Abstract
The Kaposi's sarcoma-associated herpesvirus nuclear egress complex is composed of two proteins, ORF67 and ORF69. In this study, we have recapitulated the KSHV complex by coexpression of these two proteins in insect cells using expression from recombinant baculoviruses. The proteins form a complex at the nuclear membrane as judged by live-cell analysis of protein fusions tagged with green fluorescent protein (GFP) and mCherry. Ultrastructural analysis of infected cells showed that ORF67 expression results in reduplication of the nuclear membrane. When the two proteins are expressed together, numerous virion-size nuclear membrane-derived vesicles were evident at the nuclear margins.
TEXT
Subsequent to DNA packaging of the herpesvirus capsid shell, primary envelopment of the nucleocapsid occurs at the inner nuclear membrane (INM) (7, 10, 11). All herpesviruses encode two proteins that form a complex, which plays a key role in facilitating the exit of capsids from the nucleus (7, 11). This complex is designated the nuclear egress complex (NEC). For herpes simplex virus type 1 (HSV-1), these two proteins, UL34 and UL31, participate in a molecular interaction that is required for nuclear membrane remodeling and envelopment at the INM (1, 4, 14, 19–21). UL34 is a type II membrane protein that is present in both nuclear membranes (24). UL31 is a nuclear phosphoprotein (2), which in the presence of UL34 relocalizes to the nuclear membrane (8, 20, 21). The coexpression of these two proteins of Pseudorabies virus (PRV) alone is sufficient to alter nuclear membranes, resulting in vesicle formation (8). The UL34 homolog of murine cytomegalovirus (CMV) has been shown to facilitate INM envelopment by recruiting protein kinase C to phosphorylate lamin, which potentially results in the dissolution of the nuclear lamina, thus allowing capsids to gain access to the INM (13, 15, 17). Epstein-Barr virus (EBV) encodes BFRF1 (UL34 ortholog) and BFLF2 (UL31 ortholog) gene products, which have been shown to participate in physical interactions at the nuclear membrane (5, 9) and are required for nuclear egress (3, 6). Previously, the Kaposi's sarcoma-associated herpesvirus (KSHV) NEC complex, which is encoded by ORF67 (UL34) and ORF69 (UL31), was shown by Santarelli et al. (23) to colocalize at the nuclear membrane in cotransfected 293 cells. Our goal was to determine whether we could reconstitute this complex in insect cells using recombinant baculoviruses for expression.
The ORF67 and ORF69 ORFs were PCR amplified using KSHV BAC36 (27) as a template according to protocols described previously (18). They were cloned as EcoRI-SpeI fragments into the baculovirus transfer vector pFastBac 1 (Invitrogen). We have modified this vector to encode the enhanced green fluorescent protein (EGFP), mCherry, and V5 sequences such that genes cloned into these vectors would generate a fusion protein with the fluorescent and epitope tags in frame at the C terminus of the open reading frame (Fig. 1A). All were sequence confirmed for correct amplification. Using the same methods, ORF67 and ORF69 were also cloned into pFastBac 1, encoding a C-terminal Flu hemagglutinin (HA) tag, and into the dual-expression baculovirus vector pFastBac Dual (Invitrogen). In the latter vector, the ORF67V5 (cloned EcoRI-SpeI fragment) gene was regulated by the polyhedrin promoter and the ORF69HA (cloned XhoI-KpnI fragment) gene by the p10 promoter element. The fusion sequences were transferred into the baculovirus genome using the Bac-to-Bac system (Invitrogen), and baculoviruses expressing the correct fusion protein were readily isolated and amplified (16). Insect cells (Sf9 and Sf21) and baculoviruses were propagated as described by Okoye et al. (16). Using chemiluminescent Western blot (WB) methods (enhanced chemiluminescence [ECL]; GE Healthcare) and antibodies to GFP, DsRed, and V5, we sought to confirm stable expression of the fusion proteins in infected Sf21 cells harvested 48 h postinfection. Protein lysates were prepared by lysing 1 × 106 infected Sf21 cells with EZ buffer (25) plus Halt protease inhibitor (Pierce), followed by sonication and clarification, and the soluble proteins were analyzed by the NuPage gel system (Invitrogen) and transferred to nitrocellulose membrane using the iBlot transfer system (Invitrogen). The ORF67 (29.7-kDa) and ORF69 (33-kDa) polypeptides detected were of the correct molecular weight and also displayed decreased mobility due to the addition of the 27-kDa fluorescent protein tag (Fig. 1B). Using similar methods, the coexpression of ORF67 and ORF69 was also examined (Fig. 1C). Both ORF67V5 and ORF69V5 accumulated in coinfected cells at the same levels observed in cells infected with the individual viruses. Using these lysis conditions, ORF69 accumulates in cells at levels greater than ORF67. This was also observed when two different epitope tags were used. The levels of ORF67V5 and ORF69HA were similar in both coinfected cells (ORF67V5 plus ORF69HA) and in cells infected with a dual-expression virus.
Fig 1.
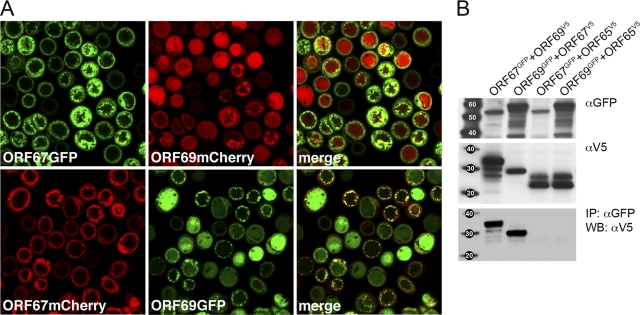
Cloning, expression, and localization of the KSHV nuclear egress complex proteins. (A) ORF67 (271 amino acids) and ORF69 (302 amino acids) were amplified and cloned into the baculovirus transfer vectors that encode C-terminal, GFP, mCherry, and V5 tags. (B) Amplified baculoviruses were used to infect Sf21 cells to confirm expression of the tagged polypeptides. The proteins were resolved on 4 to 12% Bis-Tris NuPage gels (Invitrogen), and proteins were detected using anti-V5 mouse (Invitrogen), anti-GFP rabbit (Molecular Probes), and anti-DsRed rabbit (BD Biosciences). Protein standards are shown in the left lane, and size is indicated in kilodaltons. (C) Similar infections and methods were used to examine coexpression of ORF67 and ORF69 using V5 and HA tags and Western blot procedures. Cells were infected with the individual viruses or coinfected with viruses expressing V5 and HA fusion proteins as well as a dual-expression virus vector. (D) Sf21 cells were infected with baculoviruses expressing GFP- and mCherry-tagged ORF67 and ORF69. The cells were visualized by confocal microscopy 48 h postinfection. The objective lens was 63×.
Using the GFP and mCherry tags, our goal was to determine the localization of these proteins in insect cells. Sf21 cells (1 × 106) in chamber slides were infected with the baculoviruses expressing the GFP- and mCherry-tagged proteins (Fig. 1D). When both the GFP and mCherry fusion proteins were expressed, the cellular localization observed was as expected and as reported in the literature for this family of proteins. Thus, ORF69 displayed a diffuse nuclear fluorescence and ORF67 localized to the nuclear periphery and structures adjacent to the nucleus.
Previous studies on alphaherpesvirus NEC proteins showed, using immunofluorescence of fixed cells, the relocalization of the UL31 protein to the nuclear margins in the presence of functional UL34. This was also shown for KSHV ORF67 and ORF69 using similar methods. Our goal was to use live fluorescent reporter proteins to visualize this in living cells. When Sf21 cells were coinfected with viruses such that both proteins were expressed in the same cell, relocalization of the fluorescent signal associated with ORF69 was observed (Fig. 2A). This signal (GFP or mCherry) became concentrated at the nuclear periphery and colocalized with the signal associated with ORF67. In many cells, we also saw significant alteration of the nuclear membrane as judged by the distribution of the colocalized signal. To demonstrate biochemically that the proteins physically interact, we also performed coimmunoprecipitation experiments. Infected Sf21 cells (1 × 106) were harvested at 48 h postinfection, and lysates were prepared using RIPA buffer (0.05 M Tris-HCl, pH 7.2, 0.15 M NaCl, 1% deoxycholic acid, 0.1% SDS, 1% Triton X-100). The sonicated/clarified lysates were mixed together for 30 min and then immunoprecipitated with anti-GFP antibody (1 h), followed by binding with protein-A Sepharose beads (1 h) (Sigma-Aldrich). The precipitated complexes were washed five times with RIPA buffer. The resulting immunoprecipitates following transfer to a membrane were probed with anti-V5 antibody (Fig. 2B). The results of the blot show that immunoprecipitation (IP) of either ORF67GFP or ORF69GFP coprecipitated the other protein, as judged by the V5 blot. In the control immunoprecipitation, pORF65V5 (KSHV small capsid protein) was not coprecipitated by immunoprecipitation of either ORF67GFP or ORF69GFP.
Fig 2.
ORF67 and ORF69 interactions observed visually by relocalization of ORF69 to the nuclear boundary and biochemically by coimmunoprecipitation methods. (A) Sf21 cells were coinfected with viruses expressing GFP/mCherry tagged proteins and analyzed by confocal microscopy 48 h postinfection. The objective lens was 63×. The merge of the GFP and mCherry fluorescence shows relocalization of ORF69 to the nuclear margins. (B) ORF67V5 and ORF69V5 are coprecipitated by ORF69GFP and ORF67GFP, respectively (immunoprecipitation [IP] with αGFP followed by Western blot [WB] analysis with αV5). The proteins were processed for Western blots as described in the legend to Fig. 1. The proteins present in the input lysates are shown by the αGFP and αV5 blots (top and middle). Protein standards are shown in the left lane, and size is indicated in kilodaltons.
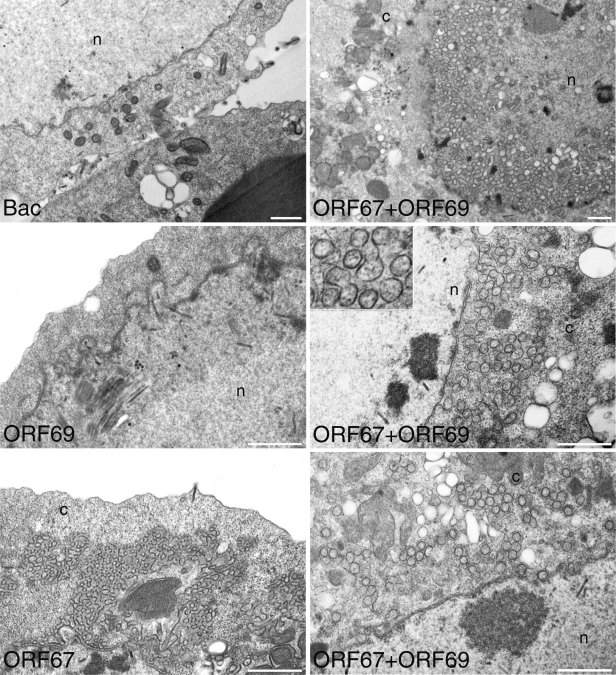
Previous studies have shown the overexpression of the EBV BFRF1 gene product results in significant nuclear membrane duplication in cells (5). When PRV UL31 and UL34 were overexpressed in mammalian cells, virus-size vesicles that were derived from the nuclear envelope were detected by similar ultrastructural analysis (8). We used transmission electron microscopy (TEM) to discover if the KSHV proteins could remodel the insect cell nuclear membrane. For these experiments, we used the viruses expressing the V5 tag. Sf21 cells (5 ×106 in 60-mm petri dishes) were infected with the baculoviruses expressing ORF67V5 or ORF69V5 or coinfected with these two viruses and processed for TEM 66 h postinfection (Fig. 3). Expression of ORF69 alone did not result in any changes within the cell. Expression of ORF67 did result in large areas of nuclear membrane stacks, similar to that observed for BFRF1 (5). When cells were infected with both ORF67- and ORF69-expressing viruses, we observed many circular vesicles that are most likely derived from reduplicated nuclear membranes. These vesicles appear to be similar in size to the herpesvirus virion. Analysis of the thin sections from these infections showed that 89 out of 100 infected cells (ORF67 alone) displayed nuclear membrane duplication events, and in 88 out of 100 cells (ORF67 and ORF69), vesicles were evident. Measurements of circular vesicles showed that they had a mean diameter of 146 nm (n = 17) or 157 nm (n = 18). The vesicles formed in the presence of PRV NEC proteins ranged in size from 130 to 160 nm. Cryotomograms of HSV-1 mature extracellular virions that contain significant tegument density were shown to have a mean diameter of 186 nm.
Fig 3.
Nuclear membrane remodeling by KSHV ORF67 and vesicle formation by the coexpression of ORF67 and ORF69. Sf21 cells were infected with the baculoviruses expressing ORF67 and ORF69 or infected with a control baculovirus. Infected cells were processed for TEM 66 h postinfection. Extensive nuclear membrane duplication was seen in BacORF67-infected cells; in cells coinfected with BacORF67 and BacORF69, numerous vesicles around the nuclear boundary were observed. These nuclear remodeling phenotypes were not seen in the control virus infections or in BacORF69-infected cells. The nucleus (n) and cytoplasm (c) compartments are indicated. Bars, 1,000 nm.
Little was known about the KSHV nuclear egress complex when we started this work. Santarelli et al. (23) reported a study on the expression and localization of ORF69. They showed that this protein is localized in the nucleus and visualized potential complex formation with ORF67 and BFRF1, both orthologs of HSV-1 UL34. Here we have used live-cell imaging of infected cells and fluorescent tags to demonstrate the correct localization of these proteins in insect cells, as well as the colocalization of ORF67 and ORF69 at the nuclear membrane. Using this expression system, we have also demonstrated that ORF67 by itself is sufficient for nuclear membrane duplication. This has only been shown previously for BFRF1 in animal cells (5), and we have also confirmed this result in insect cells using baculovirus-expressed BFRF1 (data not shown). The use of a baculovirus expression system has some advantages over plasmid transfection in animal cells. These include (i) the ability to infect the majority of the cells in culture, so the analysis is on a uniform synchronized population of cells; (ii) more-precise temporal control of the analysis because of the efficiency of transduction and replication properties of the virus; (iii) ability to coinfect many cells in culture using single- and dual-expression baculovirus vectors; and (iv) use of Escherichia coli recombineering for the rapid generation of baculovirus recombinants. The fact that we can recapitulate the KSHV NEC in insect cells and the results are similar to other studies performed in animal cells shows that this is a bona fide system of studying an essential process of a virus that is both difficult to grow and hard to genetically manipulate.
The coexpression of ORF67 and ORF69 in the same cells results in the formation of numerous virion-size circular vesicles. This phenotype was first reported by Klupp et al. (8) for the PRV orthologs, and now the results from the KSHV proteins show that they share this common mechanism, which is especially evident when overexpressed in such ex vivo systems. For PRV, the authors did not test whether UL34 was sufficient to cause membrane duplication, but nuclear membrane dissociation was demonstrated using baculovirus-expressed UL34 (26). From our data we conclude that ORF67 is responsible for membrane duplication, and when both ORF67 and ORF69 are present, the nuclear membrane is remodeled to create vesicles. We also have genetic evidence for this from mutations in ORF69, which fail to bind to ORF67 or relocalize to the nuclear margin. Cells examined by TEM display only the nuclear membrane duplication, and vesicles were absent (data not shown). This fits with studies from Roller et al. (22) that have shown that the interaction between UL31 and UL34 is important for membrane curvature around the assembled capsid. The reconstitution of this complex in insect cells makes available a tractable system to study this complex for gammaherpesviruses and to begin to determine the structure and composition of these vesicles.
ACKNOWLEDGMENTS
The studies presented here were supported by funding from the National Institutes of Health (RO1 AI 063182) and by an American Recovery and Reinvestment Act (ARRA) supplement to support undergraduate students (RO1 AI 063182S5). Additional support was also provided by an ARRA challenge grant (RC2 CA148402).
We thank S. J. Gao for providing BAC36.
Footnotes
Published ahead of print 19 October 2011
REFERENCES
- 1. Bjerke SL, Roller RJ. 2006. Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology 347:261–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang YE, Roizman B. 1993. The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein which partitions with the nuclear matrix. J. Virol. 67:6348–6356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farina A, et al. 2005. BFRF1 of Epstein-Barr virus is essential for efficient primary viral envelopment and egress. J. Virol. 79:3703–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuchs W, Klupp BG, Granzow H, Osterrieder N, Mettenleiter TC. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J. Virol. 76:364–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonnella R, et al. 2005. Characterization and intracellular localization of the Epstein-Barr virus protein BFLF2: interactions with BFRF1 and with the nuclear lamina. J. Virol. 79:3713–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Granato M, et al. 2008. Deletion of Epstein-Barr virus BFLF2 leads to impaired viral DNA packaging and primary egress as well as to the production of defective viral particles. J. Virol. 82:4042–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson DC, Baines JD. 2011. Herpesviruses remodel host membranes for virus egress. Nat. Rev. Microbiol. 9:382–394 [DOI] [PubMed] [Google Scholar]
- 8. Klupp BG, et al. 2007. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc. Natl. Acad. Sci. U. S. A. 104:7241–7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lake CM, Hutt-Fletcher LM. 2004. The Epstein-Barr virus BFRF1 and BFLF2 proteins interact and coexpression alters their cellular localization. Virology 320:99–106 [DOI] [PubMed] [Google Scholar]
- 10. Mettenleiter TC, Klupp BG, Granzow H. 2006. Herpesvirus assembly: a tale of two membranes. Curr. Opin. Microbiol. 9:423–429 [DOI] [PubMed] [Google Scholar]
- 11. Mettenleiter TC, Klupp BG, Granzow H. 2009. Herpesvirus assembly: an update. Virus Res. 143:222–234 [DOI] [PubMed] [Google Scholar]
- 12. Reference deleted. [Google Scholar]
- 13. Mou F, Forest T, Baines JD. 2007. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 81:6459–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mou F, Wills EG, Park R, Baines JD. 2008. Effects of lamin A/C, lamin B1, and viral US3 kinase activity on viral infectivity, virion egress, and the targeting of herpes simplex virus U(L)34-encoded protein to the inner nuclear membrane. J. Virol. 82:8094–8104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muranyi W, Haas J, Wagner M, Krohne G, Koszinowski UH. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297:854–857 [DOI] [PubMed] [Google Scholar]
- 16. Okoye ME, Sexton GL, Huang E, McCaffery JM, Desai P. 2006. Functional analysis of the triplex proteins (VP19C and VP23) of herpes simplex virus type 1. J. Virol. 80:929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park R, Baines JD. 2006. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J. Virol. 80:494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perkins EM, et al. 2008. Small capsid protein pORF65 is essential for assembly of Kaposi's sarcoma-associated herpesvirus capsids. J. Virol. 82:7201–7211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reynolds AE, Liang L, Baines JD. 2004. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes U(L)31 and U(L)34. J. Virol. 78:5564–5575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reynolds AE, et al. 2001. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939–8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roller RJ, Bjerke SL, Haugo AC, Hanson S. 2010. Analysis of a charge cluster mutation of herpes simplex virus type 1 UL34 and its extragenic suppressor suggests a novel interaction between pUL34 and pUL31 that is necessary for membrane curvature around capsids. J. Virol. 84:3921–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santarelli R, et al. 2008. Identification and characterization of the product encoded by ORF69 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 82:4562–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shiba C, et al. 2000. The UL34 gene product of herpes simplex virus type 2 is a tail-anchored type II membrane protein that is significant for virus envelopment. J. Gen. Virol. 81:2397–2405 [DOI] [PubMed] [Google Scholar]
- 25. White CA, Stow ND, Patel AH, Hughes M, Preston VG. 2003. Herpes simplex virus type 1 portal protein UL6 interacts with the putative terminase subunits UL15 and UL28. J. Virol. 77:6351–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ye GJ, Vaughan KT, Vallee RB, Roizman B. 2000. The herpes simplex virus 1 U(L)34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J. Virol. 74:1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou FC, et al. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]