Abstract
Dengue virus (DV) primary infection and probable secondary infection rates in relation to patient age (years) were determined for DV IgM-positive U.S. mainland residents (presumed travelers to areas of DV endemicity) and Caribbean island (area of DV endemicity) residents by evaluating IgG status and IgG avidity. Regardless of place of residence, most patients ≤20 years old exhibited primary infection and most patients >60 years old exhibited probable secondary infection. Among patients 21 to 60 years old, the primary infection rate was markedly higher in U.S. residents.
TEXT
The four serotypes of dengue virus (DV) are endemic to tropical and subtropical areas worldwide, including the Caribbean basin, where they cause illnesses of major public health concern (28). DV infections are also an important medical concern outside the tropics and subtropics, where cases in travelers returning from areas of DV endemicity have been well documented (2, 9, 12, 17, 20, 29, 31). Primary infection with any DV serotype induces an immune response that protects against later infection by that serotype; however, subsequent infection by another serotype, termed secondary DV infection, is a risk factor for dengue hemorrhagic fever, which is associated with significant morbidity and occasionally death (19, 25, 33).
DV antibody reactivity patterns serve as useful tools for classifying patients as having primary or secondary DV infection. Detection of DV IgM in the absence of DV IgG (i.e., an IgM-positive/IgG-negative [IgM+IgG−] reactivity pattern) is a clear indicator of primary DV infection (4, 11). Similarly, an IgM+IgG+ pattern combined with low IgG avidity accurately identifies primary DV infection (10–12, 15, 16, 22). An IgM+IgG+ reactivity pattern with high IgG avidity is an accurate marker of secondary infection among patients whose serum samples were collected within a month of symptom onset (10–12, 15, 16, 22); however, this reactivity pattern also characterizes patients with primary DV infection who were previously exposed to other flaviviruses (via infection or vaccination) (12). Further, based on IgG avidity maturation trends observed for other viral infections (5, 6, 14, 24), an IgM+IgG+ pattern with high IgG avidity may occur in primary DV infection patients late in the convalescent phase (several months postinfection). Thus, in the absence of information on the timing of specimen collection in relation to symptom onset, an IgM+IgG+ reactivity pattern with high avidity can only be considered a marker of probable secondary DV infection.
Epidemiological studies have shown that the likelihood of acute DV infection representing secondary infection increases with age for residents of areas of DV endemicity (18, 23, 30). However, the relationship between patient age and proportions of primary and secondary DV infections among residents of areas of nonendemicity, where DV infections are nearly always associated with foreign travel (17), has not been clearly delineated. We thus sought to employ IgM/IgG reactivity patterns and IgG avidity results to estimate the proportions of primary and probable secondary DV infections among different age groups of DV IgM-positive patients from geographically proximate areas of endemicity and nonendemicity, namely, the Caribbean islands and the U.S. mainland, respectively.
Sera included in this analysis were submitted to Focus Diagnostics for DV antibody testing between March 2009 and December 2010 and found to be DV IgM positive. Clinical information (e.g., time since onset of symptoms) was not supplied for any of the specimens. The DV IgM assay was a mu-capture enzyme-linked immunosorbent assay (ELISA), and the DV IgG assay was an indirect ELISA; both were performed as previously described (21, 22). Results were expressed as indexes, calculated by dividing the specimen absorbance value by the mean calibrator serum absorbance value; index values of >1.10 were considered positive. Most sera exhibiting a DV IgM+IgG+ reactivity pattern were further evaluated using the DV IgG avidity ELISA, performed as previously described (22). Avidity values of ≤0.39 were considered low IgG avidity, whereas values of >0.39 were considered high avidity (22). A primary infection was defined by either an IgM+IgG− reactivity pattern or an IgM+IgG+ reactivity pattern with low IgG avidity. A probable secondary infection was defined by an IgM+IgG+ reactivity pattern and high IgG avidity (4, 11, 22). Differences between proportions were evaluated by chi-square analysis (MedCalc software), with significance defined by a P value of <0.01.
A total of 2,609 DV IgM-positive patients were identified during the study period; 76 patients (2.9%) were excluded from further analysis because their age was unknown. Of the remaining 2,533 DV IgM-positive patients, 1,622 (64%) were also positive for DV IgG, and sera from 1,257 (77.5%) of these IgM+IgG+ patients were available for IgG avidity testing. When IgM+IgG+ patients not tested for avidity were compared to IgM+IgG+ patients tested for avidity, there was no bias related to patient age or place of residence; we thus assumed that within a given age and residence category, the proportion of low IgG avidity results among IgM+IgG+ sera actually tested for avidity was also applicable to IgM+IgG+ sera not tested for avidity. The numbers of IgM+IgG+ patients with low avidity and high avidity shown in Table 1 thus reflect the actual numbers observed for sera tested for avidity plus the calculated numbers for sera not tested for avidity.
TABLE 1.
Numbers of patients exhibiting various DV IgM-positive reactivity patterns and IgG avidity, segregated by age group and place of patient residence
| Age group (yr) | No. of patients with indicated reactivity pattern (avidity) residing in: |
|||||
|---|---|---|---|---|---|---|
| Caribbean islands |
U.S. mainland |
|||||
| IgM+IgG− | IgM+IgG+ (low) | IgM+IgG+ (high) | IgM+IgG− | IgM+IgG+ (low) | IgM+IgG+ (high) | |
| ≤20 | 280 | 34 | 233 | 95 | 28 | 54 |
| 21–40 | 114 | 8 | 199 | 155 | 76 | 140 |
| 41–60 | 65 | 10 | 220 | 132 | 86 | 211 |
| >60 | 38 | 15 | 158 | 32 | 30 | 120 |
| Total | 497 | 67 | 810 | 414 | 220 | 525 |
Table 1 shows the numbers of patients within each residence group, segregated by IgM/IgG reactivity patterns, IgG avidity, and age. The overall proportion of DV IgM-positive patients exhibiting a primary infection pattern was significantly higher in U.S. mainland residents (634/1,159, 54.7%) than Caribbean island residents (564/1,374, 41.0%).
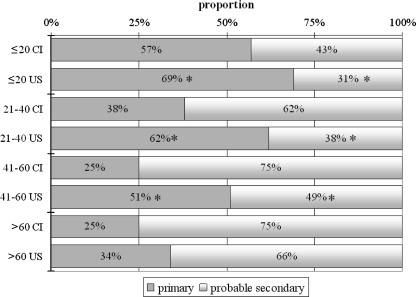
Figure 1 presents the distribution of primary and probable secondary infections among patients within a given age category in each place of residence. In patients ≤20 years old, the proportion of DV IgM-positive patients exhibiting primary infection was significantly higher (and thus the proportion exhibiting probable secondary infection was significantly lower) for U.S. mainland residents than Caribbean island residents; of particular note, however, was the observation that in both resident groups, more than 50% of patients in this age category exhibited primary infection. In patients 21 to 40 years old and patients 41 to 60 years old, the proportions of DV IgM-positive patients exhibiting primary infection were also significantly higher (and the proportions exhibiting probable secondary infection were significantly lower) in U.S. mainland residents than Caribbean island residents; the differences were more marked than the difference observed when comparing the ≤20-year-old groups by place of residence. In contrast to the observations for other age groups, the distributions of primary and probable secondary infections in patients >60 years old did not significantly differ when comparing the two resident groups; the majority of patients from both the Caribbean islands and the U.S. mainland exhibited probable secondary infections.
FIG 1.
Proportional distributions of primary and probable secondary DV infections within a given age category for each place of patient residence. Age categories (years) for Caribbean island (CI) and U.S. mainland (US) residents are indicated on the vertical axis. Asterisks indicate significant differences (P < 0.01) observed in pairwise comparisons of U.S. mainland residents and CI residents within a given age category.
Our estimates of primary infection rates among DV IgM-positive individuals represent minimum estimates. Patients previously exposed to other flaviviruses via natural infection or vaccination exhibit a DV IgM/IgG reactivity pattern and IgG avidity typical of secondary infection during their primary DV infection episode (12). Further, it is unclear if DV IgG avidity matures to levels typical of secondary infection before DV IgM falls to undetectable levels. Thus, some cases of primary DV infection were most likely misclassified by DV IgG avidity measurement and thus incorrectly placed in the probable secondary infection group. Indeed, this reasoning explains why we elected to call the IgM+IgG+ reactivity pattern with high IgG avidity a “probable” secondary infection; although it is likely that most patients in this group exhibited secondary DV infection, it remains unclear how many patients were, in fact, misclassified primary infections.
Similarly, there were undoubtedly patients with recent DV infection that we did not identify because they were in the preseroconversion window and thus negative for DV IgM (1, 13, 27). Such patients would be more likely to exhibit secondary DV infections, since IgM typically appears a few days later in secondary infections than in primary infections (26); further, approximately 5% of patients with secondary infections never exhibit detectable DV IgM (13, 32). DV nonstructural protein 1 (NS1) detection is a useful test for identifying such patients (1), although NS1 detection kits are not available in the United States. DV nucleic acid detection can also identify recently infected individuals before seroconversion (27).
Our analysis of DV infection type in relation to age and place of residence revealed that, not surprisingly, the majority of DV infections among U.S. mainland residents 21 to 60 years old were primary infections, whereas the majority of infections among Caribbean residents 21 to 60 years old were secondary infections. In contrast, the majority of DV IgM-positive individuals ≤20 years old exhibited a primary infection pattern, regardless of place of residence. Based on published reports of high DV IgG prevalence rates and secondary DV infection rates among children from other areas of endemicity (3, 7, 32, 34), we expected to find a secondary infection pattern in the majority of DV IgM-positive Caribbean children and teenagers. The reasons for this unexpected finding remain unclear; possible explanations include (i) a particularly large population of never-exposed Caribbean children and young adults due to several years of relative quiescence before the 2010 Caribbean DV outbreak (8) and (ii) increased physician awareness of DV infections linked to the outbreak, leading to more testing among febrile children and teenagers. Another unexpected finding was that the majority of DV IgM-positive individuals >60 years old exhibited a probable secondary infection pattern, regardless of place of residence. We expected this finding among IgM-positive Caribbean island residents >60 years of age (23, 30) but not among IgM-positive U.S. mainland residents >60 years old. This observation suggests that most U.S. mainland residents >60 years old with recent DV infection were exposed to DV sometime in the past, probably due to prior residence in, or travel to, an area of DV endemicity. Such individuals may be at increased risk for dengue hemorrhagic fever and should thus be followed closely by their medical care providers.
ACKNOWLEDGMENT
We thank Jay M. Lieberman for critical review of the manuscript.
Footnotes
Published ahead of print 23 November 2011
REFERENCES
- 1. Alcon S, et al. 2002. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakker RC, Veenstra J, Dingemans-Dumas AM, Wetsteyn JCFM, Kager PA. 1996. Imported dengue in The Netherlands. J. Travel Med. 3:204–208 [DOI] [PubMed] [Google Scholar]
- 3. Balmaseda A, et al. 2010. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J. Infect. Dis. 201:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blacksell SD, et al. 2008. Evaluation of the Panbio dengue virus nonstructural 1 antigen detection and immunoglobulin M antibody enzyme-linked immunosorbent assays for the diagnosis of acute dengue infections in Laos. Diagn. Microbiol. Infect. Dis. 60:43–49 [DOI] [PubMed] [Google Scholar]
- 5. Bodeus M, Feyder S, Goubau P. 1998. Avidity of IgG antibodies distinguishes primary from nonprimary cytomegalovirus infection in pregnant women. Clin. Diagn. Lab. Immunol. 9:9–16 [DOI] [PubMed] [Google Scholar]
- 6. Bottiger B, Jensen IP. 1997. Maturation of rubella IgG avidity over time after acute rubella infection. Clin. Diagn. Virol. 8:105–111 [DOI] [PubMed] [Google Scholar]
- 7. Brown MG, Vickers IE, Salas RA, Smikle MF. 2009. Patterns of dengue virus IgM and IgG antibodies in suspected cases of dengue in Jamaica, 2003-2006. Hum. Antibodies 18:29–34 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention 2011. 2010: largest dengue outbreak in Puerto Rico history. Dengue Update 3:1–2 [Google Scholar]
- 9. Cobelens FG, et al. 2002. Incidence and risk factors of probable dengue virus infection among Dutch travellers to Asia. Trop. Med. Int. Health 7:331–338 [DOI] [PubMed] [Google Scholar]
- 10. de Souza VAUF, et al. 2004. Use of an immunoglobulin G avidity test to discriminate between primary and secondary dengue virus infections. J. Clin. Microbiol. 42:1782–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Souza VAUF, et al. 2007. Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. J. Clin. Virol. 39:230–233 [DOI] [PubMed] [Google Scholar]
- 12. Domingo C, et al. 2009. Molecular and serologic markers of acute dengue infection in naïve and flavivirus-vaccinated travelers. Diagn. Microbiol. Infect. Dis. 65:42–48 [DOI] [PubMed] [Google Scholar]
- 13. Gubler DJ. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanno A, Kazuyama Y. 2002. Immunoglobulin G antibody avidity assay for serodiagnosis of hepatitis C virus infection. J. Med. Virol. 68:229–233 [DOI] [PubMed] [Google Scholar]
- 15. Matheus S, et al. 2005. Discrimination between primary and secondary dengue virus infection by an immunoglobulin G avidity test using a single acute-phase serum sample. J. Clin. Microbiol. 43:2793–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matheus S, et al. 2005. Use of four dengue virus antigens for determination of dengue immune status by enzyme-linked immunosorbent assay of immunoglobulin G avidity. J. Clin. Microbiol. 43:5785–5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohammed HP, et al. 2010. Travel-associated dengue infections in the United States, 1996-2005. J. Travel Med. 17:8–14 [DOI] [PubMed] [Google Scholar]
- 18. Monath TP. 1994. Dengue: the risk to developed and developing countries. Proc. Natl. Acad. Sci. U. S. A. 91:2395–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morens DM, Fauci AS. 2008. Dengue and hemorrhagic fever. A potential threat to public health in the United States. JAMA 299:214–216 [DOI] [PubMed] [Google Scholar]
- 20. Potasman I, Scugo I, Schwartz E. 1999. Dengue seroconversion among Israeli travelers to tropical countries. Emerg. Infect. Dis. 5:824–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prince HE, Matud JL, Lieberman JM. 2011. Dengue virus immunoglobulin M detection in a reference laboratory setting during the 2010 dengue virus outbreak on Caribbean islands. Clin. Vaccine Immunol. 18:1104–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prince HE, Yeh C, Lapé-Nixon M. 2011. Utility of IgM/IgG ratio and IgG avidity for distinguishing primary and secondary dengue virus infections using sera collected more than 30 days after disease onset. Clin. Vaccine Immunol. 18:1951–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramos MM, et al. 2008. Epidemiological and clinical observations on patients with dengue in Puerto Rico: results from the first year of enhanced surveillance — June 2005-May 2006. Am. J. Trop. Med. Hyg. 79:123–127 [PubMed] [Google Scholar]
- 24. Roque-Afonso AM, et al. 2004. Diagnostic relevance of immunoglobulin G avidity for hepatitis A virus. J. Clin. Microbiol. 42:5121–5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rothman AL, Ennis FA. 1999. Immunopathogenesis of dengue hemorrhagic fever. Virology 257:1–6 [DOI] [PubMed] [Google Scholar]
- 26. Sa-Ngasang A, et al. 2006. Specific IgM and IgG responses in primary and secondary dengue virus infections determined by enzyme-linked immunosorbent assay. Epidemiol. Infect. 134:820–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh K, et al. 2006. A prospective clinical study on the use of reverse transcription-polymerase chain reaction for the early diagnosis of dengue fever. J. Mol. Diagn. 8:613–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solomon T, Mallewa M. 2001. Dengue and other emerging flaviviruses. J. Infect. 42:104–115 [DOI] [PubMed] [Google Scholar]
- 29. Stephenson I, Roper J, Fraser M, Nicholson K, Wiselka M. 2003. Dengue fever in febrile returning travelers to a UK regional infectious diseases unit. Travel Med. Infect. Dis. 1:89–93 [DOI] [PubMed] [Google Scholar]
- 30. Tomashek KM, et al. 2009. Description of a large island-wide outbreak of dengue in Puerto Rico, 2007. Am. J. Trop. Med. Hyg. 81:467–474 [PubMed] [Google Scholar]
- 31. Vainio K, et al. 2010. Fatal and mild primary dengue virus infections imported to Norway from Africa and south-east Asia, 2008-2010. Euro Surveill. 15:19666. [DOI] [PubMed] [Google Scholar]
- 32. Vaughn DW, et al. 1997. Dengue in the early febrile phase: viremia and antibody responses. J. Infect. Dis. 176:322–330 [DOI] [PubMed] [Google Scholar]
- 33. Vaughn DW, et al. 1999. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am. J. Trop. Med. Hyg. 60:693–698 [DOI] [PubMed] [Google Scholar]
- 34. Yamashiro T, et al. 2004. Seroprevalence of IgG specific for dengue virus among adults and children in Santo Domingo, Dominican Republic. Am. J. Trop. Med. Hyg. 71:138–143 [PubMed] [Google Scholar]