Abstract
Association of pneumococcal nasopharyngeal carriage with the concentration and opsonophagocytic activity (OPA) of serum serotype-specific antibodies was determined for toddlers 1 month after immunization with a 9-valent pneumococcal conjugate vaccine. Higher anti-serotype 14 and anti-serotype 19F IgG and anti-serotype 14 IgM correlated with a lowered probability of pneumococcal acquisition. Postvaccination OPA did not correlate with pneumococcal carriage.
TEXT
Streptococcus pneumoniae is a leading cause of morbidity and mortality worldwide. The first essential step in all pneumococcal diseases is the symptomless colonization of the nasopharynx (carriage). Use of pneumococcal conjugate vaccines (PCVs) effectively prevents pneumococcal carriage of vaccine serotypes and serotype 6A (4–6, 9, 13, 14). In our previous study, an increasing serum antipolysaccharide IgG concentration after vaccination with a 9-valent PCV (PCV9) significantly decreased the probability of new acquisitions for vaccine serotypes 14 and 19F and for the vaccine-related serotype 6A (3). The objective of the present study was to update and supplement these findings by analyzing the same samples with additional, up-to-date assays. More precisely, we compared the association of postvaccination serum serotype-specific IgG and IgM and opsonophagocytic activity (OPA) with carriage of four vaccine serotypes (9V, 14, 19F, and 23F) and one vaccine-related serotype (6A) in toddlers immunized with one dose of PCV9.
The serum samples were obtained from a previous, randomized study of the effect of PCV9 on pneumococcal nasopharyngeal carriage in healthy Israeli toddlers attending day care centers (4). The vaccine used contained 2 μg each of pneumococcal serotype 1, 4, 5, 9V, 14, 18C, 19F, and 23F carbohydrates and 4 μg of serotype 6B carbohydrate coupled to the diphtheria toxin CRM197 variant (Wyeth-Lederle Vaccines [Pfizer at present]). Nasopharyngeal swabs for bacterial culture and identification of S. pneumoniae were obtained at 1- and 2-month intervals for the first and second year of life, respectively (4). Blood samples for serological assays were obtained 1 month after complete immunization. The sample set of the present study consisted of toddlers aged 18 to 35 months immunized with one dose of PCV9 (n = 81).
A modification of the serotype 22F inhibition enzyme immunoassay (EIA) applied by the WHO reference laboratory at the Institute of Child Health (London, United Kingdom) was used to measure the concentrations of IgG and IgM against pneumococcal serotypes 6A, 9V, 14, 19F, and 23F (17). These serotypes were the most frequently carried serotypes in the study population. The average IgG and IgM antibody concentrations are given as geometric mean concentrations (GMCs) with 95% confidence intervals (CIs). In our previous study (3), the antipolysaccharide IgG concentrations in the same sera were analyzed by non-serotype 22F inhibition EIA (15). The IgG measured now correlated significantly with the previous results (r = 0.83 to 0.90; P < 0.01), while the antibody concentrations tended to be slightly lower with serotype 22F EIA than with non-serotype 22F EIA. The opsonic activities of antipneumococcal antibodies against pneumococcal serotypes 6A, 9V, 14, 19F, and 23F were measured by a 4-fold multiplexed opsonophagocytic activity (MOPA4) assay (1, 18). The opsonophagocytic activities are given as geometric mean opsonic titers (GMOPTs) with 95% CIs.
We first compared the GMCs and GMOPTs of the serotype-specific antibodies in toddlers who carried S. pneumoniae of the same serotype in their nasopharynx (carriers) and those who did not (noncarriers) 1 month after PCV9 immunization (Table 1). The noncarriers had significantly higher GMCs of anti-serotype 14 and anti-serotype 19F IgG (P = 0.002 and 0.04, respectively) and anti-serotype 14 IgM (P = 0.04) than the carriers. For the other serotypes, noncarriers had slightly higher GMCs of anti-serotype 23F IgG as well as anti-serotype 6A IgM, but these differences did not reach statistical significance. The GMOPT of anti-serotype 6A tended to be slightly higher in the noncarriers than in the carriers (P = 0.05).
TABLE 1.
GMCs of serotype-specific anti-pneumococcal polysaccharide (anti-PPS) IgG and IgM and GMOPTs of anti-PPS antibodies 1 month after PCV9 immunization in toddlers who carried pneumococci of the same serotype in their nasopharynx (carriers) and those who did not carry the serotype (noncarriers)
| Variable and serotype | Carriers |
Noncarriers |
P valuea |
||
|---|---|---|---|---|---|
| n | GMC or GMOPT (95% CI) | n | GMC or GMOPT (95% CI) | ||
| IgG | |||||
| 6A | 33 | 0.34 (0.23–0.51) | 38 | 0.40 (0.26–0.62) | 0.61 |
| 9V | 6 | 2.90 (1.25–6.72) | 65 | 1.72 (1.35–2.20) | 0.22 |
| 14 | 12 | 0.51 (0.28–0.93) | 55 | 1.65 (1.20–2.26) | 0.002 |
| 19F | 31 | 1.10 (0.75–1.63) | 40 | 1.91 (1.32–2.75) | 0.043 |
| 23F | 18 | 0.63 (0.28–1.41) | 53 | 0.93 (0.60–1.45) | 0.37 |
| IgM | |||||
| 6A | 33 | 6.26 (5.00–7.84 | 38 | 7.71 (6.01–9.88) | 0.22 |
| 9V | 6 | 1.54 (0.87–2.75) | 66 | 1.54 (1.26–1.88) | 1 |
| 14 | 12 | 2.62 (1.61–4.27) | 60 | 4.47 (3.62–5.52) | 0.041 |
| 19F | 31 | 1.91 (1.38–2.66) | 40 | 2.07 (1.70–2.53) | 0.66 |
| 23F | 18 | 0.85 (0.43–1.68) | 53 | 0.93 (0.69–1.26) | 0.77 |
| MOPA | |||||
| 6A | 37 | 621 (229–1687) | 42 | 1939 (1023–3674) | 0.06 |
| 9V | 7 | 9545 (3403–26771) | 72 | 10276 (7183–14702) | 0.90 |
| 14 | 13 | 4995 (2385–10458) | 67 | 5458 (3720–8008) | 0.85 |
| 19F | 33 | 533 (250–1136) | 48 | 729 (404–1317) | 0.51 |
| 23F | 25 | 687 (185–2551) | 56 | 1811 (976–3358) | 0.12 |
Student's t test was used for statistical comparisons.
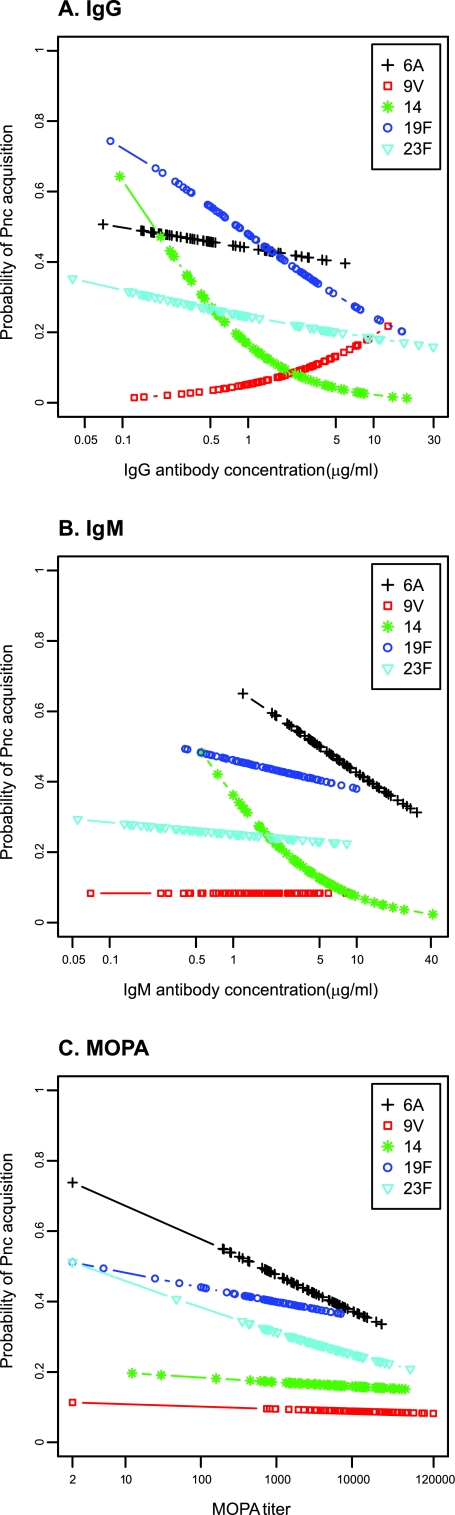
To evaluate whether the postvaccination serological variables were associated with new acquisitions of pneumococcal carriage, we used a logistic regression model reporting the odds ratio (OR) for the association between a serological variable and pneumococcal acquisition (with logarithmic IgG, IgM, or MOPA as a covariate). In this model, higher postvaccination IgG and IgM concentrations against serotype 14 and higher IgG concentrations against serotype 19F significantly reduced the probability of having a new acquisition of these serotypes (Table 2 and Fig. 1A and B). A similar but not statistically significant trend was detected for new acquisitions of serotype 6A in relation to higher anti-serotype 6A IgM concentrations (Table 2 and Fig. 1B). Higher postvaccination IgM concentrations against the other three serotypes (9V, 19F, and 23F) were not associated with the subsequent acquisition of these serotypes (Table 2 and Fig. 1B). No significant associations were found for any serotype between the postvaccination MOPA and subsequent acquisition (Table 2 and Fig. 1C).
TABLE 2.
Prediction of acquisition of postimmunization pneumococcal carriage 1 month after immunization with a 9-valent pneumococcal conjugate vaccine, with serum serotype-specific IgG and IgM antibodies and MOPA of antipneumococcal antibodies as covariates, in a logistic regression model
| Serotype | Probability of pneumococcal carriage (OR [95% CI])a |
||
|---|---|---|---|
| IgG | IgM | MOPA | |
| 6A | 0.90 (0.62–1.32) | 0.65 (0.33–1.29) | 0.83 (0.69–1.01) |
| 9V | 1.88 (0.69–5.09) | 1.00 (0.34–2.91) | 0.97 (0.59–1.60) |
| 14 | 0.39 (0.20–0.76) | 0.43 (0.19–0.99) | 0.96 (0.66–1.41) |
| 19F | 0.63 (0.40–0.996) | 0.87 (0.46–1.63) | 0.93 (0.75–1.15) |
| 23F | 0.85 (0.60–1.20) | 0.93 (0.58–1.49) | 0.87 (0.73–1.04) |
Statistically significant results are shown in bold.
FIG 1.
Probability (OR) of acquisition of pneumococcal carriage 1 month after immunization with one dose of 9-valent pneumococcal conjugate vaccine in association with postimmunization IgG (A), IgM (B), or MOPA (C). Pnc, S. pneumoniae.
While the approach of this study was similar to that reported earlier for the same sample material (3), we now used an improved EIA method with serotype 22F inhibition and also measured IgM and MOPA in addition to IgG. To our knowledge, there are no earlier reports that associate serum IgM concentration with pneumococcal carriage. A dose of PCV is able to induce a significant IgM response that is measurable 1 month after immunization in Israeli (unpublished data) and Finnish (12) toddlers, which according to our recent findings may contribute to the opsonophagocytosis of S. pneumoniae (B. Simell, A. Nurkka, K. Jousimies, S. Grönholm, N. Givon-Lavi, H. Käyhty, and R. Dagan, presented at the 7th International Symposium on Pneumococci and Pneumococcal Diseases, Tel Aviv, Israel, 14 to 18 March 2010). Small amounts of IgM are usually detected on mucosal surfaces, but this IgM is in a secretory form and thus originates from local production by mucosal plasma cells (2).
In an earlier report (3), higher IgG concentrations led to a decreasing probability of having a new acquisition, which achieved statistical significance for serotypes 14 and 19F. This observation was now confirmed in the present study. In the earlier report (3), cross-protection against serotype 6A was observed for anti-serotype 6B IgG, whereas in the present study setting anti-serotype 6A IgG was not associated with new acquisitions of serotype 6A. Besides anti-serotype 14 IgG, a higher anti-serotype 14 IgM concentration was inversely correlated with new acquisitions of this serotype. Comparison of the IgG and IgM concentrations in carrier and noncarrier children (Table 1) gave concordant results with the logistic regression model (Table 2).
We did not find any significant associations between pneumococcal carriage and postvaccination MOPA. The primary mechanism for eliminating S. pneumoniae from the host during invasive infection is opsonophagocytosis, where bacteria are opsonized with anticapsular polysaccharide antibodies followed by activation of the complement system and receptor-mediated uptake and killing of S. pneumoniae by phagocytic cells (7, 16, 20). While MOPA has been shown to be predictive for the serotype-specific efficacy of PCVs against invasive pneumococcal disease (8), the finding of MOPA not being associated with pneumococcal carriage—an event taking place on mucosal surfaces, where complement and phagocytes may be less abundant—is not surprising.
We conclude that no association could be demonstrated with antipneumococcal antibody MOPA in toddlers 1 month after immunization with PCV9 and the probability of new acquisition of pneumococcal nasopharyngeal carriage, whereas higher IgG concentrations specific to serotypes 14 and 19F and higher IgM concentrations specific to serotype 14 inversely correlated with new acquisitions of these serotypes. It was speculated recently that serum antibodies may represent merely markers of the immune response against pneumococcal colonization, while the actual effectors lie elsewhere (10, 11, 19). Optimal strategies for prevention of pneumococcal carriage and generation of herd immunity by vaccination may require the induction of both antipolysaccharide and antiprotein antibodies, the stimulation of both antibody-dependent and cell-mediated arms of the acquired immune system, and mucosal immunity.
(The data reported here were presented at the 7th International Symposium on Pneumococci and Pneumococcal Diseases, Tel Aviv, Israel, 14 to 18 March 2010.)
ACKNOWLEDGMENTS
We thank Kaisa Jousimies and Sinikka Grönholm for skilled technical assistance.
In the last 5 years, Ron Dagan has received grants/research support from Berna/Crucell, Wyeth/Pfizer, MSD, and Protea; has been a scientific consultant for Berna/Crucell, GSK Bio, Novartis, Wyeth/Pfizer, Protea, and MSD; has been a speaker for Berna/Crucell, GSK Bio, and Wyeth/Pfizer; and has been a shareholder of Protea. In the last 5 years, Helena Käyhty has provided consultancies on advisory boards for GSK Bio; has had travels paid by GSK Bio and Novartis as a scientific consultant, invited speaker, or expert at symposia; and has received honoraria from GSK Bio. Jukka Jokinen is a coinvestigator in a nationwide effectiveness study of a 10-valent pneumococcal conjugate vaccine funded mainly by GlaxoSmithKline.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Burton RL, Nahm MH. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 13:1004–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cripps AW, Gleeson M, Clancy RL. 1991. Ontogeny of the mucosal immune response in children. Adv. Exp. Med. Biol. 310:87–92 [DOI] [PubMed] [Google Scholar]
- 3. Dagan R, et al. 2005. Serum serotype-specific pneumococcal anticapsular immunoglobulin G concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J. Infect. Dis. 192:367–376 [DOI] [PubMed] [Google Scholar]
- 4. Dagan R, et al. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927–936 [DOI] [PubMed] [Google Scholar]
- 5. Dagan R, et al. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 174:1271–1278 [DOI] [PubMed] [Google Scholar]
- 6. Dagan R, Muallem M, Melamed R, Leroy O, Yagupsky P. 1997. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphtheria toxoid. Pediatr. Infect. Dis. J. 16:1060–1064 [DOI] [PubMed] [Google Scholar]
- 7. Fearon DT, Locksley RM. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50–53 [DOI] [PubMed] [Google Scholar]
- 8. Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. 2007. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 25:2518–2527 [DOI] [PubMed] [Google Scholar]
- 9. Mbelle N, et al. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180:1171–1176 [DOI] [PubMed] [Google Scholar]
- 10. McCool TL, Cate TR, Moy G, Weiser JN. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCool TL, Weiser JN. 2004. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect. Immun. 72:5807–5813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieminen T, Kayhty H, Leroy O, Eskola J. 1999. Pneumococcal conjugate vaccination in toddlers: mucosal antibody response measured as circulating antibody-secreting cells and as salivary antibodies. Pediatr. Infect. Dis. J. 18:764–772 [DOI] [PubMed] [Google Scholar]
- 13. Obaro SK, Adegbola RA, Banya WA, Greenwood BM. 1996. Carriage of pneumococci after pneumococcal vaccination. Lancet 348:271–272 [DOI] [PubMed] [Google Scholar]
- 14. O'Brien KL, et al. 2007. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J. Infect. Dis. 196:1211–1220 [DOI] [PubMed] [Google Scholar]
- 15. Quataert SA, et al. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rothlein R, Springer TA. 1985. Complement receptor type three-dependent degradation of opsonized erythrocytes by mouse macrophages. J. Immunol. 135:2668–2672 [PubMed] [Google Scholar]
- 17. Simell B, Lahdenkari M, Reunanen A, Kayhty H, Vakevainen M. 2008. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 15:1391–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simell B, et al. 2011. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 29:1929–1934 [DOI] [PubMed] [Google Scholar]
- 19. Trzcinski K, Thompson C, Malley R, Lipsitch M. 2005. Antibodies to conserved pneumococcal antigens correlate with, but are not required for, protection against pneumococcal colonization induced by prior exposure in a mouse model. Infect. Immun. 73:7043–7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walport MJ. 2001. Complement. N. Engl. J. Med. 344:1058–1066 [DOI] [PubMed] [Google Scholar]