Abstract
The host immune response is believed to tightly control viral replication of deltaretroviruses such as human T-lymphotropic virus type 1 (HTLV-1) and bovine leukemia virus (BLV). However, this assumption has not been definitely proven in vivo. In order to further evaluate the importance of the immune response in the BLV model, we studied the fate of cells in which viral expression was transiently induced. Using a dual fluorochrome labeling approach, we showed that ex vivo induction of viral expression induces higher death rates of B cells in vivo. Furthermore, cyclosporine treatment of these animals indicated that an efficient immune response is required to control virus-expressing cells.
TEXT
Human T-lymphotropic virus type 1 (HTLV-1) and leukemia virus (BLV) are related deltaretroviruses inducing inflammatory and/or lymphoproliferative diseases. These viruses are thought to transmit primarily not as free particles but as integrated proviruses by stimulating proliferation of their host cells (5, 14). Experimental evidence shows that viral factors such as the Tax protein and HBZ favor cell proliferation (4, 13), thereby providing a rationale for the driving force of mitotic expansion. Paradoxically, viral antigens can be detected neither in infected lymphocytes nor in the serum. Furthermore, only very few cells score positive by in situ hybridization (8, 9, 12). However, short-term culture of provirus-carrying cells progressively triggers viral expression (3, 8, 10), indicating a mechanism of reactivation.
With the aim of reconciling these different observations, we analyzed the kinetics of BLV-infected cell populations in which viral expression had previously been stimulated ex vivo. Our data are consistent with a tight control of virus-positive cells by the host immune response.
Our key goal was thus to assess the role of viral expression in the fate of infected cells in vivo. Although BLV transcription is almost undetectable in vivo, incubation of heparinized whole blood at 37°C is sufficient to activate viral expression (15). The most straightforward interpretation of this observation is that transcriptionally repressed proviruses are reactivated upon blood withdrawal. Although possible, this model would imply the existence of an unknown repression mechanism that would be simply relieved by mixing blood with anticoagulant. Alternatively, our working hypothesis to explain this observation is that when viral expression occurs in vivo, virus-positive cells are eliminated. In other words, viral expression could occur in a significant number of virus-positive cells in vivo that are efficiently eliminated by the host immune response. We challenged this model in BLV-infected sheep by tracing cells in which viral expression was first transiently stimulated ex vivo.
The first step relied on the determination of the optimal incubation time required to induce viral expression (experimental procedures are described elsewhere [infection of sheep with BLV {16}, determination of viral RNA levels by real-time reverse transcription-PCR {RT-PCR} {7 }, and measurement of apoptosis by flow cytometry {1}]). For this purpose, blood was collected from BLV-infected animals and incubated at 37°C for 30 min, 1 h, 2 h, and 4 h. Tax transcripts quantified by real-time RT-PCR were detectable between 1 and 2 h of incubation (Fig. 1A, upper panels). In contrast, viral expression was undetectable when cells were kept at 0°C (data not shown). On the other hand, onset of apoptosis revealed by DNA cleavage (i.e., sub-G1 peak in flow cytometry) was initiated after 4 h in cells from infected (Fig. 1A, lower panels) and control (data not shown) animals. It thus appeared that 2 h (arrows) was the optimal time providing a good compromise between induction of viral expression and occurrence of apoptosis. Having set these preliminary experimental conditions, we designed a strategy outlined in Fig. 1B. We developed a dual fluorochrome labeling strategy to study in vivo cell kinetics. Therefore, 200 ml of blood was collected by jugular venipuncture and mixed with 2,000 U of heparin. Half of the volume was chilled on ice while the other half was incubated at 37°C for 2 h. After lysis of red blood cells with 1 mM NaHCO3–150 mM NH4Cl, leukocytes were labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) or PKH26 (Sigma) accordingly to manufacturers' instructions and resuspended in 5 ml of phosphate-buffered saline (PBS). The two fractions were pooled, mixed with 40 ml of PBS prewarmed at 37°C, and reinjected into the jugular vein. Blood samples were regularly collected (5 min and 1, 2, 4, 7, and 14 days after injection), and red blood cells were lysed. Finally, B lymphocytes were labeled with anti-IgM monoclonal antibody (1H4), and the percentage of cells labeled with CFSE or PKH26 was evaluated by flow cytometry.
Fig 1.
Induction of viral expression ex vivo. (A) Blood was collected from 3 BLV-infected sheep (BLV#1, BLV#2, and BLV#3). Half of the volume was preserved at 0°C, while the other half was incubated at 37°C for 30 min or 1, 2, or 4 h and then chilled on ice. After lysis of red blood cells and RNA extraction, the tax transcripts were quantified by real-time RT-PCR. The upper graphs correspond to the Tax RNA levels normalized to actin. In parallel, B lymphocytes were labeled with anti-IgM antibodies, fixed/permeabilized with 70% ethanol, treated with RNase A, labeled with propidium iodide, and analyzed by flow cytometry. The lower graphs outline the percentages of apoptotic (i.e., sub-G1) B lymphocytes. Arrows indicate the optimal time providing a good compromise between induction of viral expression and absence of apoptosis. (B) Experimental design of the ex vivo experiment (prior to in vivo analysis corresponding to Fig. 2). Blood was collected by jugular venipuncture and mixed with heparin. Half of the volume was incubated either at 37°C or on ice for 2 h. After lysis of red blood cells, the two fractions were labeled with CFSE or PKH26, pooled, and reinjected intravenously. After lysis of red blood cells, B lymphocytes were labeled with anti-IgM antibodies and analyzed by flow cytometry. The dot plot graphs illustrate fluorescence-activated cell sorter (FACS) analyses of PKH26 or CFSE labeling (control [left], after labeling [middle], and 5 min after reinjection [right]).
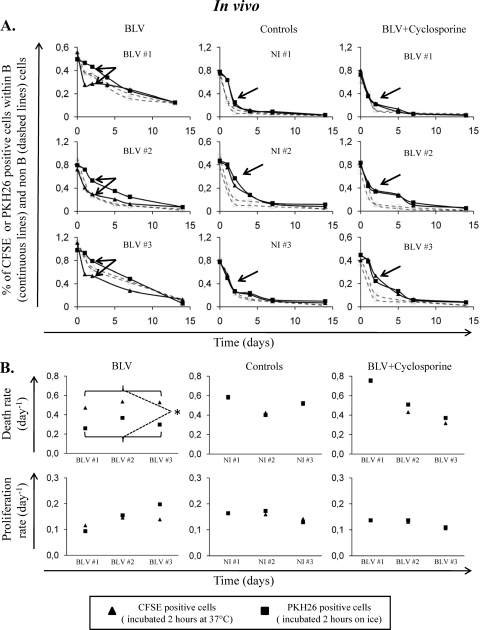
In five BLV-infected animals (with proviral loads ranging between 41 and 100% of infected B cells), CFSE-labeled B cells (i.e., previously incubated at 37°C) disappeared faster than PKH26-labeled B cells (see arrows on left panels of Fig. 2A for BLV-infected sheep BLV#1, BLV#2, and BLV#3). This difference was observed neither in the non-B cell population (dashed lines) nor in cells from 3 noninfected sheep (NI#1, NI#2, and NI#3 [controls]; Fig. 2A).
Fig 2.
In vivo cell dynamics in BLV-infected and control sheep. (A) Kinetics of CFSE- or PKH26-labeled cells. Blood was collected from 3 BLV-infected sheep and 3 controls. Half of the volume was incubated at 37°C or kept on ice for 2 h. After lysis of red blood cells, the two fractions were labeled with CFSE or PKH26, pooled, and reinjected intravenously. Blood samples were then collected at different times postinjection. After lysis of red blood cells, B lymphocytes were labeled with anti-IgM antibodies and analyzed by flow cytometry. The starting proportions of B lymphocytes were 72, 88, 78, 42, 40, and 37% for sheep BLV#1, BLV#2, BLV#3, NI#1, NI#2, and NI#3, respectively. The graphs correspond to percentages of B cells (solid lines) and non-B cells (dashed lines) labeled with CFSE or PKH26. The graphs on the right contain data for BLV-infected sheep treated for 3 weeks with an immunosuppressive dose of cyclosporine (5 mg/kg/day). Arrows indicate the differences (double arrow) or the absence of differences (single arrow) between CFSE and PKH26 profiles. (B) Death and proliferation rates of fluorochrome-labeled cells. Kinetics profiles of CFSE- or PKH26-labeled cells were analyzed with a mathematical model. Proliferation (lower panels) and death (upper panels) rates were deduced from the proportions and intensities of CFSE- or PKH26-positive cells. Statistical relevance (*, P < 0.05) of the differences in proliferation or death rates between CFSE- and PKH26-positive cells was calculated according to the two-tailed unpaired Student t test.
A reduction in the proportion of labeled lymphocytes can be due either to cell death or to label dilution below the threshold of detection due to proliferation. It is possible to quantify these two parameters, assuming that the fluorescence intensity is halved upon cell division (2). The model basically uses two sets of data from the flow cytometry analyses: the proportions of labeled cells and the mean fluorescence intensities. Compilation of these two types of experimental data allows calculation of proliferation and disappearance rates, the latter parameter including cell death and exit out of the blood. It should be mentioned that we previously demonstrated through cannulation of lymphatic vessels that BLV infection does not modify cell recirculation to lymphoid organs (6). This model was applied to experimental data and revealed that the observed difference in B cell persistence is not due to cell proliferation but rather to cell death (Fig. 2B, left panels). In contrast, incubation of whole blood at 37°C modifies neither cell death nor cell proliferation in noninfected animals (Fig. 2B, middle panels).
To control for a potential bias due to the use of a particular fluorochrome, we performed the same experiment except that the fluorochromes were swapped (i.e., CFSE and PKH26 labeling after incubation on ice and at 37°C, respectively). Results presented in Fig. S1 in the supplemental material led to the same conclusions.
We thus conclude that preincubation of a BLV-infected B cell population for 2 h at 37°C accelerates the death rates in vivo. Our working hypothesis is that the accelerated disappearance of B cells from infected sheep is due to the host immune surveillance. To address this question, infected sheep were treated with daily intravenous injections (at 5 mg/kg body weight/day for 3 weeks) of cyclosporine, an immunosuppressive drug preventing dephosphorlyation of NF-AT by binding to cyclophilin (11). Consistently, cyclosporine abrogated the differences between CFSE and PKH26 kinetics in B cells from 3 infected animals (Fig. 2A and B, right panels). Under these conditions, cyclosporine treatment inhibited interleukin 2 (IL-2) expression in peripheral blood mononuclear cells and induced a decrease in the CD4, CD8, and gamma/delta T cell counts (see Fig. S2 in the supplemental material), as expected.
Together, our results show that transient stimulation of viral transcription in BLV-infected B cells is associated with an acceleration of their disappearance in vivo. Although we found a strong relation between viral transcription and disappearance rates, we cannot formally exclude the possibility that another mechanism occurring during incubation at 37°C, such as expression of a cellular gene or posttranslational modifications (e.g., alteration of the glycosylation pattern), would affect the cell kinetics in vivo. On the other hand, viral infection also modifies a series of metabolic and signaling pathways. These indirect consequences of BLV infection thus cannot be dissociated from expression of the viral antigens themselves. However, we do favor a causal role of viral expression because (i) the onset of apoptosis and proliferation are unaltered within the 2-h time frame (Fig. 1A and data not shown), (ii) the kinetics of the non-B lymphocytes in BLV-infected sheep provides an internal reference to potential indirect effects (Fig. 2A), (iii) the B cell kinetics in noninfected animals is unaltered by incubation at 37°C (Fig. 2), and (iv) (perhaps the best evidence) an immunosuppressive dose of cyclosporine treatment reverts the phenotype in BLV-infected sheep (Fig. 2B). Our data are thus also consistent with a key role exerted by the host immune surveillance in controlling BLV-infected cells.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Fonds National de la Recherche Scientifique (FNRS), the Télévie, the Belgian Foundation against Cancer, the Sixth Research Framework Programme of the European Union (project INCA LSHC-CT-2005-018704), the Neoangio excellence program and the Partenariat Public Prive (PPP) INCA of the Direction Générale des Technologies, de la Recherche et de l'Energie/DG06 of the Walloon government, the Action de Recherche Concertée Glyvir of the Communauté Française de Belgique, the Centre Anticancéreux près ULg (CAC), the Synbiofor project of GxABT, the ULg Fonds Spéciaux pour la Recherche, and the Plan Cancer of the Service Public Fédéral. A.F., A.-B.B., and L.W. are members of the FNRS.
We are grateful to François Debande for experimental assistance. We thank Yves Beckers and André Théwis for support in animal housing facilities.
Footnotes
Published ahead of print 26 October 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Achachi A, et al. 2005. Valproate activates bovine leukemia virus gene expression, triggers apoptosis, and induces leukemia/lymphoma regression in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:10309–10314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asquith B, et al. 2006. Quantifying lymphocyte kinetics in vivo using carboxyfluorescein diacetate succinimidyl ester (CFSE). Proc. Biol. Sci. 273:1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baliga V, Ferrer JF. 1977. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc. Soc. Exp. Biol. Med. 156:388–391 [DOI] [PubMed] [Google Scholar]
- 4. Boxus M, Willems L. 2009. Mechanisms of HTLV-1 persistence and transformation. Br. J. Cancer 101:1497–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavrois M, et al. 1998. Persistent oligoclonal expansion of human T-cell leukemia virus type 1-infected circulating cells in patients with tropical spastic paraparesis/HTLV-1 associated myelopathy. Oncogene 17:77–82 [DOI] [PubMed] [Google Scholar]
- 6. Debacq C, et al. 2006. Peripheral blood B-cell death compensates for excessive proliferation in lymphoid tissues and maintains homeostasis in bovine leukemia virus-infected sheep. J. Virol. 80:9710–9719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Florins A, et al. 2009. Earlier onset of delta-retrovirus-induced leukemia after splenectomy. PLoS One 4:e6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franchini G, Wong-Staal F, Gallo RC. 1984. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc. Natl. Acad. Sci. U. S. A. 81:6207–6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gessain A, Louie A, Gout O, Gallo RC, Franchini G. 1991. Human T-cell leukemia-lymphoma virus type I (HTLV-I) expression in fresh peripheral blood mononuclear cells from patients with tropical spastic paraparesis/HTLV-I-associated myelopathy. J. Virol. 65:1628–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanon E, et al. 2000. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood 95:1386–1392 [PubMed] [Google Scholar]
- 11. Haynes RA, II, et al. 2010. Cyclosporine-induced immune suppression alters establishment of HTLV-1 infection in a rabbit model. Blood 115:815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lagarias DM, Radke K. 1989. Transcriptional activation of bovine leukemia virus in blood cells from experimentally infected, asymptomatic sheep with latent infections. J. Virol. 63:2099–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuoka M. 2010. HTLV-1 bZIP factor gene: its roles in HTLV-1 pathogenesis. Mol. Aspects Med. 31:359–366 [DOI] [PubMed] [Google Scholar]
- 14. Moulés V, et al. 2005. Fate of premalignant clones during the asymptomatic phase preceding lymphoid malignancy. Cancer Res. 65:1234–1243 [DOI] [PubMed] [Google Scholar]
- 15. Tajima S, Aida Y. 2005. Induction of expression of bovine leukemia virus (BLV) in blood taken from BLV-infected cows without removal of plasma. Microbes Infect. 7:1211–1216 [DOI] [PubMed] [Google Scholar]
- 16. Willems L, et al. 1993. In vivo infection of sheep by bovine leukemia virus mutants. J. Virol. 67:4078–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.