Abstract
Taiwan's rubella vaccination program was launched in 1986; each schoolgirl in the third grade of junior high school received one dose of rubella (RA 27/3) vaccine. We reviewed the results of 14,090 prenatal rubella tests for primiparas from three areas of Taiwan during 2002 to 2008 to investigate seronegativity rates and titer changes. In all primiparous women, the average rubella virus seronegativity rate was 6.5% (95% confidence interval [95% CI], 6.1 to 6.9%), and the average rubella virus antibody titer was 65.9 IU/ml (95% CI, 64.7 to 67.1 IU/ml). There were 1,220 women (8.7%) with weakly positive antibody titers (10 to 20 IU/ml). The rubella virus seronegativity rates, which ranged from 5.4 to 9.7%, did not exhibit a linear trend from 9 to 22 years after vaccination (P = 0.201); in contrast, a significant trend appeared in the average rubella virus IgG titer (P = 0.003), dropping from 69.9 IU/ml in the 9th year after vaccination to 54.8 IU/ml in the 22nd year. The mean annual antibody decay rate was −0.77 IU/ml. This study reveals that the level of rubella virus antibodies declined slowly in women of childbearing age who were vaccinated with RA 27/3 at junior high school age. The number of women who were seronegative or had weakly positive antibody titers was still high (15.2%). Therefore, in countries that implement a single-dose regimen in children or teenagers, it should remain an important policy to encourage voluntary immunization in seronegative women and to immunize all postpartum women who are susceptible to rubella virus infection before they leave the hospital.
INTRODUCTION
Rubella virus is a single-stranded RNA virus that is transmitted by aerosol via the respiratory tract. It generally causes a mild self-limiting disease characterized by rash, fever, and lymphadenopathy. In 1941, an Australian ophthalmologist, Norman Gregg, recognized the association between rubella virus infection in pregnancy and congenital rubella syndrome (CRS). The common manifestations of CRS include neurological deficits, hearing impairment, eye defects, and heart defects (1, 25). In 1962, a rubella pandemic began in Europe, spreading to the United States in 1963 and 1964 and to Asian countries in 1965 to 1969. During the period 1963-1964, the rubella outbreak infected 12.5 million people, caused 11,000 fetal deaths, and led to 20,000 CRS cases in the United States. This pandemic accelerated the development of rubella vaccines. Attenuated rubella vaccines were licensed in the United States and Europe in 1969; five rubella vaccines were also licensed and used in Japan during 1975 to 1980. Many developed countries implemented single-dose vaccination of target populations shortly after the vaccine first became available; at present, a two-dose measles-mumps-rubella (MMR) vaccine is provided to all children at 12 to 18 months and at preschool or school age (18, 20). These vaccination programs have successfully prevented rubella virus infection and CRS worldwide. Finland and the United States are currently documented as being free of indigenous rubella (9, 15, 17).
By the end of 2009, 130 countries had included the rubella vaccine in their national vaccination programs. Although global rubella vaccine coverage is estimated at 42% (26), many African and Asian countries have no rubella immunization programs. In Africa and Southeast Asia, the rubella vaccine coverages are estimated to be only 0.1 and 4%, respectively (26). It is believed that over 100,000 cases of CRS occur worldwide annually (19). Therefore, it remains an important public health challenge to prevent CRS in developing countries, especially in African and Southeast Asian regions.
Prior to the introduction of the vaccination program in Taiwan, four reported epidemics occurred, roughly about once every decade: in 1944, 1957-1958, 1968-1969, and 1977 (7). Since 1977, the disease appears to have become endemic, with no large-scale outbreaks of rubella. Taiwan's rubella vaccination program was launched in 1986. Schoolgirls in the third grade of junior high school each received one dose of rubella (RA 27/3) vaccine. This program was extended, with one dose of MMR (RA 27/3) vaccine being given to all junior high and elementary school students and preschool children in 1992 to 1994. The rubella vaccination programs among junior high school girls had a high coverage rate (about 98%) (21). Congenital rubella in Taiwan is currently a category 3 reportable disease, and rubella is a category 2 reportable disease; suspected cases must be reported to the Centers for Disease Control (CDC), and samples must be sent to the CDC laboratory for confirmation. The confirmed number of rubella cases has fluctuated recently, from 2 cases per year in both 1999 and 2003 to 54 cases per year in 2007. The reporting system for CRS began in 1988. Since 1994, five cases of CRS have been confirmed: three in 2001 and one each in 2007 and 2008. Two of them were indigenous cases (CDC [http://nidss.cdc.gov.tw]).
The original population of schoolgirls who received one dose of rubella (RA 27/3) vaccine in the third grade of junior high school is now of reproductive age. The persistence and waning of rubella virus antibodies have been investigated in American and Northern European countries (4, 5, 10), but we are unaware of any study of the decay of antibody titers in Asian countries. Therefore, our aim for this study was to investigate the immunity to rubella and titer changes of rubella virus antibodies in primiparous women offered rubella vaccination at 15 years of age.
MATERIALS AND METHODS
Study design.
This study involved an analysis of the results of 14,090 rubella virus antibody tests for native Taiwanese primiparas who received routine prenatal examinations at two hospitals in northern Taiwan (Taipei), one hospital in central Taiwan (Chiayi), and two hospitals in southern Taiwan (Kohsiung and Pingtung). These two medical centers and three regional hospitals specialize in obstetric health care. All of these pregnant women received routine prenatal examinations at one of the five hospitals, which supplied obstetric health care, between 2002 and 2008, when the rubella virus antibody test was a compulsory checkup item during routine prenatal examinations in Taiwan. The subjects had received the rubella (RA 27/3) or MMR (RA 27/3) vaccine when they were third-grade students in junior high school (15 years old), during 1986 to 1994. The Fooyin University Hospital Ethics Review Board approved this study protocol (FYH-IRB-97003).
Serology tests.
Rubella virus IgG antibodies were determined through a microparticle enzyme immunoassay (MEIA), using an AsXYM analyzer (Abbott Laboratories, Chicago, IL). The lower and upper detection limits for rubella virus IgG were 0.1 and 500 IU/ml, respectively. Serum IgG levels of ≥10 IU/ml were considered to be seropositive or immune; those of <10 IU/ml were considered to be seronegative, susceptible, or nonimmune.
Statistical analysis.
Seronegativity rates, geometric mean titers (GMTs), and 95% confidence intervals (CIs) are provided herein with respect to the number of years that have elapsed since the subjects were vaccinated with rubella vaccine in the third grade of junior high school. A simple linear regression analysis was used to calculate the seronegativity rates and titer changes. In calculating rubella virus antibody levels, test titers of >500 IU/ml were assigned a value of 500 IU/ml. A P value of <0.05 was regarded as statistically significant. Data were analyzed using the software SPSS 10.0 for Windows.
RESULTS
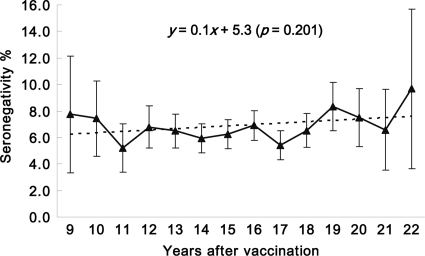
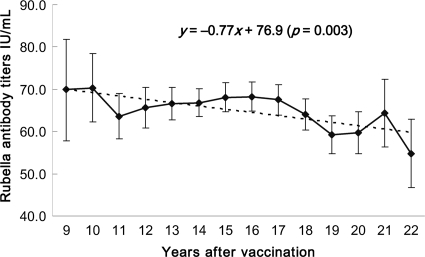
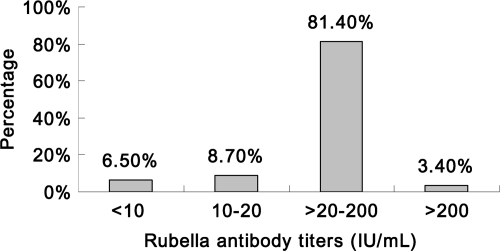
A total of 14,090 primiparas were included in this study. The women were aged 24 to 37 years; between 9 and 22 years have elapsed since they received the vaccine, when they were third-grade students in junior high school. For all primiparous women (Table 1), the average seronegativity of rubella virus was 6.5% (917/14,090 women; 95% CI, 6.1 to 6.9%), and the average rubella virus antibody titer was 65.9 IU/ml (95% CI, 64.7 to 67.1 IU/ml). The rubella virus seronegativity rates, which ranged from 5.2 to 9.7% (Fig. 1), did not exhibit a linear trend from 9 to 22 years after vaccination (P = 0.201); in contrast, the average rubella virus IgG titer displayed significant linearity (P = 0.003), dropping from 69.9 IU/ml in the 9th year to 54.8 IU/ml in the 22nd year after vaccination (Fig. 2). The mean annual antibody decay rate was −0.77 IU/ml. Figure 3 presents the rubella virus antibody titer distribution for these primiparous women. Women having rubella virus antibody titers in the ranges of <10, 10 to 20, >20 to 200, and >200 IU/ml accounted for 6.5, 8.7, 81.4, and 3.4%, respectively, of the cohort. These results suggest that among these women of childbearing age who were vaccinated in the third grade of junior high school, the rubella virus antibodies decayed but the titers remained mostly seropositive.
Table 1.
Rubella virus seronegativity rates and GMTs for primiparous women 9 to 22 years after vaccination in the third grade of junior high school
| Time (yr) after vaccination | Age (yr) | Sample size | No. of seronegative women | % Seronegativity (95% CI) | GMT (IU/ml) (95% CI) |
|---|---|---|---|---|---|
| 9 | 24 | 142 | 11 | 7.7 (3.3–12.1) | 69.9 (57.8–81.9) |
| 10 | 25 | 323 | 24 | 7.4 (4.6–10.3) | 70.3 (62.2–78.4) |
| 11 | 26 | 576 | 30 | 5.2 (3.4–7.0) | 63.6 (58.3–69.0) |
| 12 | 27 | 956 | 65 | 6.8 (5.2–8.4) | 65.6 (60.8–70.4) |
| 13 | 28 | 1,445 | 94 | 6.5 (5.2–7.8) | 66.6 (62.8–70.4) |
| 14 | 29 | 1,832 | 109 | 5.9 (4.9–7.0) | 66.8 (63.5–70.1) |
| 15 | 30 | 1,899 | 119 | 6.3 (5.2–7.4) | 68.1 (64.7–71.6) |
| 16 | 31 | 1,983 | 137 | 6.9 (5.8–8.0) | 68.2 (64.8–71.7) |
| 17 | 32 | 1,682 | 91 | 5.4 (4.3–6.5) | 67.5 (63.9–71.1) |
| 18 | 33 | 1,455 | 95 | 6.5 (5.3–7.8) | 64.0 (60.3–67.8) |
| 19 | 34 | 886 | 74 | 8.4 (6.5–10.2) | 59.3 (54.8–63.7) |
| 20 | 45 | 560 | 42 | 7.5 (5.3–9.7) | 59.7 (54.8–64.6) |
| 21 | 36 | 258 | 17 | 6.6 (3.6–9.6) | 64.4 (56.4–72.4) |
| 22 | 37 | 93 | 9 | 9.7 (3.7–15.7) | 54.8 (46.8–62.9) |
| Total | 14,090 | 917 | 6.5 (6.1–6.9) | 65.9 (64.7–67.1) |
Fig 1.
Rubella seronegativity rates with 95% confidence intervals for primiparous women 9 to 22 years after vaccination in the third grade of junior high school (at 15 years of age).
Fig 2.
Rubella virus antibody titers with 95% confidence intervals for primiparous women 9 to 22 years after vaccination in the third grade of junior high school (at 15 years of age).
Fig 3.
Distribution of rubella virus antibody titers in primiparous women 9 to 22 years after vaccination in the third grade of junior high school (at 15 years of age).
DISCUSSION
In the present study, we analyzed 14,090 test results relating to the levels of rubella virus antibodies in primiparas from five hospitals located in three areas (northern, central, and southern) of Taiwan. Rubella testing was one of the prenatal exam items of the national health insurance system in Taiwan during the period that we studied. Therefore, all test results would have been recruited into this study if a pregnant woman had visited any of the hospitals for prenatal care. We found that among all the primiparous women, the seronegativity was 6.5% (95% CI, 6.1 to 6.9%), and the average GMT was 65.9 IU/ml (95% CI, 64.7 to 67.1 IU/ml). Relative to the rubella virus seronegativity rates among local women of childbearing age who were born after the onset of the vaccination program in different areas of Taiwan (11, 21, 24), the average seronegativity from the present study was higher than those in northern and central-southern Taiwan (4.9 and 4.0%) but lower than that in southern Taiwan (7.9%). In comparing the seronegativity of rubella virus antibodies in Taiwan nationwide to the results from a previous study (12), the susceptible rate found in the current study was slightly higher than that for pregnant women born during 1971 to 1975 (6.5 versus 6.1%) and lower than that for those born after 1976 (6.5 versus 7.2%).
The susceptibility rate for rubella virus infection that we found in the present study is similar to those for women of childbearing age in many European countries, including Cyprus, Latvia, Luxembourg, England, and Wales (13). Our results revealed that the seropositivity rate remained high because the level of rubella virus antibodies declined slowly. During the past 15 years, only two local CRS cases have been confirmed by the CDC in Taiwan (2). Therefore, the school vaccination programs have been a cost-effective and successful means of preventing CRS.
The vaccine strain is the key factor influencing the persistence of rubella virus antibodies. The RA 27/3 strain is the most widely used vaccine in the world—except in Japan, where five attenuated rubella vaccines have been licensed and are now in use (23). The RA 27/3 vaccine strain generally induces higher antibody titers and produces antibody responses that most resemble natural infection. The resulting rubella immunity probably persists for a long time (>20 years). Therefore, it is the most widely used vaccine in the world. Previous studies demonstrated a 100% seroconversion rate in children who had each been vaccinated with a single dose of RA 27/3 vaccine (6, 16). From a study of the expression of interleukin-2 receptor alpha and CD45RO antigen on T lymphocytes, Toyoda and colleagues noted that T-cell-mediated immunity and humoral immunity persisted for at least 20 years after vaccination (22). Kremer and colleagues demonstrated that the minimal annual antibody decay rate for vaccinees is −2.9% (10). The concentration of antibodies significantly decayed among high-titer individuals and did not significantly decrease in the low-titer group. O'Shea et al. found that 97.9% of RA 27/3 vaccinees were seropositive 10 to 21 years after immunization (14). Horstmann and colleagues reported that the RA 27/3 vaccine provided high immunogenicity and resulted in high antibody titers; after 11 years, 95% of subjects who seroconverted were tested for hemagglutination inhibition (HI), and 100% had neutralizing antibodies (8). In this study, we found that the seronegativity rate was 9.7% for primiparous women who had been vaccinated with the RA 27/3 strain 22 years ago.
Vaccine dose is also an important point for the persistence of rubella virus antibodies. At present, to interrupt endemic virus circulation, two doses of MMR vaccine are provided to all children at 12 to 18 months and at preschool or school age. In the present study, we observed a relatively low seronegativity rate (6.5%) among women of childbearing age who had been injected with a single dose of rubella vaccine at 15 years of age; nevertheless, women with weakly positive antibody titers (10 to 20 IU/ml) accounted for 8.7% of the cohort. Based on the annual decay rate (−0.77 IU/ml), we estimate that approximately 6.4% (900/14,090 women) of women will likely become seronegative in the next decade. Therefore, we suggest that it should remain an important policy to encourage voluntary immunization of susceptible women of childbearing age.
In the present analysis, the study subjects were primiparous women, thereby reducing the interference of postpartum immunization. Some of the women might, however, have obtained immunity by checking their rubella virus antibody levels and being vaccinated prior to getting married or through natural infection. In Taiwan's national health insurance system, however, the code for rubella testing for nonpregnant women is different from that for pregnant women. We found that the number of rubella tests for nonpregnant women accounted for only 0.08% of the total number of tests during the study period in these hospitals. Thus, subjects who checked their levels of rubella virus antibodies and underwent immunization prior to marriage were rare. According to the statistics from the CDC in Taiwan, the lower annual incidence rate of rubella ranged from 0.009 to 0.235 per 100,000 individuals during the period 1999-2008 (3); nevertheless, many patients with rubella have only mild symptoms or even no symptoms, so their numbers may have been underreported. Wang et al. reported the probability of natural infection in unvaccinated women who had high titers, averaging 214 IU/ml (24). In the present study, we found that 3.4% of the primiparous women had antibody titers of >200 IU/ml; these women may have had a large immune response to a previous vaccination, had contact with the wild-type virus, or been revaccinated recently.
Our findings suggest that mandatory vaccination programs with a high coverage rate in junior high school students have been successful. Although the levels of rubella virus antibodies have declined slowly in women of childbearing age who were vaccinated with the RA 27/3 vaccine, their seronegativity rate at present is relatively low (6.5%). The number of women who were seronegative or provided weakly positive antibody titers accounted for 15.2% of the cohort. Therefore, in countries that implement a single-dose regimen in children or teenagers, it should remain an important policy to encourage voluntary immunization in seronegative women and to immunize all postpartum women who are susceptible to rubella virus infection before they leave the hospital.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Best JM. 2007. Rubella. Semin. Fetal Neonatal Med. 12: 182–192 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control, Taiwan 1985–2010. Annual reports 1985–2010. Centers for Disease Control, Taipei, Taiwan. [Google Scholar]
- 3. Centers for Disease Control, Taiwan 2000–2009. Annual reports 2000–2009. Centers for Disease Control, Taipei, Taiwan. [Google Scholar]
- 4. Christenson B, Bottiger M. 1994. Long-term follow-up study of rubella antibodies in naturally immune and vaccinated young adults. Vaccine 12: 41–45 [DOI] [PubMed] [Google Scholar]
- 5. Davidkin I, Peltola H, Leinikki P, Valle M. 2000. Duration of rubella immunity induced by two-dose measles, mumps and rubella (MMR) vaccination. A 15-year follow-up in Finland. Vaccine 18: 3106–3112 [DOI] [PubMed] [Google Scholar]
- 6. Dunlop JM, RaiChoudhury K, Roberts JS, Bryett KA. 1989. An evaluation of measles, mumps, and rubella vaccine in a population of Yorkshire infants. Public Health 103: 331–335 [DOI] [PubMed] [Google Scholar]
- 7. Grayston JT, Gale JL, Watten RH. 1972. The epidemiology of rubella on Taiwan. I. Introduction and description of the 1957-1958 epidemic. Int. J. Epidemiol. 1: 245–252 [DOI] [PubMed] [Google Scholar]
- 8. Horstmann DM, et al. 1985. Persistence of vaccine-induced immune responses to rubella: comparison with natural infection. Rev. Infect. Dis. 7: S80–S85 [DOI] [PubMed] [Google Scholar]
- 9. Icenogle JP, et al. 2006. Genetic analysis of rubella viruses found in the United States between 1996 and 2004: evidence that indigenous rubella viruses have been eliminated. Clin. Infect. Dis. 43: S133–S140 [DOI] [PubMed] [Google Scholar]
- 10. Kremer JR, Schneider F, Muller CP. 2006. Waning antibodies in measles and rubella vaccines—a longitudinal study. Vaccine 24: 2594–2601 [DOI] [PubMed] [Google Scholar]
- 11. Lin CC, Yang CY, Shih CT, Chen BH, Huang YL. 2010. Rubella seroepidemiology and catch-up immunization among pregnant women in Taiwan: comparison between women born in Taiwan and immigrants from six Asian countries. Am. J. Trop. Med. Hyg. 82: 40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin CC, et al. 2011. Rubella seroepidemiology and estimations of the catch-up immunisation rate and persistence of antibody titers in pregnant women in Taiwan. BJOG 118: 706–712 [DOI] [PubMed] [Google Scholar]
- 13. Nardone A, et al. 2008. Comparison of rubella seroepidemiology in 17 countries: progress towards international disease control target. Bull. World Health Organ. 86: 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Shea S, et al. 1998. Rubella vaccination: persistence of antibodies for 10–21 years. Lancet 332: 909. [DOI] [PubMed] [Google Scholar]
- 15. Peltola H, et al. 2000. Mumps and rubella eliminated from Finland. JAMA 284:2643–2647 [DOI] [PubMed] [Google Scholar]
- 16. Raut SK, et al. 2007. Persistence of antibodies induced by measles-mumps-rubella vaccine in children in India. Clin. Vaccine Immunol. 14: 1370–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reef SE, Cochi SL. 2006. The evidence for the elimination of rubella and congenital rubella syndrome in the United States: a public health achievement. Clin. Infect. Dis. 43: S123–S125 [DOI] [PubMed] [Google Scholar]
- 18. Robertson SE, Cutts FT, Samuel R, Diaz-Ortega JL. 1997. Control of rubella and congenital rubella syndrome (CRS) in developing countries. 2. Vaccination against rubella. Bull. World Health Organ. 75: 69–80 [PMC free article] [PubMed] [Google Scholar]
- 19. Robertson SE, Featherstone DA, Gacic-Dobo M, Hersh BS. 2003. Rubella and congenital rubella syndrome: global update. Pan Am. J. Public Health 14: 306–315 [DOI] [PubMed] [Google Scholar]
- 20. Spika JS, et al. 2003. Measles and rubella in the World Health Organization European region: diversity creates challenges. J. Infect. Dis. 187: S191–S197 [DOI] [PubMed] [Google Scholar]
- 21. Su SB, Guo HR. 2002. Seroprevalence of rubella among women of childbearing age in Taiwan after nationwide vaccination. Am. J. Trop. Med. Hyg. 67: 549–553 [DOI] [PubMed] [Google Scholar]
- 22. Toyoda M, Ihara T, Nakano T, Ito M, Kamiya H. 1999. Expression of interleukin-2 alpha and CD45RO antigen on T lymphocyte cultured with rubella virus antigen, compared with humoral immunity in rubella vaccines. Vaccine 17: 2051–2058 [DOI] [PubMed] [Google Scholar]
- 23. Ueda K. 2009. Development of the rubella vaccine and vaccination strategy in Japan. Vaccine 27: 3232–3233 [DOI] [PubMed] [Google Scholar]
- 24. Wang IJ, Huang LM, Chen HH, Hwang KC, Chen CJ. 2007. Seroprevalence of rubella infection after national immunization program in Taiwan: vaccination status and immigration impact. J. Med. Virol. 79: 97–103 [DOI] [PubMed] [Google Scholar]
- 25. WHO 2008. The immunological basis for immunization series. Module 11. Rubella. Immunization, vaccines, and biologicals. WHO, Geneva, Switzerland. [Google Scholar]
- 26. WHO Global Immunization Data Immunization surveillance, assessment and monitoring. http://www.who.int/immunization_monitoring/data/en/ Accessed 28 September 2011. [Google Scholar]