Abstract
We investigated the role of microtubules in rhesus rhadinovirus (RRV) nuclear trafficking in rhesus fibroblasts. Intact microtubules and microtubule dynamics are required for RRV trafficking to perinuclear regions. RRV trafficking was reduced by an inhibitor of the dynein motor and overexpression of dynamitin. Furthermore, RRV particles are colocalized with microtubules and dynein proteins. These results highlight the important roles of microtubules and dynein-dynactin complexes in the transport of RRV particles to nuclei during primary infection.
TEXT
Kaposi's sarcoma-associated herpesvirus (KSHV) is a gammaherpesvirus associated with Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD) (17). Because KSHV infection is restricted to humans, animal models are useful for studying KSHV and its related malignancies (34). Rhesus rhadinovirus (RRV) is closely related to KSHV (1, 7, 41). RRV naturally infects rhesus macaques and induces PEL- and MCD-like malignancies under experimental conditions (29, 36, 48).
RRV virions enter rhesus fibroblasts (RFs) primarily through clathrin-mediated endocytosis (49). Following internalization, the incoming virions have to pass the diffusional barrier in the cytoplasm in order to deliver viral genomes to nuclei (15, 43). Because of the crowded space in cytoplasm and unspecific targeting, viral trafficking is unlikely to rely on random diffusion (11, 38). Consequently, viruses often hijack cellular cytoplasmic transport machineries to achieve fast transport to nuclei during early steps of infection. In particular, a number of viruses exploit microtubules and microtubule-dependent motors to move from cell peripheries to nuclei (2, 5, 8, 24, 25, 27, 35, 42–45). KSHV infection is mediated by either actin or microtubule cytoskeletons, depending on the cell types (16, 32). Whether RRV entry and trafficking are also regulated by cytoskeletons remains unclear.
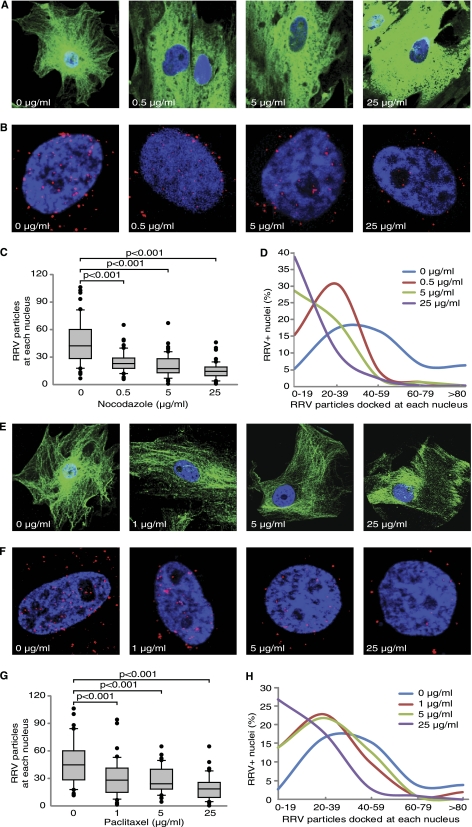
Microtubules are highly dynamic and unstable polymers undergoing rapid cycles of polymerization/depolymerization (18, 21, 30). We examined the role of microtubules in RRV trafficking following their depolymerization or stabilization with chemical inhibitors. A red fluorescent protein (RFP)-labeled RRV (RRV-RFP) was used to directly track RRV trafficking (49). Nocodazole depolymerizes microtubules by binding to β-tubulin and preventing formation of interchain bonds (19). RF cells pretreated with nocodazole (Sigma, St. Louis, MO) for 1 h were inoculated with RRV-RFP for 4 h in the presence of nocodazole. As shown in Fig. 1A, microtubules were effectively disrupted by nocodazole. The extent of microtubule disruption was intensified with increasing concentrations of nocodazole. Compared with the untreated control, nocodazole significantly inhibited RRV trafficking to the nuclei (Fig. 1B). The numbers of RRV particles docked at each nucleus significantly decreased in a dose-dependent fashion following nocodazole treatment (Fig. 1C and D).
Fig 1.
Disruption of microtubule networks with nocodazole or inhibition of microtubule dynamics with paclitaxel blocks RRV trafficking in RFs. (A and E) Effect of nocodazole and paclitaxel on microtubule networks. RFs were treated with increasing concentrations of nocodazole (A) or paclitaxel (E) and stained with an anti-β-tubulin antibody. Microtubule networks were disrupted by nocodazole but stabilized by paclitaxel. (B and F) Effect of nocodazole and paclitaxel on RRV trafficking. RFs were treated with nocodazole (B) or paclitaxel (F) for 1 h and inoculated with RRV in the presence of inhibitors, fixed at 4 h postinfection (hpi), and stained for RRV particles (red) and nuclei (blue). (C and G) Quantification of the total numbers of RRV particles docked at each nucleus following treatment with nocodazole (C) or paclitaxel (G). Images were acquired for 6 to 10 fields per coverslip to allow counting of 50 nuclei. The t test, analysis of variance, and/or Mann-Whitney tests were performed using SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA) with P < 0.05 considered significant. The numbers of RRV particles docking on a nucleus upon drug or control treatment are presented in box and whisker plots showing the median values (middle dark lines in the boxes) and the upper 75% and lower 25% quartiles (top and bottom box lines of the open boxes). The top and bottom short lines showed the ranges of the data, and outliers were represented as black dots. All experiments were performed in triplicates. (D and H) Distribution of nuclei with different numbers of RRV particles following treatment with nocodazole (D) or paclitaxel (H).
Paclitaxel (originally named “taxol”) binds to β-tubulin N terminus and promotes formation of highly stable microtubules (28). At 1 and 5 μg/ml, paclitaxel (Sigma) slightly increased the assembly of microtubules (Fig. 1E). When paclitaxel was used at 25 μg/ml, strong fibers were observed. Treatment with paclitaxel significantly inhibited RRV trafficking (Fig. 1F). The numbers of RRV particles docked at each nucleus were reduced in a dose-dependent manner following paclitaxel treatment (Fig. 1G and H). We also measured the viability of the cells using a propidium iodide (PI) labeling kit (Roche, Nutley, NJ). Neither nocodazole nor paclitaxel treatment increased the number of PI-positive cells (data no shown). Together, these results indicate that both intact microtubule networks and microtubule dynamics are required for RRV trafficking to perinuclear regions, and RRV particles might be transported along microtubules during trafficking.
Microtubules are formed with relatively stable minus ends emanating from microtubule organization center (MTOC) near nuclei and dynamic plus ends facing toward the cell membrane (33). Cargo movement on microtubules is bidirectional and is driven by microtubule-associated molecular motors, such as dynein and kinesin (18). Kinesin is a plus-end-directed microtubule motor, which moves cargos toward the cellular periphery, while dynein is minus end directed and is responsible for transporting cargos to MTOC. Both motors are ATPases, and their movements are powered by ATP hydrolysis (10, 14, 20, 23).
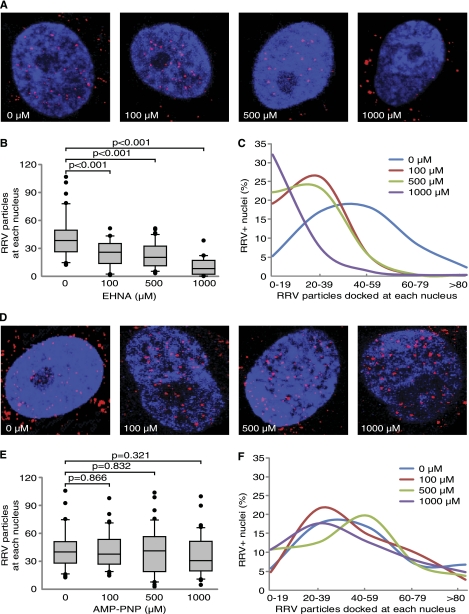
Cytoplasmic dynein is a protein complex responsible for retrograde movements of intracellular cargos and organelles, including viruses, along microtubules that serve as cellular conveyor belts. Dynein is composed of 4 major components: the heavy chain, the intermediate chain, the light intermediate chain, and the light chain (22, 46). Since dynein is involved in the trafficking of many viruses (6, 9, 13, 43, 47), we determined whether RRV transport along microtubules is dynein dependent. We used erythro-9-[3-(2-hydroxynonyl)]adenine (EHNA) (Sigma), an inhibitor of dynein ATPase, to disrupt the function of cytoplasmic dynein (37). EHNA strongly inhibited RRV intracellular trafficking (Fig. 2A). The numbers of RRV particles docked at nuclei were significantly reduced in a dose-dependent fashion in EHNA-treated cells (Fig. 2B and C). In contrast, inhibition of kinesin with 5-adenylyl-imidodiphosphate (AMP-PNP) (Sigma) had no effect on RRV trafficking (Fig. 2D to F). These results suggest that RRV trafficking in RFs is kinesin independent. Both EHNA and AMP-PNP had minimal toxicity as fewer than 2% of the cells were PI positive following the treatments (data not shown).
Fig 2.
Inhibition of dynein but not kinesin blocks RRV trafficking. (A and D) RFs were pretreated with dynein inhibitor EHNA (A) or kinesin inhibitor AMP-PNP (D) for 1 h prior to infection with RRV for 4 h and stained for RRV particles (red) and nuclei (blue). (B and E) Quantification of the total numbers of RRV particles docked at each nucleus following treatment with EHNA (B) or AMP-PNP (E). (C and F) Distribution of nuclei with different numbers of RRV particles following treatment with EHNA (C) or AMP-PNP (F). Experiments and data analyses were carried out as described in the legend to Fig. 1.
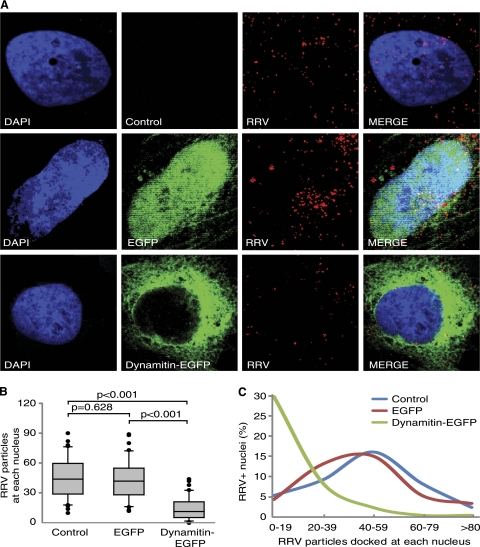
The interaction between the cytoplasmic dynein complex and cargo is often mediated by a receptor protein complex called “dynactin.” Dynactin consists of several subunits, including p150Glued, Arp1, capping protein, p62, Arp11, and dynamitin (40). Overexpression of dynamitin leads to dissociation of the subunits from the dynein-dynactin complex, resulting in disruption of dynein motor function (4). We used a dynamitin-green fluorescent protein (GFP) expression plasmid to confirm the role of the dynein-dynactin complex in RRV transport. Compared to untransfected cells, cells transfected with the vector control had no change in the numbers of RRV particles docked at nuclei. In contrast, cells transfected with dynamitin-GFP had significantly lower numbers of RRV particles docked at the nuclei (Fig. 3), indicating that intact dynein-dynactin complex is essential for RRV trafficking. Compared to vector-transfected cells, cells transfected with dynamitin-GFP had fewer cells having a clear and single MTOC; however, the presence of a focus MTOC did not appear to affect RRV infection and trafficking (data not shown).
Fig 3.
RRV trafficking is mediated by dynein. (A) Overexpression of dynamitin-GFP but not the enhanced green fluorescent protein (EGFP) vector efficiently inhibited RRV trafficking in RFs. Cells were transiently transfected with a dynamitin-GFP construct or a vector control. At 36 h posttransfection, cells were inoculated with RRV for 4 h and stained for RRV particles with a rabbit anti-RFP antibody. (B) Quantification of the total numbers of RRV particles docked at each nucleus. Images were acquired for 6 to 10 fields per coverslip to allow counting of 50 nuclei. Data were analyzed as described in the legend to Fig. 1. (C) Distribution of nuclei with different numbers of RRV particles.
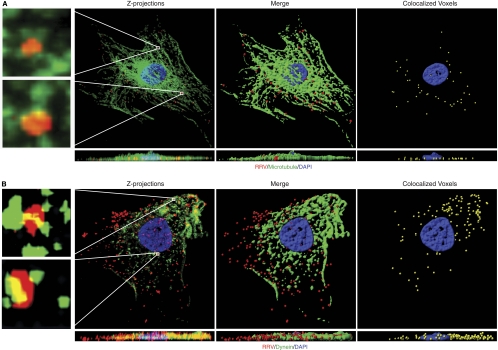
Our results so far indicate that RRV trafficking in RFs is microtubule and dynein dependent. To confirm these observations, we determined the association of RRV particles with microtubules. RFs infected with RRV-RFP were stained for RRV particles and β-tubulin. Z-stacks were acquired and used to generate three-dimensional (3D) projection XY overview images and the corresponding cross-sectional XZ images. 3D analysis indicated that more than 80% of incoming RRV particles were colocalized with microtubules (Fig. 4A; see Movie S1 in the supplemental material). A magnified 3D projection image of the individual RRV particles further illustrated their association with microtubules (insets in Fig. 4A).
Fig 4.
Colocalization of RRV particles with microtubules and the dynein motor proteins. (A) Colocalization of RRV particles with microtubules. RFs infected with RRV at a multiplicity of infection (MOI) of 50 were fixed at 1 h postinfection (hpi) and stained for RRV particles (red), microtubule cytoskeletons (green), and nuclei (blue). Z-stack images were acquired by Olympus FV1000 scanning confocal microscopy. Both the Z-projection and the XZ section showed colocalization of RRV particles with microtubules (yellow; left panel). Regions delineated by rectangle inserts were shown at higher magnifications in adjacent panels. 3D contoured images (middle and right panels) were generated with AutoQuant deconvolution (Media Cybernetics, Inc., Bethesda, MD) software and Imaris 3D image analysis software (Bitplane, Zurich, Switzerland). Corresponding XZ sections were visualized by rotating on the x axis to observe the location of virus particles. Movie S1 in the supplemental material corresponds to Fig. 4A. (B) Colocalization of RRV particles with dynein. RFs were infected with RRV for 1 h and stained for RRV particles and dynein heavy chain. Images were processed for 3D colocalization analysis. Regions delineated by rectangles are shown at higher magnifications in adjacent panels. Movie S2 in the supplemental material corresponds to Fig. 4B.
Next, we determined the association of RRV particles with dynein. By staining both RRV particles and dynein heavy chain, we observed colocalization of RRV particles with dynein (Fig. 4B; see Movie S2 in the supplemental material). These results further confirm that intact microtubule networks and microtubule dynamics are required for RRV trafficking to nuclei.
Our results show that RRV trafficking is regulated by both microtubules and dynein. Two models can explain these results. In the first model, low pH triggers the release of nucleocapsids from endosomes to cytosol. The nucleocapsids bind to microtubules through interactions with the dynein-dynactin complexes to hitch a ride along the microtubules. In the second model, virion-containing endosomes are transported along microtubules to the perinuclear regions before releasing the nucleocapsids. If the first model were correct, it would be important to identify the components of interactions between the dynein-dynactin complexes and virions. Almost all subunits in the dynein-dynactin complexes have the potential to directly bind to cargos, with the exception of dynein heavy chain (15). For example, herpes simplex virus 1 nucleocapsids bind to microtubules through the interaction between VP26 and dynein light chains RP3 and Tctex1 (12). In the second model, the interactions of the virions with microtubules and associated motor proteins are not required. Instead, the virion-containing endosomes directly bind to and travel along microtubules under the control of molecular motors such as dynein or kinesin prior to the release of nucleocapsids into the cytoplasm at low pH (3, 26, 31). In this case, examination of the colocalization of virions with intracellular endosomal markers and microtubules in real-time could define the precise stage and time and space of their association and release during virus entry.
Actin cytoskeletons are often involved in the trafficking of viruses. The involvement of either actin or microtubule cytoskeletons in KSHV trafficking is cell type dependent (16, 32, 39). In primary human umbilical vein endothelial cells, KSHV trafficking requires actin dynamics (16). However, in human fibroblasts, KSHV trafficking is mediated by microtubules (32). While we have shown that microtubules mediate RRV trafficking in RFs, it is possible that actin cytoskeletons are also involved in this process. Further investigations are required to determine the role of actin cytoskeletons in RRV trafficking.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ronald C. Desrosiers at the New England Primate Research Center for providing RRV strain RRV26-95, William Britt at the University of Alabama at Birmingham and Richard Vallee at Columbia University for the GFP-dynamitin construct, and Guangming Zhong at the University of Texas Health Science Center at San Antonio for the RFP antibody. We thank members of S.-J. Gao's laboratory for helpful discussions.
This work was supported by grants from the National Institutes of Health (CA096512, CA124332, and CA119889) to S.-J. Gao.
Footnotes
Published ahead of print 26 October 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Alexander L, et al. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amorim MJ, et al. 2011. A Rab11- and microtubule-dependent mechanism for cytoplasmic transport of influenza A virus viral RNA. J. Virol. 85:4143–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bananis E, et al. 2004. Microtubule-dependent movement of late endocytic vesicles in vitro: requirements for dynein and kinesin. Mol. Biol. Cell 15:3688–3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. 1997. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139:469–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chambers R, Takimoto T. 2010. Trafficking of Sendai virus nucleocapsids is mediated by intracellular vesicles. PLoS One 5:e10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cullen BR. 2001. Journey to the center of the cell. Cell 105:697–700 [DOI] [PubMed] [Google Scholar]
- 7. Desrosiers RC, et al. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J. Virol. 71:9764–9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. 2008. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 18:35–51 [DOI] [PubMed] [Google Scholar]
- 9. Dohner K, Nagel CH, Sodeik B. 2005. Viral stop-and-go along microtubules: taking a ride with dynein and kinesins. Trends Microbiol. 13:320–327 [DOI] [PubMed] [Google Scholar]
- 10. Dohner K, Sodeik B. 2005. The role of the cytoskeleton during viral infection. Curr. Top. Microbiol. Immunol. 285:67–108 [DOI] [PubMed] [Google Scholar]
- 11. Dohner K, et al. 2002. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell 13:2795–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Douglas MW, et al. 2004. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J. Biol. Chem. 279:28522–28530 [DOI] [PubMed] [Google Scholar]
- 13. Frampton AR, Jr, et al. 2010. Equine herpesvirus type 1 (EHV-1) utilizes microtubules, dynein, and ROCK1 to productively infect cells. Vet. Microbiol. 141:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gee MA, Heuser JE, Vallee RB. 1997. An extended microtubule-binding structure within the dynein motor domain. Nature 390:636–639 [DOI] [PubMed] [Google Scholar]
- 15. Greber UF, Way M. 2006. A superhighway to virus infection. Cell 124:741–754 [DOI] [PubMed] [Google Scholar]
- 16. Greene W, Gao SJ. 2009. Actin dynamics regulate multiple endosomal steps during Kaposi's sarcoma-associated herpesvirus entry and trafficking in endothelial cells. PLoS Pathog. 5:e1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greene W, et al. 2007. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat Res. 133:69–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gundersen GG, Cook TA. 1999. Microtubules and signal transduction. Curr. Opin. Cell Biol. 11:81–94 [DOI] [PubMed] [Google Scholar]
- 19. Hamel E. 1996. Antimitotic natural products and their interactions with tubulin. Med. Res. Rev. 16:207–231 [DOI] [PubMed] [Google Scholar]
- 20. Howard J, Hudspeth AJ, Vale RD. 1989. Movement of microtubules by single kinesin molecules. Nature 342:154–158 [DOI] [PubMed] [Google Scholar]
- 21. Hyman AA, Karsenti E. 1996. Morphogenetic properties of microtubules and mitotic spindle assembly. Cell 84:401–410 [DOI] [PubMed] [Google Scholar]
- 22. King SM. 2000. The dynein microtubule motor. Biochim. Biophys. Acta 1496:60–75 [DOI] [PubMed] [Google Scholar]
- 23. Kon T, Nishiura M, Ohkura R, Toyoshima YY, Sutoh K. 2004. Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry 43:11266–11274 [DOI] [PubMed] [Google Scholar]
- 24. Lehmann M, et al. 2009. Intracellular transport of human immunodeficiency virus type 1 genomic RNA and viral production are dependent on dynein motor function and late endosome positioning. J. Biol. Chem. 284:14572–14585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leopold PL, et al. 2000. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum. Gene Ther. 11:151–165 [DOI] [PubMed] [Google Scholar]
- 26. Loubery S, et al. 2008. Different microtubule motors move early and late endocytic compartments. Traffic 9:492–509 [DOI] [PubMed] [Google Scholar]
- 27. Lyman MG, Enquist LW. 2009. Herpesvirus interactions with the host cytoskeleton. J. Virol. 83:2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manfredi JJ, Parness J, Horwitz SB. 1982. Taxol binds to cellular microtubules. J. Cell Biol. 94:688–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mansfield KG, et al. 1999. Experimental infection of rhesus and pig-tailed macaques with macaque rhadinoviruses. J. Virol. 73:10320–10328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitchison T, Kirschner M. 1984. Dynamic instability of microtubule growth. Nature 312:237–242 [DOI] [PubMed] [Google Scholar]
- 31. Murray JW, Bananis E, Wolkoff AW. 2000. Reconstitution of ATP-dependent movement of endocytic vesicles along microtubules in vitro: an oscillatory bidirectional process. Mol. Biol. Cell 11:419–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naranatt PP, Krishnan HH, Smith MS, Chandran B. 2005. Kaposi's sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J. Virol. 79:1191–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nogales E. 2000. Structural insights into microtubule function. Annu. Rev. Biochem. 69:277–302 [DOI] [PubMed] [Google Scholar]
- 34. O'Connor CM, Kedes DH. 2007. Rhesus monkey rhadinovirus: a model for the study of KSHV. Curr. Top. Microbiol. Immunol. 312:43–69 [DOI] [PubMed] [Google Scholar]
- 35. Ogawa-Goto K, et al. 2003. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J. Virol. 77:8541–8547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orzechowska BU, et al. 2008. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: animal model for KSHV-associated malignancies. Blood 112:4227–4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Penningroth SM, Cheung A, Bouchard P, Gagnon C, Bardin CW. 1982. Dynein ATPase is inhibited selectively in vitro by erythro-9-[3-2-(hydroxynonyl)]adenine. Biochem. Biophys. Res. Commun. 104:234–240 [DOI] [PubMed] [Google Scholar]
- 38. Ploubidou A, Way M. 2001. Viral transport and the cytoskeleton. Curr. Opin. Cell Biol. 13:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Chandran B. 2009. Kaposi's sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J. Virol. 83:4895–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schliwa M, Woehlke G. 2003. Molecular motors. Nature 422:759–765 [DOI] [PubMed] [Google Scholar]
- 41. Searles RP, Bergquam EP, Axthelm MK, Wong SW. 1999. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sodeik B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8:465–472 [DOI] [PubMed] [Google Scholar]
- 43. Sodeik B, Ebersold MW, Helenius A. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Su Y, et al. 2010. Microtubule-dependent retrograde transport of bovine immunodeficiency virus. Cell. Microbiol. 12:1098–1107 [DOI] [PubMed] [Google Scholar]
- 45. Suomalainen M, et al. 1999. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 144:657–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vallee RB, Wall JS, Paschal BM, Shpetner HS. 1988. Microtubule-associated protein 1C from brain is a two-headed cytosolic dynein. Nature 332:561–563 [DOI] [PubMed] [Google Scholar]
- 47. Whittaker GR, Kann M, Helenius A. 2000. Viral entry into the nucleus. Annu. Rev. Cell Dev. Biol. 16:627–651 [DOI] [PubMed] [Google Scholar]
- 48. Wong SW, et al. 1999. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J. Exp. Med. 190:827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang W, Zhou F, Greene W, Gao SJ. 2010. Rhesus rhadinovirus infection of rhesus fibroblasts occurs through clathrin-mediated endocytosis. J. Virol. 84:11709–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.