDS | No | 340,850 | 162 | 52 |
| HIV-2 GB/1682 | 1682 | 1988 | Guinea-Bissau | PBMCs | Asymptomatic | No | NA | NA | 33 |
| HIV-2 GB/1812 | 1812 | 1988 | Guinea-Bissau | PBMCs | AIDS | No | NA | NA | 33 |
| HIV-2 A WA/1 | 12524 | 1997 | West Africae | PBMCs | AIDS | No | 7,100 | 190 | 47 |
| HIV-2 A WA/2 | 01va566 | 2001 | West Africae | PBMCs | AIDS | No | 33,700 | 30 | 47 |
| HIV-2 A WA/3 | 02va425 | 2002 | West Africae | PBMCs | AIDS | 3TC, d4T | 21,900 | 290 | 47 |
ART, antiretroviral treatment; 3TC, lamivudine; d4T, stavudine.
Isolated 37 months after seroconversion.
NA, not available.
Collected within 2 years of seroconversion.
Country in West Africa other than Guinea-Bissau.
Neutralization assay by plaque reduction.
The intra- and intertype NAc of plasma from HIV-1- and/or HIV-2-infected individuals was determined using a plaque reduction assay in U87.CD4-CCR5 cells (46). U87.CD4-CCR5 cells were grown in complete Dulbecco's modified Eagle medium (DMEM), i.e., DMEM supplemented with 10% FCS, 0.1 μg/ml streptomycin, and 0.1 U/ml penicillin (Invitrogen, Carlsbad, CA), and split 1:3 to 1:6 twice a week. Briefly, virus stocks and heat-inactivated plasma were mixed and diluted in DMEM infection medium, i.e., complete DMEM supplemented with 2 μg/ml Polybrene (Sigma-Aldrich, St. Louis, MO), to give a final concentration of virus giving 20 to 50 PFU/well and a final plasma dilution starting at 1:40. Following incubation of virus-plasma mixtures for 1 h at 37°C, 200 μl/well of virus-plasma mixtures was distributed in triplicate to 48-well microtiter plates containing U87.CD4-CCR5 cells at 50% confluence (seeded the day before). After 2 h incubation at 37°C, 300 μl/well of fresh medium was added and incubation was continued overnight. On the day after infection, medium was replaced with 1 ml of DMEM infection medium. Positive neutralization controls consisted of wells with virus and plasma with known NAc, positive virus controls consisted of wells with cells and virus without plasma, negative controls consisted of wells with virus mixed with a pool of plasma from five HIV-uninfected individuals participating in the serosurvey of HIV infection in Bissau from 2004 to 2006, and cell controls consisted of wells with cells only. The assay was terminated on day 3 by fixation with methanol-acetone (1:1). The number of PFU was determined following hematoxylin staining. NAc of the plasma was calculated by the formula [1 − (number of PFU with plasma/number of PFU without plasma)] × 100, which expresses the degree of reduction in the number of PFU in the presence of plasma relative to wells without plasma. The cutoff for neutralization in this assay was based on intra-assay variation, which was determined in three consecutive assays run on the same day and was repeatedly found to have a standard deviation (SD) of <10%. The cutoff chosen corresponded to >3 SDs, that is, a 30% reduction in the number of plaques (46). Negative-control plasma tested individually from HIV-uninfected subjects repeatedly did not result in reduction in the number of plaques by more than 30% (data not shown). A pool was prepared and used throughout the experiments. The assay has been standardized and compared with other neutralization assays, including the conventional PBMC-based HIV neutralization assay (18, 46).
Potency and breadth determination of neutralizing activity.
For analysis of the magnitude of NAc, plasma samples were titrated against five HIV-1 and five HIV-2 isolates. The magnitude of NAc in an individual plasma sample was determined by its neutralization score, defined as log-transformed titers (48). Log-transformed titers were calculated by dividing the highest dilution giving neutralization by 100 before applying a log-base 3 transformation and then adding 1 {Y = [log 3(highest dilution giving neutralization/100)] + 1}. All titers below the limit of detection were given a value of 33 for the sake of calculation of a neutralization score. The potency score was then calculated by summarizing the magnitude of the neutralization score for each plasma sample against each virus and then dividing by the total number of viruses tested (Table 2). The breadth of an individual plasma sample was defined by the number of viruses neutralized at 1:40 and 1:80 dilutions in the plaque reduction assay.
Statistical methods.
For comparisons between categorical variables, Fisher's exact test or the chi-square test was used as appropriate. Differences between subgroups of numerical variables, such as potency and breadth, were assessed by Mann-Whitney's two-sample test or the Kruskal-Wallis test as appropriate. The Spearman rank coefficient was used to assess correlations. Multivariate regression was performed and included the following variables: age group, gender, HTLV status, HIV load, CD4+ T cell count, and IgG level. Analyses were performed using STATA, version 11, and SPSS software.
Ethical considerations.
The study was approved by the Guinea-Bissau Government Ethics Committee and the Ethical Committee at the University of Lund, Lund, Sweden. Study participants were counseled and provided informed verbal consent. Participants were offered medical examination with free essential medications. Antiretroviral treatment was not available in the country at the time of plasma sampling but is now provided to this study population.
RESULTS
Comparison of intratype neutralization between HIV-1 and HIV-2 infections.
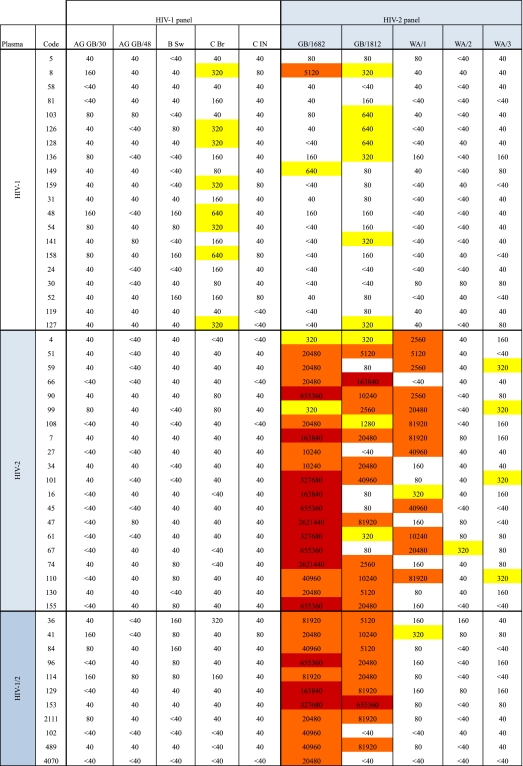
In order to study intratype NAc in HIV-1 and HIV-2 infections, we analyzed plasma from 20 HIV-1-infected individuals against a panel of five HIV-1 isolates and plasma from 20 HIV-2-infected individuals against a panel of five HIV-2 isolates. Results are shown in a heat map with reciprocal neutralization titers indicated for each individual plasma-virus combination (Table 3). Considering intratype neutralization against the most sensitive isolate, mean reciprocal titers were in the ranges of 104 and 102 for HIV-2 and HIV-1 plasma, respectively. The highest reciprocal titers encountered in the intratype neutralization with HIV-2 plasma were over 106, whereas HIV-1 reciprocal titers did not exceed 103 (Table 3). Notably, neutralization of HIV-1 isolates by HIV-1 plasma rarely exceeded 1:160, and when it did it was against only one isolate, HIV-1 C Br. In contrast, neutralization titers of HIV-2 plasma against HIV-2 isolates were ≥1:320 in 64% of tests (Table 3).
Table 3.
Reciprocal titers of neutralizing activity in individual plasma samples against individual isolates from HIV-1 and HIV-2 panelsa
The given reciprocal titers correspond to 1/dilution of plasma giving the 30% inhibitory concentration (IC30) in the plaque reduction neutralization assay applied, and boxes are color coded as follows: white, <40 to 160; yellow, 320 to 1,280; orange, 2,560 to 81,920; red, 163,840 to 2,621,440. Magnitude of neutralization was then calculated according to the formula [log 3(highest dilution giving neutralization/100) + 1] and corresponds to titers as follows: <40 = 0; 40 = 0.166; 80 = 0.797; 160 = 1.428; 320 = 2.059; 640 = 2.69; 1,280 = 3.321; 2,560 = 3.952; 5,120 = 4.582; 10,240 = 5.213; 20,480 = 5.844; 40,960 = 6.475; 81,920 = 7.106; 163,840 = 7.737; 327,680 = 8.368; 655,360 = 8.999; 2,621,440 = 10.261.
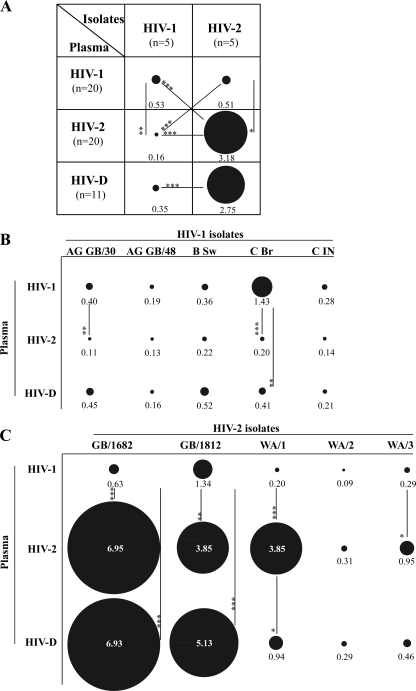
To allow quantitative comparisons between HIV-1 and HIV-2 neutralization, the magnitude of neutralization by each individual plasma sample was expressed as a neutralization score defined as log-transformed titer, i.e., [log 3(highest dilution giving neutralization/100) + 1]. (Conversions of reciprocal neutralization titers to neutralization scores appear in footnote a of Table 3.) The potency of NAc was then calculated as the mean neutralization score obtained against the different panels of tested isolates. The analysis showed that the NAc of HIV-1 plasma against HIV-1 isolates (mean potency score, 0.53) was significantly lower than the NAc of HIV-2 plasma against HIV-2 isolates (mean potency score, 3.18) (P < 0.0001) (Fig. 1A). However, it should be noted that in both virus panels, individual viruses varied in their sensitivity to neutralization and did so more in the HIV-2 panel than in the HIV-1 panel (magnitude scores, 7.5-fold and 22-fold, respectively) (Fig. 1B and C). The results indicate that HIV-2-infected individuals display a potent type-specific NAc, whereas such a strong type-specific NAc is absent in HIV-1 infection.
Fig 1.
Potency and magnitude of intra- and intertype NAc in HIV-1, HIV-2, and HIV-D plasma. (A) Mean potency scores of intra- and intertype NAcs of HIV-1 (n = 20), HIV-2 (n = 20), and HIV-D (n = 11) plasma tested against five HIV-1 and five HIV-2 isolates. (B and C) Mean magnitudes of intra- and intertype NAc in plasma against the individual isolates. The diameters of the illustrated circles correspond to the potency/magnitude scores of plasma NAc, where scores of 1, 3.1, 5.2, and 7.3 correspond to reciprocal titers of 102, 103, 104, and 105, respectively.*, P < 0.05; **, P < 0.01; ***, P < 0.001.
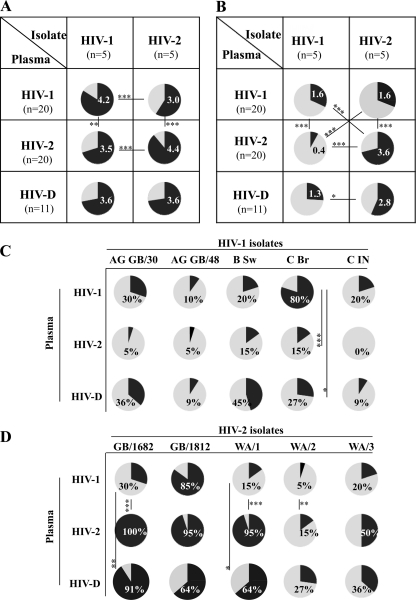
Considering the breadth of intratype NAc, we found no difference between HIV-1 and HIV-2 plasma tested at a dilution of 1:40, such that HIV-1 plasma neutralized nearly as many HIV-1 isolates as HIV-2 plasma neutralized HIV-2 isolates (on average, 4.2 and 4.45 out of the possible 5, respectively) (Fig. 2A). Due to the high percentage of neutralization in both groups at this low plasma dilution (1:40), any difference between HIV-1 and HIV-2 with regard to the breadth of neutralization might have been overlooked. Analysis was therefore repeated at the level of a 1:80 plasma dilution. In this case, we found a difference such that plasma from HIV-2-infected individuals displayed broader intratype NAc than plasma from HIV-1-infected individuals (average breadth scores, 3.6 and 1.6, respectively; P < 0.001) (Fig. 2B). In other words, only one of the HIV-1 isolates (C Br) was neutralized by more than 50% of HIV-1 plasma samples diluted 1:80, whereas four out of the five HIV-2 isolates were neutralized by 50% or more of HIV-2 plasma samples at the same dilution (Fig. 2C and D). From these results, we conclude that if the potency of NAc is taken into consideration, the breadth of the intratype NAc of HIV-1 and HIV-2 can be distinguished.
Fig 2.
Breadth of intra- and intertype NAc in HIV-1, HIV-2, and HIV-D plasma. Mean breadth of intra- or intertype NAc in HIV-1 (n = 20), HIV-2 (n = 20), and HIV-D (n = 11) plasma, where the indicated numbers correspond to the mean numbers of neutralized isolates tested against five HIV-1 and five HIV-2 isolates at 1:40 (A) and 1:80 (B) dilutions. (C and D) Breadth, here illustrated as percentage of HIV-1, HIV-2, and HIV-D plasma samples displaying NAc against individual isolates in the HIV-1 and HIV-2 panels. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Comparison of intra- and intertype neutralization in HIV-1 and HIV-2 infections.
Intertype neutralization was analyzed in three ways. First, we asked the question whether the NAc of HIV-2 plasma against HIV-1 isolates differed from the NAc of HIV-1 plasma against HIV-2 isolates (cross-analysis opposite intratype potency in Fig. 1A). The overall potency of neutralization showed that HIV-1 plasma neutralized HIV-2 isolates more potently than HIV-2 plasma neutralized HIV-1 isolates (mean potency scores, 0.51 and 0.16, respectively; P < 0.001) (Fig. 1A). Similarly to intratype neutralization, a difference in breadth of intertype neutralization between HIV-2 and HIV-1 was revealed at a 1:80 but not 1:40 plasma dilution (overall breadth scores of NAc, 1.6 and 0.4, respectively; P < 0.001) (Fig. 2A and B).
Second, we asked the question whether the NAc in plasma had type specificity (horizontal analysis in Fig. 1A and 2). Unexpectedly, we observed that HIV-1 and HIV-2 isolates were neutralized with similar potency by HIV-1 plasma (potency scores, 0.53 versus 0.51). This was in sharp contrast to HIV-2 plasma, where the difference in capacity to potently neutralize HIV-2 compared with HIV-1 isolates was highly significant (potency scores, 3.18 versus 0.16; P < 0.001) (Fig. 1A). Comparably to potency, the breadth of intratype and intertype NAc was not significantly different in HIV-1 plasma diluted 1:80. On the other hand, HIV-2 plasma, whether diluted 1:40 or 1:80, neutralized HIV-2 isolates significantly better than HIV-1 isolates, whereas type specificity of neutralization by HIV-1 plasma was revealed only at the 1:40 dilution (Fig. 2A and B).
In a third comparison, we asked whether the sensitivity of HIV-1 and HIV-2 would be different in intra- and intertype neutralization (vertical analysis in Fig. 1A and 2A and B). We found here that HIV-1 isolates in general were more sensitive to neutralization by HIV-1 plasma than by HIV-2 plasma (overall potency scores and breadth, P < 0.01 and P < 0.001, respectively) (Fig. 1A and 2B). Likewise, HIV-2 isolates were found to be more sensitive to neutralization by HIV-2 plasma than by HIV-1 plasma (overall potency scores and breadth, P < 0.05 and P < 0.001, respectively) (Fig. 1A and 2C). When each virus in the two panels was considered separately, we also noted these differences, especially when comparing the sensitivity of HIV-2 isolates to neutralization by HIV-1 and HIV-2 plasma (Fig. 1 and 2).
Taken together, comparisons of NAc of intra- and intertype neutralization suggest that HIV-2 displays a more neutralization-sensitive phenotype than HIV-1, since HIV-2 isolates were more potently neutralized by HIV-1 plasma than HIV-1 isolates were by HIV-2 plasma. The more neutralization-sensitive phenotype displayed by HIV-2 may also explain why HIV-1 plasma neutralized both types of viruses with equal potency.
HIV-1- and HIV-2-directed neutralization by HIV-D plasma.
It is to be expected that individuals dually infected with HIV-1 and HIV-2 have NAc against both types of viruses. However, it is not known to which level plasma of HIV-D-infected individuals can neutralize the two viruses compared with the level that it can neutralize viruses from individuals singly infected with either HIV-1 or HIV-2. Here we observed that HIV-D plasma neutralizes HIV-2 isolates with a significantly higher potency than HIV-1 isolates (potency scores, 2.75 versus 0.35; P < 0.001), mimicking the intratype NAc of HIV-2 plasma (Fig. 1A). When HIV-2 isolates were analyzed separately, the magnitude of NAc of HIV-D plasma was elevated against two HIV-2 isolates, GB/1682 and GB/1812, compared with HIV-1 plasma (P < 0.001 in both cases) (Fig. 1B). With all other viruses, the magnitude of neutralization by HIV-D plasma either did not differ or was intermediate between HIV-1 and HIV-2 singly infected plasma (Fig. 1B and C). In addition, overall NAc of HIV-D plasma was broader against HIV-2 than HIV-1 isolates when tested at the 1:80 but not at 1:40 dilution (Fig. 2A and B). When each isolate was considered separately, the breadth of neutralization by HIV-D plasma rarely differed from that of plasma from singly HIV-1-infected individuals, and breadth scores were most often intermediary between those of HIV-1 and HIV-2 plasma (Fig. 2C and D). Taken together, these results indicate that plasma of HIV-D-infected individuals displays a more potent type-specific NAc to HIV-2 than to HIV-1.
Modulators of neutralizing activity in HIV-1, HIV-2, and HIV-D plasma.
In order to gain insight into the mechanisms governing the potency and breadth of NAc present in plasma of HIV-1-, HIV-2-, or HIV-D-infected individuals, we studied the impact of demographic, clinical, and immunological parameters by the use of Spearman rank correlations and multivariate statistics. Analyses comprising relationships between NAc and various parameters, including age, viral load, CD4+ count/ml, and plasma IgG levels, revealed that both the potency and breadth (assayed at the 1:80 plasma dilution) of HIV-1 intratype neutralization correlated with viral load (P < 0.05) (Table 4). This was further supported by multivariate statistics, including the above-listed parameters, in addition to gender and HTLV status (P ≤ 0.02). In contrast, the potency and breadth of HIV-2 intratype NAc did not correlate with viral load. Interestingly, age was independently associated with the breadth of NAc in both HIV-1 and HIV-2 plasma (P ≤ 0.01). In addition, in the Spearman rank analyses, the potency of HIV-1-directed NAc was associated with the breadth of HIV-1 neutralization in plasma of HIV-1-, HIV-2-, as well as HIV-D-infected individuals and the potency and breadth of HIV-2 neutralization in plasma of HIV-1- and HIV-D-infected individuals. In summary, our results suggest that viral load may impact the potency and breadth of intratype HIV-1 but not HIV-2 neutralization. Moreover, age appears to influence the breadth of both HIV-1 and HIV-2 intratype neutralization.
Table 4.
Spearman rank correlates of breadth and potency of plasma neutralizing activitya
| Sample | Correlate | Potency |
Breadth |
||
|---|---|---|---|---|---|
| HIV-1 panel | HIV-2 panel | HIV-1 panel | HIV-2 panel | ||
| HIV-1 plasma | Age | 0.156 | −0.435 | 0.39 | −0.163 |
| Virus load | 0.594 | −0.316 | 0.530 | −0.205 | |
| CD4+ T cell count | −0.185 | 0.404 | 0.062 | 0.330 | |
| IgG level | 0.342 | 0.120 | 0.19 | −0.049 | |
| HIV-1 panel potency | 0.175 | 0.873 | −0.174 | ||
| HIV-2 panel potency | 0.21 | 0.666 | |||
| HIV-1 panel breadth | −0.001 | ||||
| HIV-2 plasma | Age | 0.140 | 0.121 | 0.25 | 0.347 |
| Virus load | 0.254 | −0.132 | 0.142 | −0.15 | |
| CD4+ T cell count | −0.006 | 0.143 | 0.039 | −0.003 | |
| IgG level | 0.182 | −0.034 | 0.129 | 0.025 | |
| HIV-1 panel potency | 0.379 | 0.812 | −0.08 | ||
| HIV-2 panel potency | 0.275 | 0.222 | |||
| HIV-1 panel breadth | 0.047 | ||||
| HIV-1/2 plasma | Age | −0.384 | 0.351 | −0.306 | 0.321 |
| Virus load | 0.107 | −0.108 | 0.255 | 0.472 | |
| CD4+ T cell count | 0.263 | 0.213 | 0.200 | 0.003 | |
| IgG level | 0.543 | −0.371 | 0.609 | 0.239 | |
| HIV-1 panel potency | 0.197 | 0.856 | 0.456 | ||
| HIV-2 panel potency | −0.205 | 0.804 | |||
| HIV-1 panel breadth | 0.236 | ||||
Numbers correspond to rho values, and numbers in bold indicate statistically significant (P < 0.05) correlations.
DISCUSSION
In the present work, we show that potent high-titer intratype NAc distinguishes HIV-2 infection from HIV-1 infection. The mean potency score of intratype neutralization in plasma of HIV-2-infected individuals was found to be 3.18, which was well above the potency score of NAc detected in HIV-1 plasma (mean, 0.53). This difference was even more evident considering the magnitude of intratype neutralization against the most sensitive isolate, where mean reciprocal titers in HIV-2 and HIV-1 plasma differed 100-fold. Titers of NAc for HIV-2 and HIV-1 plasma were in the ranges of 104 and 102, respectively. Instead, differences in the breadth of intratype NAc depended on the level of plasma dilution used in the analysis. At the lower plasma dilution, differences between the breadth of HIV-1 and HIV-2 plasma neutralization were not apparent, whereas differences were revealed when a higher plasma dilution was analyzed. This result shows that if the potency of NAc is taken into consideration, the breadth of the intratype NAc will also distinguish HIV-1 and HIV-2. Our findings of differences in the breadth of intratype NAc in HIV-1 and HIV-2 infection are in line with results published earlier by Rodriguez et al. (41). However, in regard to the potency of NAc, our data are at variance with the data of Rodriguez et al., in that intratype NAc was of a higher magnitude in HIV-1 plasma than in HIV-2 plasma (41). Notably, there are multiple differences between the two studies, and each of them may provide an explanation. For example, the study populations differ in sociodemographic aspects: commercial sex workers (CSW) versus the general population. Thus, it is plausible that the CSW have been regularly exposed to exogenous HIV-1 and HIV-2 variants. This antigenic stimulation may have different effects in HIV-2 and HIV-1 infections. It is also conceivable that the length of HIV-2 infection may influence the outcome of neutralization, such that prolonged exposure to antigenic stimulation may elicit neutralizing antibodies with high potency and/or breadth (51). The HIV-2-infected subjects in our study were older than those in the study of Rodriguez et al. (interquartile ranges, 55 to 68 years and 36 to 42.5 years, respectively) and were presumably infected for a longer time (41). Indeed, our multivariate analysis revealed that the breadth of both HIV-1 and HIV-2 intratype neutralization correlated with age: NAc was broader in older than younger individuals. Furthermore, another reason for the differences for intratype NAc potency could be the choice of viruses included in the neutralization assays. In our case, we noted that the neutralization sensitivity displayed a wide range, especially with HIV-2 isolates. In addition, the type of assays differed in the two studies: our study used a plaque assay with primary isolates propagated in activated donor PBMCs, whereas in the study of Rodriquez et al. (41), recombinant viruses pseudotyped with envelope glycoproteins (Env) of primary viruses were generated from transfection of 293T cells.
It has repeatedly been demonstrated that in HIV-1 infection, the higher the HIV-1 plasma viral load is, the more potent the plasma NAc is, suggesting that the amount of viral antigen drives the immune response (reviewed in reference 24). We find the same correlation for HIV-1 but, interestingly, not for HIV-2. Another difference is that HIV-1 is more prone to variation than HIV-2, with the consequence of neutralization escape. In fact, development of neutralization resistance has repeatedly been documented in HIV-1-infected subjects (1, 6, 40, 50, 53). This is not so in HIV-2 infection, where viruses remain susceptible to autologous neutralization over a prolonged period of at least 10 years (47). The emergence of new variant viruses in HIV-1 infection may provide constant antigenic stimulation, with a sustained high NAc in plasma as a consequence (24). At the same time, neutralizing antibody responses have been reported to drive the evolution of HIV-1 Env toward neutralization resistance (19). In contrast, in HIV-2 infection, high levels of NAc in plasma are concomitant with a low plasma viral load and maintained neutralization sensitivity of isolated viruses. This points to a qualitative difference in plasma NAc present in HIV-1 and HIV-2 infection due to differences in either the immunogenicity or antigenicity of the viruses.
The striking feature of our data, that the HIV-2-infected host is able to mount and sustain a potent NAc in the presence of small amounts of viral antigen, is reminiscent of features of data for a third human retrovirus, human T cell leukemia virus type 1 (HTLV-1). It has been suggested that the outcome of infection—control or disease—depends on whether the host is able to react to small or large amounts of antigen (8). If small amounts of antigen can elicit an immune response, control of infection ensues. However, if large amounts of antigen are required to evoke an immune response, considerable virus replication is needed for eliciting effective immunity. It is tempting to speculate that HIV-2 elicits a neutralizing antibody response in the absence of substantial virus replication, whereas HIV-1 replication must occur to high levels to produce a sufficient amount of antigen to trigger production of neutralizing antibodies.
In comparison to HIV-2 NAc, HIV-1 NAc was never really potent and intratype and intertype HIV-1 NAcs were equally inefficient. We hypothesize that the HIV-1 envelope glycoproteins (Env) are differently targeted by neutralizing antibodies than HIV-2 Env. It has been suggested that the conformation of HIV-2 Env is more open than that of HIV-1 Env, and thereby, HIV-2 Env is more accessible to neutralizing antibodies (47, 49). Many HIV-2 isolates can infect cells independently of CD4 through direct binding to the coreceptor (49). Such isolates are more sensitive to neutralizing antibodies, suggesting that the neutralization face of Env is more exposed in such viruses than in viruses infecting cells in a CD4-dependent manner. In fact, our most neutralization-sensitive HIV-2 isolate has previously been reported to infect cells independently of CD4 (27). Alternatively, spikes of the HIV-1 Env may be too sparse for antibodies to bind effectively, resulting in apparent neutralization resistance (25, 55). In this context, it would be interesting to know whether Env of HIV-2 have a denser placement of spikes and can more firmly bind antibodies that successfully neutralize the virus.
Importantly, we could, for the first time, evaluate NAc in HIV-D infections and observed highly potent neutralization of HIV-2 isolates (mean potency score, 2.75) but not of HIV-1 isolates (mean potency score, 0.35). Expressed in titers, NAc was over 1:160 in 49% of reactions against HIV-2 and in only one case against HIV-1. HIV-D-infected patients can thus produce NAc to HIV-2 with a potency nearly as high as that produced by singly HIV-2-infected individuals, whereas neutralization of HIV-1 remains poor. This again suggests that the difference between HIV-1 and HIV-2 infection relies on the virus itself and adds further support to the notion that HIV-1 and HIV-2 Env differ in immunogenic and/or antigenic properties.
From the dynamics of HIV-2 epidemics in Guinea-Bissau, where the spread of HIV-2 has been linked to the independence war back in the 1960s and 1970s and the HIV-1 epidemic has been documented to be a more recent event, it is likely that HIV-2 was the first to infect our HIV-D patients (unpublished data). If so, a potent intratype HIV-2 NAc had been in place when the HIV-1 infection occurred. The poor intertype NAc elicited by HIV-2 may explain why there was no protection against HIV-1 infection in these subjects. Still, HIV-2 infection may have mitigated the consequences of HIV-1 infection. Indeed, it has been reported that HIV-1 plasma viremia may be controlled following superinfection in an HIV-2-infected woman (21).
Variation of HIV-1 neutralization sensitivity is well documented, and, in fact, isolates have been classified into tiers 1 to 3, with the most neutralization-sensitive viruses assigned to tier 1 (45). In our hands, the HIV-1 C Br isolate was neutralized by all HIV-1 plasma and the magnitude of neutralization was highest in comparison with that for the other HIV-1 isolates used. In comparison to HIV-2, the neutralization sensitivity of the five HIV-1 isolates used in the present study was low across the panel. The overall neutralization sensitivity of HIV-2 isolates was higher than that of HIV-1 isolates, even though the variation of neutralization sensitivity was more striking. In a previous study involving 15 HIV-2 isolates from four patients, we proposed that the high sensitivity to neutralizing antibodies of HIV-2 compared to HIV-1 may be due to differences in the structure of the V3 domain of Env (47). We observed that HIV-2 isolates most often had only two potential glycosylation sites (PNGS) in and around the V3 loop, whereas HIV-1 isolates had four or five (47). Indeed, several studies showed that loss or removal of glycans in and around the HIV-1 V3 loop may increase the sensitivity to neutralizing antibodies (10, 31, 35, 44, 54). The difference in glycan density may confer a more open and accessible V3 domain of HIV-2 than of HIV-1 and may explain the greater neutralization sensitivity of HIV-2.
Our results may provide insight into the nature of a potently neutralizing humoral immune response. However, it is challenging to consider the possibility of changing the HIV-1 Env to make them able to elicit humoral immunity similar in potency to the immune response elicited by HIV-2.
ACKNOWLEDGMENTS
The expert technical assistance of Helen Linder, Monica Öberg, and Elzbieta Vincic is greatly appreciated.
The work was supported by grants provided from the Swedish International Development Agency/Department for Research Cooperation (Sida/SAREC), the Swedish Research Council, the European Community's Sixth and Seventh Framework Programmes, grant 037611 (EUROPRISE), and FP7/2007-2013 under grant agreement 201433 (NGIN). Grants were also provided by The Royal Physiographic Society in Lund, Sweden, the Crafoords Foundation, and the Clas Groschinskys Foundation.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Albert J, et al. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4:107–112 [DOI] [PubMed] [Google Scholar]
- 2. Albert J, Bottiger B, Biberfeld G, Fenyo EM. 1989. Replicative and cytopathic characteristics of HIV-2 and severity of infection. Lancet i:852–853 [DOI] [PubMed] [Google Scholar]
- 3. Albert J, et al. 1990. Replicative capacity of HIV-2, like HIV-1, correlates with severity of immunodeficiency. AIDS 4:291–295 [DOI] [PubMed] [Google Scholar]
- 4. Andersson S, et al. 2000. Plasma viral load in HIV-1 and HIV-2 singly and dually infected individuals in Guinea-Bissau, West Africa: significantly lower plasma virus set point in HIV-2 infection than in HIV-1 infection. Arch. Intern. Med. 160:3286–3293 [DOI] [PubMed] [Google Scholar]
- 5. Andersson S, Norrgren H, Dias F, Biberfeld G, Albert J. 1999. Molecular characterization of human immunodeficiency virus (HIV)-1 and -2 in individuals from Guinea-Bissau with single or dual infections: predominance of a distinct HIV-1 subtype A/G recombinant in West Africa. Virology 262:312–320 [DOI] [PubMed] [Google Scholar]
- 6. Arendrup M, et al. 1992. Autologous HIV-1 neutralizing antibodies: emergence of neutralization-resistant escape virus and subsequent development of escape virus neutralizing antibodies. J. Acquir. Immune Defic. Syndr. 5:303–307 [PubMed] [Google Scholar]
- 7. Ariyoshi K, et al. 1996. A community-based study of human immunodeficiency virus type 2 provirus load in rural village in West Africa. J. Infect. Dis. 173:245–248 [DOI] [PubMed] [Google Scholar]
- 8. Bangham CR. 2000. The immune response to HTLV-I. Curr. Opin. Immunol. 12:397–402 [DOI] [PubMed] [Google Scholar]
- 9. Barouch DH. 2008. Challenges in the development of an HIV-1 vaccine. Nature 455:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benjouad A, Gluckman JC, Rochat H, Montagnier L, Bahraoui E. 1992. Influence of carbohydrate moieties on the immunogenicity of human immunodeficiency virus type 1 recombinant gp160. J. Virol. 66:2473–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bjorling E, et al. 1993. Autologous neutralizing antibodies prevail in HIV-2 but not in HIV-1 infection. Virology 193:528–530 [DOI] [PubMed] [Google Scholar]
- 12. Bjorndal A, et al. 1997. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 71:7478–7487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bussmann BM, et al. 2010. Loss of HIV-specific memory B-cells as a potential mechanism for the dysfunction of the humoral immune response against HIV. Virology 397:7–13 [DOI] [PubMed] [Google Scholar]
- 14. Costa CI, Morgado MG, Santos VG, Bongertz V. 1996. HIV-1 isolation from plasma specimens. HEC/FIOCRUZ AIDS Clinical Research Group. Mem. Inst. Oswaldo Cruz 91:745–746 [DOI] [PubMed] [Google Scholar]
- 15. da Silva ZJ, et al. 2008. Changes in prevalence and incidence of HIV-1, HIV-2 and dual infections in urban areas of Bissau, Guinea-Bissau: is HIV-2 disappearing? AIDS 22:1195–1202 [DOI] [PubMed] [Google Scholar]
- 16. Doria-Rose NA, et al. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J. Virol. 84:1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esbjornsson J, et al. 2010. Frequent CXCR4 tropism of HIV-1 subtype A and CRF02_AG during late-stage disease—indication of an evolving epidemic in West Africa. Retrovirology 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fenyo EM, et al. 2009. International network for comparison of HIV neutralization assays: the NeutNet report. PLoS One 4:e4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frost SD, et al. 2005. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc. Natl. Acad. Sci. U. S. A. 102:18514–18519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gody M, Ouattara SA, de The G. 1988. Clinical experience of AIDS in relation to HIV-1 and HIV-2 infection in a rural hospital in Ivory Coast, West Africa. AIDS 2:433–436 [DOI] [PubMed] [Google Scholar]
- 21. Gunthard HF, et al. 2009. HIV-1 superinfection in an HIV-2-infected woman with subsequent control of HIV-1 plasma viremia. Clin. Infect. Dis. 48:e117–e120 [DOI] [PubMed] [Google Scholar]
- 22. Jaffar S, et al. 1997. Rate of decline of percentage CD4+ cells is faster in HIV-1 than in HIV-2 infection. J. Acquir. Immune Defic. Syndr Hum. Retrovirol. 16:327–332 [DOI] [PubMed] [Google Scholar]
- 23. Karlsson I, et al. 2003. HIV biological variability unveiled: frequent isolations and chimeric receptors reveal unprecedented variation of coreceptor use. AIDS 17:2561–2569 [DOI] [PubMed] [Google Scholar]
- 24. Klasse PJ, Sanders RW, Cerutti A, Moore JP. 20 May 2011. How can HIV-type-1-Env immunogenicity be improved to facilitate antibody-based vaccine development? AIDS Res. Hum. Retroviruses. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klein J, Bjorkman P. 2010. Few and far between: how HIV may be evading antibody avidity. PLoS Pathog. 6:e1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larsen O, et al. 1998. Declining HIV-2 prevalence and incidence among men in a community study from Guinea-Bissau. AIDS 12:1707–1714 [DOI] [PubMed] [Google Scholar]
- 27. Laurén A, Fenyö E. 2008. Implications from the SIV model in understanding HIV neutralization. Future HIV Ther. 2:47–58 [Google Scholar]
- 28. Lemey P, et al. 2003. Tracing the origin and history of the HIV-2 epidemic. Proc. Natl. Acad. Sci. U. S. A. 100:6588–6592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mansson F, et al. 2009. Prevalence and incidence of HIV-1 and HIV-2 before, during and after a civil war in an occupational cohort in Guinea-Bissau, West Africa. AIDS 23:1575–1582 [DOI] [PubMed] [Google Scholar]
- 30. Marlink RG, et al. 1988. Clinical, hematologic, and immunologic cross-sectional evaluation of individuals exposed to human immunodeficiency virus type-2 (HIV-2). AIDS Res. Hum. Retroviruses 4:137–148 [DOI] [PubMed] [Google Scholar]
- 31. McCaffrey RA, Saunders C, Hensel M, Stamatatos L. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 78:3279–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mikell I, et al. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 7:e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morner A, et al. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pereyra F, et al. 2009. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 200:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polzer S, Dittmar MT, Schmitz H, Schreiber M. 2002. The N-linked glycan g15 within the V3 loop of the HIV-1 external glycoprotein gp120 affects coreceptor usage, cellular tropism, and neutralization. Virology 304:70–80 [DOI] [PubMed] [Google Scholar]
- 36. Popper SJ, et al. 1999. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J. Infect. Dis. 180:1116–1121 [DOI] [PubMed] [Google Scholar]
- 37. Poulsen AG, et al. 1993. HIV-2 infection in Bissau, West Africa, 1987-1989: incidence, prevalences, and routes of transmission. J. Acquir. Immune Defic. Syndr. 6:941–948 [PubMed] [Google Scholar]
- 38. Poulsen AG, et al. 1989. Prevalence of and mortality from human immunodeficiency virus type 2 in Bissau, West Africa. Lancet i:827–831 [DOI] [PubMed] [Google Scholar]
- 39. Powell RL, Kinge T, Nyambi PN. 2010. Infection by discordant strains of HIV-1 markedly enhances the neutralizing antibody response against heterologous virus. J. Virol. 84:9415–9426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Richman DD, Wrin T, Little SJ, Petropoulos CJ. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 100:4144–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodriguez SK, et al. 2007. Comparison of heterologous neutralizing antibody responses of human immunodeficiency virus type 1 (HIV-1)- and HIV-2-infected Senegalese patients: distinct patterns of breadth and magnitude distinguish HIV-1 and HIV-2 infections. J. Virol. 81:5331–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rubsamen-Waigmann H, et al. 1994. Standard conditions of virus isolation reveal biological variability of HIV type 1 in different regions of the world. WHO Network for HIV Isolation and Characterization. AIDS Res. Hum. Retroviruses 10:1401–1408 [DOI] [PubMed] [Google Scholar]
- 43. Sather DN, et al. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schonning K, Jansson B, Olofsson S, Nielsen JO, Hansen JS. 1996. Resistance to V3-directed neutralization caused by an N-linked oligosaccharide depends on the quaternary structure of the HIV-1 envelope oligomer. Virology 218:134–140 [DOI] [PubMed] [Google Scholar]
- 45. Seaman MS, et al. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi Y, Albert J, Francis G, Holmes H, Fenyo EM. 2002. A new cell line-based neutralization assay for primary HIV type 1 isolates. AIDS Res. Hum. Retroviruses 18:957–967 [DOI] [PubMed] [Google Scholar]
- 47. Shi Y, et al. 2005. Evolution of human immunodeficiency virus type 2 coreceptor usage, autologous neutralization, envelope sequence and glycosylation. J. Gen. Virol. 86:3385–3396 [DOI] [PubMed] [Google Scholar]
- 48. Simek MD, et al. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 83:7337–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomas ER, Shotton C, Weiss RA, Clapham PR, McKnight A. 2003. CD4-dependent and CD4-independent HIV-2: consequences for neutralization. AIDS 17:291–300 [DOI] [PubMed] [Google Scholar]
- 50. Tremblay M, Wainberg MA. 1990. Neutralization of multiple HIV-1 isolates from a single subject by autologous sequential sera. J. Infect. Dis. 162:735–737 [DOI] [PubMed] [Google Scholar]
- 51. van Gils MJ, Schuitemaker H. 2010. Correlations between HIV-1 subtypes and HIV-1 antibody neutralization sensitivity: significant for vaccine development? Curr. HIV Res. 8:579–586 [DOI] [PubMed] [Google Scholar]
- 52. Vinner L, et al. 2011. Sequence analysis of HIV-1 isolates from Guinea-Bissau: selection of vaccine epitopes relevant in both West African and European countries. APMIS 119:487–497 [DOI] [PubMed] [Google Scholar]
- 53. Von Gegerfelt A, Albert J, Morfeldt-Manson L, Broliden K, Fenyo EM. 1991. Isolate-specific neutralizing antibodies in patients with progressive HIV-1-related disease. Virology 185:162–168 [DOI] [PubMed] [Google Scholar]
- 54. Wei X, et al. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312 [DOI] [PubMed] [Google Scholar]
- 55. Zhu P, et al. 2006. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441:847–852 [DOI] [PubMed] [Google Scholar]