Abstract
In an effort to develop an improved anthrax vaccine that shows high potency, five different anthrax protective antigen (PA)-adjuvant vaccine formulations that were previously found to be efficacious in a nonhuman primate model were evaluated for their efficacy in a rabbit pulmonary challenge model using Bacillus anthracis Ames strain spores. The vaccine formulations include PA adsorbed to Alhydrogel, PA encapsulated in liposomes containing monophosphoryl lipid A, stable liposomal PA oil-in-water emulsion, PA displayed on bacteriophage T4 by the intramuscular route, and PA mixed with Escherichia coli heat-labile enterotoxin administered by the needle-free transcutaneous route. Three of the vaccine formulations administered by the intramuscular or the transcutaneous route as a three-dose regimen induced 100% protection in the rabbit model. One of the formulations, liposomal PA, also induced significantly higher lethal toxin neutralizing antibodies than PA-Alhydrogel. Even 5 months after the second immunization of a two-dose regimen, rabbits vaccinated with liposomal PA were 100% protected from lethal challenge with Ames strain spores. In summary, the needle-free skin delivery and liposomal formulation that were found to be effective in two different animal model systems appear to be promising candidates for next-generation anthrax vaccine development.
INTRODUCTION
The Gram-positive bacterium Bacillus anthracis, the etiologic agent of anthrax, has been identified as one of the potential bioterrorist and warfare agents due to the ease of preparation and dissemination of its spores. Inhalation anthrax was the cause of death in the deliberate release of anthrax spores in the United States in October 2002. B. anthracis virulence is due to two major components, the poly-gamma-d-glutamic acid capsule and the tripartite anthrax toxin, comprised of protective antigen (PA), lethal factor (LF), and edema factor (EF). Because of the central role it plays in the formation of lethal toxin (PA+LF) and edema toxin (PA+EF), PA has been the principal target for the development of vaccines against anthrax (8, 9, 13). The current U.S.-licensed human anthrax vaccine (AVA; BioThrax) is a culture filtrate of Bacillus anthracis strain V770-NP1-R adsorbed to aluminum hydroxide that primarily consists of PA. Although this is an effective vaccine, its undefined nature, prolonged dose regimen, and reactogenicity are reasons to explore safer vaccines (8, 9, 13).
Adjuvants often are important components of a vaccine formulation because they can enhance the immunogenicity of an antigen (1). Purified recombinant PA adjuvanted with aluminum hydroxide has been suggested as an alternative to AVA. Although aluminum hydroxide is relatively safe, it sometimes causes local reactions, including subcutaneous nodules, erythema, induration, and contact hypersensitivity (5). The formulation of generic adjuvants that exhibit high levels of safety and superior immunopotency remain a major challenge in vaccinology (15).
Several adjuvant and delivery systems have been developed in our laboratories which were shown to enhance the immunogenicity of a variety of antigens. Transcutaneous immunization (TCI) is a novel needle-free skin immunization method that involves the coadministration of an adjuvant, such as Escherichia coli heat-labile enterotoxin (LT), along with an antigen(s) (6, 11, 14). Liposome-encapsulated antigens containing lipid A or liposomal lipid A-stabilized emulsions have been extensively used as potent adjuvants (2, 4, 26, 33, 34). Bacteriophage T4 is a nanoparticle antigen delivery system that allows the display of antigen(s) on the capsid surface through fusion with the outer capsid proteins, Hoc (highly antigenic outer capsid protein) and Soc (small outer capsid protein) (21, 35, 37). Although mice are very difficult to protect against lethal Ames strain spore challenge, we have previously shown that mice immunized with PA by TCI were partially protected when challenged by the intranasal route with Ames strain spores. A positive correlation between lethal toxin (LTx) neutralizing antibody titers and survival was observed (28). Currently, rabbits and nonhuman primates have been accepted as the best inhalation anthrax model systems to evaluate anthrax vaccine efficacy (9). Two rabbit anthrax inhalation models, the Dutch-belted (20, 29) and the New Zealand White rabbits, have been utilized for intranasal and bronchoscopy anthrax challenge studies, respectively (29, 30). In both models, PA-specific IgG enzyme-linked immunosorbent assay (ELISA) titers and LTx neutralization titers were identified as correlates of protection. However, for the intranasal rabbit model, LTx titers were the more predictive correlates (reviewed in reference 9).
In this study, we evaluated various PA-generic adjuvant formulations with a variety of delivery platforms and sites of immunization in New Zealand White (NZW) rabbits. The efficacy of the various PA-vaccine formulations was assessed by a pulmonary challenge model using B. anthracis Ames strain spores. The results provide insights on formulations that deserve further consideration as an alternative anthrax vaccine.
MATERIALS AND METHODS
Rabbits.
Pasteurella-free female NZW rabbits, 13 to 16 weeks old (2 to 2.5 kg), were purchased from Charles River Laboratories, individually housed, given water and food ad libitum, and maintained in a specific-pathogen-free facility that was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The study was approved by the Walter Reed Army Institute of Research Animal Safety Committee, was conducted in compliance with the Animal Welfare Act. and adhered to the principles enunciated in the Guide for the Care and Use of Laboratory Animals (27a). Rabbits were shipped in individual crates to the University of New Mexico Health Sciences Center (UNMHSC). The investigators at UNMHSC were blinded with respect to the vaccine regimen. Once the rabbits were transferred to UNMHSC, the study was conducted under a protocol approved by the UNMHSC Institutional Animal Care and Use Committee.
Following Ames spore challenge, the animals were observed twice daily for 14 days for signs of illness or morbidity. All surviving animals were humanely euthanized at the end of the study.
Adjuvant formulations.
The following vaccine formulations (Table 1) were utilized: PA-AH (Alhydrogel from EM Sergeant Pulp & Chemical Co.); L(PA+MPLA) (PA encapsulated in 50 mM liposomes containing monophosphoryl lipid A; MPLA was purchased from Avanti Polar Lipids); PA-emulsion [liposome-stabilized oil-in-water emulsion formulated with L(PA+MPLA) and 40% light mineral oil (125 mM phospholipid)]; T4-PA (PA displayed on bacteriophage T4 through Hoc and Soc); and PA+HLT (PA mixed with E. coli heat-labile enterotoxin; HLT was a kind gift from John Clements, Tulane University). Detailed procedures for the preparation of liposomes (26), liposomal emulsion (26), display of PA on bacteriophage T4 (22, 36, 37), and transcutaneous immunization (TCI) on the backs of small animals were described previously (28). To perform TCI, the backs of the rabbits were shaved (3 by 3 in.). A 2- by 2-in. gauze pad saturated with 10% glycerol in normal saline was placed on the back of the rabbit for 5 min to hydrate the skin. The gauze pad then was removed and the back blotted with dry gauze to thoroughly remove the excess saline. The area then was rubbed lightly (10 strokes) with electrocardiogram sandpaper prep strips (GE Medical Systems) to remove oil and dead epidermal cells, rehydrated again as described above, and blotted dry. PA mixed with HLT (300 μl) then was applied on the surface of the skin. Once the fluid was absorbed by the skin (approximately 15 min), the rabbit was returned to its home cage. For all of the formulations except the phage T4 nanoparticles, purified recombinant PA produced in Bacillus anthracis strain BH445 was used (a gift from Stephen Leppla, NIH) (31). For the T4 display, the recombinant PA-Hoc and Soc-PA proteins were expressed in E. coli and purified (22, 37).
Table 1.
Vaccine formulations and abbreviations
| Vaccine designation | Vaccine formulation | Administration route |
|---|---|---|
| PA-AH | PA adsorbed to Alhydrogel | i.m. |
| PA+HLT | PA mixed with E. coli heat-labile enterotoxin | TCI |
| L(PA+MPLA) | PA encapsulated in 50 mM liposomes containing monophosphoryl lipid A | i.m. |
| PA-emulsion | Liposome-stabilized oil-in-water emulsion formulated with L(PA+MPLA) and 40% light mineral oil (125 mM phospholipid) | i.m. |
| T4-PA | PA displayed on bacteriophage T4 through Hoc and Soc | i.m. |
Immunization and immunological responses.
Rabbits arrived at the facility with ear tags and were randomly placed in cages by the caretaker. The animals were immunized in groups of 5, starting with cages from the top left corner. Rabbits (5/group for vaccine formulations and 6/group for naïve animals) were immunized either by intramuscular (i.m.) or transcutaneous (TCI) routes at weeks 0, 4, and 8 or in separate experiments by the i.m. route at weeks 0 and 4 with various recombinant PA-vaccine formulations under animal biosafety level 1 (ABSL-1) conditions. All of the rabbits that were immunized by the intramuscular route received 10 μg of PA. Rabbits immunized with L(PA+MPLA) and PA-emulsion also received 100 μg of MPLA. Rabbits immunized by the transcutaneous route received 20 μg of PA and various doses of HLT (10 to 80 μg). Animals were bled at 2-week intervals from the central artery in the ear by inserting a butterfly catheter, and individual serum samples were analyzed for PA-specific IgG by ELISA and for lethal toxin (LTx) neutralizing antibodies using J774A.1 cells as described previously (20, 28) and in some detail below. Antibody titers between the different PA-adjuvant vaccine formulations were analyzed using 2-way analysis of variance (ANOVA) with Bonferroni posttests.
PA-specific IgG ELISA.
Sera from individual rabbits were assayed in triplicate for the presence of PA-specific IgG by ELISA. Briefly, 96-well flat-bottomed Nunc Maxisorp plates (VWR International, Inc.) were coated with 0.1 μg/well of PA (List Biological Laboratories, Inc.) overnight, blocked with 0.5% casein in phosphate-buffered saline (PBS) for 2 h at 37°C, washed, and incubated for 1 h at 37°C with the test serum. The plates then were washed, followed by the addition of horseradish peroxidase-labeled affinity-purified goat anti-rabbit IgG (The Binding Site Group Ltd.) for 1 h at room temperature. After the washes, the substrate (2,2′-azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt; KPL) was added for 1 h at room temperature. Plates were read at 405 nm on a SpectraMax 250 plate reader (Molecular Devices). Human sera 801 (PA-specific IgG), kindly provided by Conrad Quinn, was used as a positive control on each plate. Matched prebleed sera were used as the negative control for each animal. The data are expressed as endpoint titers defined as the highest dilution that yielded an optical density reading greater than or equal to twice that of the background values. The titers were calculated after subtracting the mean absorbance of triplicate wells lacking antigen from wells containing antigen. Data were analyzed using two-way ANOVA with Bonferroni posttests.
J774A.1 lethal toxin neutralization assay.
LTx neutralizing antibody titers were determined by the ability of the sera to neutralize the cytotoxicity of LTx in a J774A.1 macrophage cell line as described previously (18, 28). Briefly, 200,000 J774A.1 cells were plated in 96-well Costar flat-bottom plates 15 h before the start of the experiment. Individual serum samples were diluted in duplicate starting at a 1:12.5 dilution in the tube before addition to a separate plate (175 μl). Wells were serially diluted 2-fold for a total of 8 dilutions. Toxin (LTx; 0.5 μg/ml) was added to each of the wells that contained the serum, and the wells were incubated for 1 h at 37°C. The serum-toxin mixture (125 μl) from each of the wells then was transferred onto a plate that had been preseeded with J774A.1 cells and incubated for 6 h. Each plate contained wells that had cells and the toxin, but no test serum (positive control), and wells that only had cells with no toxin or test serum (negative control). These served as controls for cell death. Thiazolyl blue tetrazolium bromide (50 μl) was added to each well to a final concentration of 0.389 mg/ml and incubated at 37°C for 45 min. The solution then was aspirated and lysis buffer (50 μl) was added. The plates were read at 570 nm on a plate reader (Molecular Probes). A 4-parametric sigmoid regression curve was used to determine the dilution of antisera that resulted in a 50% reduction in toxicity (ED50) of anthrax LTx. Monoclonal anti-PA antibody (a kind gift from Steve Leppla) and human antibody 801 (a kind gift from Conrad Quinn) were used as positive controls each day to determine interassay variation. Matched prebleed sera were used as the negative control for each animal.
Pulmonary challenge.
Rabbits were transferred (in a blinded manner) to the ABSL-3/select agent facilities at UNMHSC on week 13, 18, or 25 after the first immunization. The rabbits were allowed to acclimatize for 1 week and then were anesthetized and challenged at week 14, 19, or 26 with Bacillus anthracis Ames strain spores (1.4 × 106 spores; approximately 100 LD50) delivered in a volume of 1 ml just above the bifurcation of the lung by bronchoscopy. Kaplan-Meier survival curves were prepared and evaluated using log-rank analysis.
Spore preparation.
Spore stocks were prepared in phage assay medium as previously described (7, 16), and inocula were prepared from frozen aliquots. The actual number of spores delivered was determined by culturing the inoculum on sheep blood agar plates directly from the bronchoscope. In addition, it was confirmed that the inocula consisted of spores but not vegetative organisms by comparing the average CFU of an aliquot of each postchallenge inoculum sample before and after heating at 68°C for 30 min.
RESULTS
Immunogenicity and challenge data of PA-adjuvant formulation delivered by TCI (three immunizations).
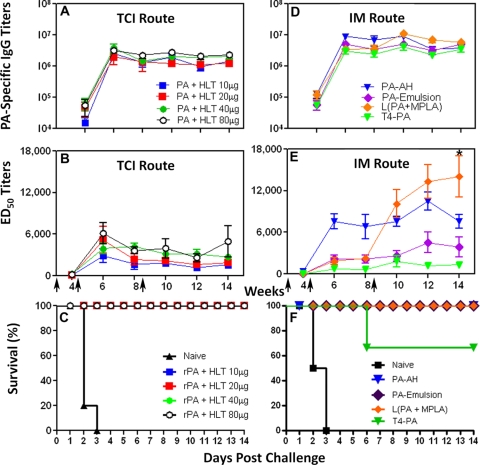
We first examined the efficacy of needle-free immunization via the transcutaneous route. Even low concentrations of the adjuvant HLT that were applied with PA on the surface of the skin by TCI on weeks 0, 4, and 8 induced high PA-specific IgG and LTx-ED50 titers (Fig. 1A and B). The PA-specific IgG titers ranged from 1.3 × 106 to 2.3 × 106 (Fig. 1A) on week 14, while the ED50 titers ranged from 1,600 to 4,900 (Fig. 1B). At week 14 of the study, rabbits were challenged by bronchoscopy with B. anthracis spores. All of the naive animals died by day 3, while 100% (20/20) of the TCI immunized rabbits were protected throughout the duration of the study (Fig. 1C).
Fig 1.
Immune responses to PA-adjuvant vaccine formulations. PA-specific IgG endpoint titers (A and D) and LTx neutralizing antibody titers (ED50) (B and E) were determined by ELISA and toxin neutralization assay, respectively, in individual serum samples of rabbits immunized with PA+HLT by the TCI route (A and B) or with PA-adjuvant vaccine formulations by the i.m. route on weeks 0, 4, and 8 (D and E). Data represent means ± standard errors of the means. Kaplan-Meier survival curves for rabbits challenged with 100 LD50 Ames strain spores on week 14 (C) or 19 (F) of the study are shown. ∗, P ≤ 0. 0.01.
Immunogenicity and challenge data of PA-adjuvant formulations delivered i.m. (three immunizations).
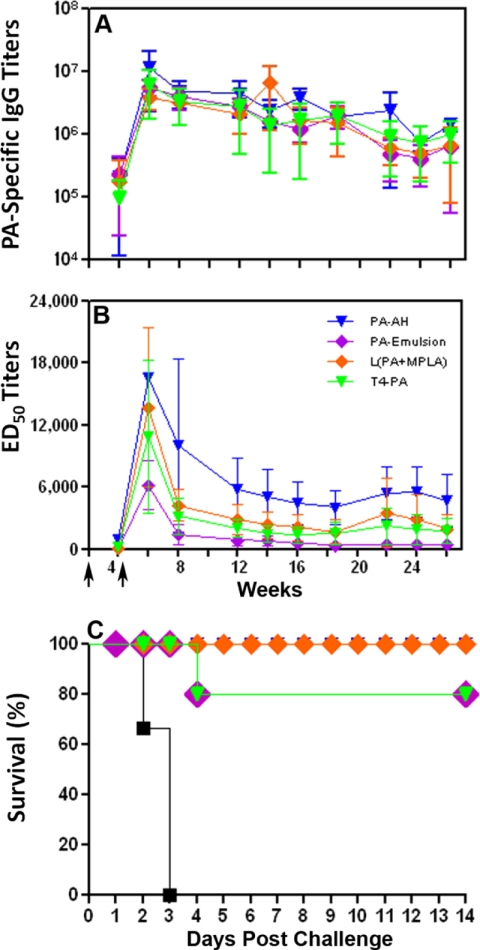
Rabbits were immunized by the i.m. route with three particulate antigen formulations using alternative adjuvants or platforms [L(PA+MPLA), PA-emulsion, and T4-PA], and the responses were compared to the immune responses obtained with PA-AH. These formulations overall induced 10-fold greater PA-specific IgG endpoint titers (Fig. 1D) than skin immunization using HLT (Fig. 1A). All four formulations induced significantly higher (P ≤ 0.05) PA-specific IgG titers at week 10 than PA+HLT. Among the formulations delivered by the i.m. route, there were no significant differences in the PA-specific IgG endpoint titers (3.5 × 106 to 5.8 × 106) between the three groups or compared to the PA-AH group (Fig. 1D). On the other hand, the L(PA+MPLA) group induced higher LTx neutralizing titers than all other groups, including PA-AH, at weeks 10, 12, and 14 following the primary immunization. The L(PA+MPLA) titer at week 14 (14,100) was significantly higher (P < 0.01) than that of PA-AH (7,600) (Fig. 1E), while the PA-emulsion and T4-PA titers were significantly lower (Fig. 1E).
The rabbits were challenged on week 19 of the study. There was 100% death in naive rabbits (2/2) on days 2 and 3 postchallenge (Fig. 1F). Although the vaccine formulations showed protection, the statistical significance between the different formulations could not be determined because of the small number of rabbits challenged. PA-AH, L(PA+MPLA), and PA-emulsion induced 100% survival (2/2, 4/4, and 3/3, respectively), whereas the T4-PA-immunized rabbits showed a 66.7% survival rate (2/3) (Fig. 1F).
Immunogenicity and challenge data of PA-adjuvant formulations delivered i.m. (two immunizations).
Since the LTx-ED50 titers with the various PA-vaccine formulations administered i.m. were significantly different from those of PA-Alhydrogel after three immunizations (Fig. 1E), we examined the effect of two i.m. immunizations (weeks 0 and 4) on the titers and on the duration of the immune response. As shown in Fig. 2A, all of the vaccine formulations induced PA-specific IgG endpoint titers ranging from 105 to 107. The titers were maintained even at week 26 of the study and ranged from 650,000 to 1.4 × 106. At week 14, the endpoint titers ranged from 1.3 × 106 to 6.7 × 106 for all of the groups (Fig. 2A), compared to endpoint titers of 3.8 × 106 to 5.7 × 106 for all of the groups that received three immunizations (Fig. 1D). The LTx-ED50 titers were maximally elevated 2 weeks after injection, but the titers were not maintained through week 26 (Fig. 2B). The titers also were significantly lower (P ≤ 0.001) in the immunized groups at week 14 in the animals that received two doses (945 to 5,000; Fig. 2B) of the vaccine formulations versus three doses (1,300 to 14,000; Fig. 1E). At week 26, L(PA+MPLA)- and PA-AH-immunized animals had LTx-ED50 titers of 2,326 and 5,015, respectively, whereas T4-PA and PA-emulsion showed lower titers, 1,470 and 945, respectively. However, the difference in LTx neutralization titers between the PA-AH and L(PA+MPLA) groups was not significant.
Fig 2.
Immune responses to PA-adjuvant vaccine formulations after two immunizations. PA-specific IgG endpoint titers (A) and LTx-ED50 titers (B) were determined through week 26 of the study in individual serum samples of rabbits immunized with PA-adjuvant vaccine formulations by the i.m. route. Data represent means ± standard errors of the means. (C) Kaplan-Meier survival curves for rabbits challenged with Ames strain spores at week 26 of the study. n = 5 rabbits/immunized group or 6/naïve group.
The immunized rabbits were challenged with B. anthracis spores (100 LD50) at week 26 of the study. All of the naive animals died by day 3 (6/6), while all of the PA-AH- and L(PA+MPLA)-immunized rabbits were 100% protected throughout the duration of the study (5/5) (Fig. 2C). In the PA-emulsion- and T4-PA-immunized groups, 80% of the rabbits were protected (4/5), with one rabbit in each group succumbing to anthrax infection on day 4. All of the blood samples from naive animals had bacteremia at levels equal to or greater than 520 CFU/ml (day 2 postchallenge), but all of the immunized groups were negative (days 2 and 8 postchallenge) except for one of the T4-PA-immunized rabbits that succumbed to anthrax challenge and had bacteremia at 20 CFU/ml (day 2 postchallenge). These results demonstrate that two doses of L(PA+MPLA) were sufficient to induce a long-lived protective immune response lasting 5.5 months after the second immunization.
DISCUSSION
It is well established that the administration of PA with aluminum hydroxide as an adjuvant confers protection to both rabbits (23) and nonhuman primates (19, 33) against a lethal pulmonary challenge with Bacillus anthracis spores. The current regimen for the licensed human anthrax vaccine, AVA, consists of 5 i.m. injections at 0, 1, 6, 12, and 18 months, followed by yearly boosters. While this has led to a decrease in the local reactions compared to those of the vaccine administered by the subcutaneous route, it also has resulted in lower anti-PA antibody titers (24). Therefore, a variety of approaches currently are being employed to develop an improved anthrax vaccine that has potent immunogenicity but low to no reactogenicity. Nonreplicating vectors, live attenuated strains, and various adjuvant and delivery systems are among some of the strategies being explored (9, 33).
In this study, the potency and efficacy of four different adjuvant and delivery systems were compared with those of PA-AH against a lethal pulmonary challenge with B. anthracis spores in NZW rabbits, and this has resulted in three significant insights. First, the delivery of PA through skin using HLT as the adjuvant induced 100% protection, even at the lowest dose tested. Considering that skin patch vaccination is a simple, easy-to-administer, noninvasive procedure and has been evaluated in several animal models as well as in humans (12, 25, 28, 33, 38), TCI might be a good alternative to i.m.-based anthrax vaccines.
Second, in a three-dose regimen, L(PA+MPLA) elicited significantly higher LTx neutralization titers than PA-AH. Furthermore, complete protection with two doses of L(PA+MPLA) as well as the durability of PA-specific antibodies for at least 5 months after the last immunization further supports this formulation as a potent anthrax vaccine candidate. However, further work is necessary to evaluate the potency of this formulation in comparison to the licensed AVA anthrax vaccine (24). The safety and immunogenicity of liposome-encapsulated antigens containing MPLA has been well documented both in animals (17, 32) and in human clinical trials (3, 10, 27). Because of its safety and potency, liposomal MPLA is an attractive adjuvant to be considered for an anthrax vaccine.
Third, these data, when combined with our studies in other animal models (25, 28, 33), demonstrate that different animal species show different responses to various PA adjuvant and delivery systems. The complete protection of NZW rabbits vaccinated with L(PA+MPLA) and PA+HLT (TCI) is consistent with the complete protection observed in nonhuman primates (rhesus macaques) immunized with the same formulations (33). On the other hand, PA-emulsion and T4-PA conferred complete protection in the nonhuman primate model (33), which was not the case in the NZW rabbit model. However, in a separate study using the Dutch-belted intranasal rabbit anthrax challenge model, PA-AH, PA+HLT (TCI), and T4-PA (i.m.) conferred 100% protection (20/20, 16/16, and 11/11, respectively; data not shown).
These differences indicate that comparative adjuvant studies in small-animal models sometimes exhibit results different from those of nonhuman primates and humans. Therefore, the animal models must be combined with the nonhuman primate studies and, where possible, with human clinical trials to assess the predictive quantitative differences in the immunogenicity between different adjuvant-vaccine formulations before the selection of the final human vaccine.
In conclusion, two anthrax vaccine formulations, L(PA+MPLA) and a noninvasive transcutaneous administration of PA+HLT, showed complete protection in rabbits. Furthermore, L(PA+MPLA) showed the durability of the PA-antibody response and protection. These data, combined with our previous anthrax vaccine work in nonhuman primates (33), makes these two formulations highly attractive candidates for further consideration for a next-generation anthrax vaccine.
ACKNOWLEDGMENTS
This research was supported by a cooperative agreement (W81XWH-07-2-0067) between the HMJ Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense. The research was funded in part by the U.S. NIAID, NIH, under NIAID grant U01-AI056443 (to V.B.R.).
We gratefully acknowledge the assistance of Elaine Morrison, Sarah McCormack, and Zhihong Zhang. We thank Stephen Leppla, John Clements, and Conrad Quinn for providing reagents.
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Department of Defense.
Footnotes
Published ahead of print 16 November 2011
REFERENCES
- 1. Alving CR. 2002. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine 20 (Suppl. 3): S56–S64 [DOI] [PubMed] [Google Scholar]
- 2. Alving CR. 1995. Liposomal vaccines: clinical status and immunological presentation for humoral and cellular immunity. Ann. N. Y. Acad. Sci. 754: 143–152 [DOI] [PubMed] [Google Scholar]
- 3. Alving CR, Koulchin V, Glenn GM, Rao M. 1995. Liposomes as carriers of peptide antigens: induction of antibodies and cytotoxic T lymphocytes to conjugated and unconjugated peptides. Immunol. Rev. 145: 5–31 [DOI] [PubMed] [Google Scholar]
- 4. Alving CR, et al. 1984. Liposomes in leishmaniasis: effects of parasite virulence on treatment of experimental leishmaniasis in hamsters. Ann. Trop. Med. Parasitol. 78: 279–286 [DOI] [PubMed] [Google Scholar]
- 5. Baylor NW, Egan W, Richman P. 2002. Aluminum salts in vaccines–US perspective. Vaccine 20(Suppl. 3): S18–S23 [DOI] [PubMed] [Google Scholar]
- 6. Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. 2004. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J. Clin. Investig. 113: 998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chand HS, et al. 2009. Discriminating virulence mechanisms among Bacillus anthracis strains by using a murine subcutaneous infection model. Infect. Immun. 77: 429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cybulski RJ, Jr, Sanz P, O'Brien AD. 2009. Anthrax vaccination strategies. Mol. Aspects Med. 30: 490–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedlander AM, Little SF. 2009. Advances in the development of next-generation anthrax vaccines. Vaccine 27(Suppl. 4): D28–D32 [DOI] [PubMed] [Google Scholar]
- 10. Fries LF, et al. 1992. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc. Natl. Acad. Sci. U. S. A. 89: 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glenn GM, Rao M, Matyas GR, Alving CR. 1998. Skin immunization made possible by cholera toxin. Nature 391: 851. [DOI] [PubMed] [Google Scholar]
- 12. Glenn GM, et al. 2000. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med. 6: 1403–1406 [DOI] [PubMed] [Google Scholar]
- 13. Grabenstein JD. 2008. Vaccines: countering anthrax: vaccines and immunoglobulins. Clin. Infect. Dis. 46: 129–136 [DOI] [PubMed] [Google Scholar]
- 14. Guebre-Xabier M, et al. 2003. Immunostimulant patch containing heat-labile enterotoxin from Escherichia coli enhances immune responses to injected influenza virus vaccine through activation of skin dendritic cells. J. Virol. 77: 5218–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guy B. 2007. The perfect mix: recent progress in adjuvant research. Nat. Rev. Microbiol. 5: 505–517 [DOI] [PubMed] [Google Scholar]
- 16. Heninger S, et al. 2006. Toxin-deficient mutants of Bacillus anthracis are lethal in a murine model for pulmonary anthrax. Infect. Immun. 74: 6067–6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heppner DG, et al. 1996. Safety, immunogenicity, and efficacy of Plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. J. Infect. Dis. 174: 361–366 [DOI] [PubMed] [Google Scholar]
- 18. Hering D, et al. 2004. Validation of the anthrax lethal toxin neutralization assay. Biologicals 32: 17–27 [DOI] [PubMed] [Google Scholar]
- 19. Ivins BE, et al. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16: 1141–1148 [DOI] [PubMed] [Google Scholar]
- 20. Li Q, et al. 2009. Anthrax LFn-PA hybrid antigens: biochemistry, immunogenicity, and protection against lethal ames spore challenge in rabbits. Open Vaccine J. 2: 92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Q, Shivachandra SB, Leppla SH, Rao VB. 2006. Bacteriophage T4 capsid: a unique platform for efficient surface assembly of macromolecular complexes. J. Mol. Biol. 363: 577–588 [DOI] [PubMed] [Google Scholar]
- 22. Li Q, Shivachandra SB, Zhang Z, Rao VB. 2007. Assembly of the small outer capsid protein, Soc, on bacteriophage T4: a novel system for high density display of multiple large anthrax toxins and foreign proteins on phage capsid. J. Mol. Biol. 370: 1006–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Little SF, et al. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22: 422–430 [DOI] [PubMed] [Google Scholar]
- 24. Marano N, et al. 2008. Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months: a randomized trial. JAMA 300: 1532–1543 [DOI] [PubMed] [Google Scholar]
- 25. Matyas GR, et al. 2004. Needle-free skin patch vaccination method for anthrax. Infect. Immun. 72: 1181–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matyas GR, Muderhwa JM, Alving CR. 2003. Oil-in-water liposomal emulsions for vaccine delivery. Methods Enzymol. 373: 34–50 [DOI] [PubMed] [Google Scholar]
- 27. McElrath MJ. 1995. Selection of potent immunological adjuvants for vaccine construction. Semin. Cancer Biol. 6: 375–385 [DOI] [PubMed] [Google Scholar]
- 27a. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 28. Peachman KK, et al. 2006. Correlation between lethal toxin-neutralizing antibody titers and protection from intranasal challenge with Bacillus anthracis Ames strain spores in mice after transcutaneous immunization with recombinant anthrax protective antigen. Infect. Immun. 74: 794–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peterson JW, et al. 2006. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 74: 1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pitt ML, et al. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19: 4768–4773 [DOI] [PubMed] [Google Scholar]
- 31. Ramirez DM, Leppla SH, Schneerson R, Shiloach J. 2002. Production, recovery and immunogenicity of the protective antigen from a recombinant strain of Bacillus anthracis. J. Ind. Microbiol. Biotechnol. 28: 232–238 [DOI] [PubMed] [Google Scholar]
- 32. Rao M, Bray M, Alving CR, Jahrling P, Matyas GR. 2002. Induction of immune responses in mice and monkeys to Ebola virus after immunization with liposome-encapsulated irradiated Ebola virus: protection in mice requires CD4(+) T cells. J. Virol. 76: 9176–9185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao M, et al. 2011. Highly effective generic adjuvant systems for orphan or poverty-related vaccines. Vaccine 29: 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richards RL, et al. 2004. Liposome-stabilized oil-in-water emulsions as adjuvants: increased emulsion stability promotes induction of cytotoxic T lymphocytes against an HIV envelope antigen. Immunol. Cell Biol. 82: 531–538 [DOI] [PubMed] [Google Scholar]
- 35. Sathaliyawala T, et al. 2006. Assembly of human immunodeficiency virus (HIV) antigens on bacteriophage T4: a novel in vitro approach to construct multicomponent HIV vaccines. J. Virol. 80: 7688–7698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shivachandra SB, et al. 2007. Multicomponent anthrax toxin display and delivery using bacteriophage T4. Vaccine 25: 1225–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shivachandra SB, et al. 2006. In vitro binding of anthrax protective antigen on bacteriophage T4 capsid surface through Hoc-capsid interactions: a strategy for efficient display of large full-length proteins. Virology 345: 190–198 [DOI] [PubMed] [Google Scholar]
- 38. Yu J, et al. 2002. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect. Immun. 70: 1056–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]