Abstract
The family Flaviviridae contains three genera of positive-strand RNA viruses, namely, Flavivirus, Hepacivirus (e.g., hepatitis C virus [HCV]), and Pestivirus. Pestiviruses, like bovine viral diarrhea virus (BVDV), bear a striking degree of similarity to HCV concerning polyprotein organization, processing, and function. Along this line, in both systems, release of nonstructural protein 3 (NS3) is essential for viral RNA replication. However, both viruses differ significantly with respect to processing efficiency at the NS2/3 cleavage site and abundance as well as functional relevance of uncleaved NS2-3. In BVDV-infected cells, significant amounts of NS2-3 accumulate at late time points postinfection and play an essential but ill-defined role in the production of infectious virions. In contrast, complete cleavage of the HCV NS2-3 counterpart has been reported, and unprocessed NS2-3 is not required throughout the life cycle of HCV, at least in cell culture. Here we describe the selection and characterization of the first pestiviral genome with the capability to complete productive infection in the absence of uncleaved NS2-3. Despite the insertion of a ubiquitin gene or an internal ribosomal entry site between the NS2 and NS3 coding sequences, the selected chimeric BVDV-1 genomes gave rise to infectious virus progeny. In this context, a mutation in the N-terminal third of NS2 was identified as a critical determinant for efficient production of infectious virions in the absence of uncleaved NS2-3. These findings challenge a previously accepted dogma for pestivirus replication and provide new implications for virion morphogenesis of pestiviruses and HCV.
INTRODUCTION
The positive-strand RNA viruses of the family Flaviviridae are subdivided into the genera Flavivirus, Hepacivirus, and Pestivirus (49), with the latter two genera displaying a significantly higher degree of similarity. Pestiviruses like classical swine fever virus (CSFV) and bovine viral diarrhea virus (BVDV-1 and -2) are important pathogens of livestock worldwide (34). Their RNA genome has a length of 12.3 kb and encodes a single polyprotein with a size of about 3,900 amino acids (aa). For pestiviruses, the sequential order of proteins in the polyprotein is NH2-Npro (N-terminal autoprotease), C, Erns (envelope protein RNase secreted), E1, E2, p7, NS2-3 (NS2 and NS3), NS4A, NS4B, NS5A, and NS5B-COOH. Npro generates its own C terminus and thereby also the N terminus of the core protein (C). All processing steps in the structural region of the pestiviral polyprotein, namely, the release of core, Erns, E1, and E2, as well as the release of p7, are mediated by endoplasmic reticulum (ER)-resident host cell proteases (7, 28). NS2 contains a cysteine autoprotease which catalyzes cleavage between NS2 and NS3 (see below) (24). NS3 is a multifunctional protein with helicase/NTPase and serine protease activities (44, 51, 52). For full serine protease activity, NS3 requires NS4A as cofactor. The NS3-4A protease mediates the processing steps at the C terminus of NS3 and at all downstream cleavage sites of the viral polyprotein (46, 53). NS5A is a Zn2+-binding phosphoprotein with so far ill-defined function (40, 48). NS5B is the RNA-dependent RNA polymerase (RdRp), which, in concert with the viral proteins NS3, NS4A, NS4B, and NS5A and an unknown number of host factors, replicates the viral genome (28).
A strict temporal regulation of the cleavage event at the NS2/3 junction is of crucial importance for the life cycle of noncytopathogenic (ncp) BVDV-1 (23, 25). ncp BVDV replicates in cell culture without induction of a macroscopic cytopathic effect (34). After infection of a bovine fetus, only the ncp but not the cytopathogenic (cp) viral biotype can establish lifelong persistence. As a consequence, about 1 to 2% of the cattle population worldwide represent a constant reservoir for ncp BVDV (19). In ncp BVDV-infected culture cells, efficient NS2-3 cleavage is restricted to the early hours of infection, and as a consequence large quantities of uncleaved NS2-3 are detected at later time points (24). NS2-3 has been shown to be crucial for the generation of infectious viral particles for BVDV as well as for CSFV (see below) (1, 26, 35). In animals persistently infected with ncp BVDV, the emergence of cp BVDV strains, often by RNA recombination, leads to onset of lethal mucosal disease (MD) (5, 8, 22, 27). In cultured cells, cp BVDV strains express high levels of NS3 which correlate with upregulation of viral RNA replication and induction of apoptosis (15, 16, 18, 24, 50, 56). The molecular bases for the high levels of NS3 found in cp BVDV-infected cells are genomic alterations in the NS2-3-coding region (5, 22). In several cp BVDV genomes, cell-derived ubiquitin-coding mRNA fragments were identified upstream of the NS3-coding sequence (5, 22, 32, 47). In the context of the viral polyprotein, the generation of the C terminus of ubiquitin (ub) by cellular ubiquitin-C-terminal hydrolases (UCHs) leads to a highly efficient release of NS3 (47). For BVDV as well as CSFV, it has been shown that an artificial insertion of ubiquitin (ub) between NS2 and NS3 led to a defect in infectious virus particle formation, most likely due to complete processing at the NS2-ub/NS3 site (1, 35). The critical role of NS2-3 for virion morphogenesis was further corroborated with bicistronic pestivirus genomes containing an internal ribosomal entry site (IRES) between the NS2 and NS3 genes. These genomes were capable of efficient RNA replication; however, for the production of infectious progeny, they depended on the presence of a helper virus or exogenous expression of NS2-3-4A (1, 26, 35). These observations are in sharp contrast to hepatitis C virus (HCV): in HCV, uncleaved NS2-3 is not required for the formation of infectious virus particles, at least in cell culture (20, 21).
Because of the high overall similarity between HCV and pestiviruses, we hypothesized that it might be possible for pestiviruses to adapt for the production of infectious virus in the absence of uncleaved NS2-3. To this end, cp BVDV strain Osloss, originally isolated from an animal with MD, served as starting point. The Osloss polyprotein carries the insertion of one monomer of bovine ubiquitin exactly at the NS2/NS3 border (31). In this ubiquitin (ub*), the C-terminal glycine residue is exchanged by serine. Since this mutation modifies the cleavage site for cellular UCHs, it had been speculated that NS2-3 cleavage of the Osloss strain is incomplete or delayed, thereby allowing infectious particle formation (47). Nevertheless, the insertion of this ubiquitin mutant also abrogated infectious particle formation in the context of BVDV-1 and CSFV strains (1, 35).
In the present study, a chimeric BVDV-1 genome encompassing the NS2-ub*-3-4A coding region of the Osloss strain gave rise to very-low-level production of infectious virions. Cell culture passage led to the generation of a mutant virus capable of high-titer virion production. The adaptive mutations identified in this virus allowed the construction of a pestivirus which replicated in the absence of uncleaved NS2-3. This unexpected result is in contrast to the established dogma that uncleaved NS2-3 is essential for pestiviral virion morphogenesis and implicates a novel parallel between virion production in pestiviruses and that in HCV.
MATERIALS AND METHODS
Cells and viruses.
Madin-Darby bovine kidney (MDBK) cells were grown in minimum essential medium (MEM) with MEM nonessential amino acids and 10% horse serum. Cells were maintained at 37°C and 5% CO2. BVDV strains NCP7, CP7, and Osloss were described previously (11, 41).
Antibodies and antisera.
For the detection of BVDV NS2-3/NS3, mouse monoclonal antibody (MAb) 8.12.7 (10) was used. Secondary species-specific antibodies were purchased from Dianova (Hamburg, Germany). Bovine polyclonal serum anti-E2 CP7 143 was obtained after immunization of a calf with lysates of insect cells infected with a recombinant baculovirus expressing E2 of BVDV-1 strain CP7.
Construction of BVDV plasmids.
Plasmids were constructed by standard cloning techniques. Their characteristics are shown in Table 1. Mutations were introduced by PCR or the QuikChange method (Stratagene, Heidelberg, Germany). Constructs were checked by restriction enzyme digestion, and PCR products were verified by sequencing. Descriptions of the constructs are presented below and in Table 1; the exact sequences and cloning strategies are available upon request. The backbone for the constructs is plasmid pNCP7-5A, encompassing the full-length BVDV NCP7 genome as cDNA (3). In this plasmid, the E2-p7-NS2-3-4A-coding region was replaced, either completely or in part, by corresponding cDNA fragments derived by reverse transcription (RT)-PCR from cells infected with BVDV Osloss. Nucleotide and amino acid positions in the chimeric infectious viruses correspond to the ones of the published CP7 genome/polyprotein (accession no. AF220247), when the CP7-specific insertion in the NS2 gene/protein is deleted (3). Furthermore, pNCP7-5A (and the chimeric plasmids derived thereof) differs from the authentic CP7 nucleotide sequence at the following bases in the 3′ untranslated region (3′UTR): C12261A, C12263T, and A12279G and in the encoded amino acid sequence by exchanges M2451V and S3336I. A minor effect of the mutations in the 3′UTR on virus growth has been reported (37).
Table 1.
Characteristics of the plasmids used in this study
| Plasmid name | Feature(s) |
|---|---|
| pN7/OsUb | Contains Osloss aa 1534-1666 (including C-terminal 56 aa of NS2) |
| pN7/OsNS2-Ub | Contains Osloss aa 1138-1666 |
| pN7/OsE2-Ub | Contains Osloss aa 694-1666 (excluding first amino acid of E2) |
| pN7/OsUb-NS4A | Contains Osloss aa 1591-2423 (including N-terminal 10 aa of NS4B) |
| pN7/OsNS2-4A | Contains Osloss aa 1138-2423 (including N-terminal 10 aa of NS4B) |
| pN7/OsE2-NS4A | Contains Osloss aa 694-2423 (excluding first amino acid of E2, including N-terminal 10 aa of NS4B) |
| pN7/OsNS2-4A(H/A,C/A) | Derivative of pN7/OsNS2-4A; active site residues histidine 1447 and cysteine 1512 replaced by alanine, leading to inactivation of NS2 protease |
| pN7/OsNS2-4A(H/A,C/A) UbWT | Derivative of pN7/OsNS2-4A(H/A,C/A); serine residue 1666 in Osloss ub* reversed to glycine → ub* sequence changed to native bovine ub |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q) | Derivative of pN7/OsNS2-4A(H/A,C/A); contains the selected mutation identified in NS2 |
| pN7/OsNS2-4A(H/A,C/A) (3′UTR) | Derivative of pN7/OsNS2-4A(H/A,C/A); contains the selected mutation identified in 3′UTR |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR) | Derivative of pN7/OsNS2-4A(H/A,C/A); contains combination of selected mutations identified in NS2 and 3′UTR |
| pN7/OsNS2-4A(H/A,C/A)deltaE2 | Derivative of pN7/OsNS2-4A(H/A,C/A) with deletion of E2 coding sequence |
| pN7/OsNS2-4A(H/A,C/A)(3′UTR)deltaE2 | Derivative of pN7/OsNS2-4A(H/A,C/A) (3′UTR) with deletion of the E2 coding sequence |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR)deltaE2 | Derivative of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR) with deletion of E2 coding sequence |
| pN7/OsNS2-4A(H/A,C/A)deltaE2-GAA | Derivative of pN7/OsNS2-4A(H/A,C/A)deltaE2 containing a GAA mutation in NS5B leading to inactivation of viral RNA-dependent RNA polymerase |
| pN7/OsNS2-4A(H/A,C/A)(R1268I,3′UTR) | Derivative of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR); R1268 in NS2 changed to isoleucine |
| pN7/OsNS2-4A(H/A,C/A)(R1268S,3′UTR) | Derivative of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR); R1268 in NS2 changed to serine |
| pN7/OsNS2-4A(H/A,C/A)(R1268A,3′UTR) | Derivative of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR); R1268 in NS2 changed to alanine |
| pN7/OsNS2-4A(H/A,C/A)(R1268Y,3′UTR) | Derivative of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR); R1268 in NS2 changed to tyrosine |
| pN7/OsNS2-4A(H/A,C/A)(R1268N,3′UTR) | Derivative of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR); R1268 in NS2 changed to asparagine |
| pN7/OsNS2-4A(H/A,C/A)(R1268E,3′UTR) | Derivative of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR); R1268 in NS2 changed to glutamate |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR)-NS2delC10 | Derivative of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR) with deletion of C-terminal 10 aa of NS2 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,C1500S,C1503S) | Derivative of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR) with mutation of putative Zn2+-binding domain in NS2 by substitution for cysteine residues 1500 and 1503 by serine |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,L1684S) | Derivative of pN7/OsNS2-4A(H/A,C/A) with a combination of mutations identified in NS2, 3′UTR, and NS3 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,Y2814H,N2984S) | Derivative of pN7/OsNS2-4A(H/A,C/A) with a combination of mutations identified in NS2, 3′UTR, and NS5A |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3-UTR,T403S,P883L,T1104I) | Derivative of pN7/OsNS2-4A(H/A,C/A) with a combination of mutations identified in NS2, 3′UTR, Ems, E2, and p7 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,T403S) | Derivative of pN7/OsNS2-4A(H/A,C/A) with a combination of mutations identified in NS2, 3′UTR, and Ems |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,P883L) | Derivative of pN7/OsNS2-4A(H/A,C/A) with a combination of mutations identified in NS2, 3′UTR, and E2 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,T1104I) | Derivative of pN7/OsNS2-4A(H/A,C/A) with a combination of mutations identified in NS2, 3′UTR, and p7 |
| pN7/OsNS2-IRES-NS3-4A(H/A,C/A)(R1268Q,3′UTR,T403S,P883L,T1104I) | Introduction of a stop codon at C terminus of NS2 and replacement of the ub*-coding sequence by IRES of EMCV derived from pCITE (Novagene) in pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,T403S,P883L,T1104I) |
Amino acid positions in the Osloss polyprotein refer to the sequence published in reference 12. In comparison to the published Osloss sequence, the Osloss isolate used in this work encoded differently at the following residues: the triplet coding for L832 was missing, H834A, W835L, S836A, E1024V, R1068A, P1069Q, V1070Y, W1071G, S1645T, H2202Q, and V2374I. Further details can be obtained on request.
In vitro transcription and electroporation.
A total of 2.5 μg of plasmid DNA linearized with SmaI at the 3′ end of the cDNA served as the template for RNA transcription using the MAXIscript SP6 kit (Ambion, Huntingdon, United Kingdom) according to the protocol of the manufacturer. The amount of RNA transcript was measured with the Quant-iT RNA assay kit and the Qubit fluorometer (Invitrogen, Karlsruhe, Germany). The RNA quality was checked by agarose gel electrophoresis. One microgram of each RNA was used to electroporate approximately 3 × 106 MDBK cells by Gene Pulser II (Bio-Rad, Hercules, California) as described previously (45).
Virus plaque purification assay.
MDBK cells were seeded into six-well dishes and subsequently infected with 10-fold serial dilutions of a virus suspension. After incubation at 37°C for 4 h, the cells were washed once with phosphate-buffered saline (PBS) and then overlaid with semisolid medium containing 0.6% low-melting-point agarose (Gibco-BRL) and 5% horse serum. Plaques were aspirated with a tip and resuspended in 400 μl cell culture medium. To obtain a biologically cloned virus, three consecutive rounds of plaque purification were applied.
Nucleotide sequencing.
Sequences obtained from Qiagen Sequencing Service were further analyzed by computer analysis using the GCG software package included in HUSAR (DKFZ, Heidelberg, Germany) (13) and Vector NTI Advance (Invitrogen, Karlsruhe, Germany).
SDS-PAGE and immunoblotting.
Proteins were separated in polyacrylamide-Tricine gels (8, 10, or 12% polyacrylamide) (43). After SDS-PAGE, proteins were transferred onto a nitrocellulose membrane (Pall, Pensacola, FL); the membrane was blocked with 3% (wt/vol) dried skim milk in PBS with 0.05% (vol/vol) Tween 20. For viral antigen detection, indicated primary monoclonal antibodies (MAbs) were detected by peroxidase-coupled species-specific secondary antibodies and Western Lightning chemiluminescence reagent Plus (Perkin Elmer, Boston, MA).
RNA isolation, RT-PCR, and direct sequencing (sequencing of the viral genome).
Total cellular RNA was prepared using the RNeasy minikit (Qiagen, Hilden, Germany). Transcriptor reverse transcriptase (Roche, Mannheim, Germany) and the Expand long-template PCR system (Roche, Mannheim, Germany) were applied to amplify cDNA fragments from viral RNA. The amplicons were subjected to agarose gel electrophoresis, purified using the Qiaquick gel extraction kit (Qiagen, Hilden, Germany), and directly sequenced with the appropriate DNA primer (Qiagen Sequencing Service).
For determination of the 5′- and 3′-terminal sequences, an RNA ligation method followed by a nested RT-PCR protocol was used (4).
Quantitative real-time RT-PCR.
For comparative quantification of viral subgenomic RNAs, MDBK cells were electroporated with 2 μg of the RNA transcript; an RNA replication-negative derivative encoding a GDD-to-GAA mutation in the viral RdRp served as control. Total cellular RNA was prepared 8 and 12 h postelectroporation (p.e.). Subsequently, photometric analyses were performed in order to determine the RNA concentration for each sample. Ten nanograms of each of the RNAs as well as five 10-fold dilutions of a pestivirus RNA standard were subjected to real-time RT-PCR analysis, including the measurements of three independent replicates. Synthesis of cDNA was carried out using random hexamers (Invitrogen) and the Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen). The quantitative real-time RT-PCR was run using the Mx 3005 P sequence detection system (Stratagene, La Jolla, CA) and the QuantiTect SYBR green RT-PCR kit (Qiagen, Hilden, Germany). For PCR amplification of BVDV-specific RNA, primer pair 324/326 (sense primer 5′-ATGCCCTTAGTAGGACTAGCA-3′ for 324 and antisense primer 5′-TCAACTCCATGTGCCATGTAC-3′ for 326) was used. For reverse transcription, the reaction mixtures were incubated for 30 min at 50°C followed by 15 min at 95°C. The cycling conditions were 40 cycles of 15 s at 95°C, 30 s at 56°C, 30 s at 72°C, and 75 s at 82°C, followed by 1 cycle of 60 s at 95°C, 30 s at 55°C, and 30 s at 95°C. To compare the increase in the amounts of the individual viral subgenomic RNAs between 8 and 12 h p.e., the viral RNA amounts measured in samples obtained 8 h p.e. were set at 100%.
IF assay.
Viral replication was detected via indirect immunofluorescence (IF) with a monoclonal antibody (MAb) directed against BVDV NS3/NS2-3 (MAb 8.12.7) (10) and visualized with a Cy3-labeled anti-mouse secondary antibody (42).
BVDV infection and virus titration.
Supernatants of electroporated or virus-infected cells were passed through a 0.2-μm-pore cellulose filter (Sartorius Stedim, Göttingen, Germany). Infection with BVDV was carried out as described previously for 1 h at 37°C (23). Endpoint titration was performed in four replicates on MDBK cells. Virus detection occurred after 72 h at 37°C by NS3-specific IF analysis. Titers were determined as 50% tissue culture infectious dose (TCID50) as described previously (23).
Determination of growth kinetics.
In triplicates, 4 × 105 MDBK cells were infected with virus at the indicated multiplicity of infection (MOI). After adsorption for 1 h at 37°C, the cells were washed six times with PBS, overlaid with medium containing 10% horse serum, and incubated at 37°C with 5% CO2. At the indicated time points, aliquots (250 μl) of the supernatant were removed and replaced by fresh medium. The supernatants of corresponding triplicates were used individually for titration on MDBK cells.
Virus neutralization assay.
Bovine polyclonal serum directed against E2 of BVDV-1 CP7 and fetal calf serum (FCS) (as a negative control) were heat inactivated at 56°C for 20 min. In quadruplicates, 10 μl of either antiserum or FCS was added to 90 μl of virus suspension in cell culture medium with a TCID50 of 1 × 103 per ml and incubated at 37°C in 5% CO2 for 1 h. MDBK cells, prepared in 96-well plates, were infected with 50 μl/well of a serial 1:3 dilution of the serum-virus or FCS-virus mixtures, respectively. Plates were incubated at 37°C with 5% CO2 for 3 days, and viral antigen was detected by IF.
RESULTS
Autonomous replication of a chimeric BVDV-1 with a genomic ubiquitin insertion between NS2 and NS3.
For the completion of their replication cycle, pestiviruses with ub* insertions between NS2 and NS3 have been shown to depend on unprocessed NS2-3 (1, 35). The cp BVDV-1 strain Osloss represents a possible exception to this dogma, since no helper virus dependency had been described. However, in the absence of an infectious cDNA representing the Osloss genome, this question could not be addressed conclusively. To challenge this working hypothesis, chimeric BVDV full-length cDNA clones containing different parts of the Osloss genome were established on the basis of the infectious ncp BVDV cDNA clone, pNCP7-5A (3). In this plasmid, the E2-p7-NS2-3-4A-coding region was replaced, either completely or in part, by corresponding cDNA fragments derived from the Osloss genome (Fig. 1). In vitro-transcribed chimeric viral genomes were electroporated into MDBK cells, and viral replication was monitored via detection of viral antigen by immunofluorescence using a MAb directed against NS3/NS2-3 (6). Supernatants of electroporated cells were harvested and tested for the presence of infectious virus.
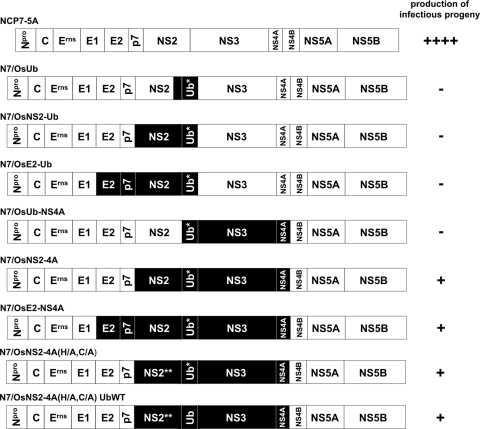
Fig 1.
Schematic drawing of the chimeric NCP7/Osloss cDNA constructs. Boxes represent the viral proteins; virus names are indicated above. The black boxes indicate coding regions derived from BVDV strain Osloss. NS2** represents an NS2 variant with exchanges H1447A and C1512A inactivating the cysteine autoprotease. Ub* represents the ubiquitin monomer of the Osloss strain in which the C-terminal glycine residue is substituted for by serine. Ub represents an authentic bovine ubiquitin monomer. In addition to the indicated Osloss-specific regions in N7/OsUb, N7/OsNS2-Ub, and N7/OsE2-Ub, the first 160 nucleotides of the NS3-coding region are derived from the Osloss genome. Amino acid sequences in this region are identical in BVDV strains NCP7 and Osloss. The capacity of each RNA transcript to give rise to infectious progeny 48 h postelectroporation is scored by minus or plus signs on the right.
While all tested chimeras were capable of RNA replication, generation of infectious progeny was restricted to the viruses carrying as a minimum the Osloss-derived sequences coding for NS2-ub*-NS3-4A and the N-terminal 10 amino acids of NS4B (Fig. 2). In the supernatants of MDBK cells electroporated with transcripts derived from pN7/OsNS2-4A, up to a few hundred infectious particles per ml were detected.
Fig 2.
The NS2 autoprotease activity and the mutation present in the Osloss-encoded ubiquitin are not essential for virion morphogenesis. MDBK cells were incubated with 500 μl of cell culture supernatant derived from MDBK cells electroporated with the indicated chimeric genome-length BVDV RNA transcripts and analyzed for infectious viral particle production 48 h p.i. by NS3-specific IF assay. Supernatants of cells electroporated with a replication-deficient RNA genome (N7/OsNS2-4A GAA) served as negative control. α-NS3, anti-NS3.
The NS2 autoprotease activity and the mutation of the C-terminal amino acid present in ub* are not essential for virion morphogenesis.
Next we addressed the possible role of the NS2 protease and the mutation present at the C terminus of the Osloss-derived ubiquitin in virion morphogenesis. Inactivation of the NS2 protease by replacement of active site residues H1447 and C1512 by alanine (H/A and C/A) in the context of virus N7/OsNS2-4A did not eliminate infectious virus production (Fig. 2). Along this line, a derivative of virus N7/OsNS2-4A encoding an authentic bovine ubiquitin monomer without the Osloss-specific mutation still produced infectious progeny (Fig. 2). Accordingly, neither NS2 protease activity nor the mutation identified in the ub* monomer of the Osloss strain is essential for the formation of infectious virions.
Selection and characterization of an efficiently spreading virus.
To select for viruses with improved virion production capacity, cells were infected with virus N7/OsNS2-4A(H/A,C/A) and serially passaged. Due to the inactivation of the NS2 protease, the ub* insertion was likely to be retained in the context of this viral genome since it is essentially required for NS3 generation and thus for viral RNA replication in the absence of a functional NS2 autoprotease. During cell culture passage, the cytopathic effect increased progressively (data not shown). A selected and biologically cloned (i.e., “sc”) cp BVD virus, termed N7/OsNS2-4A(H/A,C/A)sc, isolated from the supernatant of passage 6 produced an infectious titer of up to 3 × 107 TCID50 per ml, comparable to the one observed for other efficiently replicating BVDV strains. In cells infected with this virus, a large amount of NS3 but no NS2-3 was detectable by Western blotting, indicating that the relevant phenotype was retained (Fig. 3A). The strong increase in viral titer suggested that the virus had acquired adaptive mutations during cell culture passage. To identify these mutations, the viral genome was analyzed by RT-PCR and direct sequencing of the amplicons. Thirteen mutations were identified: 5 silent mutations in the ORF; 7 mutations leading to amino acid exchanges in Erns, E2, p7, NS2, NS3, or NS5A, respectively; and 1 mutation in the 3′UTR (Table 2). The latter nucleotide exchange, A12234C, reversed a deviation from the wild-type NCP7 sequence present in the cDNA backbone used to establish the chimeric cDNA clones and restores the wild-type structure of stem-loop 1 (SL-1) in the 3′UTR.
Fig 3.
NS2-3 cleavage analysis and growth kinetics. (A) MDBK cells infected with the indicated BVD viruses at an MOI of 1 were harvested 48 h p.i. and analyzed for NS2-3 cleavage. Lane 1, CP7; lane 2, N7/OsNS2-4A(H/A,C/A)sc (“sc” represents selected and biologically cloned virus); lane 3, N7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR); lane 4, N7/OsNS2-4A(H/A,C/A) (R1268Q, 3′UTR)(XE) (XE indicates mutations T403S, P883L, and T1104I); lane 5, uninfected MDBK cells (−). Lysates of these cells were analyzed by SDS-PAGE and by Western blotting using an NS3/NS2-3-specific monoclonal antibody. The positions of NS2-3 and NS3 are indicated by arrows. The molecular mass marker is shown on the left. (B) Growth curve analysis of the selected and biologically cloned virus N7/OsNS2-4A(H/A,C/A)sc and cDNA-derived virus N7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR)(XE). The graph represents one example of a growth curve after infection at an MOI of 0.1. Mean values and standard deviations (error bars) are indicated. Additional growth curve analyses carried out at an MOI of 3 displayed similar growth properties for both viruses (data not shown).
Table 2.
Cell culture-acquired exchanges detected in BVDV N7/OsNS2-4A(HA/CA)sca
| Nucleotide | Localization | Base exchange | Name or type of mutation |
|---|---|---|---|
| 505 | Npro | G→A | Silent |
| 1589 | Erns | A→T | T403S |
| 1717 | Erns | T→A | Silent |
| 2678 | E2 | T→C | Silent |
| 2845 | E2 | T→C | Silent |
| 3030 | E2 | C→T | P883L |
| 3693 | p7 | C→T | T1104I |
| 4185 | NS2 | G→A | R1268Q |
| 5433 | NS3 | T→C | L1608S |
| 8227 | NS4B | G→A | Silent |
| 8822 | NS5A | T→C | Y2814H |
| 9333 | NS5A | A→G | N2984S |
| 12234 | 3′UTR | A→C | 3′UTR |
Nucleotide and amino acid positions correspond to those of the published CP7-5A genome/polyprotein when the CP7-specific insertion in the NS2 gene/protein is deleted.
To identify the determinants for the increased efficiency in virion production, mutations leading to amino acid substitutions and the mutation in the 3′UTR were introduced individually or in different combinations into pN7/OsNS2-4A(H/A,C/A). Electroporation of corresponding RNA transcripts into MBDK cells revealed that the introduction of the individual mutations in the genes for NS2, NS3, or NS5A (both NS5A mutations in combination) did not lead to a substantial increase in infectious virus production in the supernatants of cells electroporated with the respective RNA transcripts (Table 3) (data not shown). The 3′UTR mutation which optimizes SL-1 displayed a 20-fold increase in virion production and thus could only partially explain the dramatic increase in infectivity observed for the passaged virus (Table 3). Intriguingly, the mutation of arginine at position 1268 to glutamine (R1268Q) identified in NS2, which did not significantly increase the efficiency of virion production on its own (Table 3), led to an increase of the viral titer of about 3 log10 TCID50 when combined with the mutation in the 3′UTR optimizing the structure of SL-1 (Table 3). A strong cytopathic effect observed after the infection of MDBK cells with this virus correlated with the increased spreading capability of this virus (Fig. 4).
Table 3.
Contribution of the selected mutations to virus productiona
| cDNA template | Virus titer 48 h p.e. (TCID50/ml) |
|---|---|
| pN7/OsNS2-4A(H/A,C/A) | 1.0 × 102 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q) | 3.1 × 102 |
| pN7/OsNS2-4A(H/A,C/A)(3′UTR) | 6.4 × 103 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR) | 3.3 × 105 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,Y2814H,N2984S) | 1.9 × 105 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,L1608S) | 4.4 × 105 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,T403S,P883L,T1104I) | 2.1 × 107 |
The table shows one representative example of several experiments in which all constructs were tested in parallel. All experiments revealed similar titer differences between the individual RNA transcripts.
Fig 4.
Determinants for efficient virion production. MDBK cells were incubated with 500 μl of cell culture supernatant derived from MDBK cells electroporated with the indicated chimeric genome-length BVDV RNA transcripts carrying selected mutations and analyzed 48 h p.e. by IF. Mutations identified in the genome of the selected and biologically cloned virus N7/OsNS2-4A(H/A,C/A)sc were introduced either alone or in various combinations, as indicated, into the parental strain N7/OsNS2-4A(H/A,C/A). A combination of the 3 mutations (T403S, P883L, and T1104I) identified in Erns, E2, and p7 is indicated by “(XE).” Note the correlation between viral fitness and virus-induced cytopathic effects (upper panel).
Upon electroporation of transcripts derived from pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR), containing the adaptive mutations in the 3′UTR and NS2, virion production still did not fully achieve the level observed for the parental virus generated by cell culture passage. The addition of the mutation in NS3 or both mutations in NS5A did not significantly alter the virion production capacity of the respective viral genomes (Table 3). In contrast, simultaneous introduction of the adaptive mutations identified in the structural genes and the p7 coding region into pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR), led to a further increase of the viral titers in the cell culture supernatants of cells electroporated with the respective RNAs by about a factor of 50 (Table 3). Insertion of the single adaptive mutations into either Erns, E2, or p7 did not lead to a similar increase in viral titers (data not shown).
In whole lysates of cells previously infected with this virus, large amounts of NS3 (but no NS2-3) were detected by Western blotting (Fig. 3A). A growth curve analysis demonstrated that the viral titers and growth kinetics of this chimeric genome are comparable to the ones of the cell-culture-selected virus (Fig. 3B).
Effect of the mutations on RNA replication efficiency.
While SL-1 is known to be an RNA element critical for pestivirus RNA replication (37, 55), a point mutation in NS2 is generally not expected to influence the efficiency of RNA replication in a pestivirus genome encoding a ubiquitin monomer directly upstream of NS3. However, synergistic effects between both mutations could not be excluded. Thus, the 3′UTR mutation was introduced individually or in combination with the NS2 mutation (R1268Q) into a pN7/OsNS2-4A(H/A,C/A)-based derivative in which the E2 gene has been deleted. Upon electroporation, the RNA transcripts from these plasmids are able to replicate but cannot generate infectious progeny. Thus, RNA replication efficiency can be determined independently from virus spread. A genomic transcript encoding an inactive viral RNA-dependent RNA polymerase, indicated by “(GAA),” served as a control. Ten nanograms of total RNA prepared from cells harvested at 8 or 12 h p.e. was analyzed by real-time RT-PCR (Fig. 5). Later time points were not analyzed due to severe differences in the cytopathic effects induced by individual replicons.
Fig 5.
Real time RT-PCR analysis. Relative amounts of accumulated viral subgenomic RNAs obtained 8 and 12 h postelectroporation (p.e.) of MDBK cells with 2 μg of the indicated RNAs. The graph (logarithmic scale) shows mean values and standard deviations (error bars) obtained by quantitative real-time RT-PCR analysis, including the measurements of three independent replicates. Mutations introduced into basic replicon N7/OsNS2-4A(H/A,C/A)deltaE2 are indicated below the bars. For each construct, the amount of accumulated viral RNA measured in samples prepared 8 h p.e. was set to 100%. The data shown represent one out of three experiments with congruent results.
To estimate the RNA replication efficiencies of the mutants, the amounts of viral RNA measured at 8 and 12 h p.e. were compared for each replicon RNA. For the pN7/OsNS2-4A(H/A,C/A)-derived replicon, the amount of RNA increased by a factor of about 7. Introduction of the 3′UTR mutation led to further increase in viral RNA amplification efficiency by about a factor of 5 and thus in a similar range to the increase in viral titer induced by this mutation (Fig. 5; Table 3). A comparable minor effect on the virus titer and plaque size has been described previously for an adaptation of the 3′UTR in pCP7-5A to the wild-type sequence of BVDV CP7 (37).
However, no further increase in RNA replication efficiency was observed for the double mutant encoding, in addition to the 3′UTR mutation, the R1268Q exchange in NS2 (Fig. 5). This strongly suggests that the dramatic increase in the viral titer induced by the NS2 mutation when combined with a wild-type SL-1 in the 3′UTR is not merely a result of increased RNA replication efficiency.
The identity of the amino acid at position 1268 is critical for virion formation.
To further characterize amino acid 1268 in NS2 with respect to virion formation, this residue was systematically mutated in the context of a derivative of pN7/OsNS2-4A(H/A,C/A) containing the 3′UTR mutation. Forty-eight hours p.e. of the respective RNA transcripts, virus titers in the supernatants were determined. For the R1268I or R1268S mutant, production of infectious progeny was comparable to that observed for the parental construct, while the R1268A or R1268Y mutant produced less than 100 TCID50/ml (Table 4). In contrast, electroporation of analogous genomes with exchange R1268E or R1268N produced numbers of infectious particles similar to those of RNAs containing the R1268Q selected mutant (Table 4). In a growth curve analysis, the virus mutants with exchange R1268Q or R1268E displayed analogous growth characteristics; the R1268N mutant reached a similar titer at 72 h p.e. but showed slightly delayed growth (data not shown).
Table 4.
Influence of the identity of the amino acid at position 1268 on the production of infectious virions
| cDNA template | Virus titer 48 h p.e. (TCID50/ml) |
|---|---|
| pN7/OsNS2-4A(H/A,C/A)(R1268,3′UTR) | 9.4 × 102 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR) | 5.4 × 104 |
| pN7/OsNS2-4A(H/A,C/A)(R1268E,3′UTR) | 2.8 × 104 |
| pN7/OsNS2-4A(H/A,C/A)(R1268N,3′UTR) | 1.6 × 104 |
| pN7/OsNS2-4A(H/A,C/A)(R1268A,3′UTR) | <102 |
| pN7/OsNS2-4A(H/A,C/A)(R1268I,3′UTR) | 1.5 × 103 |
| pN7/OsNS2-4A(H/A,C/A)(R1268S,3′UTR) | 2.0 × 103 |
| pN7/OsNS2-4A(H/A,C/A)(R1268Y,3′UTR) | <102 |
The integrity of NS2 is important for virion morphogenesis.
Next, in the context of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR) was examined whether the entire NS2 protein and/or its structural integrity is required for virion formation. To this end, either the C-terminal 10 amino acids of NS2 were deleted from the polyprotein, or the putative Zn2+-coordinating residues C1500 and C1503 were changed simultaneously to serine. Twenty-four hours after electroporation of the respective RNA transcripts, an IF analysis indicated efficient RNA replication, but no infectious virus was detected in the cell culture supernatants (Fig. 6). Accordingly, the integrity of NS2 is of critical importance for virion morphogenesis also in the context of a virus derived from pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR).
Fig 6.
The integrity of NS2 is required for virion production. (Upper) N7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR) RNA transcripts harboring either a deletion of the C-terminal 10 codons of the NS2 gene (NS2delC10) or mutations in the putative Zn2+-binding motif in NS2 (C1500S and C1503S) were electroporated into MDBK cells. Expression of viral antigen was detected by IF at 24 h p.e. (Lower) Supernatants of these cells harvested at 48 h p.e. were subsequently used for infection of naïve MDBK cells, which were analyzed by IF 48 h p.i.
Autonomous replication of a bicistronic BVDV which does not express NS2-3.
Taken together, these results suggested that the chimeric BVDV selected by cell culture passage indeed is capable of virion production in spite of undetectable levels or even in the absence of uncleaved NS2-3. To challenge the latter hypothesis rigorously, a stop codon followed by the IRES of EMCV was used to replace the ub* gene in the context of the cDNA of pN7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR,T403S,P883L,T1104I) harboring the adaptive mutations in the NS2 gene, the 3′UTR, the structural genes Erns and E2, and the p7-coding sequence.
Forty-eight hours after electroporation of cells with the bicistronic RNA transcript, cell culture supernatants contained about 2.1 × 105 TCID50 of viral particles per ml (data not shown). The infection of naïve MDBK cells by these supernatants could be completely blocked in a neutralization test by sera containing antibodies directed against E2 of BVDV CP7 (data not shown). An RT-PCR analysis of RNA derived from cells infected with these viral particles verified that the IRES sequence between the NS2 and the NS3 coding sequence has been retained in the replicating genomes (Fig. 7A). In a growth curve analysis, the bicistronic virus reached a titer of 2.4 × 104 TCID50/ml at 72 h postinfection (p.i.) (Fig. 7B).
Fig 7.
Virion production in the absence of uncleaved NS2-3. Characterization of virus N7/OsNS2-IRES-NS3-4A(H/A,C/A)(R1268Q,3′UTR)(XE) which carries an EMCV IRES between the NS2 and the NS3 coding sequences. (A) (Top panel) Schematic drawing of BVDV cDNA derivatives with the position of NS2- and NS3-specific primers used for RT-PCR analysis indicated. (Bottom panel) RT-PCR analysis of total RNA prepared from cells at 48 h after infection with either N7/OsNS2-4A(H/A,C/A(R1268Q,3′UTR) or the bicistronic derivative N7/OsNS2-IRES-NS3-4A(H/A,C/A)(R1268Q,3′UTR)(XE). The amplicon derived from N7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR) served as a size marker for a BVDV genome without IRES insertion. RNA of noninfected MDBK cells served as a negative control. α-NS3, anti-NS3. (B) Growth curve analysis comparing viruses N7/OsNS2-4A(H/A,C/A)(R1268Q,3′UTR)(XE),N7/OsNS2-4A(H/A,C/A)sc and N7/OsNS2-IRES-NS3-4A(H/A,C/A)(R1268Q,3′UTR)(XE). This figure includes the data already shown in Fig. 3. All three growth curves were generated in parallel. MDBK cells were infected with an MOI of 0.1. Virus titers in supernatants were determined at 12, 24, 48, and 72 h p.i.
These data prove that a chimeric BVDV-1 strain based on the genome of NCP7, encoding Osloss proteins NS2-ub*-3-4A and the N-terminal 10 aa of NS4B in combination with cell-culture-acquired mutations within the structural protein-coding genes, is capable of efficient virion production in the absence of uncleaved NS2-3. Moreover, these data indicate a genetic link between the structural proteins and NS2-3-4A to promote virion morphogenesis.
DISCUSSION
Pestiviruses represent important pathogens of livestock industries and a widely used surrogate model system for hepatitis C virus (HCV). The high degree of similarity between pestiviruses and HCV becomes obvious when the polyproteins and their proteolytic processing pathways are compared. While the interferon antagonists Npro and Erns are unique for the genus Pestivirus, the remainder of the polyprotein is colinear with the one of HCV (28). Analogous to pestiviruses, cleavages in the NS region of the HCV polyprotein are catalyzed by viral proteases whose active centers reside in NS2 and NS3, respectively (28). A striking difference between the processing schemes of these virus systems is the cleavage at the NS2/3 site. This cleavage appears to be highly efficient for HCV. Bicistronic HCV genomes which express NS2 and NS3 from different open reading frames produce infectious progeny, demonstrating that uncleaved NS2-3 is not required for viral replication, at least in cell culture (20, 21). In contrast, pestiviruses show a complex regulation of NS2-3 cleavage and unprocessed NS2-3 fulfills a critical but so far not defined task in the formation of infectious virus particles (1, 35). For ncp BVDV-1, it has been shown that the NS2 protease needs stoichiometric amounts of a cellular chaperone termed “Jiv” (J-domain protein interacting with viral protein) for NS2-3 cleavage (23). Low endogenous amounts of this cellular protease cofactor restrict NS2-3 cleavage and consequently by confining NS3 release also limit the efficiency of viral RNA replication. This regulation is crucial for maintaining the ncp biotype of BVDV (23). Only ncp BVDV strains are able to establish persistent infections in bovine fetuses after intrauterine infections. The resulting acquired immunotolerance protects the virus against the adaptive immune response of the host. The ncp biotype with its downregulated RNA replication efficiency seems to be a prerequisite for efficient control of the innate immune system by the viral virulence factors Npro and Erns as well as for evading induction of apoptosis (9, 30). Also for CSFV, it has recently been demonstrated that an upregulation of NS2-3 cleavage correlates with induction of the interferon system and attenuation in the host (14). Therefore, control of NS2-3 cleavage plays a crucial role in the biology of pestiviruses in general. Unprocessed NS2-3 could thus be regarded as a kind of molecular disposal for NS3 which is not proposed to build active replication complexes. However, in pestivirus infection, uncleaved NS2-3 is in addition essential for virion morphogenesis (1, 35). Consequently, the insertion of ubiquitin or other protease substrates which lead to complete cleavage between NS2 and NS3 in the polyproteins of BVDV or CSFV abolished the formation of infectious virions (1, 35). This defect is not caused merely by the resulting fusion of ubiquitin to the C terminus of NS2, since bicistronic genomes with an encephalomyocarditis virus (EMCV) IRES between the authentic NS2- and NS3-coding sequences showed the same defect. These in vitro studies are further corroborated by the observation that the vast majority of naturally emerging cp BVDV genomes with insertions exactly upstream of the NS3 gene contain large duplications providing an authentic NS2-3-4A region (5, 33). Surprisingly, those cp BVDV genomes are frequently isolated despite their enormous genetic ballast. This might indicate the existence of a significant genetic barrier for the generation of viruses which can replicate without uncleaved NS2-3. In this respect, cp BVDV strains CP14 and Osloss represent rare exceptions, since they have ubiquitin insertions between NS2 and NS3 but no genomic duplication. In the CP14 genome, an insertion comprising a polyubiquitin-mRNA fragment was identified (47). In the absence of an infectious cDNA system, cell culture experiments performed at that time strongly indicated that virion formation of this viral isolate depends on a helper virus (N. Tautz, unpublished observation), which is in agreement with recent cDNA-based studies on BVDV and CSFV (1, 35). In contrast, cp BVDV-1 strain Osloss, investigated in this study, was suspected to be able to replicate independent from a helper virus. The data obtained in the present study demonstrate that pestiviruses are indeed capable of adapting to virion morphogenesis in the absence of uncleaved NS2-3. However, the BVDV chimera containing the NS2-ub*-NS3-4A region of strain Osloss in the NCP7 backbone did produce a very small number of infectious particles. Only upon cell culture passage did this virus gain the capability to spread efficiently. Thus, the situation for the Osloss strain remains unclear. Possibly other mutations in its genome facilitate virion morphogenesis in the presence of the ub* insertion. However, it also cannot be finally excluded that the Osloss strain is helper dependent since at least in our hands, plaque-purified viruses grew only to low titers (data not shown).
Analysis of the selected, efficiently replicating NCP7/Osloss chimera identified amino acid 1268, located in the N-terminal hydrophobic third of NS2, as well as the 3′ end of the genome as highly critical determinants for virion formation. In the context of the chimeric virus, mutations in the structural proteins further optimized virion formation efficiency. These mutations might adapt the structural proteins of BVDV strain NCP7 to the Osloss-derived NS2-3-4A region and indicate that the interaction between these components is relevant for virion formation. For HCV, several studies based on intra- and intergenotype chimeras in NS2 also pointed to an important role of the N-terminal hydrophobic part of NS2 in virion formation and revealed genetic links between the structural proteins and NS2 (20, 38, 39, 54).
Furthermore, Moulin et al. have demonstrated by trans-complementation studies for CSFV that even small deletions at the N terminus of NS2-3 disrupt the functionality of NS2-3-4A in the process of virion formation. In the present study, a small deletion at the C terminus of NS2 as well as mutations destroying its putative Zn2+-binding motif abrogated virion morphogenesis, while RNA replication was not impaired. These observations indicate that the structural integrity of pestivirus NS2 plays an essential role in the production of infectious virions, analogous to observations made for NS2 of HCV (21).
For BVDV, we observed in this study that at amino acid position 1268, located in the N-terminal hydrophobic part of NS2, glutamine, asparagine, and glutamate allowed for efficient virion production. Arginine, which is conserved between the majority of pestivirus strains at this position, as well as isoleucine and serine led to inefficient generation of viral progeny, while only very few infectious virions were detected for the alanine and tyrosine mutants. Accordingly, at this site bulky amino acids with no positive charge seem to promote virion morphogenesis. In this context, data on the transmembrane topology of the pestivirus NS2 and especially the location of amino acid 1268 would be highly interesting.
As described previously for CSFV, the activity of the NS2 protease is, in the BVDV system, also not required for virion formation. Moulin et al., moreover, excluded essential functions of the helicase and NS3-4A serine protease activities, although free NS4A had to be present in addition to NS2-3 to support packaging (35). Taken together, these findings support a scaffolding function for NS2-3, as proposed for NS2 of HCV (29). For BVDV also an involvement of p7 and NS5B in virion morphogenesis has been demonstrated (2, 17, 26). Thus, as described for other members of the family Flaviviridae, structural and different nonstructural proteins are involved in the process of virion formation of BVDV (36). In addition, actively replicated viral RNA was shown to be encapsidated with higher efficiency, indicating a coupling between RNA replication and packaging (26). Along this line, it was observed in the present study that a wild-type-like SL-1 structure at the 3′ end of the genome is important for efficient virion formation. It is possible that uncleaved NS2-3 interacts either directly (e.g., via the RNA binding activity of the NS3 helicase domain) with the 3′UTR during virion morphogenesis or indirectly via interactions mediated by other components of the replication complex, such as NS5A or NS5B. In the absence of unprocessed NS2-3, the NS2 mutation and the perfect integrity of the SL-1 in the 3′UTR obviously become very important determinants for virion morphogenesis, possibly by establishing novel protein-protein or protein-RNA interactions or stabilizing existing ones. However, without further detailed studies, no conclusions regarding the underlying mechanisms can be drawn.
Compared with the monocistronic ubiquitin-encoding constructs, the bicistronic virus generated in this study grew to significantly lower titers. This observation may indicate that a continuous translation of NS2 and the downstream part of the polyprotein promotes the formation of packaging complexes. Alternatively, a short-lived NS2-3 precursor or small amounts of uncleaved NS2-ub*-NS3 could facilitate virion morphogenesis in the NCP7/Osloss chimera. Alternatively, the mutation in NS2 may lead to the formation of novel protein-protein interactions with critical relevance for virion formation. This aspect has to be addressed in future studies.
Compared to NCP7, the NCP7/Osloss chimera carries 48 amino acid exchanges. It will be interesting to define how many of those exchanges are actually essential for virion morphogenesis in the absence of uncleaved NS2-3.
The findings of the present study demonstrate the existence of an even higher degree of functional similarity between HCV and the pestivirus system. The fact that pestiviruses can adapt to virion morphogenesis in the absence of uncleaved NS2-3 leads to the question of why natural pestivirus isolates have retained their dependency on uncleaved NS2-3. Further studies also with animal hosts are required for a better understanding of the biology of this interesting virus system.
ACKNOWLEDGMENTS
This study was supported by SFB 535 “Invasionsmechanismen und Replikationsstrategien von Krankheitserregern” and Graduiertenkolleg 455 “Molekulare Veterinärmedizin” by the Deutsche Forschungsgemeinschaft. P.B. was supported by a Heisenberg professorship and a grant from the Deutsche Forschungsgemeinschaft (BE 2333/1-1 and BE 2333/2-1).
Antibody 8.12.7, directed against NS3, is a kind gift from E. J. Dubovi. We are grateful to Benjamin Ostermann for excellent technical assistance with real-time RT-PCR analysis.
Footnotes
Published ahead of print 26 October 2011
REFERENCES
- 1. Agapov EV, et al. 2004. Uncleaved NS2-3 is required for production of infectious bovine viral diarrhea virus. J. Virol. 78:2414–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ansari IH, et al. 2004. Involvement of a bovine viral diarrhea virus NS5B locus in virion assembly. J. Virol. 78:9612–9623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baroth M, Orlich M, Thiel H-J, Becher P. 2000. Insertion of cellular NEDD8 coding sequences in a pestivirus. Virology 278:456–466 [DOI] [PubMed] [Google Scholar]
- 4. Becher P, Orlich M, Thiel H-J. 2000. Mutations in the 5′ nontranslated region of bovine viral diarrhea virus result in altered growth characteristics. J. Virol. 74:7884–7894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becher P, Tautz N. 2011. RNA recombination in pestiviruses: cellular RNA sequences in viral genomes highlight the role of host factors for viral persistence and lethal disease. RNA Biol. 8:216–224 [DOI] [PubMed] [Google Scholar]
- 6. Behrens S-E, Grassmann CW, Thiel H-J, Meyers G, Tautz N. 1998. Characterization of an autonomous subgenomic pestivirus RNA replicon. J. Virol. 72:2364–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bintintan I, Meyers G. 2010. A new type of signal peptidase cleavage site identified in an RNA virus polyprotein. J. Biol. Chem. 285:8572–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brownlie J, Clarke MC, Howard CJ. 1984. Experimental production of fatal mucosal disease in cattle. Vet. Rec. 114:535–536 [DOI] [PubMed] [Google Scholar]
- 9. Charleston B, Fray MD, Baigent S, Carr BV, Morrison WI. 2001. Establishment of persistent infection with non-cytopathic bovine viral diarrhoea virus in cattle is associated with a failure to induce type I interferon. J. Gen. Virol. 82:1893–1897 [DOI] [PubMed] [Google Scholar]
- 10. Corapi WV, Donis RO, Dubovi EJ. 1990. Characterization of a panel of monoclonal antibodies and their use in the study of the antigenic diversity of bovine viral diarrhea virus. Am. J. Vet. Res. 51:1388–1394 [PubMed] [Google Scholar]
- 11. Corapi WV, Donis RO, Dubovi EJ. 1988. Monoclonal antibody analyses of cytopathic and noncytopathic viruses from fatal bovine viral diarrhea infections. J. Virol. 62:2823–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Moerlooze L, et al. 1993. Nucleotide sequence of the bovine viral diarrhoea virus Osloss strain: comparison with related viruses and identification of specific DNA probes in the 5′ untranslated region. J. Gen. Virol. 74:1433–1438 [DOI] [PubMed] [Google Scholar]
- 13. Devereux J, Haeberli P, Smithies OA. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gallei A, et al. 2008. Cytopathogenicity of classical swine fever virus correlates with attenuation in the natural host. J. Virol. 82:9717–9729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gamlen T, et al. 2010. Expression of the NS3 protease of cytopathogenic bovine viral diarrhea virus results in the induction of apoptosis but does not block activation of the beta interferon promoter. J. Gen. Virol. 91:133–144 [DOI] [PubMed] [Google Scholar]
- 16. Grummer B, Bendfeldt S, Greiser-Wilke I. 2002. Apoptosis inhibitors delay the cytopathic effect of bovine viral diarrhoea virus (BVDV). J. Vet. Med. B Infect. Dis. Vet. Public Health 49:298–303 [DOI] [PubMed] [Google Scholar]
- 17. Harada T, Tautz N, Thiel H-J. 2000. E2-p7 region of the bovine viral diarrhea virus polyprotein: processing and functional studies. J. Virol. 74:9498–9506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoff HS, Donis RO. 1997. Induction of apoptosis and cleavage of poly(ADP-ribose) polymerase by cytopathic bovine viral diarrhea virus infection. Virus Res. 49:101–113 [DOI] [PubMed] [Google Scholar]
- 19. Houe H. 2003. Economic impact of BVDV infection in dairies. Biologicals 31:137–143 [DOI] [PubMed] [Google Scholar]
- 20. Jirasko V, et al. 2008. Structural and functional characterization of nonstructural protein 2 for its role in hepatitis C virus assembly. J. Biol. Chem. 283:28546–28562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones CT, Murray CL, Eastman DK, Tassello J, Rice CM. 2007. Hepatitis C virus p7 and NS2 proteins are essential for production of infectious virus. J. Virol. 81:8374–8383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kümmerer BM, Tautz N, Becher P, Thiel H-J, Meyers G. 2000. The genetic basis for cytopathogenicity of pestiviruses. Vet. Microbiol. 77:117–128 [DOI] [PubMed] [Google Scholar]
- 23. Lackner T, Müller A, König M, Thiel H-J, Tautz N. 2005. Persistence of bovine viral diarrhea virus is determined by a cellular cofactor of a viral autoprotease. J. Virol. 79:9746–9755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lackner T, et al. 2004. Temporal modulation of an autoprotease is crucial for replication and pathogenicity of an RNA virus. J. Virol. 78:10765–10775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lackner T, Thiel H-J, Tautz N. 2006. Dissection of a viral autoprotease elucidates a function of a cellular chaperone in proteolysis. Proc. Natl. Acad. Sci. U. S. A. 103:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang D, et al. 2009. A replicon trans-packaging system reveals the requirement of nonstructural proteins for the assembly of bovine viral diarrhea virus (BVDV) virion. Virology 387:331–340 [DOI] [PubMed] [Google Scholar]
- 27. Liess B, et al. 1984. Studies on transplacental transmissibility of a bovine virus diarrhoea (BVD) vaccine virus in cattle. II. Inoculation of pregnant cows without detectable neutralizing antibodies to BVDV virus 90–220 days before parturition (51st to 190th day of gestation). Zentralblatt für Veterinärmedizin. 31:669–681 [PubMed] [Google Scholar]
- 28. Lindenbach BD, Thiel H-J, Rice CM. 2007. Flaviviridae: the viruses and their replication, p 1101–1152In ]?>Knipe DM, Howley PM. (ed), Fields virology, 5th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 29. Ma Y, et al. 2011. Hepatitis C virus NS2 protein serves as a scaffold for virus assembly by interacting with both structural and nonstructural proteins. J. Virol. 85:86–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyers G, et al. 2007. Bovine viral diarrhea virus: prevention of persistent fetal infection by a combination of two mutations affecting Erns RNase and Npro protease. J. Virol. 81:3327–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyers G, Rümenapf T, Thiel H-J. 1989. Ubiquitin in a togavirus. Nature 341:491. [DOI] [PubMed] [Google Scholar]
- 32. Meyers G, Tautz N, Dubovi EJ, Thiel H-J. 1991. Viral cytopathogenicity correlated with integration of ubiquitin-coding sequences. Virology 180:602–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meyers G, Thiel H-J. 1996. Molecular characterization of pestiviruses. Adv. Virus Res. 47:53–117 [DOI] [PubMed] [Google Scholar]
- 34. Moennig V, Plagemann PGW. 1992. The pestiviruses. Adv. Virus Res. 41:53–98 [DOI] [PubMed] [Google Scholar]
- 35. Moulin HR, et al. 2007. Nonstructural proteins NS2-3 and NS4A of classical swine fever virus: essential features for infectious particle formation. Virology 365:376–389 [DOI] [PubMed] [Google Scholar]
- 36. Murray CL, Jones CT, Rice CM. 2008. Architects of assembly: roles of Flaviviridae non-structural proteins in virion morphogenesis. Nat. Rev. Microbiol. 6:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pankraz A, Thiel HJ, Becher P. 2005. Essential and nonessential elements in the 3′ nontranslated region of bovine viral diarrhea virus. J. Virol. 79:9119–9127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phan T, Beran RK, Peters C, Lorenz IC, Lindenbach BD. 2009. Hepatitis C virus NS2 protein contributes to virus particle assembly via opposing epistatic interactions with the E1-E2 glycoprotein and NS3-4A enzyme complexes. J. Virol. 83:8379–8395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pietschmann T, et al. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl. Acad. Sci. U. S. A. 103:7408–7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reed KE, Gorbalenya AE, Rice CM. 1998. The NS5A/NS5 proteins of viruses from three genera of the family Flaviviridae are phosphorylated by associated serine/threonine kinases. J. Virol. 72:6199–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Renard A, Dino D, Martial J. 17 February 1993. Vaccines and diagnostics derived from bovine diarrhea virus. European patent 0208672 B1
- 42. Rinck G, et al. 2001. A cellular J-domain protein modulates polyprotein processing and cytopathogenicity of a pestivirus. J. Virol. 75:9470–9482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schägger H, von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368–379 [DOI] [PubMed] [Google Scholar]
- 44. Tamura JK, Warrener P, Collett MS. 1993. RNA-stimulated NTPase activity associated with the p80 protein of the pestivirus bovine viral diarrhea virus. Virology 193:1–10 [DOI] [PubMed] [Google Scholar]
- 45. Tautz N, et al. 1999. Establishment and characterization of cytopathogenic and noncytopathogenic pestivirus replicons. J. Virol. 73:9422–9432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tautz N, Kaiser A, Thiel H-J. 2000. NS3 serine protease of bovine viral diarrhea virus: characterization of active site residues, NS4A cofactor domain, and protease-cofactor interactions. Virology 273:351–363 [DOI] [PubMed] [Google Scholar]
- 47. Tautz N, Meyers G, Thiel H-J. 1993. Processing of poly-ubiquitin in the polyprotein of an RNA virus. Virology 197:74–85 [DOI] [PubMed] [Google Scholar]
- 48. Tellinghuisen TL, Paulson MS, Rice CM. 2006. The NS5A protein of bovine viral diarrhea virus contains an essential zinc-binding site similar to that of the hepatitis C virus NS5A protein. J. Virol. 80:7450–7458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thiel H-J, et al. 2005. Family Flaviviridae, p 979–996 Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. (ed), Virus taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA [Google Scholar]
- 50. Vassilev VB, Donis RO. 2000. Bovine viral diarrhea virus induced apoptosis correlates with increased intracellular viral RNA accumulation. Virus Res. 69:95–107 [DOI] [PubMed] [Google Scholar]
- 51. Warrener P, Collett MS. 1995. Pestivirus NS3 (p80) protein possesses RNA helicase activity. J. Virol. 69:1720–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wiskerchen M, Collett MS. 1991. Pestivirus gene expression: protein p80 of bovine viral diarrhea virus is a proteinase involved in polyprotein processing. Virology 184:341–350 [DOI] [PubMed] [Google Scholar]
- 53. Xu J, et al. 1997. Bovine viral diarrhea virus NS3 serine proteinase: polyprotein cleavage sites, cofactor requirements, and molecular model of an enzyme essential for pestivirus replication. J. Virol. 71:5312–5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yi M, Ma Y, Yates J, Lemon SM. 2007. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J. Virol. 81:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu H, Grassmann CW, Behrens S-E. 1999. Sequence and structural elements at the 3′ terminus of bovine viral diarrhea virus genomic RNA: functional role during RNA replication. J. Virol. 73:3638–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang G, Aldridge S, Clarke MC, McCauley JW. 1996. Cell death induced by cytopathic bovine diarrhoea virus is mediated by apoptosis. J. Gen. Virol. 77:1677–1681 [DOI] [PubMed] [Google Scholar]