Abstract
Analyses of varicella-zoster virus (VZV) protein expression during latency have been discordant, with rare to many positive neurons detected. We show that ascites-derived murine and rabbit antibodies specific for VZV proteins in vitro contain endogenous antibodies that react with human blood type A antigens in neurons. Apparent VZV neuronal staining and blood type A were strongly associated (by a χ2 test, α = 0.0003). Adsorption of ascites-derived monoclonal antibodies or antiserum with type A erythrocytes or the use of in vitro-derived VZV monoclonal antibodies eliminated apparent VZV staining. Animal-derived antibodies must be screened for anti-blood type A reactivity to avoid misidentification of viral proteins in the neurons of the 30 to 40% of individuals who are blood type A.
TEXT
Varicella-zoster virus (VZV) is a human alphaherpesvirus that establishes latency in ganglionic sensory neurons. In autopsied human ganglia, the frequency of individual VZV-positive neurons was 1.0 to 6.9% by single-cell laser capture microdissection (LCMD) and PCR (29). However, immunostaining analyses of VZV protein expression in latently infected neurons have differed significantly. Mahalingam et al. reported infrequent detection of VZV proteins in cadaver ganglia, and in positive tissues VZV protein expression was restricted to very few neurons, a finding confirmed by Kennedy et al. and our recent study (13, 16, 32). In contrast, Lungu et al. detected VZV proteins in 9 to 24% of neurons in ganglia from three of three subjects (15). Theil et al. found immediate-early 62 (IE62) proteins in ganglia from 38% of individuals and in 3 to 7% of neurons (25). Discrepancies among reports describing the frequencies of VZV protein-positive neurons in autopsied ganglia require further investigation because of their implications for understanding mechanisms of VZV latency.
Using a high-potency rabbit anti-IE63 antibody and preimmune serum control, we detected IE63 proteins in ganglia from only 1 of 18 individuals and in <2.8% of neurons (32). Subsequently, we observed cytoplasmic immunoreactivity in many neurons in ganglia from several individuals when the neurons were stained with monoclonal antibodies (MAbs) to IE62, glycoprotein E (gE), and the capsid protein ORF40 (MAB8616, MAB8612, and MAB8614, respectively; EMD Millipore, Billerica, MA); different lots had similar reactivities. These commercial reagents against IE62 and gE, known to be specific for viral proteins in cultured cells, have been used in studies that report cytoplasmic localization of VZV proteins in neurons (6, 9, 10, 25, 26, 28). The failure to confirm IE62 and gE detection with other VZV antibodies, the detection of the ORF40 capsid protein detection in latency, and the Golgi zone-like localization of all three VZV proteins suggested a possible staining artifact.
The mouse ascites Golgi (MAG) reaction results from endogenous anti-human blood type A antibodies in MAbs derived from mouse ascites (2, 5, 14, 21–23, 30, 31). Importantly, in blood type A individuals, sensory neurons express type A histo-blood group antigens (HBGAs) in Golgi zones (4, 12, 17, 18). Because Ig purification does not remove endogenous mouse antibodies, MAbs may contain murine Ig that reacts with type A HBGAs in tissues from blood type A individuals. This artifact is missed when using isotype controls not made from ascites, and type A HBGAs may be absent in cultured cells used to validate binding to specific protein targets.
Immunoreactivity in neurons stained with VZV monoclonal antibodies.
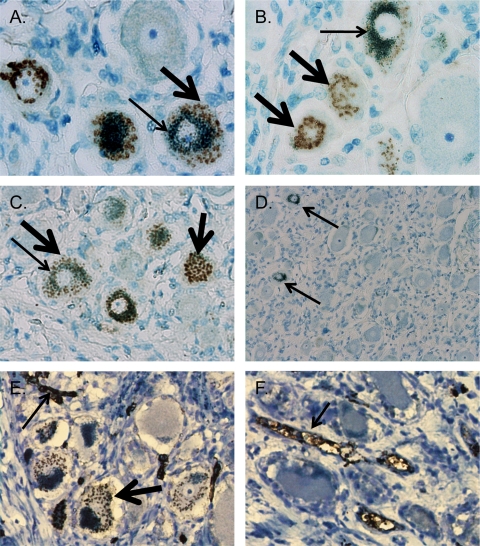
To determine whether the cytoplasmic staining found with the anti-VZV MAbs was a MAG reaction, dorsal root ganglia (DRG) from 20 subjects were randomly selected from a tissue bank under an exempt human subject protocol. Preservation of antigen was demonstrated in all 20 samples by detection of the protein neural-cell adhesion molecules (data not shown). DRG sections were immunostained with anti-gE, anti-IE62, and anti-ORF40 (EMD Millipore) using a standard immunohistochemical method. Slides were stained and examined in a blind manner (32). As summarized in Table 1, 8 of 20 DRG were scored as positive using all three anti-VZV antibodies. 3,3-Diaminobenzidine (DAB) deposits were absent in the 12 of 20 subjects who were scored as negative (data not shown). In slides scored as positive, all antibodies produced a similar staining pattern, in which DAB deposits were localized to Golgi zones (Fig. 1). No signal was detected in any of the 20 subjects with mouse Ig (Fig. 1D), isotype-matched Ig, rabbit anti-IE63 antisera or the corresponding preimmune serum, or high-titer anti-VZV human serum (data not shown). Golgi zone localization was confirmed by confocal immunofluorescence with anti-ORF40 and rabbit antibody to Golgi zone-localized synaptophysin (Invitrogen, Carlsbad, CA) (data not shown) (27).
Table 1.
Summary of immunostaining results of tissue sections from 20 DRGa
| Subject no. | VZV historyb | Results after staining with: |
Blood type | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MAb to VZV protein target |
Anti-type A antibody with:c |
Anti-type B antibody | Anti-MAG antibody | ||||||
| gE | ORF40 | IE62 | Endo | N | |||||
| 1 | Pos | − | − | − | − | − | − | − | O |
| 2 | UD | − | − | − | − | − | − | − | O |
| 3 | UD | − | − | − | − | − | − | − | O |
| 4 | UD | + | + | + | + | + | − | + | A |
| 5 | UD | − | − | − | + | − | − | − | A |
| 6 | UD | + | + | + | + | + | − | + | A |
| 7 | Pos | − | − | − | − | − | − | − | O |
| 8 | UD | − | − | − | − | − | + | − | B |
| 9 | Pos | − | − | − | + | − | − | − | A |
| 10 | UD | + | + | + | + | + | − | + | A |
| 11 | Pos | − | − | − | − | − | − | − | O |
| 12 | Pos | + | + | + | + | + | − | + | A |
| 13 | Pos | − | − | − | − | − | − | − | O |
| 14 | Pos | − | − | − | − | − | + | − | B |
| 15 | Pos | + | + | + | + | + | − | + | A |
| 16 | Pos | + | + | + | + | + | − | + | A |
| 17 | Pos | + | + | + | + | + | − | + | A |
| 18 | UD | + | + | + | + | + | − | + | A |
| 19 | UD | − | − | − | − | − | + | − | B |
| 20 | UD | − | − | − | − | − | − | O | |
Shaded rows indicate subjects with blood type A.
Pos, positive VZV history (positive for VZV DNA); UD, undetermined history (negative for VZV DNA, negative for human β-globin DNA).
Endo, endothelial staining; N, neuronal cytoplasmic staining.
Fig 1.
Apparent immunostaining of VZV proteins in human cadaver DRG using mouse ascites-derived VZV monoclonal antibodies is associated with the presence of blood type A histo-blood group antigens (HBGAs) in neuronal Golgi zones. Immunohistochemical staining of tissue sections from subject 4 using 3,3-diaminobenzidine (DAB) chromogen to detect immunoreactivity (brown deposits, thick arrows) and azure B counterstain, which colors neuronal melanin (green deposits, thin arrows). (A to C) Magnification, ×400. Stained with a 1:100 dilution of MAB8612 (anti-gE) (A), MAB8616 (anti-IE62) (B), or MAB8614 (anti-ORF40) (C). (D) Stained with tissue culture-derived mouse Ig; arrows denote neuronal melanin. Magnification, ×100. (E and F) Staining of subject 4 (E) and subject 5 (F) with anti-A antibody at a 1:10 dilution. Thin arrows denote endothelial cell staining observed in all subjects with blood type A; the thick arrow denotes staining of blood type A antigens in the neuronal cytoplasm which were present in 8 of 10 subjects and indistinguishable from the signal observed when using the VZV monoclonal antibodies.
VZV DNA can be detected in >80% of frozen, unfixed ganglia, in accordance with high seroprevalence rates (>95%) for VZV IgG antibodies (19). Using an established protocol, a prior history of VZV infection was demonstrated in all individuals for whom PCR-quality DNA could be extracted from archived paraffin blocks (10 of 20 specimens) (Table 1) (32). Five of the 10 VZV DNA-positive specimens demonstrated apparent VZV immunoreactivity.
Apparent VZV immunoreactivity correlates with expression of blood type A HBGAs in neurons.
DRG sections from all subjects were stained with anti-human blood type A and B antibodies (Abcam, Cambridge, MA) to determine blood type based on endothelial cell staining (Table 1). Blood typing was available from medical records for 10 subjects, and immunostaining results matched the typing results for all 10. All 8 individuals who had positive staining of DRG sections with the VZV MAbs were blood type A (by a χ2 test, α = 0.0003), and none of the type O (n = 7) or B (n = 3) subjects had sections with positive staining.
In blood type A subjects who showed apparent VZV immunoreactivity (8 of 10), neuronal anti-type A staining was observed in addition to the endothelial cell staining that was used to determine blood type. The staining pattern was indistinguishable from the signal that appears when using the VZV MAbs (Fig. 1E). Eighty percent of individuals with blood type A are subtype A1, and 20% are subtype A2 (24). Endogenous murine anti-A antibodies recognize carbohydrate determinants specific for the A1 subgroup, and these are not expressed in subtype A2 individuals (1, 24, 31). The absence of neuronally expressed type A HBGAs in subjects 5 and 9 indicates that they belong to subtype A2. Subtype-specific antibodies are not readily available. In any case, apparent VZV immunoreactivity is strongly associated with expression of neuronal Golgi zone-localized A antigens (by a χ2 test, α = <0.0001).
Demonstration of MAG reactivity in human neuronal Golgi zones.
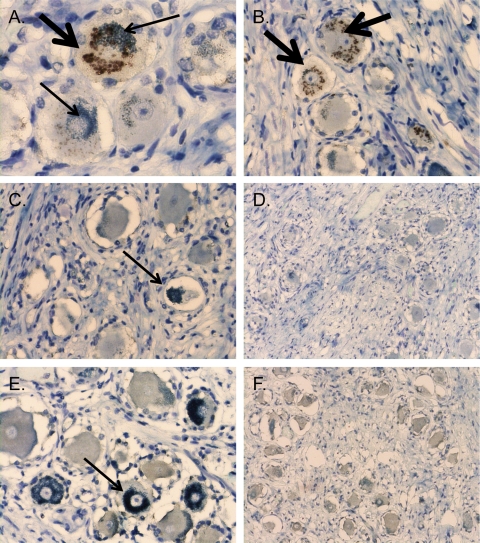
To further demonstrate that apparent VZV staining of neurons in 8 of 10 blood type A subjects was attributable to endogenous mouse anti-subtype A1 antibodies in the ascites-derived hybridoma products, sections from all 20 subjects were tested with anti-MAG antibody. Golgi zone-localized DAB (brown) deposits were observed only in sections from the eight type A individuals that showed similar reactivities using the VZV MAbs (Fig. 2). Staining was not observed in sections from individuals with no VZV MAb reactivity, which includes subjects 5 and 9 and all type B and O subjects.
Fig 2.
Apparent VZV immunoreactivity and neuronal anti-type A reactivity are associated with the mouse ascites Golgi (MAG) staining artifact. DRG tissue sections were stained with anti-MAG antibody (1:64,000 dilution), which was generated in mice by pristane priming followed by intraperitoneal injection of hybridoma cells that were not from immunized mice. The DAB antibody-specific signal is brown (thick arrows), and the melanin counterstain is green (thin arrows). Panels A and B are sections from subject 4, who is blood type A, subtype A1; panels C and D are sections from subject 5, who is blood type A, subtype A2; and panels E and F are sections from subject 1, who is blood type O. Two representative images are shown for each condition. Magnification for panels on the left side, ×400. Magnification for panels on the right side, ×200.
Elimination of immunoreactivity in neurons using tissue culture-derived VZV MAbs.
To demonstrate definitively that the apparent detection of VZV proteins in neurons using the mouse ascites-derived anti-VZV antibodies was a subtype A1-related artifact, anti-IE62 and anti-gE monoclonal antibodies were produced as hybridoma supernatants in vitro using the same cell stock that is used for peritoneal injection. Although these reagents detected their respective proteins in VZV-infected cells at a 1:1,000 dilution (data not shown), no immunoreactivity was detected in neurons stained with these reagents (Fig. 3).
Fig 3.
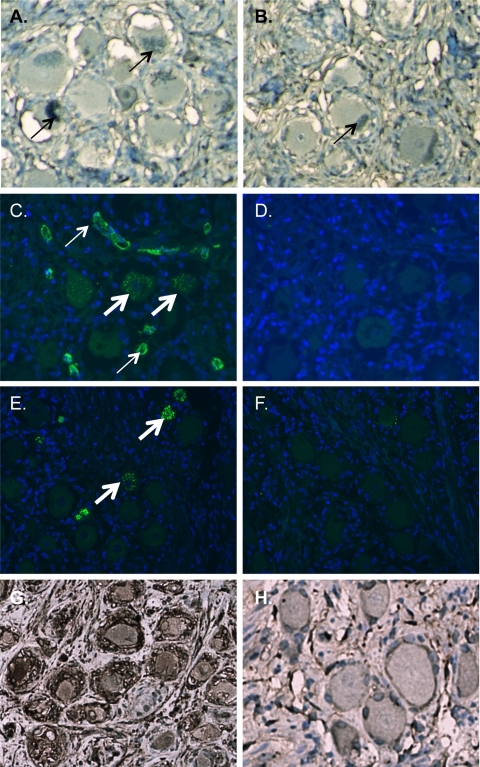
Elimination of apparent VZV immunoreactivity in neurons using tissue culture-derived VZV monoclonal antibodies or by adsorption using blood type A erythrocytes. (A and B) Immunohistochemical staining of tissue sections from a blood type A subject with purified hybridoma supernatants from anti-IE62 (A) and anti-gE (B) clones at a 1:10 dilution eliminates immunoreactivity. Sections are counterstained with azure B, which stains neuromelanin green (thin arrows). (C to F) Immunofluorescent staining using a rabbit polyclonal antibody to IE62 and Alexa Fluor 488-conjugated anti-rabbit secondary antibody (C and D) or gE mouse ascites-derived monoclonal antibody and Alexa Fluor 488-conjugated anti-mouse secondary antibody (E and F). The primary antibody was adsorbed with blood type A erythrocytes (D and F) or unadsorbed (C and E). Thin arrows denote endothelial staining, and thick arrows denote staining of neuronal histo-blood group A determinants in Golgi zones. Sections for immunofluorescent staining were pretreated with Sudan black to eliminate the signal from neuronal pigments and counterstained with DAPI (4′,6-diamidino-2-phenylindole). (G and H) Immunohistochemical staining of VZV-infected DRG (G) and uninfected DRG (H) from a SCID mouse-human xenograft model. A VZV-specific signal with gE-mouse ascites-derived monoclonal antibody after adsorption with blood type A erythrocytes demonstrates that adsorption of endogenous anti-type A antibodies does not alter VZV-specific immunoreactivity. Sections are counterstained with hematoxylin.
Endogenous anti-blood type A antibodies in rabbit anti-VZV polyclonal serum.
IE62 detection in VZV-infected cells in culture is sensitive and specific when a rabbit polyclonal serum is used (20). However, this reagent (kindly provided by Paul Kinchington, University of Pittsburgh) also showed apparent VZV protein expression in neurons and was found to contain endogenous rabbit anti-blood type A antibodies by endothelial staining and neuronal Golgi zone staining (Fig. 3). Apparent VZV staining of latently infected neurons using this rabbit anti-IE62 and the gE MAb was eliminated by adsorption by human blood type A erythrocytes (Fig. 3). Adsorption did not diminish VZV-specific staining of acutely infected neurons in a DRG xenograft model (Fig. 3) (32).
These experiments demonstrate that animal-derived antibodies specific for VZV proteins in cultured cells may contain endogenous antibodies reactive to type A HBGAs in Golgi zones of neurons. Similar staining using the reagents we tested has been identified as VZV specific but cannot be interpreted as representing VZV protein expression during latency because of the MAG artifact (9, 10, 26, 28). In blood type A1 individuals, MAG staining does not occur in all neurons, suggesting that type A HBGAs are expressed in a neuronal subpopulation. Since blood type A individuals comprise 30 to 40% of the population, our findings help explain reports that VZV protein expression during latency is common, whereas others have found that VZV protein expression is rare (7, 13, 15, 16, 25, 32). The MAG artifact may also contribute to the discrepancy between reports of high frequencies of neurons that express VZV proteins and the low frequency of neurons that contain VZV genomes based on LCMD and quantitative PCR (19, 29). In addition to the presence of neuronal pigments and immunological cross-reactivity between IE62 and neuron-specific brain-derived neurotrophic factor (BDNF), this report identifies an important new variable that confounds the accurate detection of VZV proteins in sensory neurons (8, 32).
These observations are broadly relevant to studies of human virus infections, since immunostaining methods are often used to study viral neuropathogenesis. It is important to emphasize that expression of histo-blood group antigens is tissue specific and is regulated in vivo. Therefore, the type A-related staining artifact may occur in tissues where it is not anticipated and it may be absent in cultured cells used as controls for immunostaining. Tissue culture-derived, isotyped mouse Ig does not serve as a reliable control reagent for ascites-derived monoclonal antibodies. The MAG artifact is not restricted to antibodies that recognize viral proteins and has been demonstrated with various commercially available mouse ascites antibodies (5, 23, 30). The presence of endogenous anti-blood type A antibodies in mice used to generate MAbs appears to be relatively strain specific (3, 11, 14). Since, as we observed, rabbits may also produce anti-human type A antibodies, it is necessary to test preimmune serum from the same animal as a control when evaluating protein expression using antiserum from immunized rabbits (32). To eliminate contaminating antibodies, blood type A erythrocytes may be used to adsorb contaminating rabbit or murine anti-blood type A antibodies. Golgi zone localization of a putative viral protein shown with animal-derived antibodies should raise the concern that immunoreactivity is nonspecific and due to endogenous anti-type A Ig. In summary, when neurons and other tissue types that are known to or that may express histo-blood group A antigens are evaluated by immunostaining, antibodies must be screened for anti-type A immunoglobulinin order to avoid erroneous identification of the target protein in the 30 to 40% of individuals who are blood type A.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (AI053846) and the National Cancer Institute (CA49605).
We thank Harvey J. Kliman at the Yale University School of Medicine for the anti-MAG antibody and helpful discussions.
Use of human autopsy tissues under an exempt protocol was approved by the Stanford University and University of Sydney Institutional Review Board panels.
Footnotes
Published ahead of print 19 October 2011
REFERENCES
- 1. Arend P. 2011. “Natural” antibodies and histo-blood groups in biological development with respect to histo-blood group A. Immunobiology 216:1318–1321 [DOI] [PubMed] [Google Scholar]
- 2. Arend P, Nijssen J. 1977. A-specific autoantigenic ovarian glycolipids inducing production of ‘natural’ anti-A antibody. Nature 269:255–257 [DOI] [PubMed] [Google Scholar]
- 3. Baumgarth N, Tung JW, Herzenberg LA. 2005. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 26:347–362 [DOI] [PubMed] [Google Scholar]
- 4. Dodd J, Solter D, Jessell TM. 1984. Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurones. Nature 311:469–472 [DOI] [PubMed] [Google Scholar]
- 5. Finstad CL, et al. 1991. Some monoclonal antibody reagents (C219 and JSB-1) to P-glycoprotein contain antibodies to blood group A carbohydrate determinants: a problem of quality control for immunohistochemical analysis. J. Histochem. Cytochem. 39:1603–1610 [DOI] [PubMed] [Google Scholar]
- 6. Gowrishankar K, et al. 2010. Characterization of the host immune response in human ganglia after herpes zoster. J. Virol. 84:8861–8870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grinfeld E, Sadzot-Delvaux C, Kennedy PG. 2004. Varicella-zoster virus proteins encoded by open reading frames 14 and 67 are both dispensable for the establishment of latency in a rat model. Virology 323:85–90 [DOI] [PubMed] [Google Scholar]
- 8. Hama Y, et al. 2010. Antibody to varicella-zoster virus immediate-early protein 62 augments allodynia in zoster via brain-derived neurotrophic factor. J. Virol. 84:1616–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Held K, et al. 2011. Expression of herpes simplex virus 1-encoded microRNAs in human trigeminal ganglia and their relation to local T-cell infiltrates. J. Virol. 85:9680–9685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hufner K, et al. 2006. Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia. J. Neuropathol. Exp. Neurol. 65:1022–1030 [DOI] [PubMed] [Google Scholar]
- 11. Irei T, et al. 2007. The persistent elimination of B cells responding to blood group A carbohydrates by synthetic group A carbohydrates and B-1 cell differentiation blockade: novel concept in preventing antibody-mediated rejection in ABO-incompatible transplantation. Blood 110:4567–4575 [DOI] [PubMed] [Google Scholar]
- 12. Jessell TM, Hynes MA, Dodd J. 1990. Carbohydrates and carbohydrate-binding proteins in the nervous system. Annu. Rev. Neurosci. 13:227–255 [DOI] [PubMed] [Google Scholar]
- 13. Kennedy PGE, Grinfeld E, Bell JE. 2000. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J. Virol. 74:11893–11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kliman HJ, et al. 1995. A mucin-like glycoprotein identified by MAG (mouse ascites Golgi) antibodies. Menstrual cycle-dependent localization in human endometrium. Am. J. Pathol. 146:166–181 [PMC free article] [PubMed] [Google Scholar]
- 15. Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ. 1998. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc. Natl. Acad. Sci. U. S. A. 95:7080–7085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahalingam R, et al. 1996. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc. Natl. Acad. Sci. U. S. A. 93:2122–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mollicone R, Davies DR, Evans B, Dalix AM, Oriol R. 1986. Cellular expression and genetic control of ABH antigens in primary sensory neurons of marmoset, baboon and man. J. Neuroimmunol. 10:255–269 [DOI] [PubMed] [Google Scholar]
- 18. Oriol R. 1995. ABO, Hh, Lewis, and secretion. Serology, genetics, and tissue distribution. In Cartron J, Rouger P. (ed), Blood cell biochemistry, vol. 6 Plenum, New York, NY [Google Scholar]
- 19. Pevenstein SR, et al. 1999. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J. Virol. 73:10514–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reichelt M, Brady J, Arvin AM. 2009. The replication cycle of varicella-zoster virus: analysis of the kinetics of viral protein expression, genome synthesis, and virion assembly at the single-cell level. J. Virol. 83:3904–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwartz LB, et al. 2001. Mouse ascites golgi (MAG) mucin expression and regulation by progesterone in the rat uterus. J. Soc. Gynecol. Investig. 8:216–223 [DOI] [PubMed] [Google Scholar]
- 22. Smith ZD, D'Eugenio-Gumkowski F, Yanagisawa K, Jamieson JD. 1984. Endogenous and monoclonal antibodies to the rat pancreatic acinar cell Golgi complex. J. Cell Biol. 98:2035–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spicer SS, Spivey MA, Ito M, Schulte BA. 1994. Some ascites monoclonal antibody preparations contain contaminants that bind to selected Golgi zones or mast cells. J. Histochem. Cytochem. 42:213–221 [DOI] [PubMed] [Google Scholar]
- 24. Svensson L, Rydberg L, de Mattos LC, Henry SM. 2009. Blood group A(1) and A(2) revisited: an immunochemical analysis. Vox Sang. 96:56–61 [DOI] [PubMed] [Google Scholar]
- 25. Theil D, et al. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 163:2179–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Theil D, et al. 2003. Dually infected (HSV-1/VZV) single neurons in human trigeminal ganglia. Ann. Neurol. 54:678–682 [DOI] [PubMed] [Google Scholar]
- 27. Tixier-Vidal A, Faivre-Bauman A, Picart R, Wiedenmann B. 1988. Immunoelectron microscopic localization of synaptophysin in a Golgi subcompartment of developing hypothalamic neurons. Neuroscience 26:847–861 [DOI] [PubMed] [Google Scholar]
- 28. Verjans GM, et al. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. U. S. A. 104:3496–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang K, Lau TY, Morales M, Mont EK, Straus SE. 2005. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J. Virol. 79:14079–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weinstein RS, et al. 1990. ABO blood type predicts the cytolocalization of anti-P-glycoprotein monoclonal antibody reactivity in human colon and ureter. Hum. Pathol. 21:949–958 [DOI] [PubMed] [Google Scholar]
- 31. White T, et al. 1990. Murine monoclonal antibodies directed to the human histo-blood group A transferase (UDP-GalNAc:Fuc alpha 1–2Gal alpha 1-3-N-acetylgalactosaminyltransferase) and the presence therein of N-linked histo-blood group A determinant. Biochemistry 29:2740–2747 [DOI] [PubMed] [Google Scholar]
- 32. Zerboni L, et al. 2010. Expression of varicella-zoster virus immediate-early regulatory protein IE63 in neurons of latently infected human sensory ganglia. J. Virol. 84:3421–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]