Abstract
A triple α1,3 glucan synthase mutant of Aspergillus fumigatus obtained by successive deletions of the three α1,3 glucan synthase genes (AGS1, AGS2, and AGS3) has a cell wall devoid of α1,3 glucans. The lack of α1,3 glucans affects neither conidial germination nor mycelial vegetative growth and is compensated by an increase in β1,3 glucan and/or chitin content.
INTRODUCTION
In Aspergillus fumigatus, α1,3 glucans are a major amorphous cell wall polysaccharide, accounting for 35 to 40% of the mycelial cell wall and 20 to 25% of the conidial cell wall (21). α1,3 glucans are also a major cell wall component of the yeast form of the human pathogens Paracoccidioides brasiliensis, Histoplama capsulatum, Blastomyces dermatitidis, and Cryptococcus neoformans and of the nonpathogenic model yeast Schizosaccharomyces pombe (14, 15, 24, 25, 27). In these species, the concentrations reported vary from 28% in S. pombe to 35 to 46% in the virulent yeast forms (13). In all pathogenic species studied to date, α1,3 glucans are important for virulence. In addition, in S. pombe, α1,3 glucans are essential for fungal viability. For A. fumigatus, it was shown that α1,3 glucans have a major adhesive role in the interactions between hyphae or germinating conidia (3, 8).
α1,3 glucans are synthesized by α1,3 glucan synthases, which are transmembrane enzymes with very high molecular masses (>200 kDa). The number of genes coding for Ags proteins varies between fungi. Only one AGS gene was found in H. capsulatum and C. neoformans, whereas five AGS genes were identified in S. pombe (14, 24, 25). In A. fumigatus, α1,3 glucans are synthesized by three α1,3 glucan synthases (Ags1p [AFUA_3G00910], Ags2p [AFUA_2G11270], and Ags3p [AFUA_1G15440]) (21). All three A. fumigatus AGS genes are expressed constitutively (21). Three single AGS mutants have been constructed in A. fumigatus. The ags1Δ mutant had a 50% reduction in the cell wall α1,3 glucan content of the mycelium (2). In spite of this cell wall defect, deletion of AGS1 did not reduce the virulence of the strain in an experimental murine model of invasive aspergillosis (2). In contrast to the case for the ags1Δ mutant, the mycelial cell walls of ags2Δ and ags3Δ mutants had α1,3 glucan levels similar to that of the parental strain. In addition, no modification of the α1,3 glucan content of the conidial cell wall was seen in all single agsΔ mutants. Compensatory expression of the other members of the AGS family, which has been seen in all single agsΔ mutants, could explain the lack of a significant phenotype for each single agsΔ mutant (2, 21). For example, AGS3 and AGS2 showed increased expression in the ags1Δ mutant, and AGS1 expression compensated for the lack of AGS3 in the ags3Δ mutant (21).
Understanding the functional role of α1,3 glucans in A. fumigatus required the construction of a triple ags1Δ ags2Δ ags3Δ mutant devoid of α1,3 glucans. We report here the construction and growth phenotype of this triple mutant. As expected for the triple AGS deletion, the cell wall of the ags1Δ ags2Δ ags3Δ mutant did not contain any α1,3 glucans, but surprisingly, the mutant did not show any reduction in fungal viability and growth in vitro.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
The AkuBku80 pyrGΔ strain was used as the parental strain (6). The AkuBku80 pyrGΔ strain and mutants were maintained on 2% malt agar slants. Conidia were produced on 2% malt agar slants for 5 days at 37°C and recovered by vortexing with 0.05% (vol/vol) Tween 20 aqueous solution. For DNA extraction, mycelium was grown for 16 h at 37°C in a Sabouraud liquid medium supplemented with 10 mM uracil. For transformation experiments, complete medium supplemented with 100 μg/ml hygromycin (Sigma) was used for screening of the single mutant strain (ags1Δ::HPH), with 5 μg/ml phleomycin (Invitrogen) added for the double deletion mutant strain (ags1Δ::HPH ags2Δ::BLE), and minimal medium was used for the selection of the triple deletion mutant strain (ags1Δ::HPH ags2Δ::BLE ags3Δ::PYRG; named the ags1Δ ags2Δ ags3Δ strain in this study). For complementation, minimal medium supplemented with 50 μg/ml sulfonylurea (chlorimuron-ethyl; Sigma) or 10 μg/ml pyrithiamine (sigma) was used for screening of the transformants (10, 17, 29). For cell wall analysis, the mycelium was grown for 24 h at 37°C in Brian's medium (4). For nikkomycin Z (Sigma) susceptibility testing, 1% yeast extract medium was used.
Deletion of the AGS genes and construction of a triple AGS mutant.
Genomic DNA was extracted as described by Girardin et al. (12). For Southern blot analysis, 10 μg of digested genomic DNA was size fractionated in 0.7% agarose and blotted onto a positively charged nylon membrane (Hybond-N+; GE Healthcare).
Deletion cassettes were constructed by joining both 5′- and 3′-flanking sequences of each gene to be deleted with the positively selectable marker HPH, BLE, or PYRG, using the overlap method and the primers described in Table S1 in the supplemental material (18). Upstream and downstream AGS1, AGS2, or AGS3 sequences were amplified from AkuBku80 genomic DNA. Hygromycin, phleomycin, and PYRG resistance cassettes were amplified from pAN 7.1, pAN 8.1, and pAB4-1, respectively (7, 20, 23). The ags1Δ::HPH (ags1Δ) single mutant was constructed by replacing AGS1 with the HPH gene (conferring resistance to hygromycin). The ags1Δ::HPH ags2Δ::BLE (ags1Δ ags2Δ) double deletion mutant was constructed by replacing the AGS2 open reading frame (ORF) with the BLE gene (conferring resistance to phleomycin) in the ags1Δ background. In the ags1Δ::HPH ags2Δ::BLE ags3Δ::PYRG (ags1Δ ags2Δ ags3Δ) triple deletion mutant, the AGS3 ORF was replaced with the PYRG gene from Aspergillus niger (conferring the ability to grow on medium without uracil and uridine) (28) in the double deletion mutant (ags1Δ ags2Δ) background (see Fig. S1). Transformations were achieved by following an electroporation or protoplast protocol (2, 19). The following day, drugs were added to the plates. The plates were incubated for 1 week at 20°C for the ags1Δ and ags1Δ ags2Δ strains and at 37°C for the ags1Δ ags2Δ ags3Δ strain.
For each transformation, transformants obtained under appropriate selective conditions were screened by PCR amplification using three pairs of primers (see Table S1 in the supplemental material). The first pair consisted of one primer designed to target outside the 5′ end of the deletion cassette coupled with one primer designed to target inside the resistance gene. The second pair of primers consisted of one primer designed to target outside the 3′ end of the deletion cassette coupled with one primer designed to target inside the resistance gene. The third pair of primers was designed to target inside the AGS gene. Positive amplicons obtained with only the first two pairs of primers indicated that the full-length deletion cassette was incorporated at the appropriate locus and that the AGS gene was deleted in these transformants. To confirm the results obtained by PCR, genomic DNA was digested by appropriate enzymes and analyzed by Southern blotting (see Fig. S1).
Complementation of the triple mutant.
Strategies to complement the triple AGS mutant are available in the supplemental material.
Phenotypic analysis of mutants.
The carbohydrate compositions of the cell walls of conidia and mycelia were determined as described previously, using three different batches of culture (21, 22). The alkali-insoluble (AI) and alkali-soluble (AS) fractions were extracted from the lyophilized cell wall (22). Monosaccharide composition was analyzed by gas chromatography after hydrolysis with 4 N trifluoroacetic acid for hexoses and 8 N HCl for hexosamines for 4 h at 100°C, followed by reduction and peracetylation of the alkali-insoluble and alkali-soluble fractions (22). α- and β1,3 glucans were determined by measuring the reducing sugar released by recombinant α- and β1,3 glucanases (2). An aggregation assay of germinating conidia was done as previously described (8). Nikkomycin Z susceptibility was tested following an adaptation of the resazurin method described by Clavaud et al. (5).
RESULTS AND DISCUSSION
Construction of a triple AGS mutant.
A triple AGS mutant was generated through successive deletions of the three AGS genes (see Fig. S1 in the supplemental material). Deleting AGS genes in A. fumigatus has always been difficult. Originally, single mutants were obtained in a CBS144-89 wild-type background, with a transformation efficiency of <1% (2, 21). In spite of many attempts, no triple mutants were obtained using this parental strain. In order to improve the transformation efficiency, an AkuBku80 derivative of the same strain, deficient for nonhomologous end joining and favoring homologous recombination, was used (6). Even though the transformation efficiency was improved, the total number of transformants per transformation remained very low (<6/transformation). Four ags1Δ mutants were obtained after 3 transformation experiments; 2 double mutant ags1Δ ags2Δ transformants were selected after 5 transformation experiments. It was verified that the phenotype of the ags1Δ mutant was the same in AkuBku80 and CBS144-89. The ags1Δ ags2Δ mutant had the same phenotype (growth, sporulation, and cell wall composition) as the ags1Δ mutant (data not shown). Finally, after 3 transformations, a unique clone was isolated subsequent to the integration of the ags3Δ cassette at the AGS3 position in the ags1Δ ags2Δ strain to create the triple ags1Δ ags2Δ ags3Δ mutant. The correct integration of the resistance marker at the right locus was verified by Southern blotting.
The triple AGS mutant of A. fumigatus is totally deficient in α1,3 glucans.
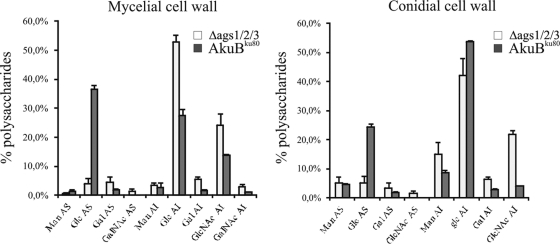
The chemical composition of the A. fumigatus cell wall was investigated in the parental and triple AGS mutant strains. The ratios of AI concentration to AS concentration in the mycelial and conidial cell walls of the ags1Δ ags2Δ ags3Δ mutant were increased in comparison to those of the AkuBku80 parental strain. Ratios of 10 and 2.6 were seen for the ags1Δ ags2Δ ags3Δ and parental strains, respectively, for the mycelium, with ratios of 8 and 2.2, respectively, for the conidia. The high alkali-insoluble/alkali-soluble hexose ratio was due to a reduction in the glucose content of the alkali-soluble fraction of the ags1Δ ags2Δ ags3Δ mutant: there was 4% glucose in the ags1Δ ags2Δ ags3Δ mycelium and 38% glucose in the AkuBku80 mycelium, while there was 5% glucose in the ags1Δ ags2Δ ags3Δ conidia and 24% glucose in the AkuBku80 conidia (Fig. 1). Unexpectedly, a small amount of glucan was found in the AS fraction of the ags1Δ ags2Δ ags3Δ mutant. However, after α1,3 glucanase treatment, the mycelial AS fraction of the parental strain also contained 4% glucan and the conidial AS fraction contained 7% glucan, similar to the amounts encountered in the triple AGS mutant. The remaining glucan in the α1,3 glucanase-treated mycelial and conidial AS fractions of the ags1Δ ags2Δ ags3Δ mutant and parental strains was totally degraded by β1,3 glucanase (data not shown). This result showed that the remaining glucan was a β1,3 glucan. The origin and structure of this small amount of β1,3 glucan in the alkali-soluble fraction remain unknown. This result confirmed, however, that the triple AGS mutant was totally devoid of α1,3 glucans.
Fig 1.
Cell wall compositions of the ags1Δ ags2Δ ags3Δ mutant and the AKuBku80 parental strain. The monosaccharide compositions of the alkali-soluble (AS) and alkali-insoluble (AI) fractions are shown. Glc, glucose; Man, mannose; Gal, galactose; GlcNac, N-acetylglucosamine; GalNac, N-acetylgalactosamine.
The decrease in the α1,3 glucan in the alkali-soluble fraction was compensated by an increase in the galactosamine present in the alkali-soluble and alkali-insoluble fractions (Fig. 1). Polygalactosamine constituted the galactosaminogalactan, which is also an amorphous polysaccharide of the A. fumigatus cell wall (9). β1,3 glucans and chitin were also increased in the mycelial alkali-insoluble fraction, whereas only chitin was increased in the conidial alkali-insoluble fraction (Fig. 1). Similarly, analysis of the cell wall of a C. neoformans ags1Δ mutant demonstrated that the loss of α1,3 glucans was accompanied by a compensatory increase in the chitin/chitosan concentration and a redistribution of β1,3 glucans between the cell fractions (26). The loss of α1,3 glucans in the cell wall of the ags1Δ ags2Δ ags3Δ mutant was compensated by an increase in the alkali-insoluble polysaccharide content (Fig. 1). The compensatory increase of the alkali-insoluble polysaccharide amount showed that A. fumigatus needed to reinforce the rigidity of the cell wall affected by the loss of α1,3 glucans. These compensatory reactions explain why the thicknesses of the cell walls of the triple mutant and parental strains observed by transmission electron microscopy were similar (data not shown). The increase in the amount of chitin in the cell wall of the ags1Δ ags2Δ ags3Δ mutant was correlated with a decrease in the susceptibility of the mutant to the chitin synthase inhibitor nikkomycin Z. MIC values for nikkomycin were 25 μg/ml and >200 μg/ml for AkuBku80 and the ags1Δ ags2Δ ags3Δ mutant, respectively. Seven chitin synthases were found in the A. fumigatus genome (1).
α1,3 glucans are not essential in A. fumigatus.
Unexpectedly, the growth of the triple mutant was similar to that of the AkuBku80 strain in Sabouraud's, Brian's, complete, and minimal media at all temperatures and pHs tested (Fig. 2; data not shown). This result showed that α1,3 glucans were not essential in A. fumigatus and suggested that alkali-soluble polysaccharides of A. fumigatus did not play any structural role in the cell wall of this fungal species. Similarly, a strain of H. capsulatum that did not contain any α1,3 glucans in the cell wall grew like wild-type strains that contained more than 35% α1,3 glucans in the cell wall (16). In other fungi, such as S. pombe and C. neoformans, α1,3 glucans are essential for yeast morphogenesis (14, 26). Similar results were also obtained for other dimorphic pathogenic fungi in which the switch between β1,3 glucans and α1,3 glucans is associated with the changes between mycelium and yeast forms (15, 27). In S. pombe, the content of α1,3 glucans is 28%. Five AGS genes are present in S. pombe, but only AGS1 of S. pombe is an essential gene (14). At a semipermissive temperature, the thermosensitive ags1.1 mutant contains only 7% α1,3 glucans in its cell wall (14). The cells are rounded and pear-shaped, and the cell wall becomes looser and thicker than that of the parental strain.
Fig 2.
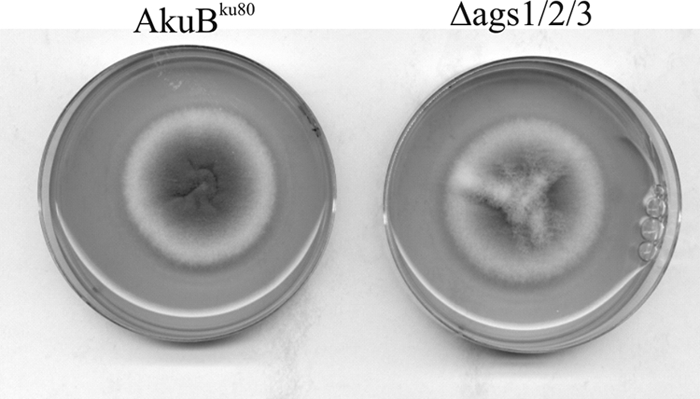
Colonies of the triple AGS mutant (ags1Δ ags2Δ ags3Δ) and the AkuBku80 parental strain grown for 48 h at 37°C on Sabouraud agar medium.
The conidiogenesis of the triple mutant was slightly decreased, as found for the single ags1Δ and ags2Δ mutants, compared to that of the parental strain (2). The viability of the conidia was not affected, and the germination level of the triple AGS mutant was similar to that of the parental strain (data not shown). However, the analysis of the aggregation phenotype of the germinating conidia also confirmed that the triple AGS mutant did not contain α1,3 glucans in the cell wall. As reported earlier, conidia of the parental strain started to aggregate after 90 min of incubation in a culture medium at 37°C, as soon as α1,3 glucans emerged on the cell wall surface. After 3 h, large aggregates containing more than 95% of germinating conidia were seen for the parental AkuBku80 strain (Fig. 3). Previous studies (8) have demonstrated that this aggregation depends exclusively on α1,3 glucan-α1,3 glucan interactions. In contrast, and in agreement with the lack of α1,3 glucans in the cell wall, no aggregation was observed with the germinating conidia of the ags1Δ ags2Δ ags3Δ mutant (Fig. 3).
Fig 3.
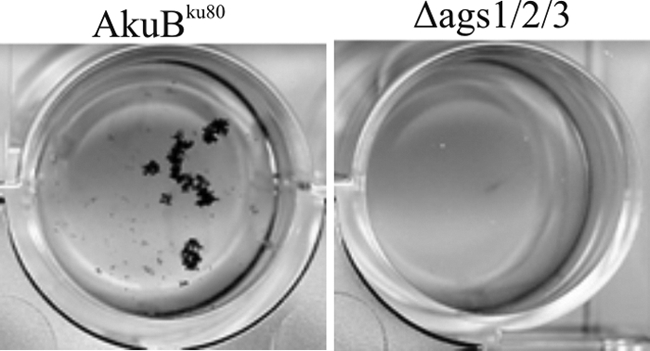
Conidial aggregation after 2 h of incubation at 37°C with shaking (150 rpm). Note the lack of conidial aggregation for the triple ags1Δ ags2Δ ags3Δ mutant.
Unfortunately, in spite of many transformation experiments using the various strategies summarized above, it was impossible to complement the triple AGS mutant. Reasons for these repeated failures remain unknown, but they could be due to the large size of the genes (>8 kb), their in vivo three-dimensional conformation, or the presence of one of these AGS genes (AGS1) in the subtelomeric region of a chromosome (chromosome 3), which is always difficult to manipulate genetically (11). Nevertheless, the lack of a growth phenotype in a triple AGS mutant totally devoid of α1,3 glucans showed definitively that the α1,3 glucans that are the major cell wall component are fully dispensable for A. fumigatus vegetative growth.
Supplementary Material
Footnotes
Published ahead of print 4 November 2011
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Alcazar-Fuoli L, et al. 2011. Functional analysis of the fungal/plant class chitinase family in Aspergillus fumigatus. Fungal Genet. Biol. 48:418–429 [DOI] [PubMed] [Google Scholar]
- 2. Beauvais A, et al. 2005. Two alpha(1-3) glucan synthases with different functions in Aspergillus fumigatus. Appl. Environ. Microbiol. 71:1531–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beauvais A, et al. 2007. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell. Microbiol. 9:1588–1600 [DOI] [PubMed] [Google Scholar]
- 4. Brian PW, et al. 1961. Phytotoxic compounds produced by Fusarium equiseti. J. Exp. Bot. 12:1–12. [Google Scholar]
- 5. Clavaud C, Beauvais A, Barbin L, Munier-Lehman H, Latgé JP. The composition of the culture medium influences the β1,3 glucan metabolism of Aspergillus fumigatus and the antifungal activity of inhibitors of β1,3 glucan synthesis tested alone or in combination. Antimicrob. Agents Chemother., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. da Silva Ferreira ME, et al. 2006. The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. d'Enfert C. 1996. Selection of multiple disruption events in Aspergillus fumigatus using the orotidine-5′-decarboxylase gene, pyrG, as a unique transformation marker. Curr. Genet. 30:76–82 [DOI] [PubMed] [Google Scholar]
- 8. Fontaine T, et al. 2010. Cell wall alpha1-3 glucans induce the aggregation of germinating conidia of Aspergillus fumigatus. Fungal Genet. Biol. 47:707–712 [DOI] [PubMed] [Google Scholar]
- 9. Fontaine T, et al. 2011. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 7:e1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fudal I, Collemare J, Bohnert HU, Melayah D, Lebrun MH. 2007. Expression of Magnaporthe grisea avirulence gene ACE1 is connected to the initiation of appressorium-mediated penetration. Eukaryot. Cell 6:546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibbons JG, et al. 2012. Global transcriptome changes underlying colony growth in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot. Cell 11:68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Girardin H, Latge JP, Srikantha T, Morrow B, Soll DR. 1993. Development of DNA probes for fingerprinting Aspergillus fumigatus. J. Clin. Microbiol. 31:1547–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grun CH, et al. 2005. The structure of cell wall alpha-glucan from fission yeast. Glycobiology 15:245–257 [DOI] [PubMed] [Google Scholar]
- 14. Hochstenbach F, et al. 1998. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc. Natl. Acad. Sci. U. S. A. 95:9161–9166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hogan LH, Klein BS. 1994. Altered expression of surface alpha-1,3-glucan in genetically related strains of Blastomyces dermatitidis that differ in virulence. Infect. Immun. 62:3543–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klimpel KR, Goldman WE. 1988. Cell walls from avirulent variants of Histoplasma capsulatum lack alpha-(1,3)-glucan. Infect. Immun. 56:2997–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kubodera T, Yamashita N, Nishimura A. 2002. Transformation of Aspergillus sp. and Trichoderma reesei using the pyrithiamine resistance gene (ptrA) of Aspergillus oryzae. Biosci. Biotechnol. Biochem. 66:404–406 [DOI] [PubMed] [Google Scholar]
- 18. Lamarre C, Ibrahim-Granet O, Du C, Calderone R, Latge JP. 2007. Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Genet. Biol. 44:682–690 [DOI] [PubMed] [Google Scholar]
- 19. Lambou K, Perkhofer S, Fontaine T, Latge J-P. 2010. Comparative functional analysis of the OCH1 mannosyltransferase families in Aspergillus fumigatus and Saccharomyces cerevisiae. Yeast 75:910–923 [DOI] [PubMed] [Google Scholar]
- 20. Mattern IE, Punt PJ, Van den Hondel CAMJJ. 1988. A vector of Aspergillus transformation conferring phleomycin resistance. Fungal Genet. Newsl. 35:25 [Google Scholar]
- 21. Maubon D, et al. 2006. AGS3, an alpha(1-3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet. Biol. 43:366–375 [DOI] [PubMed] [Google Scholar]
- 22. Mouyna I, et al. 2010. Members of PMT family in Aspergillus fumigatus differentially affect growth, morphogenesis, and viability. Mol. Microbiol. 76:1205–1221 [DOI] [PubMed] [Google Scholar]
- 23. Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CA. 1987. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117–124 [DOI] [PubMed] [Google Scholar]
- 24. Rappleye CA, Engle JT, Goldman WE. 2004. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol. Microbiol. 53:153–165 [DOI] [PubMed] [Google Scholar]
- 25. Reese AJ, Doering TL. 2003. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol. Microbiol. 50:1401–1409 [DOI] [PubMed] [Google Scholar]
- 26. Reese AJ, et al. 2007. Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol. Microbiol. 63:1385–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. San-Blas F, San-Blas G, Cova LJ. 1976. A morphological mutant of Paracoccidioides brasiliensis strain IVIC Pb9. Isolation and wall characterization. J. Gen. Microbiol. 93:209–218 [DOI] [PubMed] [Google Scholar]
- 28. Weidner G, d'Enfert C, Koch A, Mol PC, Brakhage AA. 1998. Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5′-monophosphate decarboxylase. Curr. Genet. 33:378–385 [DOI] [PubMed] [Google Scholar]
- 29. Zhang S, Fan Y, Xia YX, Keyhani NO. 2010. Sulfonylurea resistance as a new selectable marker for the entomopathogenic fungus Beauveria bassiana. Appl. Microbiol. Biotechnol. 87:1151–1156 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.