Abstract
The transporter associated with antigen processing (TAP) delivers the viral proteolytic products generated by the proteasome in the cytosol to the endoplasmic reticulum lumen that are subsequently recognized by cytotoxic T lymphocytes (CTLs). However, several viral epitopes have been identified in TAP-deficient models. Using mass spectrometry to analyze complex human leukocyte antigen (HLA)-bound peptide pools isolated from large numbers of TAP-deficient vaccinia virus-infected cells, we identified 11 ligands naturally presented by four different HLA-A, HLA-B, and HLA-C class I molecules. Two of these ligands were presented by two different HLA class I alleles, and, as a result, 13 different HLA-peptide complexes were formed simultaneously in the same vaccinia virus-infected cells. In addition to the high-affinity ligands, one low-affinity peptide restricted by each of the HLA-A, HLA-B, and HLA-C class I molecules was identified. Both high- and low-affinity ligands generated long-term memory CTL responses to vaccinia virus in an HLA-A2-transgenic mouse model. The processing and presentation of two vaccinia virus-encoded HLA-A2-restricted antigens took place via proteasomal and nonproteasomal pathways, which were blocked in infected cells with chemical inhibitors specific for different subsets of metalloproteinases. These data have implications for the study of the effectiveness of early empirical vaccination with cowpox virus against smallpox disease.
INTRODUCTION
The eradication of smallpox, a disease caused by variola major virus, was made possible by early empirical, cross-protective vaccination with cowpox virus and later through the massive worldwide production and administration of vaccinia virus (VACV) vaccines (18). The Orthopoxvirus vaccinia virus is a widely used tool for research and vaccine development (47), and recent concerns about bioterrorism and emerging infectious diseases have elicited renewed interest in VACV and other poxviruses (31). Vaccination induces a strong humoral response leading to viral clearance, and the role of cellular responses in this cross-protection is well documented (21, 56). In recent years, studies using both vaccinated humans and human histocompatibility complex (HLA)-transgenic mouse models have allowed the identification of more than 70 VACV-derived epitopes restricted by various HLA molecules (reviewed in references 30 and 32).
In cellular immunity, recognition and killing of infected cells by CD8+ cytolytic T lymphocytes (CTLs) first require viral proteins to be proteolytically degraded (73). Antigen (Ag) processing generates short peptides that are translocated to the endoplasmic reticulum (ER) lumen by the transporter associated with antigen processing (TAP) and then assembled with a newly synthesized β2-microglobulin and HLA class I heavy chain. Despite initial assumptions that the multicatalytic and ubiquitous proteasome was the only protease fully capable of generating peptide ligands for presentation on HLA class I molecules, several studies have demonstrated that a growing number of alternative pathways contribute to endogenous antigen processing (reviewed in references 13 and 27).
Individuals with mutations in the TAP gene that generate nonfunctional TAP complexes have been previously described (reviewed in reference 9). Patients with this HLA class I deficiency may appear asymptomatic for long periods of their lives. TAP-deficient (TAP−) patients do not seem particularly susceptible to viral infections or neoplasms. Therefore, their immune systems must be reasonably efficient, and antibodies (Abs), NK cells, CD8+ γδ T cells, and the reduced cytolytic CD8+ αβ T subpopulation that is specific for TAP-independent antigens may all contribute to immune defenses that protect against severe viral infections in such individuals. In addition, several strains of viruses, including cowpox virus (1), have found ways to obstruct TAP expression or function in order to prevent CTLs from identifying infected cells (reviewed in reference 36); therefore, the TAP-independent pathways must be important for killing cells infected with these viruses. The identification of TAP-independent epitopes that are conserved among orthopoxviruses could also be relevant to the study of the mechanisms of early empirical vaccination against smallpox disease as performed with cowpox virus.
Although TAP-independent viral epitopes are known (reviewed in reference 36), there has been a marked absence of methodical studies of TAP-independent epitopes and ligands restricted by different HLA molecules in cells infected with a single virus. Moreover, in all previous studies, very limited antigen processing capacity in TAP-deficient cells has been reported. Therefore, is only one TAP-independent ligand or epitope restricted by a single HLA molecule exposed on the cell membrane surface, as suggested by these studies? Conversely, could a TAP-deficient cell simultaneously bind several viral ligands to different HLA molecules? We are interested in the identification of viral ligands presented by several common HLA antigens in TAP-deficient infected cells. Using immunoproteomic analysis, we compared HLA ligands isolated from large numbers of healthy or VACV-infected cells. This study identified 11 TAP-independent, naturally processed ligands from eight different VACV proteins in infected cells that were mostly conserved among the members of the Orthopoxviridae family, including cowpox virus.
MATERIALS AND METHODS
Mice.
H-2 class I knockout HLA-A*0201-transgenic mice (20) were bred in our animal facilities in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals and Spanish government regulations (accreditation 28079-34A). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Institute of Health “Carlos III” (permit PI-283). All surgery was performed under conditions of sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Cell lines.
T2 cells, a line of TAP-deficient human cells that express HLA-A2, HLA-B51, and HLA-Cw1 class I molecules on their surface (57), were transfected with HLA-B27 (a gift from David Yu, University of California, Los Angeles, CA). The mouse cell lines RMA (TAP positive [TAP+]) and RMA-S (TAP−) were transfected with HLA-A*0201 α1α2 domains, and the mouse H-2Db α3 transmembrane and cytoplasmic domains have been previously described (52). The RMA-S transfectant cells expressing HLA-B*2705 have been previously described (66). All cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 5 μM β-mercaptoethanol (β-ME).
Synthetic peptides.
Peptides were synthesized in a peptide synthesizer (model 433A; Applied Biosystems, Foster City, CA) and purified by reverse-phase high-performance liquid chromatography (HPLC). The correct molecular mass of the peptides was established by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), and their correct composition was determined by quadrupole ion trap micro-HPLC.
Inhibitors.
Brefeldin A (BFA) and all protease inhibitors were purchased from Sigma-Aldrich, except for leupeptin (LEU) (Amersham-UBS), pepstatin (PEPST) (Boehringer Mannheim), Z-VAD.fmk (Enzyme System Products, Livermore, CA), (z-LL)2 ketone (Merck), and lactacystin (E. J. Corey, Harvard University). The specificity and activity of all inhibitors used in this study are summarized in Table 1. For control of activity of the protease inhibitors, RMA-HLA-A*0201 cells (1 × 108) were disrupted by sonication for 15 min at 4°C and centrifuged as previously reported (40). A supernatant aliquot corresponding to 1 × 107 cells was directly frozen (nondegraded control). Equivalent aliquots were incubated in the presence of individual inhibitors at 200 μM, and digestion by cellular proteases was allowed to proceed for 5 days at 37°C in phosphate-buffered saline (PBS). Inhibitors were renewed daily. A sample incubated without inhibitors was used as the degraded control. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) separation and Coomassie blue staining of these samples, the overall protein content of each lane was quantitated by densitometry using the TINA 2.09e program (Isopenmeβgeräte GmbH, Germany). Percent inhibition of protein degradation caused by each inhibitor was calculated as follows: 100 × (sample with inhibitor − degraded sample)/(nondegraded sample − degraded sample).
Table 1.
Specificity and activity of the inhibitors used in this study
| Inhibitor | Abbreviation | Specificity | Reference(s) | Concn | % Inhibition of degradationa |
|---|---|---|---|---|---|
| Brefeldin A | BFA | Vesicle transport | 48, 71 | 5 μg/ml | ND |
| Lactacystin | LC | Proteasome | 19, 49 | 10 μM | ND |
| (z-LL)2 ketone | z-LL2 | Signal peptide peptidase | 68, 69 | 100 μM | 13 ± 4 |
| Leupeptin | LEU | Trypsin-like proteases and cysteine proteases | 65 | 100 μM | 38 ± 18 |
| Pepstatin | PEPST | Aspartic proteases | 34, 65 | 100 μM | 50 ± 5 |
| 1-10 Phenanthroline | PHE | Metalloproteases and caspase-1 | 34, 64 | 50 μM | ND |
| Leucinethiol | LeuSH | Metalloaminopeptidases, including ERAAP | 61 | 30 μM | ND |
| Benzyl succynil acid | BENZ | Metallocarboxypeptidases A and B | 34 | 100 μM | −10 ± 8 |
| Captopril | CAPT | ACEb and ACE-like proteases | 34 | 100 μM | 25 ± 2 |
| Phosphoramidon | PHOSP | All bacterial metalloendopeptidases but few of mammalian origin | 22, 34 | 100 μM | 15 ± 4 |
| z-VAD.fmk | z-VAD | Caspases | 62 | 100 μM | NDc |
The activity of these inhibitors was measured as their ability to prevent proteolytic degradation in cellular extracts by the method described in reference 40. The amount of protein still present after incubation in the case of the degraded control sample was considered to represent 0% inhibition of degradation, and the nondegraded unincubated sample was taken to represent 100 % inhibition. Data represent the means of the results of two independent experiments. The negative value indicates that there was enhanced degradation in the presence of the compound. ND, not done.
ACE, angiotensin-converting enzyme.
The compound was found to block apoptosis (data not shown).
Infection of the T2-B27 cell line by VACV.
A total of 1 × 109 to 3 × 109 T2-B27 cells were infected with VACV at a multiplicity of infection of 10 PFU/cell in 10 ml, incubated for 2 h at 37°C, and then washed. The cells were then cultured for 24 h and stained with Omnitope antiserum-fluorescein isothiocyanate (FITC), which recognizes VACV-purified virions. Samples were analyzed by fluorescence-activated cell sorting (FACS). Later, the cells were frozen.
Isolation of HLA-bound peptides.
HLA-bound peptides were isolated from 4 × 1010 healthy or VACV-WR-infected T2-B27 transfectant cells. Cells were lysed in 1% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate; Sigma)–20 mM Tris-HCl buffer–150 mM NaCl (pH 7.5) in the presence of a protease inhibitor cocktail (24). HLA-peptide complexes were isolated via affinity chromatography of the soluble fraction of cell extracts with the following monoclonal antibodies (MAbs), used sequentially: PA2.1 (anti-HLA-A2) (51), ME1 (anti-HLA-B27) (14), and W6/32 (specific for a monomorphic HLA class I determinant) (6) (Fig. 1). HLA-bound peptides were eluted at room temperature with 0.1% aqueous trifluoroacetic acid (TFA), separated from the large subunits, and concentrated using a Centricon 3 column (Amicon, Beverly, MA) exactly as previously described (24).
Fig 1.
Diagram of sequential immunoprecipitation. Healthy or VACV-infected T2-B27 transfectant cells (4 × 1010) were lysed. HLA-peptide complexes were isolated via affinity chromatography of the soluble fraction of cell extracts with the following mAbs, used sequentially: PA2.1 (anti-HLA-A2), ME1 (anti-HLA-B27), and W6/32 (specific for a monomorphic HLA class I determinant).
Electrospray-ion trap mass spectrometry analysis.
Peptide mixtures recovered after the ultrafiltration step were concentrated using Micro-Tip reversed-phase columns (C18; Harvard Apparatus, Holliston, MA) (200 μl) (24). Each C18 tip was equilibrated with 80% acetonitrile–0.1% TFA, washed with 0.1% TFA, and then loaded with the peptide mixture. The tip was then washed with an additional volume of 0.1% TFA, and the peptides were eluted with 80% acetonitrile–0.1% TFA. Peptide samples were then concentrated to about 18 μl using vacuum centrifugation (24).
HLA class I peptides immunoprecipitated with each HLA-specific MAb were analyzed in three different HPLC runs by micro-liquid chromatography-tandem MS (μLC-MS/MS) using an Orbitrap XL mass spectrometer (Thermo Electron, San Jose, CA) fitted with a capillary HPLC column (Eksigent, Dublin, CA) (24). The peptides were resolved on homemade Reprosil C18 capillary columns (75 μm inner diameter) (25) with a 7 to 40% acetonitrile gradient for 2 h in the presence of 0.1% formic acid. The seven masses exhibiting the greatest intensity and single-, double-, and triple-charge states were selected for fragmentation from each full mass spectrum by collision-induced dissociation (CID).
Database searches.
Sequest 3.31 software (Thermo-Fisher) (15) was used for peak-list generation of the μLC-MS/MS data. The peaks were identified by using Proteome Discoverer 1.0 SP1 software (Thermo-Fisher), combining the results obtained with Sequest 3.31 and Bioworks Browser 3.3.1 SP1 (Thermo-Fisher) (15), and using the human and virus parts of the NCBI database (January 2009), which included 656,486 proteins. The search was not limited by enzymatic specificity, the peptide tolerance was set to 0.005 Da, and the fragment ion tolerance was set to 0.5 Da (24, 43). This search was not limited by any methodological bias (selection of individual protein, use of HLA consensus-scoring algorithms, etc.). Identified peptides were selected when the following criteria were met: Sequest Xcorr > 1.4 for singly, > 2.2 for doubly, and > 2.9 for triply charged peptides; P(pep) less than 1 × 10−3; and mass accuracy of 0.005 Da (24, 43). When the MS/MS spectra fitted two or more peptides, only the highest-scoring peptide was analyzed. No peptides were found in a search of a reversed database. The purpose of the filtering criteria was to identify candidate vaccinia virus peptide MS/MS scans for further manual inspection to determine whether the MS/MS fragment ion fingerprint matched the identified peptide sequence. In addition, the corresponding synthetic peptide was made, and its MS/MS spectrum was used to confirm the assigned sequence.
MHC-peptide stability assays.
The following synthetic peptides were used as controls in complex stability assays: KPNA2 (GLVPFLVSV, HLA-A2 restricted) (23), influenza virus NP (SRYWAIRTR, HLA-B27 restricted) (67), hepatitis B virus (HBV) HBc19-27 (LPSDFFPSV, HLA-B51 restricted) (8), cytomegalovirus (CMV) pp657-15 (RCPEMISVL, HLA-Cw1 restricted) (33), and C4CON (QYDDAVYLK, HLA-Cw4 restricted) (17). Either RMA-S transfectants or T2 cells expressing small amounts of major histocompatibility complex (MHC) class I on the cell surface were incubated at 26°C for 16 h in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS). This allowed the expression of empty MHC class I molecules (without antigenic peptide) that are stable only at 26°C, and not at 37°C, on the cell membrane. The cells were washed and incubated for 2 h at 26°C with various concentrations of peptide in the same medium. Afterward, the cells were kept at 37°C and collected after 4 h for flow cytometry analysis. This assay allows for internalization of empty MHC class I molecules and can therefore discriminate between bound and unbound peptides. HLA expression levels were measured using monoclonal PA2.1 (anti-HLA-A2), monoclonal ME1 (anti-HLA-B27), polyclonal H00003106-B01P (specific for HLA-B class I molecules) (Abnova, Taipei, Taiwan), and polyclonal SC-19438 (specific for HLA-C class I molecules) (Santa Cruz Biotechnology, Santa Cruz, CA) Abs as previously described (41). Samples were acquired on a FACSCanto flow cytometer (BD Biosciences, San Jose, CA) and analyzed using CellQuest Pro 2.0 software (BD Bioscience). Cells incubated without peptides had peak fluorescence intensities close to those of background staining with secondary Ab alone. The fluorescence index (FI) was calculated as the ratio of the mean channel fluorescence of the sample to that of control cells incubated without the peptides. The binding of peptides was also expressed as the 50% effective concentration (EC50), which is the molar concentration of the peptides producing 50% of the maximum fluorescence obtained at a concentration range between 0.001 and 100 μM.
Magnetic antigen cell separation (MACS).
Mouse T lymphocytes were isolated by the depletion of non-T CD8+ cells (negative selection) and the use of a T CD8a+ cell isolation kit (Miltenyi Biotec GmbH, Gladbach, Germany) according to the manufacturer's specifications. The purity of the cell preparations recovered after negative selection was verified by fluorescence-activated cell sorting (FACS) and found to be higher than 95% for CD8+ T lymphocytes (3).
Ex vivo ICS.
Intracellular cytokine staining (ICS) assays were performed as described previously (58). Purified CD8+ T lymphocytes were obtained from HLA-A*0201-transgenic mice up to 30 days (memory) postintraperitoneal (post-i.p.) infection with 1 × 107 PFU of VACV-WR, stimulated for 2 h with either RMA or RMA-S HLA-A*0201 cells infected with VACV-WR, and incubated overnight in the presence of BFA (5 μg/ml). Later, cells were incubated with FITC-conjugated anti-CD8 MAb (ProImmune, Oxford, United Kingdom) for 30 min at 4°C, fixed with Intrastain kit (DakoCytomation, Glostrup, Denmark) reagent A, and incubated with phycoerythrin (PE)-conjugated anti-gamma interferon (IFN-γ) MAb (BD PharMingen, San Diego, CA) in the presence of Intrastain kit permeabilizing reagent B for 30 min at 4°C. Events were acquired and analyzed as described for the MH-peptide stability assays.
T cell lines, cytotoxicity assays, and ICS.
Polyclonal SIINFEKL or VACV peptide-monospecific CTLs were generated by immunizing mice i.p. with 1 × 107 PFU of VACV-OVA257-264 (encoding the miniprotein MSIINFEKL) or VACV-WR, respectively, as previously described (40, 45). Splenocytes from immunized mice were restimulated in vitro with mitomycin C-treated spleen cells pulsed with a 10−6 M concentration of the respective peptide and cultured in alpha-minimal essential medium (α-MEM) supplemented with 10% FBS–1 × 10−7 M peptide–1% 2-ME. Recombinant human interleukin-2 for the long-term propagation of peptide-specific CTL lines was generously provided by Hoffmann-LaRoche. The RMA-HLA-A*0201 cells were used as target cells in standard 4-h cytolytic assays (40).
ICS assays to detect the recognition of infected cells by polyclonal CTL cell lines were performed as previously described (10). CTL lines were stimulated for 4 h in the presence of BFA (5 μg/ml) and target cells that had been infected overnight with VACV or VACV-OVA257-264. When protease inhibitors were used, all drugs were added 15 min before the virus and kept at a 5-fold-higher concentration during the 1-h adsorption period than throughout the infection. After the virus inoculum was washed, the inhibitors were kept at the concentrations indicated for the individual experiments. The inhibitors were not toxic at the indicated concentrations, since they affected neither antigen presentation by either of the A10L688-696 and A17L9-17 epitopes (see below) or VACV infection when the Omnitope antiserum with specificity for VACV proteins from purified virions (ViroStat Inc., Portland, ME) was used (see Fig. S1 in the supplemental material). The ICS was performed with polyclonal CTL as described for ex vivo ICS.
In vivo cytotoxicity assay.
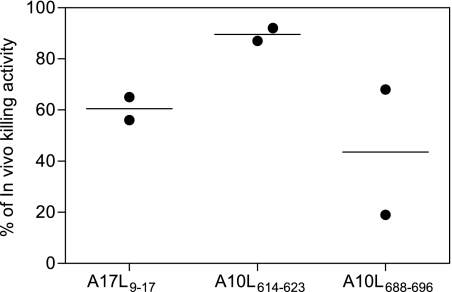
In vivo cytotoxicity assays were performed as previously published (45). Spleens were obtained, erythrocytes were removed, and HLA-A*0201 splenocytes were split into two populations and labeled with either a high concentration (5 μM) or a low concentration (0.5 μM) of carboxyfluorescein succinimidyl ester(CFSE). After excess CFSE was removed by washing, CFSEhigh spleen cells were pulsed with 10−6 M VACV peptides for 30 min at 37°C. Excess peptide was washed at least twice, and CFSEhigh peptide-pulsed cells were mixed with equal numbers of CFSElow cells. A total of 8 × 106 cells of the spleen cells mixed suspension were i.p. injected into each HLA-A*0201-transgenic mouse that had been left uninfected or had been i.p. infected with VACV-WR (1 × 107 PFU) 7 days earlier. Two days later, the peritoneal cavity was subjected to lavage, spleens were extracted, and the cells were analyzed by flow cytometry using a FACSCanto flow cytometer to measure in vivo killing. Data were analyzed using CellQuest Pro 2.0 software. Specific lysis was calculated as previously published (45) according to the following formula: [1 − (ratio unprimed/ratio primed) × 100], where the ratio unprimed values represent % CFSElow/% CFSEhigh cells remaining in control uninfected recipients and the ratio primed values represent % CFSElow/% CFSEhigh cells remaining in experimentally infected recipients.
RESULTS
VACV-specific CD8+ T cells recognize TAP-deficient HLA-A*0201-transfected cells.
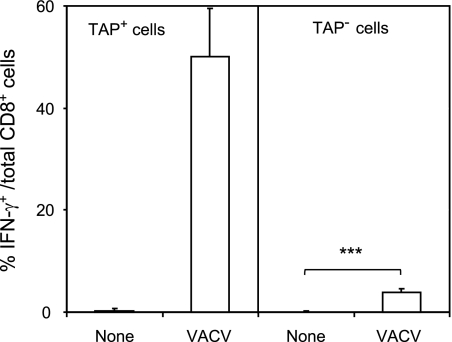
As the first step in the study of TAP-independent HLA-restricted responses to vaccinia virus, HLA-A*0201-transgenic mice were immunized with the virus. Next, the VACV-specific CD8+ response was evaluated using intracellular cytokine staining (ICS) assays. A strong ex vivo response (i.e., 50.1% ± 9.4% of CD8+ cells secreted IFN-γ) specific for this virus was detected in TAP-positive (TAP+) target cells (Fig. 2, left panel). Additionally, a small fraction of VACV-specific CD8+ T lymphocytes recognized infected TAP-deficient cells (3.8 ± 0.8% of CD8+ cells secreted IFN-γ) (Fig. 2, right panel). These data indicate the existence of a TAP-independent antigen-processing pathway(s) of some vaccinia virus epitopes in infected cells that could be recognized by specific CD8+ T lymphocytes.
Fig 2.
Recognition of TAP+ and TAP− cell lines by VACV-specific CD8+ T lymphocytes. HLA-A*0201 TAP+ cells (RMA; left panel) and TAP− cells (RMA-S; right panel) were infected with VACV at a multiplicity of infection of 40 PFU/cell and analyzed by ICS for CD8+ T cell activation. The results are calculated as the means ± standard deviations (SD) of the results of three or four independent experiments. ***, P < 0.0001.
Physiological processing generates three different viral HLA-A2 ligands in human TAP-deficient vaccinia virus-infected cells.
HLA-A2-bound peptide pools were isolated from large numbers of either healthy or VACV-infected human TAP-deficient cells (110 ± 20 mean fluorescence intensity [MFI] in VACV-infected cells versus 15 ± 5 in healthy cells stained with an anti-VACV antiserum). These peptide mixtures were subsequently separated by reverse-phase HPLC and analyzed by mass spectrometry. According to the results observed with bioinformatics tools, three fragmentation spectra present in the VACV-infected HLA-A2-bound peptide pool, but absent from the control uninfected pool, were resolved with high-confidence parameters as peptides of vaccinia virus proteins. A human proteome database search failed to identity any of these spectra as human protein fragments, confirming the viral origin of these peptides. The first ion peak, with an m/z of 926.4, was assigned to the viral amino acid sequence MLDDFSAGA, spanning residues 9 to 17 of the A17L protein of vaccinia virus (see Fig. S2 in the supplemental material, upper panel). In addition, two different ion peaks at m/z 974.6 and 514.8 were assigned to peptides of the same viral protein. These ion peaks corresponded to the SPEGEETII peptide (see Fig. S2 in the supplemental material, middle panel) and ILDRIITNA peptide (see Fig. S2 in the supplemental material, lower panel), which span residues 614 to 623 and residues 688 to 696, respectively, of the A10L protein. Virtually all significant fragments of the three MS/MS spectra were assigned as daughter ions of the putative peptidic sequences (see Fig. S2 in the supplemental material). This theoretical assignment was confirmed by determination of identity with the MS/MS spectrum of the corresponding synthetic peptide (see Fig. S2 in the supplemental material). Therefore, these results indicate that a total of three TAP-independent HLA-A2 ligands were endogenously processed and presented in the VACV-infected cells.
Binding affinity of TAP-independent vaccinia virus ligands for the A*0201 molecule.
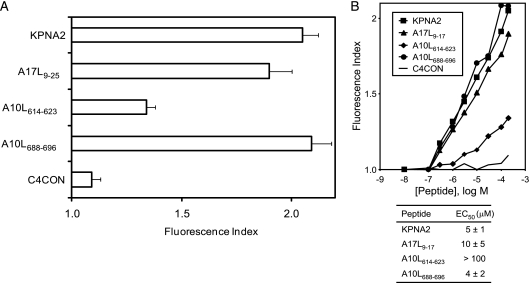
The classical anchor motifs for HLA-A*0201 binding, Leu or Met at position 2 (P2) and aliphatic C-terminal residues (SYFPEITHI database; http://www.syfpeithi.de [54]), were present in two of the three TAP-independent viral ligands detected. In contrast, the A10L614-623 ligand presented Pro at P2, although it coimmunoprecipitated with an HLA-A2-specific MAb and thus could have represented an unusual HLA-A2-restricted ligand. To confirm that HLA-A*0201 was the MHC class I molecule that presented these ligands, MHC-peptide complex stability assays were performed using TAP-deficient RMA-S cells transfected with the HLA-A*0201 molecule (Fig. 3). The two viral ligands with HLA-A2 anchor motifs, A10L688-696 and A17L9-17, bound to HLA-A*0201 class I molecules with EC50s in the range commonly found among other natural high-affinity ligands (Fig. 3B). In contrast, HLA affinity for the A10L614-623 ligand was substantially lower, as the absence of HLA-A2 anchor motifs suggested; therefore, this peptide must be considered a low-affinity ligand. These data confirm that all ligands detected in vaccinia virus-infected cells were endogenously presented in association with the A*0201 molecule.
Fig 3.
HLA-A*0201 stabilization assay with synthetic VACV ligands. (A) The stability of HLA-A*0201-peptide complexes on the surface of RMA-S transfectant cells was measured by flow cytometry. The indicated peptides were used at 200 μM. The mAb used was PA2.1. (B) The titration curves for synthetic VACV A17L9-17, A10L614-623, and A10L688-696 peptides with HLA-A*0201 are depicted. The C4CON and KPNA2 peptides were used as negative and positive controls, respectively. The results, calculated as fluorescence index values (see panel A), represent the means of the results of three or four independent experiments. The calculated EC50s ± SD are shown in panel B.
Three viral HLA-B*2705 ligands were endogenously processed in human TAP-deficient vaccinia virus-infected cells.
To date, about 60 human TAP-independent MHC class I ligands are known (36, 70), and these are mostly restricted by HLA-A2 and derived from the cleavage of signal sequences generated by the signal peptidase (SPase) complex. The A*0201-restricted CTL epitopes of vaccinia virus that we detected in TAP-deficient cells could therefore be exceptional, and TAP-independent antigen-processing pathways might be unable to generate vaccinia virus peptides that could bind to other HLA class I molecules. HLA-B27, which was previously described as an allele with high TAP dependency (2), was used to obtain peptide pools from either healthy or VACV-infected TAP-deficient cells, as used to identify the HLA-A*0201 ligands. Again, three fragmentation spectra present in the VACV-infected, HLA-B27-bound peptide pool, but absent from the control uninfected pool, were also resolved as peptides of vaccinia virus proteins. The human proteome database search failed to identify these spectra as representing human protein fragments, confirming the viral origin of these HLA-B27-bound peptides. The first ion peak, with an m/z of 428.8, was assigned to the viral amino acid sequence SRGYFEHMKK, which spans residues 867 to 876 of the A10L protein of vaccinia virus (see Fig. S3 in the supplemental material, upper panel), indicating that antigen processing of this protein could generate several viral ligands bound to two different HLA class I molecules. The second ion peak, at m/z 567.6, was assigned to the peptidic sequence YRLQGFTNAGIVAYK (see Fig. S3 in the supplemental material, middle panel), which spans residues 16 to 30 of the K2L protein. Finally, the third ion peak, at m/z 471.8, corresponded to a WQTMYTN peptide (see Fig. S3 in the supplemental material, lower panel), which spans residues 53 to 59 of the B8R protein. Figure S3 in the supplemental material shows that all significant fragments of these three MS/MS spectra were assigned as daughter ions of the putative peptidic sequences. As HLA-A2 ligands, these assignments were confirmed by identity with the MS/MS spectrum of the corresponding synthetic peptide (see Fig. S3 in the supplemental material). In addition, the K2L16-30 ligand was also identified as a molecular ion at m/z +2 (see Fig. S5 in the supplemental material). Collectively, these results indicate that similar numbers of TAP-independent ligands were endogenously processed and presented by HLA-A2 and HLA-B27 class I molecules in the same vaccinia virus-infected cells.
Binding affinity of TAP-independent vaccinia virus ligands for the B*2705 molecule.
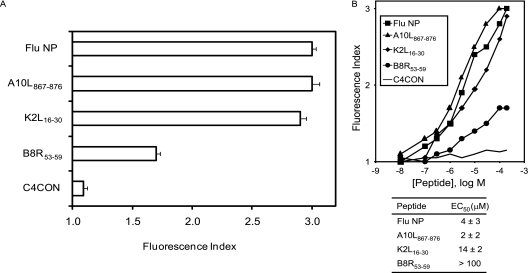
The A10L867-876 and K2L16-30 (but not B8R53-59) peptides have the known anchor motifs for HLA-B*2705 binding of Arg at P2 and basic or aliphatic C-terminal residues (SYFPEITHI database [54]). As the B8R53-59 peptide was also coimmunoprecipitated with an HLA-B27-specific MAb, it could be an unusual HLA-B27-restricted ligand. To confirm that HLA-B*2705 is the MHC class I molecule that presents these ligands, MHC-peptide complex stability assays were performed using TAP-deficient RMA-S cells transfected with the HLA-B*2705 molecule (Fig. 4). The two viral ligands with HLA-B27 anchor motifs, A10L867-876 and K2L16-30, bound to HLA-B*2705 class I molecules with EC50s to similar those of other natural high-affinity ligands (Fig. 4B). In contrast, the HLA affinity was substantially lower for the B8R53-59 ligand, as suggested by the absence of HLA-B27 anchor motifs, and this peptide could be considered a low-affinity ligand. These data confirm that the ligands detected in vaccinia virus-infected cells were endogenously presented in association with the B*2705 molecule. In summary, either HLA-A*0201 or B*2705 class I molecules can bind both high- and low-affinity ligands derived from different vaccinia virus proteins.
Fig 4.
HLA-B*2705 stabilization assay with synthetic VACV ligands. (A) Stability of HLA-B*2705-peptide complexes on the surface of RMA-S transfectant cells measured by flow cytometry. The indicated peptides were used at 200 μM. The mAb used was ME1. (B) The titration curves for synthetic VACV A10L867-876, K2L16-30, and B8R53-59 peptides with HLA-B*2705 are depicted. The C4CON and influenza virus (Flu) NP peptides were used as negative and positive controls, respectively. The results, as in Fig. 2, represent the means of the results of four independent experiments.
Six vaccinia virus ligands were endogenously presented by HLA-B51 and/or HLA-Cw1 class I molecules in human TAP-deficient cells.
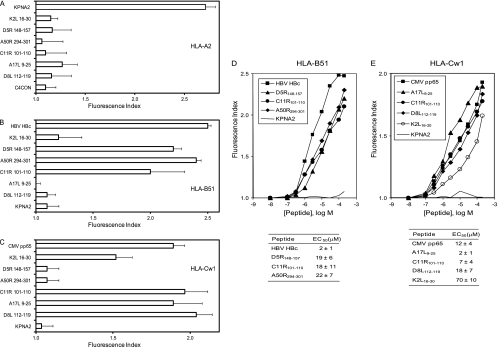
Given that several TAP-independent viral ligands were identified in association with either HLA-A*0201 or B*2705 class I molecules (see above), we investigated the possibility of new vaccinia virus ligands being presented by other HLA class I molecules expressed in the same T2 cells. As in the previous two analyses, six fragmentation spectra were found in the VACV-infected peptide pool that were absent from the uninfected control pool, and these spectra were also resolved as peptides of vaccinia virus proteins. Furthermore, a human proteome database search also failed to identify these spectra as human protein fragments, confirming the viral origin of these HLA-bound peptides. Supplemental Fig. S4 and S5 show the experimentally obtained MS/MS spectra and their respective assignments. Each putative peptidic sequence was confirmed according to its identity with the MS/MS spectrum of the corresponding synthetic peptide (see Fig. S4 and S5 in the supplemental material). Five of these peptides, IAMKRTLLEL (D5R148-157), LPFGSLGI (A50R294-301), IPSPGIMLV (C11R101-110), MLDDFSAGAGVLDKDL (A17L9-25), and DGLIIISI (D8L112-119), represented new viral sequences. Surprisingly, the K2L16-30 ligand previously detected in association with HLA-B*2705 (see Fig. S3 in the supplemental material) was also immunoprecipitated in the third round with the W6/32 Ab. As HLA-A2, HLA-B51, and HLA-Cw1 present peptides with similar anchor motifs (SYFPEITHI database [54]), HLA-peptide complex stability assays were performed to confirm that the sequential immunoprecipitation was performed correctly and to exclude the possibility that residual HLA-A2-bound ligands that were not fully immunoprecipitated with the PA2.1 (anti-HLA-A2) Ab in the first round would be immunoprecipitated in the third round with the W6/32 Ab (specific for a monomorphic HLA class I determinant). Figure 5A shows that, in contrast to the positive-control KPNA2 peptide results, binding of HLA-A2 complexes to these six ligands was not detected. Therefore, these viral ligands do not bind to HLA-A2, which validates the experimental strategy used. In addition, to identify the HLA restriction of these ligands, new HLA-peptide complex stability assays using TAP-deficient T2 cells with specific anti-HLA-B or anti-HLA-C Abs were performed. The numbers of HLA-peptide surface complexes induced by the IAMKRTLLEL (D5R148-157), LPFGSLGI (A50R294-301), and IPSPGIMLV (C11R101-110) synthetic peptides were similar to those induced by a well-known HLA-B51 ligand, HBV HBc19-27 (Fig. 5B), by the use of the anti-HLA-B Ab, indicating that these peptides were restricted by the HLA-B51 class I molecule. HLA stabilization was detected using anti-HLA-C (Fig. 5C) Ab with IPSPGIMLV (C11R101-110), MLDDFSAGAGVLDKDL (A17L9-25), and DGLIIISI (D8L112-119), indicating that these peptides were restricted by the HLA-Cw1 allele. In addition, the K2L16-30 ligand also bound to HLA-B*2705 (see Fig. S3 in the supplemental material and Fig. 4) presented efficient binding to HLA-Cw1 class I molecule, justifying their dual immunoprecipitation (Fig. 5). Five of these six viral ligands bound to HLA-B51 (Fig. 5D) or HLA-Cw1 (Fig. 5E) class I molecules with EC50s similar to those of other natural high-affinity ligands. The only exception was the K2L16-30 peptide, which showed a substantially lower affinity for HLA-Cw1, indicating that this peptide could be considered a low-affinity ligand (Fig. 5E).
Fig 5.
HLA class I stabilization assay of synthetic VACV ligands. (A, B, and C) The stability of HLA-A2 (A), HLA-B51 (B), and HLA-Cw1 (C) at the cell surface of T2 TAP-deficient cells was measured by flow cytometry. The indicated peptides were used at 200 μM. (D and E) Titration curves for the indicated synthetic VACV peptides, immunoprecipitated with W6/32 Ab, with HLA-B51 (D) or HLA-Cw1 (E). The KPNA2 peptide was used as negative control (solid line). The HBV HBc19-27 and CMV pp657-15 peptides were used as positive controls for binding to the HLA-B51 and HLA-Cw1 alleles, respectively. The Abs used were monoclonal PA2.1 (anti-HLA-A2; see panel A), polyclonal H00003106-B01P (anti-HLA-B class I molecules; see panels B and D), and polyclonal SC-19438 (anti-HLA-C class I molecules; see panels C and E). The results, as in Fig. 2, represent the means of the results of four to six independent experiments.
Thirteen natural peptide-HLA class I complexes were formed simultaneously in the same infected TAP-deficient cells.
A total of 11 viral ligands that bound to the four HLA class I molecules expressed in the T2 cells were identified (summarized in Table 2). Similar numbers (3 or 4) of ligands were identified in association with HLA class I molecules previously described as showing low (HLA-A2), high (HLA-B27), or unknown (HLA-B51 and HLA-Cw1) TAP dependency. The same N-terminal core peptide, MLDDFSAGA, was found in two different ligands, A17L9-17 and A17L9-25, bound to HLA-A2 and HLA-Cw1, respectively. Ten sequences represented new vaccinia HLA ligands. Most were restricted by a single HLA allele, but two ligands, K2L16-30 and C11R101-110, were found to be associated with two different alleles each, alleles HLA-B27 and HLA-Cw1 and alleles HLA-B51 and HLA-Cw1, respectively. This implies that 13 different natural peptide-HLA class I complexes were formed simultaneously in the same infected TAP-deficient cells.
Table 2.
Summary of HLA molecules bound by vaccinia virus epitopes
| Ligand | Sequence | MAb useda | Molecule detection resultb |
|||
|---|---|---|---|---|---|---|
| HLA-A2 | HLA-B27 | HLA-B51 | HLA-Cw1 | |||
| A17L9-17 | MLDDFSAGA | PA2.1 | +c | ND | ND | ND |
| A10L614-623 | SPEGEETII | PA2.1 | + | ND | ND | ND |
| A10L688-696 | ILDRIITNA | PA2.1 | + | ND | ND | ND |
| A10L867-876 | SRGYFEHMKK | ME1 | ND | + | ND | ND |
| B8R53-59 | WQTMYTN | ME1 | ND | + | ND | ND |
| K2L16-30 | YRLQGFTNAGIVAYK | ME1/W6-32 | − | + | − | + |
| D5R148-157 | IAMKRTLLEL | W6-32 | − | ND | + | − |
| A50R294-301 | LPFGSLGI | W6-32 | − | ND | + | − |
| C11R101-110 | IPSPGIMLV | W6-32 | − | ND | + | + |
| A17L9-25 | MLDDFSAGAGVLDKDL | W6-32 | − | ND | − | + |
| D8L112-119 | DGLIIISI | W6-32 | − | ND | − | + |
The MAbs used for the sequential immunoprecipitations were PA2.1 (specific for HLA-A2), ME1 (specific for HLA-B27), and W6-32 (specific for a monomorphic HLA-A, HLA-B, or HLA-C determinant).
ND, not done.
Significant difference (P < 0.001) compared with the negative control results.
Conservation of ligands among members of the Orthopoxvirus family.
The sequences of 11 vaccinia virus ligands identified in the WR strain were compared with homologs derived from various poxviruses. This comparison included the Copenhagen and MVA strains of VACV, two strains of human poxvirus (variola major and variola minor), and other mammalian poxviruses, such as camelpox, cowpox, ectromelia virus, horsepox, monkeypox, rabbitpox, and taterapox. This study revealed a high degree of conservation of the ligands among orthopoxviruses (Table 3). Ten of these 11 ligands are almost fully conserved in the variola major and minor viruses, with a minor substitution in the P7 position of the C11R101-110 sequence. Only 60% of the previously described TAP+ vaccinia virus epitopes are conserved in the variola proteome (46, 50, 53), indicating that TAP-independent vaccinia ligands are more highly conserved between immunogenic and pathological poxviruses than TAP-dependent epitopes.
Table 3.
Conservation of viral HLA ligands in several orthopoxvirusesa
| Poxvirus | A17L9-17 (HLA-A2) | A10L614-623 (HLA-A2) | A10L688-696 (HLA-A2) | A10L867-876 (HLA-B27) | B8R53-59 (HLA-B27) | K2L16-30 (HLA-B27, -Cw1) | D5R148-157 (HLA-B51) | A50R294-301 (HLA-B51) | C11R101-110 (HLA-B51, -Cw1) | A17L9-25 (HLA-Cw1) | D8L112-119 (HLA-Cw1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VACV WR | MLDDFSAGA | SPEGEETII | ILDRIITNA | SRGYFEHMKK | WQTMYTN | YRLQGFTNAGIVAYK | IAMKRTLLEL | LPFGSLGI | IPSPGIMLV | MLDDFSAGAGVLDKDL | DGLIIISI |
| VACV Copenhagen | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | --------- | ---------------- | -------- |
| VACV MVA | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | --------- | ---------------- | -------- |
| Variola major | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | ------V-- | ---------------- | -------- |
| Variola minor | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | ------V-- | ---------------- | -------- |
| Camelpox | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | ------V-- | ---------------- | -------- |
| Cowpox | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | ------V-- | ---------------- | --I--VA- |
| Ectromelia virus | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | --------- | ---------------- | -------- |
| Horsepox | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | --------- | ---------------- | -------- |
| Monkeypox | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | ---L--V-- | ---------------- | --I---A- |
| Rabbitpox | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | ------V-- | ---------------- | -------- |
| Taterapox | --------- | --------- | --------- | ---------- | ------- | --------------- | ---------- | -------- | ------V-- | ---------------- | -------- |
The sequences used were obtained from the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Low hydrophobicity in TAP-independent vaccinia virus ligands.
Eight TAP-independent epitopes, restricted by four different HLA class I molecules, were previously characterized with respect to their Epstein-Barr virus (EBV) CTL response (reviewed in reference 38). For this virus, only peptides from the BRLF1 and LMP2 proteins with high hydrophobicity are TAP independent (37, 38). Therefore, we tested this correlation in our vaccinia virus system. The hydrophobicity values for all TAP-independent EBV epitopes were determined over a range that reached a maximum of 2.1 on the grand average of hydropathicity (GRAVY) scale, with the maximum value found for the EBV LMP2 LLWTLVVLL peptide (Table 4). All three HLA-B*2705 ligands and the A10L614-623 HLA-A*0201-restricted ligand identified in our study were hydrophilic, with negative GRAVY values (Table 4). Only the vaccinia virus HLA-Cw1-restricted D8L112-119 epitope, with a GRAVY value of 2.1, was hydrophobic. The other six ligands showed low positive GRAVY values, indicating low hydrophobicity (Table 4). The GRAVY mean value for these 11 vaccinia ligands was only 0.3, which was very different from the value for the EBV epitopes and similar to the value for the TAP-dependent vaccinia epitopes (Table 4). These results show that hydrophobicity is not a necessary condition for the TAP-independent presentation of ligands and epitopes from other viruses such as vaccinia virus.
Table 4.
TAP-independent ligands and epitopes and their hydrophobicity
| Ligand(s) or epitope(s) | Protein | Virus | Hydrophobicitya | Reference(s) or source |
|---|---|---|---|---|
| LLWTLVVLL | LMP2 | EBV | 2.9 | 38 |
| CLGGLLTMV | LMP2 | EBV | 2.1 | 38 |
| 8 TAP− epitopes (mean ± SD) | EBV | 2.5 ± 0.2 | 38 | |
| MLDDFSAGA | A17L9-17 | Vaccinia virus | 0.4 | This study |
| SPEGEETII | A10L614-623 | Vaccinia virus | −0.6 | This study |
| ILDRIITNA | A10L688-696 | Vaccinia virus | 0.8 | This study |
| SRGYFEHMKK | A10L867-876 | Vaccinia virus | −1.7 | This study |
| WQTMYTN | B8R53-59 | Vaccinia virus | −1.2 | This study |
| YRLQGFTNAGIVAYK | K2L16-30 | Vaccinia virus | −0.1 | This study |
| IAMKRTLLEL | D5R148-157 | Vaccinia virus | 0.7 | This study |
| LPFGSLGI | A50R294-301 | Vaccinia virus | 1.5 | This study |
| IPSPGIMLV | C11R101-110 | Vaccinia virus | 1.6 | This study |
| MLDDFSAGAGVLDKDL | A17L9-25 | Vaccinia virus | 0.3 | This study |
| DGLIIISI | D8L112-119 | Vaccinia virus | 2.1 | This study |
| 11 TAP− ligands (mean ± SD) (P < 0.0001) | Vaccinia virus | 0.3 ± 1.2 | This study | |
| 79 TAP+ epitopes (mean ± SD) | Vaccinia virus | 0.4 ± 1.0 | 26, 46 |
Values correspond to the grand average of hydropathicity (GRAVY) scale (ProtParam tool [ExPASy Proteomics Server; http://www.expasy.ch).
Recognition of HLA-A*0201 ligands by specific CD8+ T cells in HLA-transgenic mice immunized with vaccinia virus.
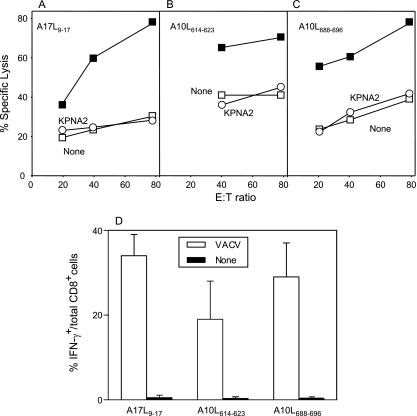
To study the immunogenicity of the identified HLA-A*0201 viral ligands, transgenic HLA-A*0201-positive mice were immunized with VACV. Later, physiological measurement of the functional in vivo activity of CD8+ T lymphocytes against HLA-A2 viral ligands as identified by mass spectrometry was carried out. HLA-A*0201-transgenic mice eliminated VACV peptide-pulsed CFSEhigh cells when they had been previously immunized but not when uninfected control mice were used (Fig. 6). In addition, up to 30 days postimmunization, polyclonal CTL lines were generated that were monospecific for each HLA-A2 viral ligand identified by mass spectrometry. These CTL lines specifically recognized peptide-pulsed cells (Fig. 7), indicating that the HLA ligands were all A*0201-restricted CTL epitopes and that they were simultaneously recognized as part of the long-term memory response to vaccinia virus. Also, these CD8+ effector lines specifically recognized VACV-infected cells (Fig. 7D).
Fig 6.
In vivo killing activity of VACV peptide-specific CTLs in HLA-A*0201-transgenic mice. HLA-A*0201-transgenic mice were infected i.p. with VAVC. One week later, they were injected i.p. with syngeneic splenocytes that were either labeled with a low level of CFSE and not subjected to pulsing or pulsed with the indicated VACV peptides and labeled with a high level of CFSE. Two days later, the remaining CFSE-labeled target cells in the peritoneal cavity were lavaged and analyzed by flow cytometry. Percent in vivo lysis values for individual animals (circles) ± SD (lines) are shown.
Fig 7.
Recognition by CTLs of VACV epitopes presented by the HLA-A*0201 class I molecule. (A to C) RMA-HLA-A*0201 target cells prepulsed with 10−5 M A17L9-17 (A), A10L614-623 (B), and A10L688-696 (C) synthetic peptides (filled squares) were tested in a cytolytic assay with their respective memory peptide-specific CTL lines obtained from HLA-A*0201-transgenic mice immunized up to 30 days before with VACV (upper panels). Negative controls used were unpulsed cells (open squares) and pulsed cells with the KPNA2 peptide (open circles). The data represent the means of the results of at least three independent experiments. (D) HLA-A*0201 TAP+ cells were infected with VACV (open bars) or not (filled bars) at a multiplicity of infection of 40 PFU/cell and analyzed by ICS for CD8+ T cell activation with the HLA-A*0201 memory peptide-specific CTL lines. The data represent the means of the results of 4 to 6 independent experiments.
Several attempts to induce CTL responses to the identified viral B*2705-restricted ligands in HLA-B*2705-transgenic mice were unsuccessful. This was also the case for the influenza NP epitope previously described in a study of influenza virus-infected B27+ humans (67) that we used as a CTL-positive control. The mouse model used carried HLA-B27 and endogenous murine H2 class I molecules (28), in contrast to the HLA-A2-transgenic mouse knockout used previously for these mouse molecules (52). This may have reduced the efficiency of HLA-restricted Ag-specific responses to undetectable levels (11), although the possibility that the T cell repertoire was a limiting factor could not be discounted. Unfortunately, HLA-B27-transgenic mice deficient for H2 class I expression (11) were not available. Therefore, the study of HLA-B*2705-restricted CTL responses to vaccinia virus ligands was not feasible.
Three different antigen-processing pathways were involved in the presentation of A10L688-696 and A17L9-17 epitopes in infected TAP-sufficient cells.
To study the antigen-processing pathways involved in the endogenous generation of the A10L688-696 and A17L9-17 viral epitopes, we investigated the presentation of these epitopes to specific CTLs in the presence of diverse protease inhibitors in VACV-infected TAP-sufficient cells.
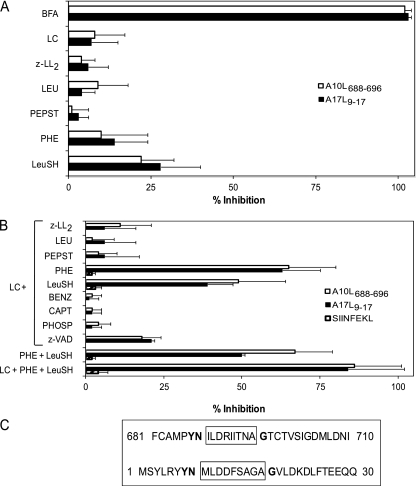
First, to demonstrate that these HLA-A2-restricted epitopes required endogenous processing, their presentation was analyzed in the presence of BFA. This drug blocks class I export beyond the cis-Golgi compartment (39), thus preventing the surface expression of newly assembled HLA class I-peptide complexes from an endogenous origin (Table 1 summarizes the specificity of all inhibitors used). The complete inhibition of specific lysis in both specific CTL lines caused by the addition of BFA during vaccinia infection (Fig. 8A) demonstrated that the two relevant epitopes were indeed generated from proteins endogenously processed in infected cells.
Fig 8.
Lactacystin, a Streptomyces metabolite (19, 49), was used to study the involvement of the proteasome in the presentation of these epitopes. This drug had no effect on the specific recognition by A10L688-696- or A17L9-17-specific CTLs of target cells infected with VACV (Fig. 8A). Although the proteasome may be involved in the antigen processing of these epitopes, these data suggest that the lactacystin-inhibitable proteasome activity is not absolutely required.
Because several endogenous TAP-independent HLA-2 class I ligands are derived from cleavage of the signal sequences generated by the signal peptidase (SPase) complex (36, 70) and because no specific inhibitor of this enzymatic activity was available, the involvement of the SPase complex in A17L9-17 ligand production could not be studied directly. SPase-processed peptides need further cleavage by signal peptide peptidase (SPPase) (42, 68, 69). Therefore, the involvement of SPPase in antigen presentation was tested by treating target cells with the SPPase-specific inhibitor (z-LL)2 ketone (68, 69). In similarity to lactacystin results, the inhibition of specific presentation was not detected with this drug (Fig. 8A).
To identify proteases distinct from the proteasomes that could contribute to antigen processing of both HLA-A2 epitopes, experiments with several specific protease inhibitors were performed. Leupeptin (LEU) (65), pepstatin (PEPST) (34, 65) and 1,10-phenanthroline (PHE) (34, 64) were initially tested because they specifically inhibit different protease families (Table 1), thereby covering a wide range of proteases. Figure 8A shows that these three inhibitors had no effect on the specific recognition of target cells infected with VACV. In addition, because the activity of ERAAP, an enzyme involved in antigen processing (59, 72), is not fully blocked by PHE at the concentration used in this study, leucinethiol (LeuSH) (Table 1) (61) was also included. Like the other protease inhibitors, LeuSH did not inhibit the recognition of infected cells (Fig. 8A), indicating that ERAAP cannot be involved in the generation of these TAP-independent epitopes.
In summary, the inhibitors used did not block the presentation of the two viral epitopes tested. The two most likely explanations for these results are as follows. First, these epitopes could have been processed by a protease(s) that was not blocked by the collection of inhibitors used in Fig. 8A. This explanation is not likely, because the inhibitors were chosen to cover a wide range of protease classes. Alternatively, these epitopes could have been independently processed in parallel by different proteases, meaning that the different antigen-processing pathways would need to be inhibited at the same time to produce an effect. To test this hypothesis, the effects of combinations of several inhibitors on antigen presentation in vaccinia virus-infected cells were tested.
Because most class I epitopes are generated by proteasome activity, the presentation of these epitopes was analyzed in the presence of lactacystin, together with each other inhibitor in turn. Figure 8B shows that the combination of lactacystin with (z-LL)2 ketone, LEU, or PEPST did not block presentation in infected cells. In contrast, a partial blocking of presentation was observed in target cells treated with lactacystin and PHE (Fig. 8B). These results demonstrate that the A10L688-696 and A17L9-17 peptides were processed by both metalloproteases and proteasomes in vivo. Additionally, the antigen presentation of both epitopes was partially inhibited by the combination of lactacystin and LeuSH, indicating that ERAAP or other metalloaminopeptidases were involved in the generation of these viral epitopes. Metallopeptidases can be divided into aminopeptidases, endopeptidases, carboxypeptidases, and carboxydipeptidases, among other peptidase categories, based on their cleavage mechanisms (reviewed in reference 55). Some of these groups can be distinguished by the use of different specific inhibitors (summarized in Table 1). To more precisely identify the metallopeptidase group involved in both A10L688-696 and A17L9-17 antigen processing, target cells were infected with VACV and treated with a mixture of lactacystin and different specific inhibitors (Table 1). The caspase-1-specific inhibitor z-VAD.fmk was also included in view of the sensitivity of this cysteine protease to PHE. Remarkably, none of the different combinations of inhibitors tested prevented antigen presentation to specific CTLs (Fig. 8B). Because phosphoramidon is able to inhibit bacterial endopeptidases but does not block all metalloendopeptidases of mammalian origin, the most likely explanation for our results is that mammalian metalloendopeptidases that are not blocked by phosphoramidon are involved in the antigen processing of these viral epitopes. Over 100 different well-characterized metalloendoproteases of higher vertebrates are resistant to this drug (7); therefore, positive identification of the peptidase involved the processing of TAP-independent vaccinia epitopes awaits further characterization.
To demonstrate that two different types of metalloproteases, aminopeptidases and endopeptidases, were independently involved in the generation of the A10L688-696 and A17L9-17 epitopes, their presentation was analyzed in the presence of a mixture of PHE and LeuSH. The incomplete blocking detected in the presentation indicated that aminometalloproteases and metalloendopeptidases are independently needed to process both the A10L688-696 and A17L9-17 epitopes (Fig. 8B). Finally, the recognition of infected cells by specific CTL was abrogated in the presence of three inhibitors: lactacystin, PHE, and LeuSH (Fig. 8B). To exclude the possibility that the inhibitory effect of lactacystin, PHE, or LeuSH was due to toxic effects on target cells or on recombinant vaccinia virus (rVV) replication rather than to a specific blocking of the respective proteases, experiments similar to those shown in Fig. 8B were performed in parallel using the same target cells and VACV-OVA257-264, which codes for the miniprotein MSIINFEKL. Specific recognition by SIINFEKL-specific CTLs of target cells infected with the VACV-OVA257-264 virus (64% ± 11% of CD8+ cells secreted IFN-γ) was detected. In contrast, VACV-OVA257-264-infected target cells incubated with all combinations of these three inhibitors were efficiently recognized by SIINFEKL-specific CTL and no inhibition was detected (hatched bars in Fig. 8B). These data indicate that inhibition of the A10L688-696 and A17L9-17 epitopes by addition of lactacystin, PHE, and LeuSH drugs is formally due to specific blockage of the respective proteases and not to blocking of rVV replication or other toxic effects.
In summary, the proteasomes, aminometalloproteases, and metalloendopeptidases are all independently involved in the antigen processing of both A10L688-696 and A17L9-17 epitopes. The existence of identical neighboring residues around both the A10L688-696 and A17L9-17 sequences (Fig. 8C) could explain the similarity of the antigen-processing pathways identified in these two different HLA-restricted epitopes.
DISCUSSION
The results reported here show an exceptional diversity of TAP-independent ligands, with 11 ligands simultaneously processed and presented as part of 13 different HLA-peptide complexes in VACV-infected cells. All three of the identified HLA-A2 ligands generated long-term CTL memory responses to vaccinia virus in a transgenic mouse model. Proteasomal and nonproteasomal pathways were involved in the processing and presentation of two vaccinia virus-encoded HLA-A2-restricted antigens.
Identification of viral HLA ligands by mass spectrometry analysis contributes to a better understanding of the cellular antiviral immune response. However, the mass spectrometry strategies designed to identify such ligands have not become routine because of the difficulty in selecting and identifying the very limited number of viral sequences among the large number of self-peptides bound to HLA class I molecules. Therefore, the number of studies on viral HLA ligands is still limited. To date, only two studies have identified vaccinia virus ligands by mass spectrometry (26, 46). Here, we identified three to four TAP-independent ligands that were processed and presented by each of the four class I molecules expressed in infected cells. Neither HLA-specific elution nor CTL responses have previously been reported in TAP+ cells for 10 of the ligands identified in our study. However, as all three TAP-independent HLA-A*0201 ligands also generated responses in TAP+ HLA-A*0201-transgenic mice, these epitopes must also be generated in wild-type cells.
We detected HLA ligands from viral gene products expressed in the three gene expression temporality clusters of the viral life cycle (see Table S1 in the supplemental material), namely, the early (A50R, B8R, C11R, and D5R), early/late (K2L), and late (A10L, A17L, and D8L), in agreement with a previous study investigating the CTL response in the same virus strain (53). In similarity to the TAP+ response, the TAP-independent response also sampled proteins from the entire viral life cycle of the VACV WR strain. Other authors reported the absence of presentation of peptides derived from late viral Ags to specific T cells by infected mouse target cells (29, 46), but a different VACV strain was used in their studies. The sequences we identified were derived from eight different vaccinia virus proteins (see Table S1 in the supplemental material). Five of the proteins present signal sequence or transmembrane domains in their respective amino acid sequences and are therefore accessible to HLA-containing compartments where they could be processed by resident proteases. In contrast, for three vaccinia virus proteins, A10L, A50R, and D5R, no obvious TAP-independent antigen presentation could be predicted. However, vaccinia virus DNA replication is associated with the cytoplasmic side of the rough ER, and when the replication proceeds, ER membranes are recruited (reviewed in reference 60). Late in infection, the ER envelope is disassembled, and these membranes are recycled to the ER (reviewed in reference 60). During this complex process, which includes deep membrane reorganization, some molecules of A10L, A50R, and D5R proteins could gain access to a TAP-independent antigen-processing pathway.
In most cases, the identified natural MHC class I ligands had the canonical anchor motifs, and their respective antigenicity and MHC class I binding affinity data were correlated. This suggests that only high-affinity peptides are recognized by CTLs and that epitopes of low affinity may be immunologically irrelevant. We have demonstrated that cytotoxic responses are targeted against both high- and low-affinity HLA-A*0201 ligands of VACV; therefore, the TCRs of individual CTLs specific for low-affinity epitopes must display a compensatory high affinity. In addition, it has been reported that low-affinity self-peptides in autoimmunity (reviewed in reference 16) or tumor peptides (4) generate specific responses. These findings suggest that the range of peptides that can generate CTLs is broader than was formerly thought and that the role of low-affinity epitopes in antiviral responses must be evaluated in future studies.
Different MHC class I alleles have different TAP dependencies. The widespread HLA-A2 allele is considered to be the least TAP dependent (63). Alleles such as HLA-B7 and HLA-B8 can also bind ligands that are dependent on mechanisms other than TAP transport, while other MHC class I molecules, including HLA-A3, HLA-A24, and HLA-B27, have been described as mainly TAP dependent (2). In the present report, several TAP-independent ligands were identified for alleles with different TAP requirements. Thus, the overall expression of MHC class I molecules with endogenous bound peptides is not indicative of specific TAP-independent cellular responses, which must be studied individually for each specific virus.
Although TAP-independent viral epitopes are known (reviewed in references 13, 27, and 36), only one epitope has been identified as the target of a specific antiviral CTL response in most of the cases studied. No systematic studies of TAP-independent pathways with a single virus and different HLA molecules have been reported. The exception is EBV, for which CTLs from different donors recognize several viral epitopes from two different viral proteins restricted by several HLA class I molecules in TAP-negative cell backgrounds (reviewed in reference 38). Here, we report a second case with 11 ligands from eight different viral proteins presented by four different HLA class I molecules in the same TAP-deficient vaccinia virus-infected cells. The simultaneous presentation of the elements of this broad complexity of viral peptide-MHC complexes can help to explain why TAP-deficient patients do not seem particularly susceptible to viral infections and may appear asymptomatic for long periods of their lives. In addition, the existence of multiple TAP-independent ligands in two very different viruses, a gammaherpesvirus and an orthopoxvirus, suggests that these pathways could represent an extended but perhaps secondary mechanism that forms part of the multiple layers of defense against viral infection and that the significance of these alternative processing pathways in vivo needs to be further studied.
The involvement of the following proteases in the processing of endogenously synthesized antigens in a manner independent of that seen with the classical proteasome pathway has been reported previously (reviewed in reference 12). In the present study, two HLA-A2-restricted epitopes were processed in parallel by aminometallopeptidases as well as by metalloendoproteases and a classic proteasomal pathway.
In addition to the A17L9-17 epitope previously described in a study performed using TAP-sufficient cells (5), two distinct peptide epitopes recognized by human HLA-A2-restricted CD8+ T cells and eight HLA-B27, HLA-B51, and/or HLA-Cw1 ligands were newly detected in this study. Nine of the viral ligands identified are conserved in all three vaccinia virus strains, seven orthopoxviruses (including cowpox virus), and two variola strains. In contrast, only 60% of the previously described vaccinia virus epitopes in the variola virus proteome are identical (46, 50, 53). Therefore, TAP-independent vaccinia ligands are more highly conserved between immunogenic and pathological poxviruses than TAP-dependent epitopes and could be more specific targets for immunization. Cowpox virus, the first component of early vaccines, specifically inhibits TAP-dependent peptide translocation (1); therefore, TAP-independent epitopes conserved between variola virus and cowpox virus are probably responsible for the initial cross-protection. Additionally, truncated gene fragments similar to those encoding the TAP-blocking CPXV12 protein are widely found in poxvirus genomes (Poxvirus Bioinformatics Resources Center; http://www.poxvirus.org) and frequently represent loss-of-function phenotypes, but this “genetic debris” may acquire entirely new, unanticipated functions. Therefore, the conservation of epitopes between different vaccine strains and pathogens is relevant for vaccine design and suggests that ligands from TAP-independent pathways could be interesting candidates for inclusion in immunization protocols. This idea is also important with respect to bioterrorism, because some countries did not participate in the WHO smallpox eradication program and, at this time, no information about the elimination of their samples of the pandemic virus has been provided.
Computational methods designed for predicting MHC-peptide binding are increasingly being used to identify epitopes for vaccine design and to monitor T cell responses (reviewed in references 35 and 44). The prediction of MHC-peptide binding is far from perfect, as our results indicate. The prediction by bioinformatics tools of HLA class I molecules presenting the nine vaccinia virus ligands identified in the current study showed several different mistakes, including incomplete HLA identifications (K2L16-30 and C11R101-110 ligands), ambiguous HLA restrictions (D5R148-157 and A17L9-25 ligands), and wrong assignations (A10L614-623 and D8L112-119 ligands) (Table 5). In summary, more than half of these ligands showed inconsistencies between the computational predictions and experimentally detected HLA restriction. These results reveal the limitations of predictive methods for identifying natural MHC class I ligands and T cell epitopes. The current analytical algorithms may not be sufficiently accurate and should be used with caution.
Table 5.
Comparison between predicted and experimentally detected binding of vaccinia virus ligands to HLA molecules
| Ligand | Sequence | HLA |
|
|---|---|---|---|
| Predicteda | Experimentally detected | ||
| A17L9-17 | MLDDFSAGA | A2 | A2 |
| A10L614-623 | SPEGEETII | B51 | A2 |
| A10L688-696 | ILDRIITNA | A2 | A2 |
| A10L867-876 | SRGYFEHMKK | B27 | B27 |
| B8R53-59 | WQTMYTN | B27 | B27 |
| K2L16-30 | YRLQGFTNAGIVAYK | B27 | B27/Cw1 |
| D5R148-157 | IAMKRTLLEL | B51/Cw1 | B51 |
| A50R294-301 | LPFGSLGI | B51 | B51 |
| C11R101-110 | IPSPGIMLV | B51 | B51/Cw1 |
| A17L9-25 | MLDDFSAGAGVLDKDL | A2/Cw1 | Cw1 |
| D8L112-119 | DGLIIISI | B51 | Cw1 |
Data are from the SYFPEITHI (http://www.syfpeithi.de), BIMAS (http://www-bimas.cit.nih.gov), and IEDB (http://www.immuneepitope.org) databases.
Collectively, the results in the current report highlight the importance of analyzing natural peptides that result from the endogenous processing of viral proteins and demonstrate the complexity and plasticity of MHC-peptide interactions. This analysis is of fundamental importance for gaining a detailed understanding of MHC class I-restricted immunity and for future vaccine design.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. A. López de Castro (Centro de Biología Molecular Severo Ochoa, Madrid, Spain) for the cell lines. Recombinant human interleukin-2 was generously provided by Hoffmann-LaRoche.
This work was supported by grants to D.L. from the Programa Ramón y Cajal, Ministerio de Ciencia e Innovación and the FIPSE Foundation and to A.A. from the ISF 9916/05.
The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicting financial interests.
Footnotes
Published ahead of print 26 October 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Alzhanova D, et al. 2009. Cowpox virus inhibits the transporter associated with antigen processing to evade T cell recognition. Cell Host. Microbe 6:433–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson KS, Alexander J, Wei M, Cresswell P. 1993. Intracellular transport of class I MHC molecules in antigen processing mutant cell lines. J. Immunol. 151:3407–3419 [PubMed] [Google Scholar]
- 3. Angel Rico MA, et al. 2009. Human respiratory syncytial virus infects and induces activation markers in mouse B lymphocytes. Immunol. Cell Biol. 87:344–350 [DOI] [PubMed] [Google Scholar]
- 4. Apostolopoulos V, Karanikas V, Haurum JS, McKenzie IF. 1997. Induction of HLA-A2-restricted CTLs to the mucin 1 human breast cancer antigen. J. Immunol. 159:5211–5218 [PubMed] [Google Scholar]
- 5. Assarsson E, et al. 2007. A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J. Immunol. 178:7890–7901 [DOI] [PubMed] [Google Scholar]
- 6. Barnstable CJ, et al. 1978. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell 14:9–20 [DOI] [PubMed] [Google Scholar]
- 7. Barrett AJ. 2004. Metallopeptidases, p. 231–1047 InBarrett A. J., Rawlings N. D., Woessner J. F. (ed.), Handbook of proteolytic enzymes. Academic Press, London, United Kingdom [Google Scholar]
- 8. Bertoni R, et al. 1997. Human histocompatibility leukocyte antigen-binding supermotifs predict broadly cross-reactive cytotoxic T lymphocyte responses in patients with acute hepatitis. J. Clin. Invest. 100:503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cerundolo V, de la Salle H. 2006. Description of HLA class I- and CD8-deficient patients: insights into the function of cytotoxic T lymphocytes and NK cells in host defense. Semin. Immunol. 18:330–336 [DOI] [PubMed] [Google Scholar]
- 10. Chen W, Anton LC, Bennink JR, Yewdell JW. 2000. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 12:83–93 [DOI] [PubMed] [Google Scholar]
- 11. Cheuk E, et al. 2002. Human MHC class I transgenic mice deficient for H2 class I expression facilitate identification and characterization of new HLA class I-restricted viral T cell epitopes. J. Immunol. 169:5571–5580 [DOI] [PubMed] [Google Scholar]
- 12. Del Val M, Iborra S, Ramos M, Lázaro S. 2011. Generation of MHC class I ligands in the secretory and vesicular pathways. Cell. Mol. Life Sci. 68:1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del-Val M, López D. 2002. Multiple proteases process viral antigens for presentation by MHC class I molecules to CD8+ T lymphocytes. Mol. Immunol. 39:235–247 [DOI] [PubMed] [Google Scholar]
- 14. Ellis SA, Taylor C, McMichael A. 1982. Recognition of HLA-B27 and related antigen by a monoclonal antibody. Hum. Immunol. 5:49–59 [DOI] [PubMed] [Google Scholar]
- 15. Eng JK, McCormack Al, Yates JR., III 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Amer. Soc. Mass. Spectrom. 5:976–989 [DOI] [PubMed] [Google Scholar]
- 16. Fairchild PJ, Wraith DC. 1996. Lowering the tone: mechanisms of immunodominance among epitopes with low affinity for MHC. Immunol. Today 17:80–85 [DOI] [PubMed] [Google Scholar]
- 17. Fan QR, et al. 1996. Direct binding of a soluble natural killer cell inhibitory receptor to a soluble human leukocyte antigen-CW4 class I major histocompatibility complex molecule. Proc. Natl. Acad. Sci. U. S. A. 93:7178–7183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi I. 2004. Smallpox and its eradication. WHO, Geneva, Switzerland [Google Scholar]
- 19. Fenteany G, et al. 1995. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268:726–731 [DOI] [PubMed] [Google Scholar]
- 20. Firat H, et al. 1999. H-2 class I knockout, HLA-A2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur. J. Immunol. 29:3112–3121 [DOI] [PubMed] [Google Scholar]
- 21. Freed ER, Duma RJ, Escobar MR. 1972. Vaccinia necrosum and its relationship to impaired immunologic responsiveness. Am. J. Med. 52:411–420 [DOI] [PubMed] [Google Scholar]
- 22. Howell S, Caswell AM, Kenny AJ, Turner AJ. 1993. Membrane peptidases on human osteoblast-like cells in culture: hydrolysis of calcitonin and hormonal regulation of endopeptidase-24.11. Biochem. J. 290:159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunt DF, et al. 1992. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science 255:1261–1263 [DOI] [PubMed] [Google Scholar]
- 24. Infantes S, et al. 2010. Multiple, non-conserved, internal viral ligands naturally presented by HLA-B27 in human respiratory syncytial virus-infected cells. Mol. Cell Proteomics 9:1533–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishihama Y, Rappsilber J, Andersen JS, Mann M. 2002. Microcolumns with self-assembled particle frits for proteomics. J. Chromatogr. A 979:233–239 [DOI] [PubMed] [Google Scholar]
- 26. Johnson KL, Ovsyannikova IG, Mason CJ, Bergen HR, III, Poland GA. 2009. Discovery of naturally processed and HLA-presented class I peptides from vaccinia virus infection using mass spectrometry for vaccine development. Vaccine 28:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnstone C, Del Val M. 2007. Traffic of proteins and peptides across membranes for immunosurveillance by CD8+ T lymphocytes: a topological challenge. Traffic 8:1486–1494 [DOI] [PubMed] [Google Scholar]
- 28. Kalinke U, Arnold B, Hammerling GJ. 1990. Strong xenogeneic HLA response in transgenic mice after introducing an alpha 3 domain into HLA B27. Nature 348:642–644 [DOI] [PubMed] [Google Scholar]
- 29. Kastenmuller W, et al. 2007. Cross-competition of CD8+ T cells shapes the immunodominance hierarchy during boost vaccination. J. Exp. Med. 204:2187–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kennedy R, Poland GA. 2007. T-Cell epitope discovery for variola and vaccinia viruses. Rev. Med. Virol. 17:93–113 [DOI] [PubMed] [Google Scholar]
- 31. Kennedy RB, Ovsyannikova I, Poland GA. 2009. Smallpox vaccines for biodefense. Vaccine 27(Suppl 4):D73–D79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kennedy RB, Ovsyannikova IG, Jacobson RM, Poland GA. 2009. The immunology of smallpox vaccines. Curr. Opin. Immunol. 21:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kondo E, et al. 2004. Identification of novel CTL epitopes of CMV-pp65 presented by a variety of HLA alleles. Blood 103:630–638 [DOI] [PubMed] [Google Scholar]
- 34. Kozlowski S, et al. 1993. Multiple pathways are involved in the extracellular processing of MHC class-I-restricted peptides. J. Immunol. 151:4033–4044 [PubMed] [Google Scholar]
- 35. Lafuente EM, Reche PA. 2009. Prediction of MHC-peptide binding: a systematic and comprehensive overview. Curr. Pharm. Des. 15:3209–3220 [DOI] [PubMed] [Google Scholar]
- 36. Larsen MV, Nielsen M, Weinzierl A, Lund O. 2006. TAP-independent MHC class I presentation. Curr. Immunol. Rev. 2:233–245 [Google Scholar]
- 37. Lautscham G, et al. 2001. Processing of a multiple membrane spanning Epstein-Barr virus protein for CD8+ T cell recognition reveals a proteasome-dependent, transporter associated with antigen processing-independent pathway. J. Exp. Med. 194:1053–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lautscham G, Rickinson A, Blake N. 2003. TAP-independent antigen presentation on MHC class I molecules: lessons from Epstein-Barr virus. Microbes Infect. 5:291–299 [DOI] [PubMed] [Google Scholar]
- 39. Lippincott-Schwartz J, et al. 1990. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell 60:821–836 [DOI] [PubMed] [Google Scholar]
- 40. López D, Gil-Torregrosa BC, Bergmann C, Del Val M. 2000. Sequential cleavage by metallopeptidases and proteasomes is involved in processing HIV-1 ENV epitope for endogenous MHC class I antigen presentation. J. Immunol. 164:5070–5077 [DOI] [PubMed] [Google Scholar]
- 41. López D, Samino Y, Koszinowski UH, Del Val M. 2001. HIV envelope protein inhibits MHC class I presentation of a cytomegalovirus protective epitope. J. Immunol. 167:4238–4244 [DOI] [PubMed] [Google Scholar]
- 42. Lorente E, Garcia R, Lopez D. 2011. Allele-dependent processing pathways generate the endogenous human leukocyte antigen (HLA) class I peptide repertoire in TAP-deficient cells. J. Biol. Chem. 286:38054–38059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lorente E, et al. 2011. TAP-independent human histocompatibility complex-Cw1 antigen processing of an HIV envelope protein conserved peptide. AIDS 25:265–269 [DOI] [PubMed] [Google Scholar]
- 44. Lundegaard C, Lund O, Buus S, Nielsen M. 2010. Major histocompatibility complex class I binding predictions as a tool in epitope discovery. Immunology 130:309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Medina F, et al. 2009. Furin-processed antigens targeted to the secretory route elicit functional TAP1−/− CD8+ T lymphocytes in vivo. J. Immunol. 183:4639–4647 [DOI] [PubMed] [Google Scholar]
- 46. Meyer VS, et al. 2008. Long-term immunity against actual poxviral HLA ligands as identified by differential stable isotope labeling. J. Immunol. 181:6371–6383 [DOI] [PubMed] [Google Scholar]
- 47. Moss B. 1991. Vaccinia virus: a tool for research and vaccine development. Science 252:1662–1667 [DOI] [PubMed] [Google Scholar]
- 48. Nuchtern JG, Bonifacino JS, Biddison WE, Klausner RD. 1989. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature 339:223–226 [DOI] [PubMed] [Google Scholar]
- 49. Omura S, et al. 1991. Lactacystin, a novel microbial metabolite, induces neuritogenesis of neuroblastoma cells. J. Antibiot. (Tokyo) 44:113–116 [DOI] [PubMed] [Google Scholar]
- 50. Oseroff C, et al. 2005. HLA class I-restricted responses to vaccinia recognize a broad array of proteins mainly involved in virulence and viral gene regulation. Proc. Natl. Acad. Sci. U. S. A. 102:13980–13985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parham P, Bodmer WF. 1978. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature 276:397–399 [DOI] [PubMed] [Google Scholar]
- 52. Pascolo S, et al. 1997. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J. Exp. Med. 185:2043–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pasquetto V, et al. 2005. HLA-A*0201, HLA-A*1101, and HLA-B*0702 transgenic mice recognize numerous poxvirus determinants from a wide variety of viral gene products. J. Immunol. 175:5504–5515 [DOI] [PubMed] [Google Scholar]
- 54. Rammensee HG, Bachmann J, Emmerich NPN, Bachor OA, Stevanovic S. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213–219 [DOI] [PubMed] [Google Scholar]
- 55. Rawlings ND, Barrett AJ. 1995. Evolutionary families of metallopeptidases. Methods Enzymol. 248:183–228 [DOI] [PubMed] [Google Scholar]
- 56. Redfield RR, et al. 1987. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N. Engl. J. Med. 316:673–676 [DOI] [PubMed] [Google Scholar]
- 57. Salter RD, Cresswell P. 1986. Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. EMBO J. 5:943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Samino Y, López D, Guil S, de León P, Del Val M. 2004. An endogenous HIV envelope-derived peptide without the terminal NH3+ group is physiologically presented by major histocompatibility class I molecules. J. Biol. Chem. 279:1151–1160 [DOI] [PubMed] [Google Scholar]
- 59. Saric T, et al. 2002. An IFN-γ-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat. Immunol. 3:1169–1176 [DOI] [PubMed] [Google Scholar]
- 60. Schramm B, Locker JK. 2005. Cytoplasmic organization of POXvirus DNA replication. Traffic 6:839–846 [DOI] [PubMed] [Google Scholar]
- 61. Serwold T, González F, Kim J, Jacob R, Shastri N. 2002. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature 419:480–483 [DOI] [PubMed] [Google Scholar]
- 62. Slee EA, et al. 1996. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem. J. 315:21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Spies T, DeMars R. 1991. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature 351:323–324 [DOI] [PubMed] [Google Scholar]
- 64. Thornberry NA. 1994. Interleukin-1 beta converting enzyme. Methods Enzymol. 244:615–631 [DOI] [PubMed] [Google Scholar]
- 65. Umezawa H. 1976. Structures and activities of protease inhibitors of microbial origin. Methods Enzymol. 45:678–695 [DOI] [PubMed] [Google Scholar]
- 66. Villadangos JA, Galocha B, Lopez de Castro JA. 1994. Unusual topology of an HLA-B27 allospecific T cell epitope lacking peptide specificity. J. Immunol. 152:2317–2323 [PubMed] [Google Scholar]
- 67. Wang M, et al. 2007. CTL epitopes for influenza A including the H5N1 bird flu; genome-, pathogen-, and HLA-wide screening. Vaccine 25:2823–2831 [DOI] [PubMed] [Google Scholar]
- 68. Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B. 2002. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science 296:2215–2218 [DOI] [PubMed] [Google Scholar]
- 69. Weihofen A, Lemberg MK, Ploegh HL, Bogyo M, Martoglio B. 2000. Release of signal peptide fragments into the cytosol requires cleavage in the transmembrane region by a protease activity that is specifically blocked by a novel cysteine protease inhibitor. J. Biol. Chem. 275:30951–30956 [DOI] [PubMed] [Google Scholar]
- 70. Weinzierl AO, et al. 2008. Features of TAP-independent MHC class I ligands revealed by quantitative mass spectrometry. Eur. J. Immunol. 38:1503–1510 [DOI] [PubMed] [Google Scholar]
- 71. Yewdell JW, Bennink JR. 1989. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science 244:1072–1075 [DOI] [PubMed] [Google Scholar]
- 72. York IA, et al. 2002. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat. Immunol. 3:1177–1184 [DOI] [PubMed] [Google Scholar]
- 73. York IA, Goldberg AL, Mo XY, Rock KL. 1999. Proteolysis and class I major histocompatibility complex antigen presentation. Immunol. Rev. 172:49–66 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.