Abstract
Here we demonstrate that a combination of tenofovir, emtricitabine, and raltegravir effectively suppresses peripheral and systemic HIV replication in humanized BLT mice. We also demonstrate that antiretroviral therapy (ART)-treated humanized BLT mice harbor latently infected resting human CD4+ T cells that can be induced ex vivo to produce HIV. We observed that the levels of infected resting human CD4+ T cells present in BLT mice are within the range of those observed circulating in patients undergoing suppressive ART. These results demonstrate the potential of humanized BLT mice as an attractive model for testing the in vivo efficacy of novel HIV eradication strategies.
TEXT
Antiretroviral therapy (ART) is critical for improved quality of life and long-term survival in most human immunodeficiency virus (HIV)-infected patients. Unfortunately, ART is a lifelong commitment, because it is unable to eradicate HIV reservoirs and ART interruption is accompanied by viremia rebound (22, 23). Thus, replication-competent HIV persists in infected individuals despite ART-induced reduction in plasma viral RNA. This persistent HIV infection in patients is thought to be predominantly due to the presence of latently infected resting CD4+ T cells that survive despite long-term ART (8, 16, 29, 32, 38). Most analyses of latent but replication-competent HIV-1 in humans have been limited to the recovery of virus from resting CD4+ T cells in peripheral blood (1, 5, 14, 16, 20, 26, 27, 33–35, 37, 38). A few studies have analyzed virus recovered from lymph node samples (5, 26, 34, 37). However, it is clear that other anatomical sites are involved in the persistence of HIV during ART (7, 39). Successful eradication of the HIV that persists during ART hinges on an improved understanding of the nature of the latent viral reservoir, and validated animal models that recapitulate key aspects of the human condition are critical to this effort (4, 17, 30). Peripheral blood and lymphoid and nonlymphoid tissues of nonhuman primates receiving ART have been evaluated for the presence of latently infected resting CD4+ T cells (9, 13, 25, 31). These important studies involving simian immunodeficiency virus (SIV) have provided significant insight into the location of productively infected cells in ART treated animals. Here we describe the implementation of a novel in vivo system to study HIV infection of resting human T cells, the humanized bone marrow/liver/thymus (BLT) mouse model. Specifically, we first demonstrated the ability of anti-HIV drugs to efficiently suppress HIV replication and then determined the presence of HIV infected resting CD4+ T cells in BLT mice. Our results demonstrate that BLT mice represent an outstanding in vivo model of HIV latency that can be utilized to evaluate HIV eradication interventions.
Humanized BLT mice are individually bioengineered by bone marrow transplant of CD34+ hematopoietic progenitor cells into immunodeficient mice previously implanted with autologous liver and thymus tissue (3, 10–12, 18, 19, 24, 28, 36). Human hematopoietic cells are present in all tissues of BLT mice, including peripheral blood, primary lymphoid tissues, secondary lymphoid tissues, and mucosal tissues. BLT mice were prepared for these experiments essentially as previously described (3, 10–12, 18, 19, 24, 28, 36). Briefly, preconditioned NOD/SCID-gamma chain null (NSG) mice (The Jackson Laboratories) implanted with thymus and liver tissue (Advanced Bioscience Resources) were transplanted with 2 × 105 to 3 × 105 autologous human fetal liver CD34+ cells and monitored for human engraftment. Mice were maintained at the Animal Resources Center of UT Southwestern Medical Center at Dallas or the Division of Laboratory Animal Medicine at the University of North Carolina at Chapel Hill in accordance with protocols approved by each institution's Institutional Animal Care and Use Committee. Prior to HIV infection, all the BLT mice (n = 20) used for these experiments were characterized for human reconstitution. The peripheral blood of these mice contained an average of 51% (±17% [standard deviation {SD}]) human CD45+ cells of which an average of 83% (±7% [SD]) were human CD4+ T cells.
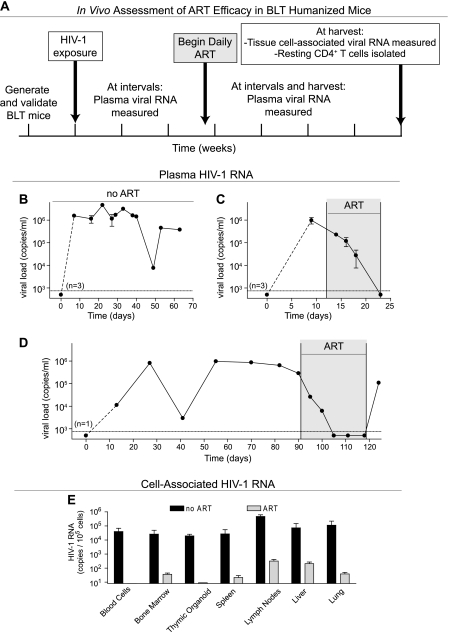
The general approach used to conduct the experiments described below is outlined in Fig. 1A. Humanized BLT mice are susceptible to vaginal, rectal, and intravenous HIV infection (10–12, 36), which results in systemic viral dissemination and robust viral replication in peripheral blood (3, 10–12, 36) (Fig. 1B). The course of HIV infection in BLT mice closely mimics that observed in humans, and currently prescribed therapeutic antiretrovirals exhibit efficacy in humanized BLT mice (10–12, 36). We infected BLT mice with CCR5-tropic HIV (JR-CSF or SF162) as previously described (10–12, 36) and monitored their plasma viral loads as previously described (12), with a limit of detection of 750 copies per ml of mouse plasma. The low sensitivity of our standard assay compared to that of the standard clinical assay (50 copies per ml) is due to the lower volume of plasma that can be obtained on a regular basis from mice. Infected BLT mice received ART consisting of daily intraperitoneal injections of emtricitabine (FTC; 140 to 200 mg/kg body weight), tenofovir disoproxil fumarate (TDF; 146 to 208 mg/kg), and raltegravir (RAL; 56 to 80 mg/kg). ART was very efficient at reducing plasma viral loads to below the limit of detection in as few as 11 days (Fig. 1C). We also noted that, similar to what occurs in patients taking ART (6, 15, 21), during structured treatment interruption, there is a rapid rebound of plasma viremia to levels comparable to those detected prior to therapy (Fig. 1D).
Fig 1.
Human therapeutic antiretroviral drugs suppress HIV replication in BLT mice. (A) The in vivo assessment of ART efficacy in BLT mice is depicted. HIV-positive BLT mice were treated daily with ART consisting of FTC, TDF, and raltegravir. Viral RNA was measured in plasma throughout the experiment and in tissues at harvest. (B through D) Day 0 corresponds to the time of virus exposure. Days during ART are shaded gray. (B) Plasma viral loads from three infected BLT mice were followed in the absence of ART to demonstrate sustained viremia without treatment. The three mice in this experiment were followed for 33, 38, and 68 days postexposure. (C) Plasma viral loads from three infected BLT mice were followed pre- and post-ART initiation to demonstrate the ability of ART to rapidly suppress viremia below detection. (D) Plasma viral load from an infected BLT mouse rapidly dropped below detection and remained undetectable for the duration of ART. As seen in humans, viremia rebounded to a level similar to that observed pretreatment following a structured treatment interruption. (E) Cell-associated HIV-1 RNA levels at harvest were determined in multiple tissues of an infected BLT mouse that received ART for 58 days (followed for a total of 77 days postexposure) as well as in a pair of untreated, infected BLT mice (followed for a total of 69 and 70 days postexposure), and triplicate RNA determinations for each are presented with standard deviations. RNA was isolated from the total cell population, and the data are presented as RNA copies per 1 × 105 mononuclear cells on a logarithmic scale to facilitate visualization of the low levels of HIV RNA present in the tissues of the treated animal.
In addition to the evaluation of plasma viremia during ART, we also assessed the effect of ART on systemic virus production in BLT mice. Tissues harvested from one infected BLT mouse receiving ART and two untreated infected controls were examined for the presence of viral RNA (quantitative real-time PCR [qRT-PCR]). Since RNA was isolated from total mononuclear cells, the viral RNA detected was being produced by all the productively infected cells in each tissue and not latently infected cells (2). ART dramatically reduced viral RNA levels in all the tissues examined compared to the case in the control animals (Fig. 1E), and the greatest reductions were observed in peripheral blood and thymocytes. The least pronounced reductions in viral RNA levels were noted in lymph nodes and liver (Fig. 1E). It should be noted that the viral RNA levels in the control mice reflect both the relative levels of humanization and the CCR5 expression on T cells in each tissue (10, 36). Together, these data establish that ART consisting of a well-characterized combination of FDA-approved therapeutic antiretroviral drugs is capable of suppressing HIV replication in BLT mice and demonstrate that, like in humans and nonhuman primates, the infection persists for the duration of the treatment.
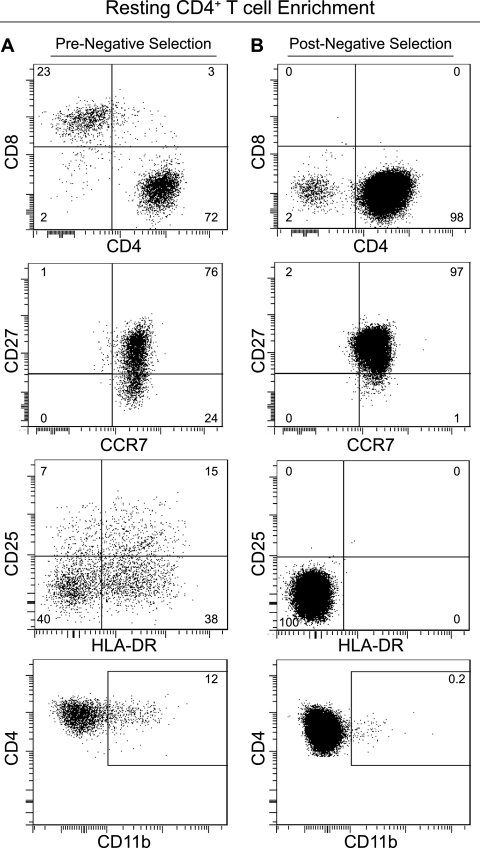
HIV persistence during ART can be due to latently infected resting CD4+ T cells in either preintegration or postintegration phases of infection (2). As preintegration latency does not persist during long-term ART, we sought to identify postintegration latency in infected resting CD4+ T cells in BLT mice. We first demonstrated the presence of resting CD4+ T cells in naive BLT mice. For this analysis, we isolated mononuclear cells from peripheral blood, bone marrow, spleen, lymph nodes, liver, and lung according to published protocols (10, 11, 24, 36). All mononuclear cells from individual mice were combined and resting human CD4+ T cells were negatively selected by magnetic separation (Stemcell Technologies, Vancouver, Canada) essentially using the same approach used to isolate resting cells from human leukapheresis product (1). Briefly, cells were incubated with antibodies specific for mouse CD45 and TER119 and for human CD8, CD14, CD16, CD19, CD56, glycophorin A, CD41, HLA-DR, CD25 (35 μl/ml) (Stem Cell Technologies), and CD31 and CD105 (0.5 μg/ml each) (eBiosciences). Antibody-bound cells were removed using magnetic selection and the flowthrough fraction containing the purified resting cells was collected. This negative selection approach resulted in a >97% pure resting CD4+ T cell population as defined by expression of CD4, CCR7, and CD27 and lack of expression of CD8, CD25, HLA-DR, and CD11b (Fig. 2).
Fig 2.
Resting human CD4+ T cells are present in BLT mice. (A) Flow cytometry analysis of pooled BLT mouse mononuclear cells prior to negative selection showed the presence of CD4+ and CD8+ T cells. Within the CD4+ T cell population, a heterogeneous population of cells expressing variable levels of CD27, CD25, HLA-DR, and CD11b was observed. (B) Following negative selection, flow cytometry analysis showed a homogeneous population of human CD4+ CCR7+ CD27+ CD8− CD25− HLA-DR− CD11b− resting T cells. Results for one representative BLT mouse of two are shown.
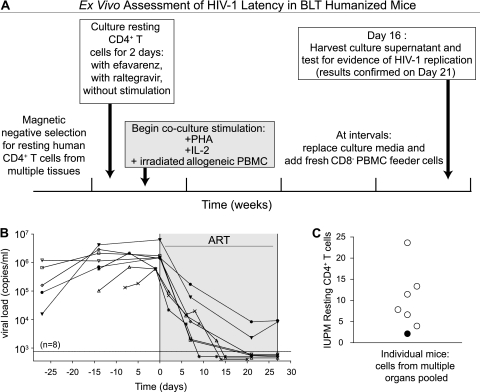
After establishing that resting CD4+ T cells are present in BLT mice and that they can be isolated with the same procedure used to obtain similar cells from human peripheral blood, we determined whether a latent reservoir of HIV-infected cells is present in infected BLT mice on ART. The general approach used to accomplish this objective is described in Fig. 3A. Administration of ART to infected BLT mice resulted in an average 3.2-log reduction (±0.5 [SD]; n = 8) in plasma viral load (Fig. 3B). Mononuclear cells from peripheral blood, lymph nodes, human thymic organoid, spleen, bone marrow, lungs and liver were collected and all cells from each individual mouse were pooled for resting cell isolation. Resting CD4+ T cell enrichment was performed on each pool of cells, as described above (Fig. 2). The average yield of resting cells per BLT mouse was 3.0 × 106 (±1.3 × 106 [standard error of the mean {SEM}]; n = 10 [including 2 control mice that did not receive ART]). To limit the potential contribution of nonintegrated HIV DNA to the outgrowth assay results, the enriched population of resting cells was cultured in the presence of 15 nM efavirenz and 1 μM raltegravir for 2 days prior to any stimulation for viral outgrowth (1, 20). The lack of ongoing virion production in resting cells maintained in efavirenz and raltegravir was confirmed prior to coculture and stimulation of viral outgrowth as previously published (1). The frequency of resting cell infection was determined using a maximum likelihood method, and the results were expressed as infectious units per million (IUPM) resting CD4+ T cells (1). The coculture assay results yielded an average of 9.9 (±2.7 [SEM]) IUPM resting CD4+ T cells per mouse (Fig. 3C). These results demonstrate that HIV infection of humanized mice results in the establishment of a population of resting cells latently infected with HIV that can be readily isolated and induced to express HIV ex vivo, recapitulating key aspects of the human condition. Because of the relatively short frame of time necessary to observe ART efficacy in this model, preclinical evaluation of successful HIV eradication interventions can be rapidly performed prior to more extensive evaluation in nonhuman primates and humans.
Fig 3.
Latent HIV infection occurs in BLT mouse resting human CD4+ T cells. (A) The ex vivo assessment of HIV-1 latency in humanized BLT mice is depicted. Daily ART dosing consisted of FTC, TDF, and raltegravir. PHA, phytohemagglutinin; IL-2, interleukin 2; PBMC, peripheral blood mononuclear cells. (B) Plasma viral loads from eight infected BLT mice were followed pre- and post-ART initiation, and then mice were harvested between days 20 and 27 of ART. Day 0 corresponds to the time at which ART was initiated, and days during ART are shaded gray. (C) At harvest, resting CD4+ T cells were isolated from the pooled cells from individual mice and then placed into coculture according to clinical protocols to determine the frequency of latently infected cells in each animal during ART. Outgrowth assay determinations of IUPM resting CD4+ T cells from six animals with nondetectable viral loads at harvest are plotted as open circles, while one mouse with detectable viral load at harvest is represented with a filled circle. The second mouse with detectable viral loads at harvest is not represented in the graph, due to an unusually low yield of resting CD4+ T cells.
In summary, we have established a novel application of the humanized BLT mouse model for the study of HIV latency in vivo. Specifically, we have demonstrated that a combination of well-characterized human antiretroviral drugs is capable of effectively controlling viral replication. We demonstrated the presence of latently infected resting human CD4+ T cells in ART-treated BLT mice that can be induced ex vivo to produce HIV. In these experiments, we observed that the frequency of infected resting human CD4+ T cells present in tissues from BLT mice is within the range observed circulating in patients undergoing suppressive ART. Overall these results demonstrate that humanized BLT mice are an attractive model for testing the in vivo efficacy of novel HIV eradication strategies.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants AI096113, AI073146, AI071940, AI082608, AI082637 (J.V.G.), AI081613 (S.K.C.), and 5T32AI005284 (J.F.K.), the UNC Center for AIDS Research grant P30 AI50410, a Foundation for AIDS Research (amfAR) Fellowship (107752-44-RFRL) (P.W.D.), the Augustinus Foundation, the Danish AIDS Foundation, Danielsen's Foundation (R.O.), and a Research Fellowship of the Japan Society for the Promotion of Science (T.N.). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
We thank I. Chen for providing the JR-CSF plasmid via the AIDS Research and Reference Reagent Program and former and current lab members and veterinary technicians at UNC Division of Laboratory Animal Medicine for their assistance with aspects of this work.
Footnotes
Published ahead of print 19 October 2011
REFERENCES
- 1. Archin NM, et al. 2008. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS 22:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blankson JN, et al. 2000. Biphasic decay of latently infected CD4+ T cells in acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 182:1636–1642 [DOI] [PubMed] [Google Scholar]
- 3. Brainard DM, et al. 2009. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 83:7305–7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choudhary SK, Margolis DM. 2011. Curing HIV: pharmacologic approaches to target HIV-1 latency. Annu. Rev. Pharmacol. Toxicol. 51:397–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chun TW, et al. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183–188 [DOI] [PubMed] [Google Scholar]
- 6. Chun TW, et al. 2010. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS 24:2803–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chun TW, et al. 2008. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J. Infect. Dis. 197:714–720 [DOI] [PubMed] [Google Scholar]
- 8. Chun TW, et al. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 94:13193–13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deere JD, Schinazi RF, North TW. 2011. Simian immunodeficiency virus macaque models of HIV latency. Curr. Opin. HIV AIDS 6:57–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denton PW, et al. 2008. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 5:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Denton PW, et al. 2010. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS One 5:e8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denton PW, et al. 2011. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J. Virol. 85:7582–7593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dinoso JB, et al. 2009. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J. Virol. 83:9247–9257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dornadula G, et al. 1999. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 282:1627–1632 [DOI] [PubMed] [Google Scholar]
- 15. El-Sadr WM, et al. 2006. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 355:2283–2296 [DOI] [PubMed] [Google Scholar]
- 16. Finzi D, et al. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300 [DOI] [PubMed] [Google Scholar]
- 17. Han Y, Wind-Rotolo M, Yang HC, Siliciano JD, Siliciano RF. 2007. Experimental approaches to the study of HIV-1 latency. Nat. Rev. Microbiol. 5:95–106 [DOI] [PubMed] [Google Scholar]
- 18. Kim SS, et al. 2010. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol. Ther. 18:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. 2006. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108:487–492 [DOI] [PubMed] [Google Scholar]
- 20. Lehrman G, et al. 2005. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet 366:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lori F, Lisziewicz J. 2001. Structured treatment interruptions for the management of HIV infection. JAMA 286:2981–2987 [DOI] [PubMed] [Google Scholar]
- 22. Maldarelli F. 2011. Targeting viral reservoirs: ability of antiretroviral therapy to stop viral replication. Curr. Opin. HIV AIDS 6:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Margolis DM. 2011. Eradication therapies for HIV infection: time to begin again. AIDS Res. Hum. Retroviruses 27:347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melkus MW, et al. 2006. Humanized mice mount specific adaptive and innate immune response to EBV and TSST-1. Nat. Med. 12:1316–1322 [DOI] [PubMed] [Google Scholar]
- 25. North TW, et al. 2010. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol. 84:2913–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pantaleo G, et al. 1991. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc. Natl. Acad. Sci. U. S. A. 88:9838–9842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prins JM, et al. 1999. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS 13:2405–2410 [DOI] [PubMed] [Google Scholar]
- 28. Rajesh D, et al. 2010. Th1 and Th17 immunocompetence in humanized NOD/SCID/IL2rgammanull mice. Hum. Immunol. 71:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richman DD. 2011. Introduction: challenges to finding a cure for HIV infection. Curr. Opin. HIV AIDS 6:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richman DD, et al. 2009. The challenge of finding a cure for HIV infection. Science 323:1304–1307 [DOI] [PubMed] [Google Scholar]
- 31. Shen A, et al. 2003. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J. Virol. 77:4938–4949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen L, Siliciano RF. 2008. Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J. Allergy Clin. Immunol. 122:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siliciano JD, et al. 2007. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J. Infect. Dis. 195:833–836 [DOI] [PubMed] [Google Scholar]
- 34. Stellbrink HJ, et al. 2002. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial). AIDS 16:1479–1487 [DOI] [PubMed] [Google Scholar]
- 35. Strain MC, et al. 2003. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc. Natl. Acad. Sci. U. S. A. 100:4819–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun Z, et al. 2007. Intrarectal transmission, systemic infection and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204:705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong JK, et al. 1997. Reduction of HIV-1 in blood and lymph nodes following potent antiretroviral therapy and the virologic correlates of treatment failure. Proc. Natl. Acad. Sci. U. S. A. 94:12574–12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wong JK, et al. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291–1295 [DOI] [PubMed] [Google Scholar]
- 39. Yukl SA, et al. 2010. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J. Infect. Dis. 202:1553–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]