Abstract
Binding to heparan sulfate is essential for baculovirus transduction of mammalian cells. Our previous study shows that gp64, the major glycoprotein on the virus surface, binds to heparin in a pH-dependent way, with a stronger binding at pH 6.2 than at 7.4. Using fluorescently labeled peptides, we mapped the pH-dependent heparin-binding sequence of gp64 to a 22-amino-acid region between residues 271 and 292. Binding of this region to the cell surface was also pH dependent, and peptides containing this sequence could efficiently inhibit baculovirus transduction of mammalian cells at pH 6.2. When the heparin-binding peptide was immobilized onto the bead surface to mimic the high local concentration of gp64 on the virus surface, the peptide-coated magnetic beads could efficiently pull down cells expressing heparan sulfate but not cells pretreated with heparinase or cells not expressing heparan sulfate. Interestingly, although this heparin-binding function is essential for baculovirus transduction of mammalian cells, it is dispensable for infection of Sf9 insect cells. Virus infectivity on Sf9 cells was not reduced by the presence of heparin or the identified heparin-binding peptide, even though the peptide could bind to Sf9 cell surface and be efficiently internalized. Thus, our data suggest that, depending on the availability of the target molecules on the cell surface, baculoviruses can use two different methods, electrostatic interaction with heparan sulfate and more specific receptor binding, for cell attachment.
INTRODUCTION
The baculovirus Autographa californica nuclear polyhedrosis virus (AcMNPV) is a large enveloped insect virus widely used for recombinant protein production (31). After packing its double-stranded DNA genome into a rod-shaped nucleocapsid, the budded virus (BV) is released from the infected insect cells with gp64 glycoprotein forming peplomers at one end of the virus particle (2, 29, 39). In recent years, BV has also been exploited for gene therapy applications, as it can efficiently transduce many types of mammalian cells with little cytotoxicity (21, 45, 47, 49). Heparan sulfate, the highly negatively charged polysaccharide on the surface of mammalian cells, serves as the major binding molecule during baculovirus transduction (9).
gp64 of baculovirus mediates both the initial binding to insect cells and the subsequent endosome escape step (3, 13, 25). As a class III fusion protein, it exists as trimers in both the pre- and postfusion states and does not undergo proteolytic cleavage during the pH-dependent conformational change (20). The postfusion crystal structure of gp64 has been solved, and it closely resembles those of vesicular stomatitis virus G glycoprotein (VSVG) and glycoprotein B of herpes simplex virus 1 (HSV-1) in the same class, with 5 domains similarly positioned in the elongated molecule (14, 20, 33). gp64 has been used to pseudotype lentivirus, and the resulting virus can efficiently transduce a wide variety of mammalian cells (22, 34). This suggests that gp64 also mediates virus binding to the surface of mammalian cells. Further study on a soluble form of gp64 without the transmembrane domain demonstrated that gp64 binds to heparin in a pH-dependent manner, with a stronger binding at pH 6.2 than at pH 7.4 (48).
Heparin-binding sites are rich in basic amino acids (5, 24, 35, 43). Examining the sequence of gp64, we found that the central region of gp64 from residues 271 to 292 is also rich in basic amino acids. In the crystal structure of gp64, this central region consists of a disordered segment and surrounding loop regions which form a linker between domains II and III (20). B12D5, a monoclonal antibody against gp64, has a binding epitope of KKRPPTWRHNV which lies within the disordered segment (26). These pieces of evidence suggest that the central basic region is exposed on the surface of gp64 protein and may be involved in binding to heparin. In this study, we demonstrated the heparin-binding activity of this central region and mapped the sequence that closely resembles the binding pattern of soluble gp64 protein. The difference in requirement for heparan sulfate between mammalian and insect cells during virus attachment was also investigated.
MATERIALS AND METHODS
Cell lines.
Spodoptera frugiperda (Sf9) insect cells were maintained in spinner flasks using Sfm-900 II serum-free medium (SFM; Invitrogen, Carlsbad, CA). Human U251 glioma cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/ml), and streptomycin (100 μg/ml). Jurkat T cells were grown in suspension in RPMI 1640 medium containing 10% FBS, 100 units/ml of penicillin, and 100 μg/ml of streptomycin.
Construction of recombinant baculoviruses.
CMV-Luc and CMV-EGFP baculoviruses with a luciferase or enhanced green fluorescent protein (EGFP) reporter gene, respectively, driven by the human cytomegalovirus (CMV) early promoter have been described previously (44). VSVG-CMV-EGFP baculovirus, with the vesicular stomatitis virus G glycoprotein (VSVG) gene under the control of the strong viral polyhedrin promoter (pPH) and EGFP under the CMV promoter, was constructed using the BAC-to-BAC baculovirus expression system according to the supplier's manual (Invitrogen, Carlsbad, CA). In brief, a CMV-EGFP expression cassette was inserted downstream of the p10 promoter in the pFastBac Dual plasmid (1). The VSVG gene was cut out from pLP/VSVG plasmid (Invitrogen, Carlsbad, CA) using EcoRI restriction enzyme and inserted into the modified pFastBac Dual vector. Bacmid DNA generated from this transfer vector was used to transfect Sf9 cells. The resulting recombinant baculovirus was further amplified in Sf9 cells at a multiplicity of infection (MOI) of 0.1.
Heparin-agarose bead binding assay.
Three fluorescein isothiocyanate (FITC)-labeled peptides encompassing the central region of gp64 from residues 272 to 287, GV18 (GGVEHRVKKRPPTWRHNV), GT24 (GGKVEHRVKKRPPTWRHNVRAKYT), and KT24 (KRKVEHRVKKRPPTWRHNVRAKYT), were purchased from GL Biochem (Shanghai, China). One milliliter of heparin-agarose beads (Sigma-Aldrich, St. Louis, MO) was equilibrated with 10 ml of 0.3× phosphate-buffered saline (PBS), pH 6.2. Fifteen micrograms of fluorescently labeled peptides was diluted in 500 μl of 0.3× PBS, pH 6.2, and loaded onto the column. After being washed with 6 ml of 0.3× PBS, pH 6.2, the peptides were eluted sequentially with 6 ml of 1× PBS, pH 6.2; 1× PBS, pH 7.4; and 2× PBS, pH 7.4. The eluted fractions were collected and measured for fluorescence intensity using a Tecan GENios Pro (Tecan Group Ltd., Männedorf, Switzerland).
Transduction assay.
U251 cells were seeded into 24-well plates at 2 × 105 cells per well. One day later, normal cell culture medium was replaced with a mixture of 50% FBS and 50% 1× PBS, pH 7.4 or 6.2. Increasing amounts of GT24 peptide were added to the medium and incubated at 37°C. Thirty minutes later, CMV-Luc baculovirus was added to the cells at an MOI of 20 and incubated at 37°C for 4 h. The cells were washed once with normal cell culture medium before being returned to the incubator. For the heparin inhibition assay, U251 cells were treated in a similar way expect that the cell culture medium was not replaced before virus was added. One day later, cells were washed once with 1× PBS and lysed by freeze-thaw in lysis reporter buffer (Promega, Madison, WI). The luciferase activity was measured on a Berthold Lumat LB 9507 luminometer (Berthold Detection System, Pforzheim, Germany). The relative light units (RLU) were normalized to the protein concentration measured with the Bio-Rad protein assay kit. (Promega, Madison, WI).
Jurkat T cells were incubated with CMV-EGFP or VSVG-CMV-EGFP baculovirus at an MOI of 200 at 37°C for 24 h. The cells were washed twice with 1× PBS, pH 7.4, and their EGFP expression levels were measured on a FACSCalibur using CellQuest (BD, Franklin Lakes, NJ).
Cell-peptide binding assay.
U251 cells were trypsinized and resuspended in a mixture of 50% FBS and 50% 1× PBS, pH 7.4 or 6.2. FITC-labeled peptides were added to a final concentration of 1 μM. After a 30-min incubation at room temperature, cells were washed twice with 1× PBS, pH 7.4 or 6.2. Flow cytometry analysis of the labeled cells was carried out on a FACSCalibur cytometer using CellQuest. The normalized mean fluorescence intensity was calculated by subtracting the mean fluorescence intensity of the untreated control cells from the measured mean fluorescence intensity of treated cells.
Sf9 cells were seeded in 24-well plates in insect cell culture medium. FITC-labeled peptides were added to a final concentration of 10 μM. After a 5-hour incubation at 4°C or a 2-hour incubation at room temperature, the cells were washed three times with 1× PBS, pH 6.2, and observed under a fluorescence microscope.
Plaque assay.
Sf9 insect cells were seeded in 6-well plates at 50% confluence, and 1 h later the medium was replaced with 1 ml of fresh Sfm-900 II medium containing GT24 peptide or heparin. After a 30-min incubation at room temperature, 100 μl of serially diluted CMV-Luc baculovirus was added to each well and incubated for 1 h at room temperature. The cells were washed once with fresh Sfm-900 II medium before 1% nutrient agarose overlay was added. Plaques were counted 7 days later.
Immobilization of biotinylated GT24 peptide onto streptavidin-coupled Dynabeads.
Fifty microliters of Dynabeads MyOne Streptavidin T1 (Invitrogen, Carlsbad, CA) was diluted with 200 μl of 1× PBS, pH 7.4. One hundred micrograms of biotinylated GT24 peptide was added to Dynabeads and incubated at room temperature for 30 min. After 7 washes with 1× PBS containing 0.1% bovine serum albumin (BSA), the beads were resuspended in 200 μl of 1× PBS containing 0.1% BSA. The zeta potential of the GT24-coated Dynabeads was measured on a Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, United Kingdom).
Cell pulldown assay using GT24-Dynabeads.
Cells were resuspended in 150 μl of culture medium in 0.6-ml tubes. Ten microliters of GT24-Dynabeads was added to each cell-containing tube and incubated at room temperature for 30 min with brief vortexing every 10 min. Dynabeads with bound cells were separated from supernatant by placing a strong magnet behind the tubes for 3 min. Supernatant was carefully pipetted out and transferred to a new tube. Bead-bound cells were resuspended in 150 μl of medium. Cells were counted on a hemocytometer under a phase-contrast microscope.
Heparinase treatment.
One million trypsinized U251 cells were resuspended in 150 μl of PBS containing 0.1% BSA. Two units of heparinase II (Sigma-Aldrich, St. Louis, MO) was added to cells and incubated at room temperature for 1 h with brief vortexing every 15 min. Cells were spun down at 200 × g for 4 min and resuspended in 150 μl of 1× PBS, pH 7.4, containing 0.1% BSA before GT24-Dynabeads were added.
RESULTS
Binding of GV18, GT24, and KT24 to heparin-agarose beads.
Our previous study shows that binding of soluble gp64 to heparin-agarose beads is pH dependent. Soluble gp64 binds to heparin at pH 6.2 and can be eluted out at pH 7.4 (48). The central region of gp64 is rich in basic amino acids and contains a disordered region surrounded by loops as shown in the crystal structure (20). To test if this central region can bind to heparin and to map the exact sequence responsible for pH-dependent binding of gp64 to heparin, we designed three FITC-labeled peptides (Fig. 1A). GV18 contains only the disordered segment from gp64, and GT24 contains both the disordered segment and the surrounding loop regions. In KT24, the two-glycine spacer in GT24 was replaced with two basic amino acids that are upstream of the loop region and buried inside gp64. At pH 6.2 and a salt concentration of 0.3× PBS, all three peptides could bind to heparin-agarose beads. When the salt concentration was increased to 1× PBS, GV18 could be eluted out. When the pH value was further increased to 7.4, GT24 could also be eluted out. However, KT24 could be eluted out only at an even higher salt concentration, 2× PBS (Fig. 1B). The elution profile of GT24 is very similar to that of soluble gp64, which is also eluted out at pH 7.4 and 150 mM NaCl (48). This suggests that the KVEHRVKKRPPTWRHNVRAKYT sequence in GT24 is the pH-sensitive heparin-binding region on gp64.
Fig 1.
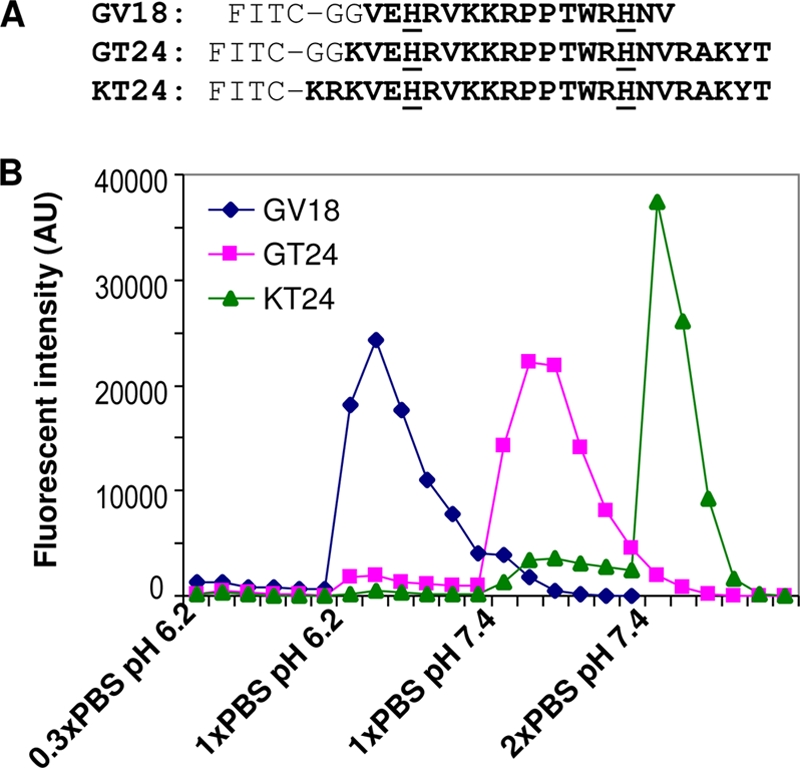
Binding of peptides to heparin-agarose beads. (A) Sequences of the three peptides tested in this study for heparin binding. All peptides were labeled with FITC at the N termini. Residues from gp64 protein are shown in bold letters, and the two histidines are underlined. The two glycines serve as a spacer. GV18 contains only the disordered segment in the crystal structure of gp64. GT24 extends the sequence to the surrounding loop regions. In KT24, the two glycines in GT24 are replaced with two basic amino acids buried in the crystal structure of gp64. (B) FITC-labeled peptides were loaded onto a 1-ml heparin-agarose bead column and eluted with different elution buffers. Fractions of the elution were collected as 1 ml per fraction, and their fluorescence intensities were measured. AU, arbitrary units.
Binding of peptides to human U251 glioma cells under different pH conditions.
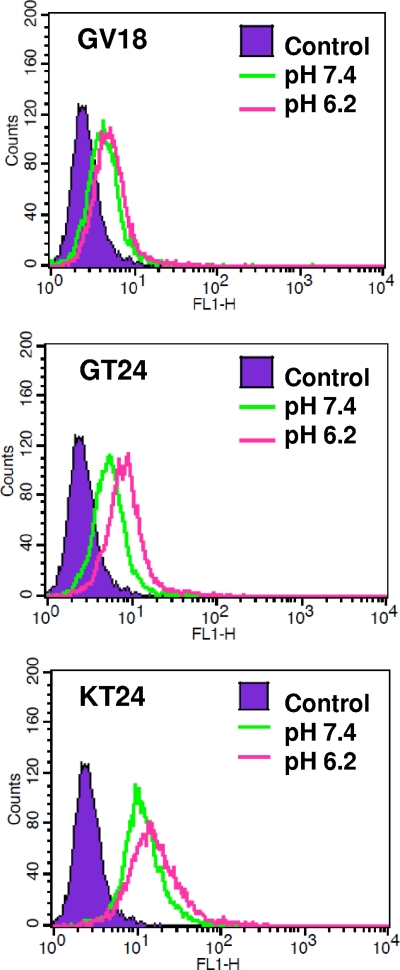
To test whether the binding of three peptides to mammalian cells is pH dependent, U251 glioma cells were incubated with FITC-labeled peptides in 1× PBS at pH 6.2 or 7.4. After extensive washing, the amounts of cell-bound peptides were analyzed using fluorescence-activated cell sorting (FACS) (Fig. 2). Binding of GV18 to U251 cells was quite weak under both pH conditions, with a normalized mean fluorescence intensity around 2.3. For GT24, the binding was weak at pH 7.4 but increased by more than 2-fold at pH 6.2 (the normalized mean fluorescence intensity increased from 2.5 to 5.2). For KT24, its binding at pH 7.4 was already very strong, with a fluorescence intensity higher than that of GT24 at pH 6.2. However, its fluorescence intensity increased only slightly when pH was further lowered to 6.2 (the normalized mean fluorescence intensity increased from 8.4 to 11.5). These results show that binding of GT24 to mammalian cells is also pH dependent.
Fig 2.
Binding of peptides to U251 cells under different pH conditions. FITC-labeled peptides were incubated with U251 cells for 30 min at pH 6.2 or 7.4. After extensive washing, cells were analyzed by flow cytometry for the presence of fluorescently labeled peptides.
Competition binding of virus to U251 cells in the presence of GT24 peptide.
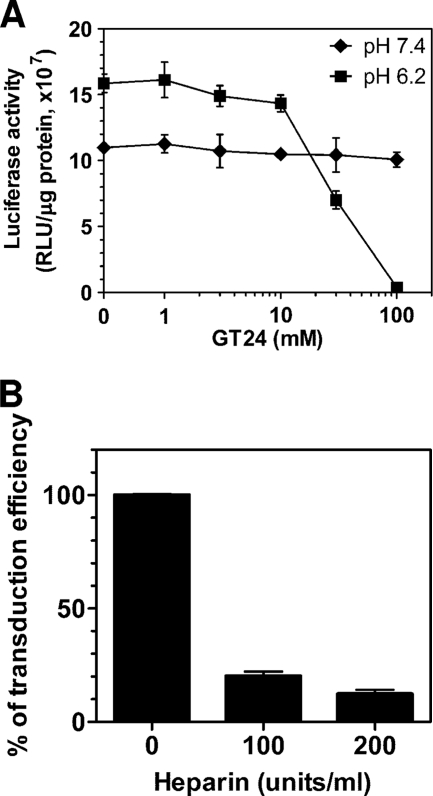
Since the binding of GT24 to mammalian cell surface is pH dependent, we wondered if GT24 could block the binding of baculovirus to mammalian cells in a pH-dependent manner. To test this, U251 cells were transduced with CMV-Luc baculovirus in the presence of GT24 at pH 6.2 or pH 7.4 (Fig. 3A). At pH 6.2, there was little decrease in transduction efficiency with GT24 concentrations lower than 10 μM. When the GT24 concentration was increased to 30 μM, the transduction efficiency started to decrease sharply by more than 50%. At 100 μM GT24, the transduction efficiency dropped further to about 2% of the level without GT24. However, when transduction assays were performed at pH 7.4, the transduction efficiency was only slightly inhibited by GT24, even at 100 μM GT24, the highest concentration tested. This demonstrates that GT24 can efficiently compete with baculovirus for binding to mammalian cells at pH 6.2. Interestingly, the transduction efficiency without GT24 at pH 7.4 is only about 70% of that at pH 6.2, suggesting that baculovirus transduction of mammalian cells is also pH dependent.
Fig 3.
Effects of GT24 peptide and heparin on baculovirus transduction of U251 cells. (A) Effects of GT24 peptide on baculovirus transduction efficiency under different pH conditions. U251 cells were transduced with CMV-Luc baculovirus in the presence of increasing amounts of GT24 peptide at pH 6.2 or pH 7.4. One day later, luciferase activities of the transduced cells were measured and expressed as relative light units per milligrams of cellular protein. (B) Inhibition effects of heparin on baculovirus transduction of U251 cells. U251 cells were transduced with CMV-Luc baculovirus in the presence of heparin. One day later, luciferase activities of the transduced cells were measured and compared to those without heparin treatment. Data represent mean values with standard deviations of three experiments.
Binding of GT24-Dynabeads to U251 cells.
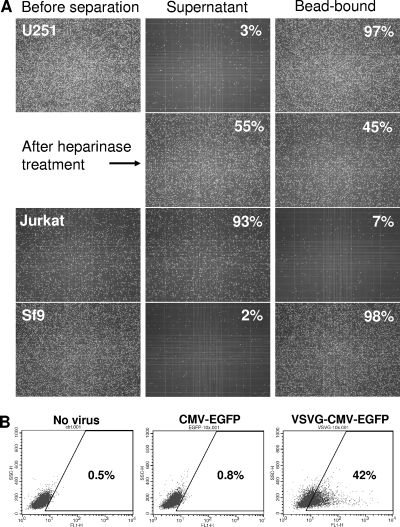
gp64 protein forms trimers and concentrates at one pole of baculovirus. To mimic this high local concentration distribution of gp64, biotinylated GT24 peptide was linked to streptavidin-coupled Dynabeads T1 and tested for binding to U251 cells. The magnetic Dynabeads used in this study have a diameter of 1 μm, with a binding capacity up to 2 × 105 peptides/bead particle. The original beads were slightly negatively charged, with a zeta potential of −10 mV. After beads were coated with GT24 peptide and resuspended in 1× PBS, pH 7.4, containing 0.1% BSA, the zeta potential of Dynabeads dropped to −20 mV. This may be caused by the absorption of negatively charged BSA to the bead surface. Despite their negative surface charge, GT24-coated Dynabeads efficiently pulled down U251 cells with very few cells left in the supernatant (Fig. 4A). This demonstrates that multiple GT24 molecules immobilized on the bead surface still retained their binding activity to U251 cells.
Fig 4.
Binding of GT24-Dynabeads to heparan sulfate on the cell surface. (A) Pulldown of cells using GT24-Dynabeads. Biotinylated GT24 peptide was immobilized onto streptavidin-coupled Dynabeads. GT24-Dynabeads were incubated with cells for 30 min at room temperature and separated from supernatant using a magnet. Binding assays for U251 and Jurkat cells were carried out in 1× PBS, pH 7.4, while binding assays for Sf9 insect cells were performed in insect cell culture medium with a pH of 6.2. Bead-bound cells were resuspended in the same volume of the supernatant and counted on a hemocytometer. Images of cells before separation and those remaining in the supernatant were also taken. (B) Transduction of Jurkat T cells with CMV-EGFP and VSVG-CMV-EGFP baculovirus. VSVG-CMV-EGFP baculovirus expresses both VSVG and the endogenous gp64 on the virus surface while CMV-EGFP virus has only gp64. One day after transduction, the EGFP-positive Jurkat cells were analyzed using flow cytometry.
Effect of heparinase II treatment on binding of GT24-Dynabeads to U251 cells.
To test whether GT24 binds to the cell surface through interaction with heparan sulfate, U251 cells were pretreated with heparinase before the GT24-Dynabeads binding assay was carried out. Without heparinase treatment, almost all U251 cells could be pulled down with GT24-Dynabeads. After 1 h of pretreatment with heparinase II, which digests both heparan sulfate and heparin, only about 45% of U251 cells could be pulled down using GT24-Dynabeads (Fig. 4A). This is quite similar to the reported 50% reduction of baculovirus transduction efficiency on mammalian cells after heparinase treatment (9). The drop in GT24-Dynabead binding after heparinase treatment demonstrates that GT24 indeed binds to heparan sulfate on the cell surface. The remaining binding of GT24-Dynabeads to heparinase-treated cells may be due to the incomplete removal of heparan sulfate on the cell surface, as heparinases recognize only a subset of linkage from the highly heterogeneous polysaccharides (23).
Binding of GT24-Dynabeads to Jurkat T cells.
Jurkat T cells express very little heparan sulfate proteoglycan on the cell surface (32). They could be transduced by baculovirus displaying vesicular stomatitis virus G glycoprotein (VSVG) but not by baculovirus with only endogenous gp64 (Fig. 4B). After Jurkat cells were incubated with GT24-Dynabeads, very few Jurkat cells could be pulled down with a magnet and the majority of cells still remained in the supernatant (Fig. 4A). This further confirms that GT24-Dynabeads bind to the cell surface through interaction with heparan sulfate.
Binding of peptides to Sf9 cells.
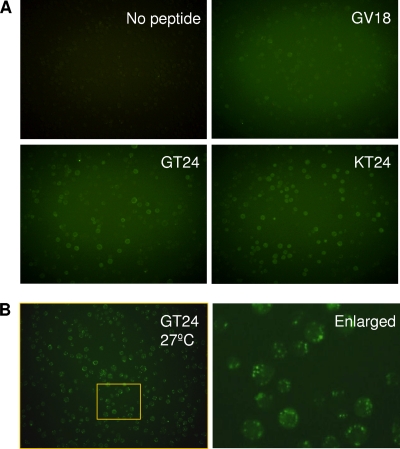
Having identified and demonstrated the importance of the pH-sensitive heparin-binding sequence during virus transduction of mammalian cells, we wondered whether this sequence interacts with insect cells in a similar way. When Sf9 cells were incubated with GV18, GT24, and KT24 peptides at a concentration of 10 μM at 4°C, GV18 showed only very weak binding, but the binding of GT24 and KT24 to Sf9 cells was much stronger, as indicated by the green fluorescence around the cells (Fig. 5A). Furthermore, GT24-Dynabeads could also efficiently pull down Sf9 cells (Fig. 4A). These data demonstrate that the identified pH-sensitive heparin-binding sequence in GT24 could efficiently mediate binding to Sf9 cells under normal insect cell culture conditions (pH 6.2). When Sf9 cells were incubated with GT24 at 27°C to allow endocytosis, multiple green fluorescent vesicles could be observed inside cells (Fig. 5B), suggesting that cell surface-bound GT24 can be efficiently endocytosed.
Fig 5.
Binding of peptides to Sf9 insect cells. (A) Sf9 cells were incubated with FITC-labeled peptides in insect culture medium (pH 6.2) at 4°C to prevent endocytosis. Five hours later, cells were washed and observed under a fluorescence microscope. (B) Sf9 cells were incubated with FITC-labeled GT24 in insect culture medium (pH 6.2) at 27°C to allow endocytosis. Two hours later, cells were washed and observed under a fluorescence microscope with a 20× objective. The right panel shows the enlarged image of the area marked by the yellow rectangle in the left panel. Note the multiple green fluorescent vesicles inside cells.
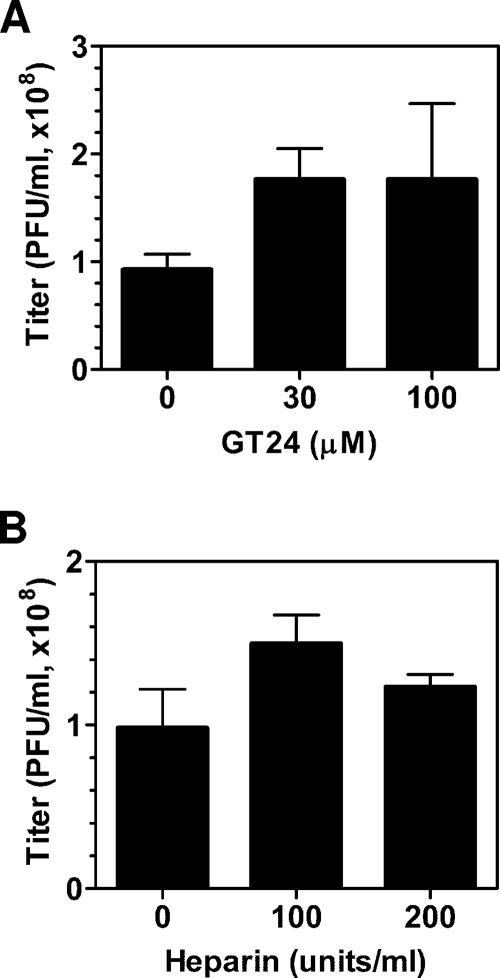
Effect of GT24 on baculovirus infection of Sf9 insect cells.
At pH 6.2, excess amounts of GT24 could block baculovirus transduction of mammalian cells. To test if GT24 also inhibits baculovirus infection of insect cells, plaque assays were carried out in the presence of GT24. Sf9 cells were preincubated with GT24 for 30 min, and the subsequent virus attachment step was also performed in the presence of GT24. GT24 concentrations of 30 μM and 100 μM were chosen, as these concentrations showed a strong inhibitory effect on virus transduction of mammalian cells (Fig. 3A). Unexpectedly, GT24 did not reduce baculovirus infection of Sf9 cells; it actually increased the infectivity slightly (Fig. 6A). The inability of GT24 to inhibit infection of Sf9 suggests that specific receptors other than heparan sulfate on the cell surface can serve as attachment molecules for virus entry into Sf9 cells.
Fig 6.
Baculovirus titers determined by plaque assay in the presence of GT24 peptide (A) and heparin (B). Sf9 cells were preincubated with GT24 peptide (A) or heparin (B) for 30 min before baculovirus was added. Plaques were counted 7 days later and reported as mean values with standard deviations of three experiments.
Effects of heparin on baculovirus transduction of U251 cells and infection of Sf9 insect cells.
If baculovirus can use the specific receptor other than heparan sulfate on the Sf9 cell surface as an attachment molecule for virus entry, one would expect the addition of heparin not to abolish virus infectivity. This is exactly what we observed. Similarly to a previous report (9), the transduction efficiency of baculovirus on U251 cells was greatly inhibited by heparin at a concentration of 100 or 200 units/ml (Fig. 3B). However, virus titers measured at these heparin concentrations were not reduced; they even increased slightly (Fig. 6B). This confirmed that heparan sulfate is the attachment molecule for transduction of mammalian cells, while other specific receptors can be used for cell attachment during infection of insect cells.
DISCUSSION
Using three peptides encompassing a disordered segment in the crystal structure of gp64, we have confirmed that residues from 271 to 292 with a sequence of KVEHRVKKRPPTWRHNVRAKYT are responsible for the binding of gp64 to heparin. This sequence closely resembled the binding pattern of soluble gp64 to heparin, as demonstrated by the strong binding of GT24 at pH 6.2 and very weak binding at pH 7.4. GV18 peptide, containing only the disordered segment, could not bind to heparin even at pH 6.2. With the addition of two extra basic amino acids to the N terminus of GT24, the resulting KT24 peptide required a higher salt concentration to be eluted out than did gp64.
Based on the sequence analysis of heparin-binding proteins, binding motifs such as BBXB and BBBXXB have been identified, where B's are basic residues and X's are other residues (5). The identified heparin-binding sequence in gp64 contains HRVK, which resembles the BBXB consensus heparin-binding motif. However, other amino acids also play a great role in binding to heparin, as GV18 also contains this motif but its binding to heparin is much weaker. A previous study on concatemers of heparin-binding motifs shows that more than 3 copies of binding motifs are required for a strong binding (40). This is in good agreement with our observation. KT24 with two extra basic amino acids at its N terminus showed a stronger binding, probably due to the newly formed KRKVEHR sequence, which resembles the BBBXXB motif. Since the binding of KT24 to heparin is stronger than that of gp64, it is likely that the two basic residues buried inside gp64 in the postfusion crystal structure are also buried inside in the prefusion state and thus do not participate in binding to heparin.
The identified heparin-binding sequence contains two histidines which may provide the pH-sensitive binding property. Since these two histidines are located in the disordered segment of gp64, their exact pKa values cannot be calculated due to lack of structural information. However, given that they are exposed on the surface of the protein and are involved in binding to heparin and antibodies (26, 51), it is very likely that they are not interacting with other residues and thus maintain a pKa of around 6.0, the original pKa of the imidazole side chain of histidine. At pH 6.2, the optimal pH for Sf9 cell growth (12), the two histidines in the identified heparin-binding sequence of gp64 will be positively charged and participate in binding to the negatively charged heparin. The calculated overall net charges for GV18, GT24, and KT24 under this low-pH condition are 5.3, 8.3, and 10.3, respectively. But at pH 7.4, the physiological pH for mammalian cells, the two histidines are not charged and the calculated net charges for GV18, GT24, and KT24 drop to 4, 7, and 9, respectively. The decreased positive charge of the peptides will result in a weaker binding to the negatively charged heparin and heparan sulfate. The importance of histidine residues has also been demonstrated in other pH-sensitive heparin-binding proteins such as selenoprotein P and histidine-proline-rich glycoprotein (HPRG) (4, 15). Replacing the two histidines in the heparin-binding sequence with arginine or lysine should result in peptides or viruses with greater affinity for heparan sulfate on the cell surface. On the other hand, it may be possible to design heparin-binding peptides with better pH sensitivity by introducing more histidines.
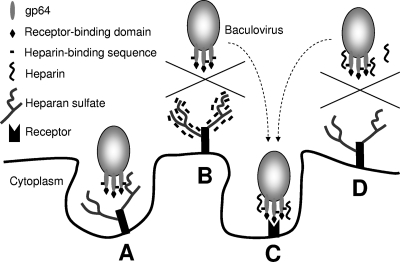
We also tested the binding of the three peptides to U251 glioma cells under different pH conditions. As expected, binding of GT24 to cells was weak at pH 7.4 and increased when pH is lowered to 6.2. Furthermore, GT24 could efficiently compete with baculovirus for binding to U251 cells at pH 6.2 but not at pH 7.4. These data confirm that GT24 bound to the mammalian cell surface in a pH-dependent manner. However, when plaque assays were performed on Sf9 insect cells in the presence of GT24, the measured virus titer actually increased slightly. This is not due to the lack of binding of GT24 to Sf9 cells, as we could still detect binding of GT24 to the Sf9 cell surface. It has been well known that many types of viruses, including adenovirus, herpesvirus, flavivirus, retrovirus, and papillomavirus, use heparan sulfate on the cell surface as an attachment factor (6, 8, 11, 16, 19, 36, 38). For baculovirus, it seems that the virus can use both receptor binding and heparan sulfate binding as two independent ways to attach to Sf9 cells. This hypothesis is supported by several lines of evidence. First, the receptor binding domain has been mapped to the N terminus of gp64 from amino acid 21 to 159, which is different from the identified heparin-binding region. Point mutations in the receptor binding domain cannot completely abolish virus binding to Sf9 cells (50), indicating the existence of another type of binding. Second, B12D5 antibody, with a binding epitope within the heparin-binding sequence, is not a neutralizing antibody (26, 51). AcV1, another monoclonal antibody against an epitope extensively overlapping with the heparin-binding region, does not inhibit virus binding to the cell surface (41). Lastly, this heparin-binding region can tolerate insertions of and substitutions by a variety of foreign sequences without affecting virus titers, suggesting that the heparin-binding function is dispensable for infection of Sf9 cells (10, 37, 51). Our results for the effects of GT24 and heparin on virus infectivity also support this hypothesis. A recent study on single-chain variable fragment (scFv) anti-heparan sulfate antibodies shows that not all heparan sulfate binding events can induce endocytosis (46). Thus, when GT24 or heparin blocks the nonspecific electrostatic interaction between gp64 and heparan sulfate on the cell surface, more viruses will attach to cells through the specific binding between gp64 and its receptor, leading to more endocytosed viruses and higher infectivity (Fig. 7). In vivo, this heparan sulfate binding function may be utilized for the successful infection of insect cells that do not express the specific receptor on their surface, prompting the systemic spreading of virus inside the insect body. The supplement of this nonspecific heparan sulfate binding could also contribute to the broad host range of AcMNPV.
Fig 7.
A schematic illustration showing the interactions of baculovirus with its target molecules on the cell surface. (A) Baculovirus binds to heparan sulfate and is being endocytosed. (B) Heparin-binding peptide binds to heparan sulfate on the cell surface, blocking the virus interaction with heparan sulfate. (C) Baculovirus binds to the receptor through its receptor binding domain and is being endocytosed. (D) Heparin binds to the heparin-binding region on gp64 and prevents virus binding to heparan sulfate. On the surface of Sf9 cells, blocking the interaction with heparan sulfate will not inhibit virus entry, since baculovirus can still bind to its specific receptor as shown in panel C.
The heparin-binding sequence forms a linker between domains II and III in the structure of gp64. The flexibility of this linker may be crucial for the pH-induced conformational change during membrane fusion. Unlike the neutralizing antibody AcV1, which imposes a specific structural constraint upon binding to this region (51), the bound heparin does not inhibit the pH-dependent conformational switch, probably due to the small diameter and the flexibility of the polysaccharide chain. It is interesting that H1781, a neutralizing monoclonal antibody against glycoprotein B of HSV-1, also binds to the disordered linker between domains II and III in glycoprotein B (14).
gp64 is not evenly distributed on the virus surface. It is concentrated at one pole of the virus particle, resulting in a high local concentration of gp64. This explains why the binding of baculovirus to heparin beads is much stronger than that of free soluble gp64 (48). To increase the local concentration of GT24, multiple copies of GT24 were immobilized to the surface of magnetic Dynabeads, with a density of up to 2 × 105 peptides/bead. The GT24-coated beads efficiently pulled down Sf9 and U251 cells but not heparinase-treated U251 cells or Jurkat cells, which express very little heparan sulfate on the cell surface. These findings indicate that GT24-coated beads can be used for cell separation based on the heparan sulfate density on the cell surface. For example, most tumor cells can be transduced by baculovirus, presumably through interacting with heparan sulfate, while hematopoietic cell types express little heparan sulfate and cannot be transduced by gp64-pseudotyped lentivirus (18, 19, 34, 42). This difference of heparan sulfate content on the cell surface may be utilized to capture circulating tumor cells from the blood, as well as exosomes secreted by tumor cells.
Transduction of mammalian cells by baculovirus is not sensitive to the presence of serum proteins. Similarly, GT24 peptide or GT24-coated Dynabeads could also bind to heparan sulfate-expressing cells in a serum-containing environment. In contrast, commonly used nonviral gene delivery systems such as poly-l-lysine/DNA and polyethyleneimine (PEI)/DNA complexes are highly sensitive to serum due to the interaction with serum proteins (7, 17, 28). Since GT24 could bind to the cell surface in the presence of serum and be efficiently endocytosed, it will be useful to incorporate this heparin-binding sequence into other nanoparticles for efficient in vivo gene/drug delivery. It will also be possible to enhance transduction efficiency or broaden the tropism of other types of viruses by displaying this sequence on the virus surface, either as a fusion protein or as a short stand-alone protein.
gp75 envelope protein of Thogoto virus, a tick-transmitted orthomyxovirus, is closely related to gp64 of baculovirus, with 28% amino acid sequence identity (27, 30). However, the corresponding central region of gp75 shows no obvious similarity to the heparin-binding sequence of gp64. Whether Thogoto virus also interacts with heparan sulfate on the cell surface requires more detailed studies.
In summary, we have mapped the heparin-binding sequence of gp64 to a 22-amino-acid region between residues 271 and 292. Similarly to gp64, binding of this region to heparin and heparan sulfate on the cell surface was pH sensitive, with a stronger binding at pH 6.2 than at 7.4. This heparin-binding function is essential for baculovirus transduction of mammalian cells but is dispensable for infection of Sf9 insect cells.
ACKNOWLEDGMENT
This work was funded by the Institute of Bioengineering and Nanotechnology (Biomedical Research Council, Agency for Science, Technology and Research, Singapore).
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Balani P, et al. 2009. High mobility group box2 promoter-controlled suicide gene expression enables targeted glioblastoma treatment. Mol. Ther. 17:1003–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blissard GW. 1996. Baculovirus-insect cell interactions. Cytotechnology 20:73–93 [DOI] [PubMed] [Google Scholar]
- 3. Blissard GW, Wenz JR. 1992. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J. Virol. 66:6829–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borza DB, Morgan WT. 1998. Histidine-proline-rich glycoprotein as a plasma pH sensor. Modulation of its interaction with glycosaminoglycans by pH and metals. J. Biol. Chem. 273:5493–5499 [DOI] [PubMed] [Google Scholar]
- 5. Cardin AD, Weintraub HJ. 1989. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9:21–32 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, et al. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866–871 [DOI] [PubMed] [Google Scholar]
- 7. Dash PR, Read ML, Barrett LB, Wolfert MA, Seymour LW. 1999. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Ther. 6:643–650 [DOI] [PubMed] [Google Scholar]
- 8. Dechecchi MC, Tamanini A, Bonizzato A, Cabrini G. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268:382–390 [DOI] [PubMed] [Google Scholar]
- 9. Duisit G, et al. 1999. Baculovirus vector requires electrostatic interactions including heparan sulfate for efficient gene transfer in mammalian cells. J. Gene Med. 1:93–102 [DOI] [PubMed] [Google Scholar]
- 10. Ernst WJ, Spenger A, Toellner L, Katinger H, Grabherr RM. 2000. Expanding baculovirus surface display. Modification of the native coat protein gp64 of Autographa californica NPV. Eur. J. Biochem. 267:4033–4039 [DOI] [PubMed] [Google Scholar]
- 11. Giroglou T, Florin L, Schafer F, Streeck RE, Sapp M. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grace TDC. 1962. Establishment of four strains of cells from insect tissue grown in vitro. Nature 195:788–789 [DOI] [PubMed] [Google Scholar]
- 13. Hefferon KL, Oomens AG, Monsma SA, Finnerty CM, Blissard GW. 1999. Host cell receptor binding by baculovirus GP64 and kinetics of virion entry. Virology 258:455–468 [DOI] [PubMed] [Google Scholar]
- 14. Heldwein EE, et al. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220 [DOI] [PubMed] [Google Scholar]
- 15. Hondal RJ, Ma S, Caprioli RM, Hill KE, Burk RF. 2001. Heparin-binding histidine and lysine residues of rat selenoprotein P. J. Biol. Chem. 276:15823–15831 [DOI] [PubMed] [Google Scholar]
- 16. Ibrahim J, Griffin P, Coombe DR, Rider CC, James W. 1999. Cell-surface heparan sulfate facilitates human immunodeficiency virus type 1 entry into some cell lines but not primary lymphocytes. Virus Res. 60:159–169 [DOI] [PubMed] [Google Scholar]
- 17. Ignatovich IA, et al. 2003. Complexes of plasmid DNA with basic domain 47–57 of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J. Biol. Chem. 278:42625–42636 [DOI] [PubMed] [Google Scholar]
- 18. Jarousse N, Chandran B, Coscoy L. 2008. Lack of heparan sulfate expression in B-cell lines: implications for Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 infections. J. Virol. 82:12591–12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones KS, Petrow-Sadowski C, Bertolette DC, Huang Y, Ruscetti FW. 2005. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 79:12692–12702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kadlec J, Loureiro S, Abrescia NG, Stuart DI, Jones IM. 2008. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat. Struct. Mol. Biol. 15:1024–1030 [DOI] [PubMed] [Google Scholar]
- 21. Kost TA, Condreay JP. 2002. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol. 20:173–180 [DOI] [PubMed] [Google Scholar]
- 22. Kumar M, Bradow BP, Zimmerberg J. 2003. Large-scale production of pseudotyped lentiviral vectors using baculovirus GP64. Hum. Gene Ther. 14:67–77 [DOI] [PubMed] [Google Scholar]
- 23. Lohse DL, Linhardt RJ. 1992. Purification and characterization of heparin lyases from Flavobacterium heparinum. J. Biol. Chem. 267:24347–24355 [PubMed] [Google Scholar]
- 24. Margalit H, Fischer N, Ben-Sasson SA. 1993. Comparative analysis of structurally defined heparin binding sequences reveals a distinct spatial distribution of basic residues. J. Biol. Chem. 268:19228–19231 [PubMed] [Google Scholar]
- 25. Markovic I, Pulyaeva H, Sokoloff A, Chernomordik LV. 1998. Membrane fusion mediated by baculovirus gp64 involves assembly of stable gp64 trimers into multiprotein aggregates. J. Cell Biol. 143:1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monsma SA, Blissard GW. 1995. Identification of a membrane fusion domain and an oligomerization domain in the baculovirus GP64 envelope fusion protein. J. Virol. 69:2583–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morse MA, Marriott AC, Nuttall PA. 1992. The glycoprotein of Thogoto virus (a tick-borne orthomyxo-like virus) is related to the baculovirus glycoprotein GP64. Virology 186:640–646 [DOI] [PubMed] [Google Scholar]
- 28. Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. 1999. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 6:595–605 [DOI] [PubMed] [Google Scholar]
- 29. Oomens AG, Monsma SA, Blissard GW. 1995. The baculovirus GP64 envelope fusion protein: synthesis, oligomerization, and processing. Virology 209:592–603 [DOI] [PubMed] [Google Scholar]
- 30. Pearson MN, Rohrmann GF. 2002. Transfer, incorporation, and substitution of envelope fusion proteins among members of the Baculoviridae, Orthomyxoviridae, and Metaviridae (insect retrovirus) families. J. Virol. 76:5301–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pennock GD, Shoemaker C, Miller LK. 1984. Strong and regulated expression of Escherichia coli beta-galactosidase in insect cells with a baculovirus vector. Mol. Cell. Biol. 4:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pinon JD, et al. 2003. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J. Virol. 77:9922–9930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roche S, Bressanelli S, Rey FA, Gaudin Y. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187–191 [DOI] [PubMed] [Google Scholar]
- 34. Schauber CA, Tuerk MJ, Pacheco CD, Escarpe PA, Veres G. 2004. Lentiviral vectors pseudotyped with baculovirus gp64 efficiently transduce mouse cells in vivo and show tropism restriction against hematopoietic cell types in vitro. Gene Ther. 11:266–275 [DOI] [PubMed] [Google Scholar]
- 35. Sobel M, Soler DF, Kermode JC, Harris RB. 1992. Localization and characterization of a heparin binding domain peptide of human von Willebrand factor. J. Biol. Chem. 267:8857–8862 [PubMed] [Google Scholar]
- 36. Spear PG, Longnecker R. 2003. Herpesvirus entry: an update. J. Virol. 77:10179–10185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spenger A, Grabherr R, Tollner L, Katinger H, Ernst W. 2002. Altering the surface properties of baculovirus Autographa californica NPV by insertional mutagenesis of the envelope protein gp64. Eur. J. Biochem. 269:4458–4467 [DOI] [PubMed] [Google Scholar]
- 38. Spillmann D. 2001. Heparan sulfate: anchor for viral intruders? Biochimie 83:811–817 [DOI] [PubMed] [Google Scholar]
- 39. Summers MD, Volkman LE. 1976. Comparison of biophysical and morphological properties of occluded and extracellular nonoccluded baculovirus from in vivo and in vitro host systems. J. Virol. 17:962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verrecchio A, et al. 2000. Design of peptides with high affinities for heparin and endothelial cell proteoglycans. J. Biol. Chem. 275:7701–7707 [DOI] [PubMed] [Google Scholar]
- 41. Volkman LE, Goldsmith PA. 1985. Mechanism of neutralization of budded Autographa californica nuclear polyhedrosis virus by a monoclonal antibody: inhibition of entry by adsorptive endocytosis. Virology 143:185–195 [DOI] [PubMed] [Google Scholar]
- 42. Vongchan P, Linhardt RJ. 2007. Expression of human liver HSPGs on acute myeloid leukemia. Clin. Immunol. 122:194–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vyas AA, et al. 1997. Analysis of binding of cobra cardiotoxins to heparin reveals a new beta-sheet heparin-binding structural motif. J. Biol. Chem. 272:9661–9670 [DOI] [PubMed] [Google Scholar]
- 44. Wang CY, et al. 2006. Recombinant baculovirus containing the diphtheria toxin A gene for malignant glioma therapy. Cancer Res. 66:5798–5806 [DOI] [PubMed] [Google Scholar]
- 45. Wang CY, Wang S. 2005. Adeno-associated virus inverted terminal repeats improve neuronal transgene expression mediated by baculoviral vectors in rat brain. Hum. Gene Ther. 16:1219–1226 [DOI] [PubMed] [Google Scholar]
- 46. Wittrup A, et al. 2009. ScFv antibody-induced translocation of cell-surface heparan sulfate proteoglycan to endocytic vesicles: evidence for heparan sulfate epitope specificity and role of both syndecan and glypican. J. Biol. Chem. 284:32959–32967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu C, et al. 2009. Combinatorial control of suicide gene expression by tissue-specific promoter and microRNA regulation for cancer therapy. Mol. Ther. 17:2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu C, Soh KY, Wang S. 2007. Ion-exchange membrane chromatography method for rapid and efficient purification of recombinant baculovirus and baculovirus gp64 protein. Hum. Gene Ther. 18:665–672 [DOI] [PubMed] [Google Scholar]
- 49. Zeng J, Du J, Zhao Y, Palanisamy N, Wang S. 2007. Baculoviral vector-mediated transient and stable transgene expression in human embryonic stem cells. Stem Cells 25:1055–1061 [DOI] [PubMed] [Google Scholar]
- 50. Zhou J, Blissard GW. 2008. Identification of a GP64 subdomain involved in receptor binding by budded virions of the baculovirus Autographa californica multicapsid nucleopolyhedrovirus. J. Virol. 82:4449–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou J, Blissard GW. 2006. Mapping the conformational epitope of a neutralizing antibody (AcV1) directed against the AcMNPV GP64 protein. Virology 352:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]