Abstract
The immunogenicity of adenovirus serotype 5 (Ad5) vectors has been shown to be suppressed by neutralizing antibodies (NAbs) directed primarily against the hexon hypervariable regions (HVRs). However, the role of NAbs directed against other capsid components, particularly the adenovirus fiber, remains unclear. Here we show that Ad5 NAbs target both hexon and fiber following vaccination and natural infection. Utilizing neutralization assays with capsid chimeric vectors, we observed that NAb responses to hexon appeared dominant and NAb responses against fiber were subdominant in sera from vaccinated mice, vaccinated humans, and naturally exposed humans. A novel chimeric Ad5 vector in which both the hexon HVRs and the fiber knob were exchanged nearly completely evaded Ad5-specific NAbs both in vitro and in vivo.
TEXT
Preexisting adenovirus serotype 5 (Ad5) immunity in human populations has been shown to suppress the immunogenicity of Ad5-based vaccine and gene therapy vectors (10–13). It is therefore important to understand the functionally relevant determinants of Ad5 immunity and to develop novel Ad vectors that circumvent Ad5-specific neutralizing antibodies (NAbs). The adenovirus capsid consists of three major structural proteins: hexon, penton, and fiber. Our laboratory and others have reported that Ad5-specific NAbs are directed primarily against the hexon (12, 15), and we previously showed that a chimeric Ad5 vector with the seven hexon hypervariable regions (HVRs) exchanged from the corresponding regions of Ad48, termed Ad5HVR48(1-7) (or simply Ad5HVR48), evaded the majority of anti-Ad5 immunity in vivo (10). However, Ad5-specific NAbs against the fiber knob and penton base have also been described, although the extent to which they are functionally relevant remains unclear (3, 5, 6, 12). A recent study suggested that Ad5-specific NAbs following natural infection were directed primarily against fiber, whereas Ad5-specific NAbs following Ad5 vector vaccination were directed primarily against hexon (3).
To evaluate the functional relevance of fiber knob-specific NAbs in terms of suppression of vaccine vector immunogenicity, we constructed chimeric Ad5 and Ad5HVR48 vectors in which the fiber knob was exchanged with that of the chimpanzee adenovirus AdC68 (Pan 9), which has been shown to have low seroprevalence in humans in Africa (14) and also utilizes the same primary cellular receptor as Ad5, the coxsackievirus adenovirus receptor (CAR) (4). Ad5-based vectors with the hexon HVRs and/or the fiber knob exchanged were then evaluated in NAb assays and immunogenicity studies to assess the relative role of hexon- and fiber-specific NAbs following both vaccination and natural infection.
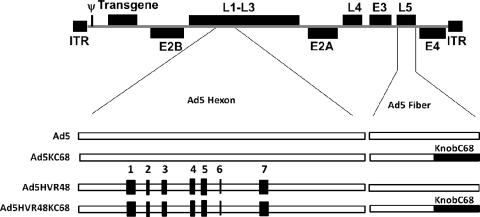
We constructed the capsid-chimeric Ad vectors Ad5KC68 and Ad5HVR48KC68 (Fig. 1), in which the Ad5 fiber knob was replaced with that of AdC68 in the Ad5 and Ad5HVR48 vectors, respectively (Fig. 1). Recombinant Ad5 fiber gene fragments were synthesized and cloned into the Ad5 cosmid pWE.Ad5.Aflii-rITR.dE3 or HVR cosmid pWE.Ad5HVR48.Aflii-rITR.dE3. E1/E3-deleted, replication-incompetent Ad5 vectors containing chimeric hexon and/or fiber knob genes were then produced essentially as described previously (13). The Ad5KC68 and Ad5HVR48KC68 vectors were produced to high titers and exhibited analytical and performance characteristics similar to those of the Ad5 and Ad5HVR48 vectors in terms of yield, purity, expression, and specific infectivity.
Fig 1.
Generation of Ad5KC68 and Ad5HVR48KC68 vectors. Hexon HVRs derived from Ad48 and fiber knob sequences derived from AdC68 are highlighted in black. All four vectors shown were produced to high titers. ITR, inverted terminal repeat.
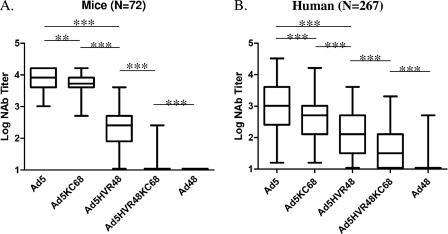
We evaluated NAb responses against Ad5, Ad5KC68, Ad5HVR48, Ad5HVR48KC68, and Ad48 expressing luciferase in both murine and human serum samples by utilizing a luciferase-based virus neutralization assay as described previously (13). To generate high levels of Ad5-specific immunity, mice were preimmunized with two injections of 1010 virus particles (vp) of Ad5-Empty separated by 4 weeks. Sera from Ad5-preimmunized C57BL/6 mice (n = 72) were analyzed for NAb titers to these viruses, defined as the serum dilution that neutralized 90% of luciferase activity (Fig. 2A). High Ad5 NAb titers (median log titer, 3.9) were detected in all vaccinated mice. Ad48 NAb titers were not observed, as expected. Intermediate NAb titers against the chimeric vectors Ad5KC68 and Ad5HVR48 were evident. Median Ad5HVR48 NAb titers (median log titer, 2.4) were 1.5 logs lower than the median Ad5 NAb titers (P < 0.0001; Wilcoxon signed-rank test) (Fig. 2A), similar to our previous data (10). Ad5KC68 NAb titers (median log titer, 3.6) proved 0.3 logs lower than Ad5 NAb titers (P = 0.0016) but 1.2 logs higher than Ad5HVR48 NAb median titers (P < 0.0001) (Fig. 2A). In contrast, Ad5HVR48KC68 NAb titers were low. These data indicate that Ad5 NAbs in vaccinated mice were directed primarily against the hexon HVRs and secondarily against the fiber knob. The fact that the Ad5HVR48KC68 vector evaded Ad5 NAbs nearly completely suggests that the Ad5 NAbs that were directed against non-HVR epitopes targeted primarily the fiber knob.
Fig 2.
NAb responses to hexon- and fiber-chimeric Ad5 vectors. (A) Median log Ad5, Ad5KC68, Ad5HVR48, Ad5HVR48KC68, and Ad48 NAb titers in 72 C57BL/6 mice preimmunized with Ad5 are represented as box-and-whisker plots showing the full range, 25% to 75% interquartile range (box), and median (bar). ***, P < 0.0001; **, P = 0.0016. (B) Median log Ad5, Ad5KC68, Ad5HVR48, Ad5HVR48KC68, and Ad48 NAb titers in 267 South African serum samples are similarly represented as box-and-whisker plots. ***, P < 0.0001.
We next evaluated NAb responses against Ad5, Ad5KC68, Ad5HVR48, Ad5HVR48KC68, and Ad48 in serum from 267 healthy adults from South Africa (Fig. 2B) (2). High Ad5 NAb titers were detected in these samples (median log titer, 3.0), indicating high levels of NAbs against Ad5, presumably as a result of natural Ad5 exposure (2). As expected, Ad48 NAb titers were low (2). Median Ad5HVR48 NAb titers (median log titer, 2.1) were 1.0 log lower than median Ad5 NAb titers (P < 0.0001) (Fig. 2B), indicating that approximately 90% of Ad5 NAb activity was directed against epitopes located in the seven hexon HVRs. Ad5KC68 NAb titers (median log titer, 2.7) were 0.3 logs lower than Ad5 titers (P < 0.0001) but 0.6 logs higher than Ad5HVR48 NAb titers (P < 0.0001) (Fig. 2B). In contrast, Ad5HVR48KC68 NAb titers (median log titer, 1.5) were both 1.5 logs lower than Ad5 titers (P < 0.0001) and 0.6 logs lower than Ad5HVR48 titers (P < 0.0001). These data confirm our observations in vaccinated mice and show that NAbs against Ad5 were directed primarily against HVR epitopes and secondarily against fiber knob epitopes in a large cohort of naturally Ad5-infected humans from sub-Saharan Africa. These findings also suggest that the Ad5HVR48KC68 vector largely evaded Ad5 NAbs in humans with preexisting Ad5 immunity. The evasion of NAbs in humans was less complete than in mice, perhaps reflecting higher baseline Ad48 NAb titers.
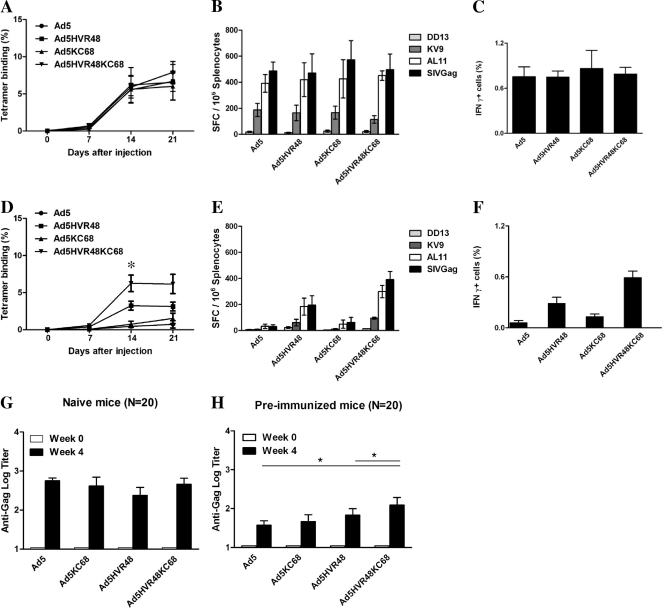
We assessed the immunogenicity of Ad5, Ad5HVR48, Ad5KC68, and Ad5HVR48KC68 vectors expressing simian immunodeficiency virus (SIV) Gag in C57BL/6 mice to evaluate if swapping the fiber knob would improve evasion of anti-Ad5 immunity in vivo. We previously reported that Ad5HVR48 evaded the majority of preexisting Ad5 immunity in preclinical studies in mice and rhesus monkeys (10), but the extent of this evasion was incomplete. We therefore investigated whether the Ad5HVR48KC68 would prove more immunogenic than Ad5HVR48 in mice with preexisting Ad5 immunity. To induce high levels of anti-Ad5 immunity, mice were preimmunized intramuscularly twice, separated by a 4-week interval, with 1010 vp Ad5-Empty in 100 μl sterile phosphate-buffered saline (PBS) (10). Naïve as well as Ad5-preimmunized C57BL/6 mice (median log Ad5 NAb titer, 3.9) (8 mice/group) were intramuscularly immunized once with 109 vp of the vectors Ad5, Ad5HVR48, Ad5KC68, and Ad5HVR48KC68. Tetrameric H-2Db complexes folded around the immunodominant SIV Gag AL11 epitope (AAVKNWMTQTL) (7) were prepared and used to measure SIV Gag-specific CD8+ T lymphocyte responses on days 0, 7, 14, and 21 postimmunization. CD8+ T lymphocytes from naïve mice exhibited <0.1% tetramer staining. In naïve mice, the kinetics and magnitudes of AL11-specific CD8+ T lymphocyte responses of all four vectors proved comparable (Fig. 3A). To evaluate functional responses, splenocytes from day 28 were utilized in gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) and intracellular cytokine staining (ICS) assays as described previously (7). IFN-γ ELISPOT responses to overlapping Gag peptides, the dominant CD8+ T cell epitope AL11 (AAVKNWMTQTL), the subdominant CD8+ T cell epitope KV9 (KSLYNTVCV), and the CD4+ T cell epitope DD13 (DRFYKSLRAEQTD) (7) were comparable among all four vectors in naïve mice (Fig. 3B). IFN-γ ICS responses also proved similar among these vectors (Fig. 3C).
Fig 3.
Cellular immune responses induced by Ad5KC68 and Ad5HVR48KC68 vectors. Naïve (A to C) or Ad5-preimmunized (D to F) C57BL/6 mice (8/group) were immunized intramuscularly with 109 vp of the chimeric vectors Ad5, Ad5HVR48, Ad5KC68, and Ad5HVR48KC68 expressing SIV Gag. AL11-specific tetramer responses at multiple time points, IFN-γ ELISPOT assays using splenocytes from the mice on day 28 postimmunization, and ICS assays were utilized to measure SIV Gag-specific cellular immune responses. SFC, spot-forming cells. *, P < 0.05. Gag-specific antibodies were also assessed in naïve (G) or Ad5-preimmunized (H) mice (20/group) at weeks 0 and 4 by ELISA. * = P < 0.05.
In mice with high baseline Ad5 NAb titers (median titer, 3.9), the immunogenicity of Ad5-SIV Gag was abrogated as expected, while the immunogenicity of Ad5HVR48-SIV Gag was partially preserved (Fig. 3D to F), consistent with our previous data (10). The immunogenicity of Ad5KC68-SIV Gag was largely suppressed (Fig. 3D to F), indicating that evasion of only fiber knob-specific NAbs was insufficient to circumvent high Ad5 NAb titers in this system. Interestingly, the CD8+ T lymphocyte responses elicited by Ad5HVR48KC68-SIV Gag were nearly completely preserved and proved higher than responses induced by Ad5HVR48-SIV Gag (P < 0.05; Wilcoxon signed-rank test). We also measured Gag-specific antibody responses in both naïve mice (Fig. 3G) and Ad5-preimmunized mice (Fig. 3H) by enzyme-linked immunosorbent assay (ELISA) at week 4 following immunization. Comparable titers of Gag-specific antibodies were observed in naïve mice immunized with all four vectors. In Ad5-preimmunized mice, significantly higher titers of Gag-specific antibodies were detected in mice immunized with Ad5HVR48KC68-SIV Gag than in those immunized with Ad5-SIV Gag and Ad5HVR48-SIV Gag (P < 0.05; Mann-Whitney test) (Fig. 3H). These data indicate that NAbs against fiber were functionally relevant but less important than NAbs against hexon. Moreover, exchanging both the hexon HVRs and the fiber knob resulted in an improved degree of evasion of preexisting Ad5 immunity relative to just swapping the hexon HVRs. These observations are consistent with the efficient evasion of NAb responses by the Ad5HVR48KC68 vector in vitro in virus neutralization assays using both murine and human sera (Fig. 2A and B). However, Gag-specific antibody responses elicited by Ad5HVR48KC68 were still lower in mice with baseline Ad5 NAbs than in naïve mice, suggesting a partial role of vector-specific cellular immunity as well (12).
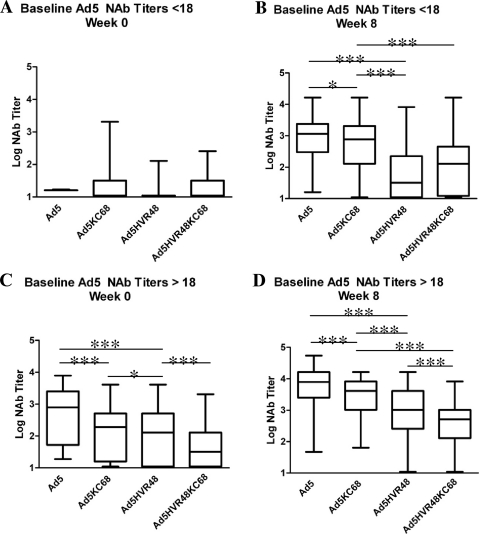
In order to evaluate further the contributions of hexon- and fiber-specific NAbs in the context of both natural Ad5 immunity and vaccine-elicited Ad5 immunity in humans, we analyzed serum samples from 116 subjects vaccinated with the Merck Ad5-Gag vaccine in the phase 1 studies that preceded the STEP study (Fig. 4A to D) (8, 9). We assessed NAb responses against Ad5, Ad5KC68, Ad5HVR48, and Ad5HVR48KC68 in serum obtained at week 0 (baseline) and week 8 (4 weeks following the second vaccination). In individuals with baseline Ad5 NAb titers of <18, NAbs to all 4 viruses were low to undetectable at week 0, as expected, given the relatively low seroprevalences of Ad48 and AdC68 (Fig. 4A). Following vaccination, these individuals exhibited high levels of Ad5-specific NAbs (median log titer, 3.0), which were only 0.3 logs higher than those induced against Ad5KC68 (median log titer, 2.7) (P = 0.0016) but were 1.5 logs higher than those against Ad5HVR48 (median log titer, 1.5) (P < 0.0001) (Fig. 4B). These data indicate that in baseline Ad5-naïve individuals, the majority of Ad5-specific NAbs induced by vaccination were directed primarily against the hexon, although low levels of fiber-specific and other NAbs were also detected.
Fig 4.
NAb responses in serum from Ad5-seropositive and Ad5-seronegative individuals pre- and post-Ad5 vector vaccination. (A) Log NAb titers in Ad5-seronegative individuals at week 0 prior to vaccination. (B) Log NAb titers in Ad5-seronegative individuals at week 8 following Ad5 vector vaccination. (C) Log NAb titers in Ad5-seropositive individuals at week 0 prior to vaccination. (D) Log NAb titers in Ad5-seropositive individuals at week 8 following Ad5 vector vaccination. ***, P < 0.0001; *, P = 0.0016.
In individuals with baseline Ad5 NAb titers of >18, high-level Ad5-specific NAbs were detected (median log titer, 2.9) at week 0 (Fig. 4C). Consistent with the results of prior experiments, the Ad5-specific NAbs were 0.4 logs higher than those against Ad5KC68 (median log titer, 2.5) (P < 0.0001), 0.6 logs higher than those against Ad5HVR48 (P < 0.0001), and 1.4 logs higher than those against Ad5HVR48KC68 (P < 0.0001) (Fig. 4C). Following vaccination, a similar stepwise hierarchy of NAb titers was observed (Fig. 4D), suggesting that the patterns of dominant HVR-specific and subdominant fiber knob-specific NAbs were similar among vaccinated mice, naturally infected humans, and vaccinated humans.
These data confirm and extend previous studies showing that Ad5-specific NAbs were directed primarily against the hexon HVRs (10). However, Ad5-specific NAbs against the fiber knob also exist and appear to be functionally relevant, although secondary in nature to NAbs against hexon (1, 3, 5, 6, 12). Replacing only the Ad5 fiber knob with that of a heterologous Ad serotype was insufficient to evade high levels of Ad5-specific NAbs in mice. However, replacing the fiber knob in addition to the seven hexon HVRs resulted in a chimeric vector that effectively evaded preexisting Ad5 immunity even better than did Ad5HVR48. These observations are consistent with the dramatic reduction of NAb titers to Ad5HVR48KC68 in both vaccinated mice and naturally infected humans (Fig. 2).
A previous study reported that Ad5-specific NAbs induced by natural infection were primarily directed against Ad5 fiber, whereas Ad5-specific NAbs elicited by vaccination were primarily directed against nonfiber capsid components (3). In the present study, we similarly detected NAb responses to the fiber in both vaccinated and naturally infected individuals. However, we observed that Ad5-specific NAbs were directed primarily against hexon in both vaccinated and naturally infected individuals utilizing two large cohorts of human subjects from sub-Saharan Africa and the United States (Fig. 2B and 4C). Our data demonstrate that there is no fundamental differential targeting of Ad5 NAbs by vector vaccination compared with natural infection, although subtle differences may exist (Fig. 4). The differences between these two studies may relate to different sample sizes, study populations, and chimeric vector characteristics.
Identification of key neutralizing epitopes on the adenovirus capsid will lead to a better understanding of adenovirus immunology as well as improved vectors for both vaccination and gene therapy. Our findings indicate that dominant vector-specific NAbs, whether induced by natural exposure or by vector vaccination, are directed primarily against the hexon HVRs, whereas subdominant but still functionally relevant NAbs are directed against the fiber knob. The efficient evasion of Ad5 NAbs by Ad5HVR48KC68 both in vitro and in vivo warrants further investigation of this novel candidate vector.
ACKNOWLEDGMENTS
We thank S. Clark, A. SanMiguel, D. Casimiro, and M. Robertson for technical assistance, advice, and reagents.
We acknowledge support from NIH/NIAID (grants AI078526, AI066924, and AI066305), the Bill & Melinda Gates Foundation, and the Ragon Institute of MGH, MIT, and Harvard.
We report no financial conflicts of interest.
Footnotes
Published ahead of print 9 November 2011
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Barouch DH, et al. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290–6297 [DOI] [PubMed] [Google Scholar]
- 2. Barouch SVK, et al. 2011. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29:5203–5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng C, et al. 2010. Differential specificity and immunogenicity of adenovirus type 5 neutralizing antibodies elicited by natural infection or immunization. J. Virol. 84:630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen CJ, et al. 2002. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol. 83(Part 1):151–155 [DOI] [PubMed] [Google Scholar]
- 5. Gahéry-Ségard H, et al. 1998. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J. Virol. 72:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong SS, Habib NA, Franqueville L, Jensen S, Boulanger PA. 2003. Identification of adenovirus (ad) penton base neutralizing epitopes by use of sera from patients who had received conditionally replicative ad (addl1520) for treatment of liver tumors. J. Virol. 77:10366–10375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, et al. 2006. Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies. J. Virol. 80:11991–11997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Brien KL, et al. 2009. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat. Med. 15:873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Priddy FH, et al. 2008. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 46:1769–1781 [DOI] [PubMed] [Google Scholar]
- 10. Roberts DM, et al. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239–243 [DOI] [PubMed] [Google Scholar]
- 11. Shiver JW, et al. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331–335 [DOI] [PubMed] [Google Scholar]
- 12. Sumida SM, et al. 2005. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 174:7179–7185 [DOI] [PubMed] [Google Scholar]
- 13. Vogels R, et al. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263–8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiang Z, et al. 2006. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 12:1596–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Youil R, et al. 2002. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum. Gene Ther. 13:311–320 [DOI] [PubMed] [Google Scholar]