Abstract
The plaque reduction neutralization test (PRNT) is used widely to measure the neutralization activity of anti-dengue virus (DENV) antibodies, but it is time-consuming and labor-intensive and has low sample throughput. For fast and convenient measurement of neutralizing antibodies, especially in evaluating the efficiency of the DENV vaccines on a large scale, a new method is needed to replace PRNT. In recent decades, several microneutralization assays have been developed to overcome the limitations of PRNT. In the present study, we evaluated one of these, the enzyme-linked immunospot microneutralization test (ELISPOT-MNT), in comparison with PRNT. ELISPOT-MNT is performed in 96-well format, and the plaques are developed after 2 to 4 days using an ELISA to transform them into spots, which are detected automatically with an ELISPOT instrument. The assay is faster than PRNT, has a high throughput, and is more objective. We used 10 monoclonal antibodies (MAbs) against domain III of the DENV envelope protein (EDIII) to evaluate the two assays; all of these MAbs cross-react with all four serotypes of DENV as measured by immunofluorescence assay. The two neutralization assays were performed simultaneously to measure the 50% inhibitory concentration (IC50) of these MAbs. Using PRNT as the reference and treating IC50 values higher than 50 μg/ml of MAbs as negative, ELISPOT-MNT showed a sensitivity of 95.6% and specificity of 88.24% when 10 MAbs were tested against four DENV serotype strains. A good correlation (R2 = 0.672; P = 0.000) was observed between the two assays, making ELISPOT-MNT a potentially valuable method for measure of neutralizing antibodies against DENV.
INTRODUCTION
Dengue virus (DENV) is a mosquito-borne virus that belongs to the Flavivirus genus in the Flaviviridae family (11). DENV has four known serotypes: DENV-1, DENV-2, DENV-3, and DENV-4. Infection with any of the four serotypes can cause a spectrum of diseases ranging from dengue fever (DF) to dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) (4). In the absence of effective vaccines or specific treatments, dengue has become a major public health problem throughout the tropical and subtropical areas of the world (18). Antibodies elicited by one primary DENV serotype infection are not strongly protective against the other three; conversely they may lead to the development of DHF or DSS because the cross-reactivity may facilitate viral infection through Fc receptor-mediated binding to monocytes (5, 6). For this reason, any dengue vaccine produced must be evaluated for its ability to induce long-term and simultaneous protection against all four serotype DENV, in order to avoid antibody-dependent enhancement (ADE) of viral infection. Therefore, in vaccine research, the protective ability of each antibody needs to be evaluated.
The plaque reduction neutralization test (PRNT) has been considered the gold standard for detecting the neutralization activity of antibodies against DENV since it was first introduced in 1967 (14). Although WHO has developed a standard protocol for PRNT (19), the method is time-consuming and labor-intensive and is not applicable to all DENV serotype strains, especially some clinical isolates (16). For most primary clinical isolates, PRNT does not form clear plaques or does not have a visible cytopathic effect (CPE) on cell monolayers. Moreover, it is not well suited to high-throughput screening (12), which is needed for vaccine evaluation. Therefore, a fast, convenient, and efficient method should be established.
Recently, Shanaka et al. (15) developed an enzyme-linked immunospot-based microneutralization assay (ELISPOT-MNT) to detect the viral antigen in infected cells and a 96-well enzyme-linked immunospot readout instrument to measure the spots produced in an indirect immunostaining method. The extent of infection can be observed easily in ELISPOT-MNT by counting the spots; this is comparable to counting the plaques developed in the classic PRNT, but the former test offers an automated and high-throughput way for measuring neutralizing antibodies, which is also more objective. In this study, our aim to compare ELISPOT-MNT and PRNT by using a panel of monoclonal antibodies (MAbs) raised against domain III of the DENV envelope protein (EDIII); these MAbs with cross-reactivity toward all four DENV serotypes were used to evaluate the two assays.
MATERIALS AND METHODS
Virus and cell lines.
Four DENV serotype strains (DENV-1, Hawaii; DENV-2, New Guinea-C; DENV-3, Guanxi-80-2; and DENV-4, H241) used in this study were kindly provided by the Center for Disease Control and Prevention of Guangzhou, China (3). They were propagated in Aedes albopictus cells (C6/36, ATCC CRL-1660) and titrated in continuous African green monkey kidney cells (Vero-E6, ATCC CRL-1586) with a plaque assay.
Preparation of MAbs with neutralization.
All of the monoclonal antibodies (MAbs) used in this paper were produced in our laboratory (unpublished data), as described briefly below. Purified recombinant EDIII protein of DENV-1, DENV-2, DENV-3, and DENV-4 (1), separately or mixed together, were used to immunize BALB/c mice as described previously (2). The hybridoma cell lines were screened by both indirect ELISA using the EDIII protein from each dengue serotype as coating antigens and immunofluorescence assay (IFA) that detected MAb binding on C6/36 cells infected with each dengue serotype. The MAbs that cross-reacted with all four DENV serotypes were isolated and cloned according to their abilities to bind to EDIII protein and/or infected C6/36 cells from each DENV serotype. The MAbs were purified by using protein G column chromatography (Amersham-Pharmacia, Uppsala, Sweden) according to the manufacturer's instructions, filtrated through a 0.2-μm-pore-size membrane, and stored at −80°C in 50% glycerol.
Plaque reduction neutralization test in Vero E6 cells.
The PRNT was performed using the protocol from WHO (19) with modification. Vero-E6 cells were seeded in six-well plates at a density of 4 × 105 cells/well and incubated overnight at 37°C in 5% CO2 until the cells were approximately 80% to 90% confluent. The test antibodies were serially diluted 3-fold from 100 μg/ml in minimum essential medium (MEM; Gibco) containing 1% fetal bovine serum (FBS). An equal volume of virus preparation, diluted to give approximately 50 PFU per well, was added to the diluted antibodies (initial concentration of 50 μg/ml). After incubation at 37°C in 5% CO2 for 1 h, the virus-plus-antibody mixture was added to the Vero E6 cells and incubated for 1.5 h. The plates were rocked gently every 15 min to distribute the inoculum. Each antibody dilution plus virus was performed in duplicate or triplicate and three controls, a virus dose control (virus plus diluent only), a blank control (diluent only), and an unrelated MAb control (anti-influenza A virus nucleocapsid protein antibody), were tested simultaneously. Each entire experiment was replicated. After incubation, the cell monolayers were overlaid with semisolid medium consisting of 1.2% methylcellulose (Sigma), 2% heat-inactivated FBS, MEM, and 50 μg/ml gentamicin (Gibco) and then incubated at 37°C in 5% CO2 for 7 to 10 days. Next, 2 ml of 1% formaldehyde solution was added to each well and the cells were fixed for 1 h. Plaques were stained with 0.8% crystal violet (Sigma) for 10 min after removing the overlay. The PRNT data for each antibody were used to calculate percent reduction in plaques, determined as follows: % reduction = 100 × [1 − (average number of plaques for each dilution/average number of the virus dose control plaques)].
Enzyme-linked immunospot based microneutralization test.
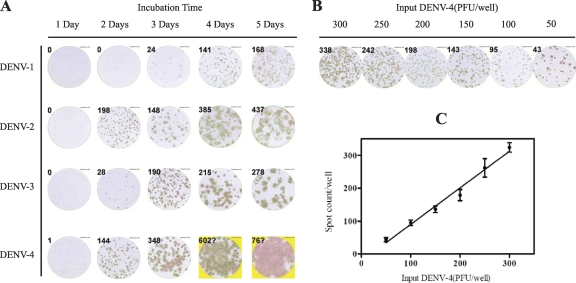
The enzyme-linked immunospot based microneutralization test (ELISPOT-MNT), first described by Shanaka et al. (15), was performed with minor modifications according to our optimal experiments (Fig. 1). Each well of 96-well plates was seeded with 2 × 104 Vero-E6 cells and incubated overnight at 37°C in 5% CO2 until the cells were approximately 90% confluent. The antibodies were diluted as described for PRNT and mixed with an equal volume of virus, diluted to give about 200 PFU per well. Each antibody dilution plus virus was inoculated in triplicate wells (200 μl/well), and each entire experiment was duplicated or triplicated. After incubation and infection as described for PRNT, the cell monolayers were overlaid with semisolid medium consisting of 1.2% methylcellulose (Sigma), 5% heat-inactivated FBS, 50 μg/ml gentamicin, and MEM and then incubated at 37°C in 5% CO2 for 2 to 4 days. The optimal incubation time for each virus was determined in preliminary experiments, based on the visible stained spot (plaque) formation (Fig. 1). After the incubation, the semisolid medium was removed and the cells were fixed for 20 min at −20°C with methanol: acetone (3:7). The fixed cells were treated with an anti-DENV nonstructural protein 1 (NS1) monoclonal antibody (5F10A7) that recognizes equally all four DENV serotypes (3), and the spots were developed with AEC solution (SK4200; Vector Laboratories) in an indirect immunostaining assay. The spots were detected by ELISPOT instrument (S5 VERSA; Cellular Technology Ltd.) and analyzed using the smart count software on the machine (CTL ImmunoSpot Academic Software Version 5.0). The percent reduction in plaques of ELISPOT-MNT was calculated in the same manner as for PRNT.
Fig 1.
Optimization of incubation time for visibly stained spot formation in Vero-E6 infected by four serotype DENVs and the efficiency of DENV-4 plaque formation in Vero E6 cells. Each DENV was added to Vero E6 cell monolayers (2.0 × 104 cells/well) in five 96-well plates and then incubated for 1 to 5 days. After incubation, virus plaques (spots) were detected by indirect immunostaining with a cross-reactive mouse anti-DENV NS1 monoclonal antibody (5F10A7) and counted using the ELISPOT instrument (S5 VERSA, Cellular Technology Ltd.). (A) Change in size and definition of spots from days 1 to 5. The optimal incubation times are 4 days for DENV-1, 2 days for DENV-2 and -4, and 3 days for DENV-3 based on the spots that are clear enough to distinguish but not diffuse. (B) Graded amounts of DENV-4 were added to Vero E6 cells in 96-well plates; the figure shows representative wells from an assay performed four times. (C) Efficiency of DENV-4 infection. Error bars show the standard deviation of spot counting in four measurements. There is a good relation between the amount of virus and the spot count (R2 = 0.9646; P < 0.01).
Data analysis.
The 50% inhibitory concentration of each antibody (IC50), which is the concentration that gave 50% plaque reduction, was calculated by nonlinear, dose-response regression analysis with GraphPad Prism 5.04 software. The results of two assay methods were compared by a paired-sample t test, agreement was assessed by McNemar's test, and the correspondence between the two methods was analyzed by linear regression using SPSS 13.0 software. A P value of <0.05 was considered statistically significant for all parameters, and the confidence interval (CI) was 95%.
RESULTS
Specificity of the MAbs.
To develop MAbs with cross-reactivity toward all four DENV serotypes, we selected 10 MAbs that cross-reacted with domain III from DENV-1 to -4 as measured by ELISA and/or that recognized cells infected with different serotypes of DENV as measured by IFA from more than 100 MAbs that are available in our laboratory (unpublished data). The results are shown in Table 1. It was observed that some MAbs did not react with heterotypic EDIII by ELISA, but all MAbs cross-reacted with four DENV serotypes as measured by IFA, irrespective of the recombinant EDIII protein that was used as the immunogen. The discrepancy may be due to the fact that epitopes recognized by some MAbs do not exist in heterotypic EDIII or some MAbs may be directed against conformational or discontinuous epitopes on EDIII.
Table 1.
Specificity of MAbsa
| Immunogen | MAb | Isotype | Indirect ELISA of EDIII result |
IFA result |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | DENV-1 | DENV-2 | DENV-3 | DENV-4 | |||
| DENV-1 EDIII | 5E7A6 | IgG1 | + | + | + | + | + | + | + | + |
| DENV-1 EDIII | 5A12A7 | IgG1 | + | + | + | + | + | + | + | + |
| DENV-2 EDIII | 4C58A2 | IgG1 | + | + | + | + | + | + | + | + |
| DENV-3 EDIII | 2D73A7 | IgG1 | − | − | + | − | + | + | + | + |
| DENV-3 EDIII | 4A19A2A33 | IgG1 | + | + | + | + | + | + | + | + |
| DENV-3 EDIII | 2D13A12 | IgG2b | + | + | + | + | + | + | + | + |
| DENV-4 EDIII | 1E1A7 | IgG1 | + | + | + | + | + | + | + | + |
| DENV-1-4 EDIII | 3E31A4 | IgG1 | + | + | + | + | + | + | + | + |
| DENV-1-4 EDIII | 5E56A5 | IgG1 | + | − | + | + | + | + | + | + |
| DENV-1-4 EDIII | 2B11A35 | IgG1 | + | − | + | − | + | + | + | + |
The reactivity of each MAb was determined using indirect immunofluorescence assay (IFA) on C6/36 cells infected with DENV and indirect ELISA of EDIII using recombinant EDIII protein from the four DENV serotypes.
Inhibition profiles of the MAbs in two neutralization assays.
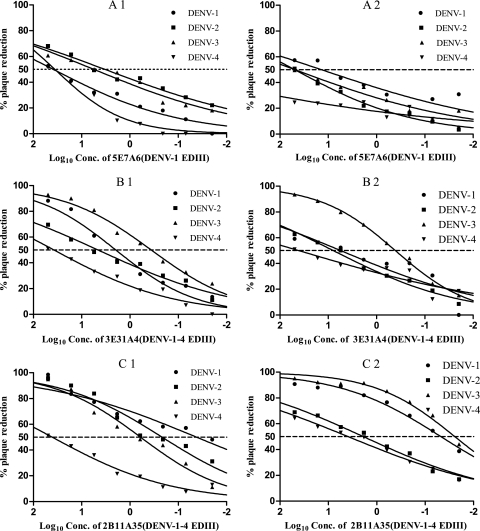
PRNT and ELISPOT-MNT assays were performed simultaneously to measure the neutralizing abilities of these MAbs against the four DENV serotypes in Vero E6 cells. The results (Fig. 2) were used to calculate the IC50 by nonlinear, dose-response regression analysis. The mean values determined from two or three independent experiments are reported. The three representative MAbs shown in Fig. 2 each produce a concentration-dependent plaque reduction for all DENV serotypes in both of the two assay methods. However, in some cases, less than 50% inhibition was reached at the highest MAb concentration tested (50 μg/ml). In these cases, the IC50 is shown in Table 2 as “>50,” and they were designated a negative result. This designation produces some differences from those obtained using IFA, where it was observed that all MAbs can bind to each DENV serotype. MAbs with an IC50 below 50 μg/ml were regarded as positive. From the data in Table 2, two MAbs (3E31A4 and 2B11A35) have a positive reaction with all four DENV serotypes with both assay methods, and one MAbs (5E7A6) neutralizes all four DENV serotypes in PRNT, but three serotypes expect DENV-4 in ELISPOT-MNT. For the subsequent statistical analysis, IC50 values of >50 μg/ml were taken as equal to 50 μg/ml.
Fig 2.
PRNT and ELISPOT-MNT in Vero E6 cells. These data represent the extent of plaque reduction compared to a virus dose control as a function of the concentration of various MAbs that show cross-reaction toward four DENV serotypes. A1, B1, and C1 were measured by PRNT, while A2, B2, and C2 were assayed by ELISPOT-MNT.
Table 2.
Comparison of PRNT and ELISPOT-MNT using MAbs raised against recombinant DENV EDIII proteina
| Immunogen | MAb | Isotype | Resultb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DENV-1 |
DENV-2 |
DENV-3 |
DENV-4 |
|||||||
| PRNT | ELISPOT-MNT | PRNT | ELISPOT-MNT | PRNT | ELISPOT-MNT | PRNT | ELISPOT-MNT | |||
| DENV-1 EDIII | 5E7A6 | IgG1 | 38.91 | 13.96 | 3.33 | 49.74 | 5.63 | 49.67 | 36.11 | >50 |
| DENV-1 EDIII | 5A12A7 | IgG1 | 5.2 | 46.51 | >50 | >50 | >50 | >50 | >50 | >50 |
| DENV-2 EDIII | 4C58A2 | IgG1 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| DENV-3 EDIII | 2D73A7 | IgG1 | >50 | >50 | 0.09 | 0.16 | 0.86 | 0.18 | >50 | 18.82 |
| DENV-3 EDIII | 4A19A2A33 | IgG1 | >50 | >50 | 2.01 | 7.87 | 4.02 | 15.2 | >50 | >50 |
| DENV-3 EDIII | 2D13A12 | IgG2b | >50 | >50 | 10.45 | 6.28 | 2.44 | 10.31 | >50 | >50 |
| DENV-4 EDIII | 1E1A7 | IgG1 | 36.83 | 33.9 | >50 | >50 | 5.47 | 3.38 | 43.86 | 38.7 |
| DENV-1-4 EDIII | 3E31A4 | IgG1 | 1.98 | 5.55 | 4.64 | 7.89 | 0.37 | 0.41 | 37.79 | 40.9 |
| DENV-1-4 EDIII | 5E56A5 | IgG1 | >50 | 13.96 | >50 | >50 | 2.02 | 32.84 | >50 | >50 |
| DENV-1-4 EDIII | 2B11A35 | IgG1 | 0.04 | 0.05 | 0.22 | 1.89 | 0.61 | 0.02 | 40.99 | 3.91 |
PRNT, plaque reduction neutralization test; ELISPOT-MNT, enzyme-linked immunospot based microneutralization test.
The neutralization concentration for each MAbs was determined concurrently with two assay methods, and the data shown are the mean 50% inhibitory concentrations (IC50) (in μg/ml). “>50” indicates less than 50% inhibition at the highest MAb concentration of 50 μg/ml. All IC50 values were calculated by nonlinear, dose-response regression analysis with GraphPad Prism 5.04 software.
Correlation between PRNT and ELISPOT-MNT.
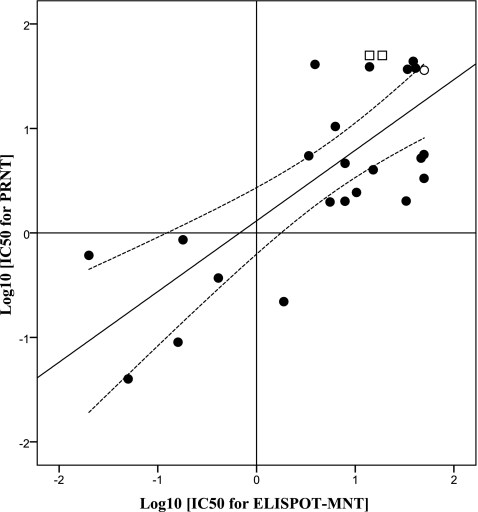
PRNT is generally regarded as the standard assay method, so we judged the success of the microneutralization tests against this reference. All of the results were compared by a paired-sample t test with SPSS 13.0 software, which showed that there was no significant difference (P = 0.365) and a good relationship between the two assay methods (R2 = 0.672; P = 0.000). As shown in Table 3, there are one false negative and two false positives for ELISPOT-MNT. The method showed a sensitivity of 95.6% (22/23) and a specificity of 88.24% (15/17) when these 10 MAbs were tested against four DENV serotype strains. McNemar's association test was used to measure the disagreement between the two assay methods. There was no significant (P = 1.000) disagreement between them. All 25 positive results from at least one of the two assay methods were further analyzed by linear regression (SPSS 13.0 software) to compare the agreement between IC50 values. As shown in Fig. 3, the two assay methods agree reasonably well (R2 = 0.555; P = 0.000), despite the scatter in the data.
Table 3.
Assay disagreement by McNemar's test comparing two assays (n = 40)c
| ELISPOT-MNT result | PRNT result (no.) |
Total no. of results | |
|---|---|---|---|
| Positive | Negative | ||
| Positivea | 22 | 2 | 24 |
| Negativeb | 1 | 15 | 16 |
| Total | 23 | 17 | 40 |
IC50, ≤50 μg/ml.
IC50, >50 μg/ml.
Significance level (P = 1.000) is a statistical measure of the assay disagreement determined using McNemar's test. Using the PRNT as a reference, the sensitivity and specificity of ELISPOT-MNT were 95.6% (22/23) and 88.24% (15/17), respectively.
Fig 3.
Scatter plot of the IC50 values from PRNT and ELISPOT-MNT (log-log transformation). The middle line is the best fit to the data by linear regression (R2 = 0.555; P = 0.000); the dashed lines show the 95% confidence interval. The hollow square and circle are the false-positive and false-negative results compared to PRNT, taking the value >50 μg/ml as 50 μg/ml.
DISCUSSION
DENV is a member of the family Flaviviridae and has four serotypes, with each serotype capable of causing DF and DHF/DSS. Both humoral and cellular immunity contribute to the protection and clearance of DENV, but the neutralization by antibodies is regarded as the key mechanism by which protection against DENV is achieved (13). To develop such a vaccine, one condition is particularly desirable: a laboratory measurement of the immune response that can reliably and unambiguously predict protective immunity (7). A large number of vaccine-induced neutralizing antibodies against DENV are likely to be generated, necessitating an accurate and efficient assay to measure their neutralization activity (12).
Although PRNT has achieved widespread use in DENV research, neither the methodology nor the required critical reagents were standardized and harmonized between laboratories. It is known that many variables, such as virus passage, cell lines, and the use of complement, have an impact on the assay results (16). To improve its reproducibility, WHO established a guideline for standardizing the method (19). Nevertheless, the conventional PRNT remains time-consuming and labor-intensive, with low throughput. To overcome these deficiencies of PRNT, newer tests assessing dengue virus neutralization are being developed, such as a microfocus reduction neutralization test (mFRNT) (8), microneutralization assays based on an ELISA (17), flow cytometry (10), and ELISPOT (15). However, any new approach to titration of dengue virus neutralizing antibodies will need to be validated against the standard PRNT. Microneutralization assays are performed in a 96-well format, use much less sample, and can be applied to high-throughput screening, potentially overcoming the major limitations of PRNT. Furthermore, assays based on microreadout format can be automated, making them less subjective than any manual method. Flow cytometry shares the advantages of microneutralization but has some drawbacks (9, 10). First, intra-assay variability is high, especially when the same sample is tested on different days. Second, it is difficult to achieve conditions of antibody excess because the amount of virus used in flow cytometry is 10- to 100-fold higher than in PRNT. Another microneutralization assay, ELISA-based microneutralization, has been developed to measure the anti-DENV antibodies in serum samples obtained from patients with DENV infection (17). This assay reduces the incubation time to 5 days, is less expensive, and is more conducive to testing large numbers of samples. However, it has been reported that the results obtained from PRNT and the ELISA-based microneutralization assay are not comparable when the serum samples are obtained from patients experiencing a second DENV infection (17).
In the present work, ELISPOT-MNT was used to assay 10 MAbs against DENV EDIII and the results were compared with PRNT. The ELISPOT-MNT assay was developed in 2009 from an ELISA-based neutralization test (15). This microneutralization plaque assay utilizes a 96-well ELISPOT readout instrument for detection and quantitation, opening up the possibility of automated, high-throughput measurement of dengue plaques. The results of our comparison of PRNT and ELISPOT-MNT show that there was significant agreement (R2 = 0.672; P = 0.000) between the two methods. The only problem that we encountered is that care must be taken during the multiple plate washings; otherwise, cells can be flushed from the plate, which then causes an underestimate in the spot count.
Treating the results of PRNT as the standard, ELISPOT-MNT gives similar results apart from two false positives and one false negative. We suggest that this is not a defect but, rather, that the apparent false positives are evidence that this assay is somewhat more sensitive than PRNT. The reason for the higher sensitivity of the assay may be that the instrument can detect the relatively small spots formed after incubating for a shorter time than in PRNT.
Our experience with PRNT showed that it is very time-consuming while ELISPOT-MNT is fast, gives results that are more objective due to the use of automation, and correlates well with PRNT. Therefore, ELISPOT-MNT shows potential as a primary method for screening neutralization activities of large numbers of antibodies, prior to using PRNT for confirmation.
ACKNOWLEDGMENTS
We thank Ronald G. Duggleby for his assistance in editing this paper.
Funding: this work was supported by grant 30725031 from the National Outstanding Young Scientist Foundation of China, grant 2009ZX10004-306 of the National Science and Technology Major Project of China, and grant 30671874 of the Research Program of the National Natural Science Foundation of China.
Footnotes
Published ahead of print 23 November 2011
REFERENCES
- 1. Cai JP, et al. 2010. Characterization and secreted expression of dengue virus type I-IV envelope glycoprotein domain III in Pichia pastoris. Zhonghua Yu Fang Yi Xue Za Zhi 44: 721–725 (In Chinese.) [PubMed] [Google Scholar]
- 2. Che XY, et al. 2004. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory symdrome. J. Clin. Microbiol. 42: 2629–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding X, et al. 2011. Full serotype- and group-specific NS1 capture enzyme-linked immunosorbent assay for rapid differential diagnosis of dengue virus infection. Clin. Vaccine Immunol. 18: 430–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. George R, Lum LCS. 1997. Clinical spectrum of dengue infection, p 89–114 In Gubler DJ, Kuno G. (ed), Dengue and dengue hemorrhagic fever, 3rd ed CAB International Press, London, United Kingdom [Google Scholar]
- 5. Halstead SB. 2002. Dengue hemorrhagic fever: two infections and antibody dependent enhancement, a brief history and personal memoir. Rev. Cuba. Med. Trop. 54: 171–179 [PubMed] [Google Scholar]
- 6. Halstead SB. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239: 476–481 [DOI] [PubMed] [Google Scholar]
- 7. Jin X, Block OT, Rose R, Schlesinger J. 2009. Dengue vaccine development and dengue viral neutralization and enhancement assays. Antivir. Ther. 14: 739–749 [DOI] [PubMed] [Google Scholar]
- 8. Jirakanjanakit N, Sanohsomneing T, Yoksan S, Bhamarapravati N. 1997. The micro-focus reduction neutralization test for determining dengue and Japanese encephalitis neutralizing antibodies in volunteers vaccinated against dengue. Trans. R. Soc. Trop. Med. Hyg. 91: 614–617 [DOI] [PubMed] [Google Scholar]
- 9. Kraus AA, Messer W, Haymore LB, de Silva AM. 2007. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J. Clin. Microbiol. 45: 3777–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lambeth CR, White LJ, Johnston RE, de Silva AM. 2005. Flow cytometry-based assay for titrating dengue virus. J. Clin. Microbiol. 43: 3267–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindenbach BD, Thiel HJ, Rice CM. 2007. Flaviviridae: the viruses and their replication, p 1103–1113 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 12. Martin NC, et al. 2006. An immunocytometric assay based on dengue infection via DC-SIGN permits rapid measurement of anti-dengue neutralizing antibodies. J. Virol. Methods 134: 74–85 [DOI] [PubMed] [Google Scholar]
- 13. Murphy BR, Whitehead SS. 2011. Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 29: 587–619 [DOI] [PubMed] [Google Scholar]
- 14. Russell PK, Nisalak A. 1967. Dengue virus identification by the plaque reduction neutralization test. J. Immunol. 99: 291–296 [PubMed] [Google Scholar]
- 15. Shanaka WW, et al. 2009. An automated Dengue virus microneutralization plaque assay performed in human Fcγ receptor-expressing CV-1 cells. Am. J. Trop. Med. Hyg. 80: 61–65 [PubMed] [Google Scholar]
- 16. Thomas SJ, et al. 2009. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: how alterations in assay conditions impact performance. Am. J. Trop. Med. Hyg. 81: 825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vorndam V, Beltran M. 2002. Enzyme-linked immunosorbent assay-format microneutralization test for dengue viruses. Am. J. Trop. Med. Hyg. 66: 208–212 [DOI] [PubMed] [Google Scholar]
- 18. Whitehead SS, Blaney JE, Durbin AP, Murphy BR. 2007. Prospects for a dengue virus vaccine. Nature reviews. Microbiology 5: 518–528. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization Department of Immunization Vaccines Biologicals 2007. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. World Health Organization, Geneva, Switzerland: WHO/IVB/07.07. http://whqlibdoc.who.int/hq/2007/who_ivb_07.07_eng.pdf [Google Scholar]