Abstract
The second-generation MVista Blastomyces antigen enzyme immunoassay was not quantitative; therefore, specimens obtained previously were tested in the same assay as new specimens to assess the change in antigen levels. Furthermore, the sensitivity in serum had not been fully evaluated. The purpose of this study was to evaluate a quantitative Blastomyces antigen assay and detection of antigen in serum. Calibrators containing known concentrations of Blastomyces galactomannan were used to quantify antigen in urine and serum from patients with proven blastomycosis and from controls. Paired current and previously obtained urine specimens were tested to determine if quantification eliminated the need for concurrent testing to assess change in antigen. Pretreatment of serum with EDTA at 104°C was evaluated to determine if dissociation of immune complexes improved detection of antigenemia. Antigenuria was detected in 89.9% of patients with culture- or histopathology-proven blastomycosis. Specificity was 99.0% in patients with nonfungal infections and healthy subjects, but cross-reactions occurred in 95.6% of patients with histoplasmosis. Change in antigen level categorized as increase, no change, or decrease based on antigen units determined in the same assay agreed closely with the category of change in ng/ml determined from different assays. Pretreatment increased the sensitivity of detection of antigenemia from 35.7% to 57.1%. Quantification eliminated the need for concurrent testing of current and previously obtained specimens for assessment of changes in antigen concentration. Pretreatment increased the sensitivity for detection of antigenemia. Differentiation of histoplasmosis and blastomycosis is not possible by antigen detection.
INTRODUCTION
Antigen detection is a useful method for diagnosis of blastomycosis. The sensitivity has been reported to be over 90% and specificity has been reported to be 100% in healthy subjects, but cross-reactions occur in most patients with histoplasmosis (6). In a recent report of 59 patients with proven blastomycosis (4), culture was positive in 86%, histopathology in 81%, antigen in 74%, cytopathology in 38%, and immunodiffusion for antibody in 32% (4). Antigen detection was considered to be a “reliable method to make an accurate and rapid diagnosis of blastomycosis, particularly when a large burden of disease is present, and when cytological analysis is performed at less-experienced centers” (4). Blastomyces antigen detection was also reported to be useful for diagnosis of blastomycosis in solid organ transplant patients (7).
Because of interassay variability, specimens obtained earlier have been tested simultaneously with current specimens to assess the change in antigen levels. In a Histoplasma antigen assay, quantification of galactomannan antigen eliminated the need to test the previously obtained specimen with the current specimen to determine change in antigen level (5). In a recent study, quantification and improved detection of antigen in serum following EDTA treatment at 104°C in the third-generation MVista Blastomyces antigen assay were described (3). The present study assessed quantification and EDTA-heat treatment in a larger group of patients to determine whether quantification eliminates the need for testing current and previous specimens concurrently to quantify the change in antigen concentration.
MATERIALS AND METHODS
Patients.
Patients had proven blastomycosis based upon isolation of the organism or demonstration of broad-budding yeast by histochemical staining. These included previously described cases (3) for which clinical and other laboratory data were available from a retrospective multicenter study approved by the institutional review committees at three institutions (n = 40) and cases identified during routine clinical testing with limited information that was provided by their physicians for which institutional review was not obtained (n = 49). Specimens were obtained at the time of initial diagnosis in these 89 cases.
Additionally, 178 paired urine samples from 89 patients, previously submitted for Blastomyces antigen testing, were tested on two different days, and results were calculated as enzyme units (EU) by comparison to the negative control or as nanograms per milliliter by extrapolation from the standard curve. These sample pairs were chosen to include a full spectrum of antigen levels and changes in levels between current and previous specimen pairs, including pairs showing an increase in antigen of at least 4 units (n = 13), a decrease of at least 4 units (n = 21), or no change (n = 55).
Control specimens were obtained from 131 patients evaluated at Indiana University Medical Center in which the diagnosis of a fungal infection was not established, 68 healthy commercial donors from SeraCare Life Sciences (Milford, MA), and 90 patients with proven disseminated histoplasmosis from a multicenter study (9).
Quantitative Blastomyces antigen enzyme immunoassay.
The concentration of antigen was determined by comparison to calibrators containing known amounts of Blastomyces dermatitidis galactomannan antigen, prepared from a pool of urine specimens from patients with blastomycosis and quantified by comparison to galactomannan purified from B. dermatitidis mold, as described previously for Histoplasma capsulatum galactomannan (5). Calibrators contained 14.7, 9.7, 5.7, 3.0, 1.6, 0.7, 0.4, and 0.2 ng/ml of Blastomyces galactomannan.
Pretreatment of serum at 104°C with EDTA.
The pretreatment procedure has been described for detection of Histoplasma antigen (11). A total of 200 μl of 4% EDTA was added to 600 μl of serum, vortex mixed, and placed in a heat block (Fisher Scientific) at 104°C for 6 min. The specimen was then centrifuged for 10 min at 10,000 × g, and the supernatant was removed for testing in the antigen enzyme immunoassay (EIA).
Statistical analysis.
The cutoff for positivity was determined by receiver operating characteristic (ROC) curve analysis. Comparisons between the semiquantitative and quantitative assay were evaluated by linear regression analysis. To test the hypothesis that quantification eliminated the need to include the previously obtained specimen in the same assay, changes in antigen levels determined using results for paired current and previously obtained specimens from the same assay were compared to those using current and previously obtained results from different assays. t test was used to compare the mean antigen concentrations. A chi-square test was used to compare the proportions between the different tests. A paired t test and McNemar's test were used when paired results were compared. 95% confidence intervals for the sensitivity and proportions were calculated using the Wilson score method for small samples with asymmetrical distribution (1).
RESULTS
Patients.
Of 89 total cases, 40 were from the multicenter study (3) and 49 from clinical testing. All had positive cultures and/or pathology. Forty-six (51.7%) exhibited pulmonary disease, 26 (29.2%) exhibited combined pulmonary and extrapulmonary disease, and 17 (19.1%) exhibited extrapulmonary disease only.
ROC analysis.
Urine specimens from 89 patients with proven blastomycosis and 199 controls, including 68 healthy subjects and 131 patients without fungal infection, were tested simultaneously. The receiver operating characteristic (ROC) curve indicated that an optical density (OD) of 0.053 best discriminated cases from controls (Fig. 1). At this cutoff, the sensitivity was 91.7%, specificity was 99.0%, area under the curve (AUC) was 0.962, the 95% confidence interval was 0.932 to 0.981, and P was 0.0001.
Fig 1.
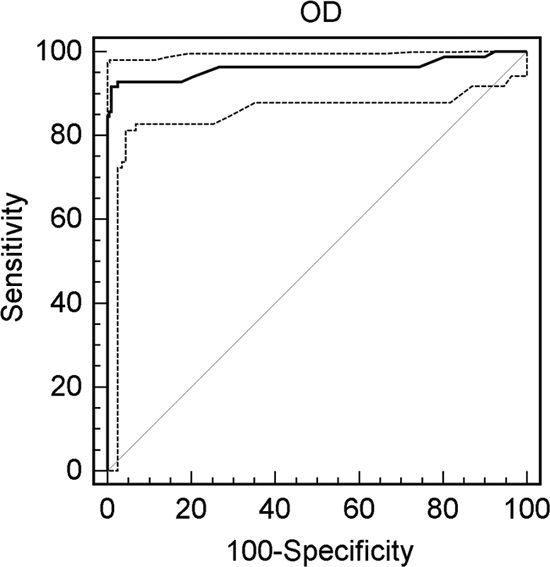
ROC curve for determination of cutoff. With a cutoff OD of 0.053, the sensitivity is 91.7% and the specificity is 99.0%. The dashed lines represent the 95% confidence interval.
Sensitivity.
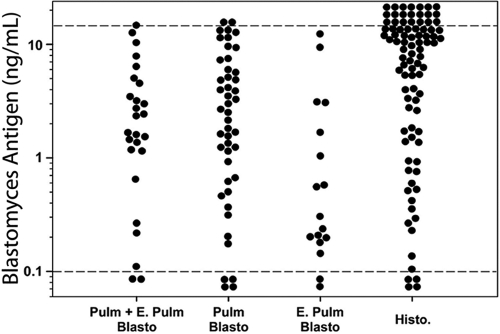
Antigenuria was detected in 80 of 89 (89.9%) cases, including 41 of 46 (91.3%) with isolated pulmonary involvement, 24 of 26 (92.3%) with combined pulmonary and extrapulmonary involvement, and 15 of 17 (88.2%) with extrapulmonary involvement alone. Results for individual cases are shown in Fig. 2. Mean antigen concentration was highest in patients with pulmonary manifestations (4.3 ng/ml), intermediate in those with combined pulmonary and extrapulmonary manifestations (3.4 ng/ml) and lowest in those with extrapulmonary manifestations only (1.9 ng/ml) (P = 0.148). Antigenuria was present in 24 of 27 (88.9%) patients with positive pathology compared to 12 of 14 (85.7%) with negative pathology, at similar concentration, 4.1 ng/ml and 3.1 ng/ml, respectively (P = 0.564). Antigenuria was higher in patients with acute respiratory distress syndrome (ARDS) (n = 5) than in other subjects (n = 84), 8.8 ng/ml versus 3.3 ng/ml, respectively (P = 0.004).
Fig 2.
Antigenuria in patients with blastomycosis and controls. Pulm, pulmonary; E. Pulm, extrapulmonary; Blasto, blastomycosis; Histo, histoplasmosis; Pulm + E. Pulm indicates both pulmonary and extrapulmonary, Pulm indicates pulmonary only, and E. Pulm indicates extrapulmonary only. The cutoff for positivity is indicated by the lower dashed line. The upper dashed line indicates the upper limit of quantification.
Specificity.
Antigen was not detected in 197 of 199 (99.0%) controls with nonfungal diseases or healthy subjects. Cross-reactions occurred in 86 of 90 (95.6%) of patients with proven disseminated histoplasmosis. The antigenuria concentration for the histoplasmosis cases was 12.6 ng/ml in the Histoplasma antigen assay and 8.4 ng/ml in the Blastomyces antigen assay (P < 0.001). In addition, Blastomyces antigenuria in patients with pulmonary and/or extrapulmonary blastomycosis was lower than Histoplasma antigenuria in patients with disseminated histoplasmosis, 3.6 ng/ml and 12.6 ng/ml, respectively (P < 0.001).
Antigenemia.
Antigenemia was determined with and without pretreatment of serum with EDTA at 104°C. Antigenemia was detected in 10 of 28 (35.7%) cases without and 16 of 28 (57.1%) with EDTA-heat pretreatment (P = 0.097). Among 10 specimens positive with and without treatment, the concentration increased from 4.1 ng/ml to 6.3 ng/ml with pretreatment (P = 0.019). Among 20 patients for whom urine and serum were tested, both were positive for 12 (60%), urine only was positive for six (30%), and neither was positive for two (10%); for the 12 in which both were positive, concentrations in urine and serum were similar, 5.1 ng/ml and 4.2 ng/ml, respectively (P = 0.402).
Interassay agreement in paired current and previously obtained specimens.
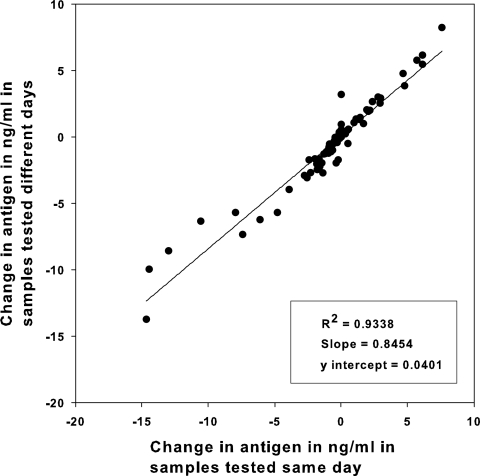
Paired current and previously obtained urine specimens were tested twice. Change in antigen levels between paired samples showed excellent agreement when calculated as ng/ml on different days or the same day: R2 = 0.974 (Fig. 3).
Fig 3.
Interassay agreement of change in 89 blastomycosis cases with paired current and previously obtained specimens, comparing results determined in the same assay (same day) with that determined in different assays (different days). Data below the 0 value represent pairs in which the concentration declined, and those above 0 represent those in which the concentration increased. Changes of 2 ng/ml or more were considered significant.
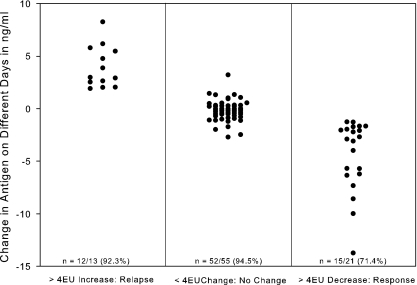
Cases were categorized into three groups based upon same-day differences in EIA units: ≥4-unit increases, ≥4-unit decreases, or no change (<4-unit increase or decrease), as previously reported for histoplasmosis (5). The difference in concentration (ng/ml) between the current and previously obtained specimens tested on different days was analyzed according to the same-day category: ≥4-unit increase, ≥4-unit decrease, or no change (<4-unit increase or decrease). A 2.0-ng/ml or greater increase occurred in 12 of 13 (92.3%) of cases with an increase in antigen levels of 4 or more EIA units, while a similar decrease occurred in 15 of 21 (71.4%) cases with a 4-unit or greater decrease (Fig. 4). Of those with a <4-unit change, 52 of 55 (94.5%) did not change by more than 2 ng/ml. The numbers of days between the previous and current specimens for the group with the >4-unit increase were as follows: mean, 76 days; standard deviation, 98 days; median, 31 days; range, 8 to 357 days. Values for the group with the >4-unit decrease were as follows: mean, 119 days; standard deviation, 125 days; median, 56 days; range, 15 to 539 days. Values for the no-change (<4-unit-change) group were as follows: mean, 68 days; standard deviation, 54 days; median, 54 days; range, 15 to 343 days.
Fig 4.
Change in antigen concentration in 89 patients between a current and previously obtained specimen according to category of change in antigen units in the same assay. The values (number/total and percent) at the bottom of each column show agreement between category of change (in ng/ml) as determined from different days' assays with the change in antigen units determined from the same assay. For determination of agreement, a significant change was defined as >2.0 ng/ml.
Precision and reproducibility.
The intra-assay precision was determined by testing three aliquots of eight reference samples that spanned the reportable range in the same assay, and the interassay precision was determined by testing three aliquots of those eight reference samples in 10 assays performed over 10 days. For assessment of interassay precision, the mean concentration for the eight reference samples was 3.94 ng/ml, the standard deviation was 0.42 ng/ml, and the coefficient of variation was 14.37%; for intra-assay precision, the mean concentration was 3.94 ng/ml, the standard deviation was 0.38 ng/ml, and the coefficient of variation was 13.30%. The results of the initial and repeat testing of urine specimens from patients with blastomycosis were reproducibly positive or negative in 91 of 93 cases (97.8%) and initial with repeat result correlated closely by linear regression (Passing and Bablock method); slope, 1.0; y intercept, 0.01; P < 0.01.
DISCUSSION
The sensitivity of the quantitative Blastomyces antigen assay for detection of antigenuria was 90%, similar to results in previous reports (85% to 93%) (3, 6). Antigenuria concentrations were higher in patients with pulmonary blastomycosis alone or combined with extrapulmonary blastomycosis than in those with isolated extrapulmonary blastomycosis and highest in patients with ARDS. Antigenuria was present in 86% of cases in which microscopy was negative. Specificity was 99% in healthy subjects and patients with nonfungal disease, but cross-reactions occurred in 96% of patients with histoplasmosis. Blastomyces antigenuria in patients with proven extrapulmonary blastomycosis was lower than Histoplasma antigenuria in patients with proven disseminated histoplasmosis: mean concentrations, 3.3 ng/ml and 12.6 ng/ml, respectively. It is noteworthy that this was not a prospective study but rather used stored specimens from proven cases of blastomycosis and histoplasmosis. Bias toward selection of antigen-positive cases may have overestimated the sensitivity of antigen detection.
EDTA-heat treatment improved the sensitivity for detection of antigenemia. Sensitivity was 57%, lower than previously reported (82%) (3). More cases were tested in the current report than the previous report: 28 and 11, respectively. However, antigenemia was detected less frequently in patients with blastomycosis than in those with histoplasmosis: 90% in patients with disseminated histoplasmosis (11) and 80% in those with acute pulmonary histoplasmosis (12).
Less antigen appears to be produced in blastomycosis than in histoplasmosis. A larger fungal burden is expected in histoplasmosis, since multiple reticuloendothelial organs are commonly involved, in contrast to isolated lesions in the skin and subcutaneous tissue, bones, and joints in most patients with blastomycosis. Additionally, fungemia is rare in extrapulmonary blastomycosis but common in disseminated histoplasmosis: no cases of fungemia were found in this study or in 59 recently reported cases (4) compared to 50% of cases of disseminated histoplasmosis (2).
The change in antigen units measured in the same assay correlated well with the change in concentration in different assays, as reported for the MVista Histoplasma quantitative antigen assay (5). To date there is meager evidence for use of the antigen assay for monitoring therapy for blastomycosis. Antigen persistence was reported for a patient who failed itraconazole therapy, compared to steady clearance in two others who responded to therapy (13). Antigenemia and antigenuria steadily declined during itraconazole therapy in dogs with blastomycosis (10). Antigen cleared more rapidly in serum than in urine, similar to what was observed in humans with histoplasmosis (8). The findings of the present study and these other reports support the rationale for a larger study using antigen to monitor therapy in humans.
Several limitations of this study should be recognized in the use of the information for patient management. First, a retrospective design may bias case finding, causing overestimation of sensitivity. Second, less than half of cases were derived from an observational study for which medical records were reviewed, hampering the correlation of antigen results with clinical and other diagnostic laboratory findings. Third, cases in which serum was available were insufficient for determination of the role of antigenemia testing. Fourth, the role of testing sequential specimens for monitoring treatment has not been established in cases with known outcome.
In conclusion, quantification is reproducible and permits assessment of antigen clearance without testing current and previously obtained specimens in the same assay. EDTA-heat treatment improved the sensitivity for detection of antigenemia.
Footnotes
Published ahead of print 23 November 2011
REFERENCES
- 1. Agresti A, Coull BA. 2009. Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 52: 119–216 [Google Scholar]
- 2. Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. 2007. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine (Baltimore) 86: 162–169 [DOI] [PubMed] [Google Scholar]
- 3. Bariola JR, et al. 2011. Detection of Blastomyces dermatitidis antigen in patients with newly diagnosed blastomycosis. Diagn. Microbiol. Infect. Dis. 69: 187–191 [DOI] [PubMed] [Google Scholar]
- 4. Carlos WG, et al. 2010. Blastomycosis in Indiana: digging up more cases. Chest 138: 1377–1382 [DOI] [PubMed] [Google Scholar]
- 5. Connolly PA, Durkin MM, LeMonte AM, Hackett EJ, Wheat LJ. 2007. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 14: 1587–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Durkin M, Witt J, LeMonte A, Wheat B, Connolly P. 2004. Antigen assay with the potential to aid in diagnosis of blastomycosis. J. Clin. Microbiol. 42: 4873–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grim SA, et al. 2011. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl. Infect. Dis. [Epub ahead of print.] doi: 10.1111/j.1399–3062.2011.00658.x [DOI] [PubMed] [Google Scholar]
- 8. Hage CA, et al. 2011. Histoplasma antigen clearance during treatment of histoplasmosis in patients with AIDS determined by a quantitative antigen enzyme immunoassay. Clin. Vaccine Immunol. 18: 661–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hage CA, et al. 2011. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin. Infect. Dis. 53: 448–454 [DOI] [PubMed] [Google Scholar]
- 10. Spector D, et al. 2008. Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J. Vet. Intern. Med. 22: 839–843 [DOI] [PubMed] [Google Scholar]
- 11. Swartzentruber S, et al. 2009. Improved detection of Histoplasma antigenemia following dissociation of immune complexes. Clin. Vaccine Immunol. 16: 320–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swartzentruber S, et al. 2009. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin. Infect. Dis. 49: 1878–1882 [DOI] [PubMed] [Google Scholar]
- 13. Tarr M, et al. 2007. Blastomyces antigen detection for monitoring progression of blastomycosis in a pregnant adolescent. Infect. Dis. Obstet. Gynecol. 2007: 89059. [DOI] [PMC free article] [PubMed] [Google Scholar]