Abstract
Extrapulmonary tuberculosis may be due to underlying immune compromise. Immunosuppressive regulatory T cells (Treg cells), and CD4+ T lymphocytes in general, are important in the host immune response to Mycobacterium tuberculosis. We evaluated T lymphocytes from patients after recovery from extrapulmonary tuberculosis, which may reflect conditions before M. tuberculosis infection. A case-control study was conducted among HIV-uninfected adults with previously treated extrapulmonary tuberculosis and 3 sets of controls: (i) subjects with previously treated pulmonary tuberculosis, (ii) close tuberculosis contacts with M. tuberculosis infection, and (iii) close tuberculosis contacts with no infection. Monocyte-depleted peripheral blood mononuclear cells (PBMC-M) were stained for CD4+ CD25hi CD127low FoxP3+ cell (Treg cell) and T lymphocyte activation. Both characteristics were compared as continuous variables between groups with the Kruskal-Wallis test. There were 7 extrapulmonary tuberculosis cases, 18 pulmonary tuberculosis controls, 17 controls with M. tuberculosis infection, and 18 controls without M. tuberculosis infection. The median Treg cell proportion was highest among persons with previous extrapulmonary tuberculosis (1.23%) compared to subjects with pulmonary tuberculosis (0.56%), latent M. tuberculosis infection (0.14%), or no M. tuberculosis infection (0.20%) (P = 0.001). The median proportion of CD4+ T lymphocytes that expressed the activation markers HLA-DR and CD38 was highest for CD4+ T lymphocytes from persons with previous extrapulmonary tuberculosis (0.79%) compared to subjects with pulmonary tuberculosis (0.44%), latent M. tuberculosis infection (0.14%), or no M. tuberculosis infection (0.32%) (P = 0.005). Compared with controls, persons with previously treated extrapulmonary tuberculosis had the highest Treg cell frequency, but also the highest levels of CD4+ T lymphocyte activation. Immune dysregulation may be a feature of individuals at risk for extrapulmonary tuberculosis.
INTRODUCTION
Of the estimated 2 billion people infected with Mycobacterium tuberculosis, only 5 to 10% will develop tuberculosis (TB) (14, 28). Although most persons with TB have pulmonary disease, approximately 20% have extrapulmonary involvement (8). Extrapulmonary tuberculosis may be a marker of underlying immune compromise, as illustrated by the increased risk of such disease among HIV-infected persons and young children (31, 35). We previously demonstrated decreased CD4+ T lymphocyte numbers and a decreased ability of unstimulated peripheral blood mononuclear cells (PBMC) to produce cytokines among HIV-uninfected adults who have recovered from extrapulmonary tuberculosis compared to controls with pulmonary tuberculosis or latent M. tuberculosis infection (1, 47). The factors that predispose individuals to extrapulmonary disease may provide insights into the risk factors for progression to all forms of active tuberculosis after M. tuberculosis infection.
The increased incidence of tuberculosis, specifically extrapulmonary tuberculosis, among individuals with HIV infection (31) or individuals receiving tumor necrosis factor alpha (TNF-α) inhibitors (32) demonstrates the importance of cell-mediated immune responses for the containment of M. tuberculosis infection. Activated effector T lymphocytes migrate to granulomas and presumably control infection through the release of cytokines and through cytolytic function (34). These immune responses appear to be modulated through the recruitment of regulatory T lymphocytes (Treg cells) to the sites of active infection (22). This suggests that Treg cells may play a significant role in the host immune response to M. tuberculosis infection, specifically a role in determining the site of tuberculosis disease (22, 43).
Treg cells are a subset of CD4+ T lymphocytes and constitute 1 to 5% of all circulating CD4+ cells (40). Their main function is to prevent autoimmunity and maintain self-tolerance (18, 55). Treg cells also play a role in the immune response to infections, where they minimize excessive tissue destruction from adaptive immune responses via cell-cell contact and secretion of cytokines such as transforming growth factor beta (TGF-β) (6, 42, 51). However, by limiting the adaptive immune response, Treg cells may allow persistence and establishment of chronic infections. Depletion of Treg cells has been shown to increase in vitro immune responses to pathogens that cause chronic infections, such as Helicobacter pylori (38), HIV (33), hepatitis C virus (HCV) (7, 48), and M. tuberculosis (22, 43). The role of Treg cells in the pathogenesis of M. tuberculosis is not known. Treg cells could possibly be a response to the generalized immune activation that occurs in chronic infections such as HIV infection and tuberculosis (11, 44, 50), and they may dampen the immune response directed against M. tuberculosis (43); however, the relationship of Treg cells and immune activation to the site of tuberculosis disease is not clear. To date, studies of Treg cells and immune activation have been performed in persons with active tuberculosis disease (10). However, active tuberculosis is characterized by aberrations in the host immune system (5, 26) and may not be an accurate depiction of the immune dysregulation that leads to active tuberculosis.
To determine the immune response characteristics that may predispose individuals to extrapulmonary tuberculosis, we measured the frequency of Treg cells and the extent of CD4+ and CD8+ T lymphocyte activation in peripheral blood among HIV-seronegative adults who completed treatment for either extrapulmonary or pulmonary tuberculosis or latent M. tuberculosis infection. The optimal surface and intracellular markers to identify Treg cells continue to evolve. Based on previous studies that have found Treg cells to have high-level expression of CD25 (2, 3) and low-level expression of CD127 (36) and to be regulated by FoxP3 (17, 27, 57), we defined Treg cells as CD4+ CD25hi CD127low FoxP3+ cells in the current study. These measurements were performed for a subset of persons that were recruited for a study evaluating the in vitro immune response of monocyte-derived macrophages to infection with M. tuberculosis in persons with different manifestations of tuberculosis (16).
MATERIALS AND METHODS
Subjects.
Case subjects were defined as persons with previously treated extrapulmonary TB. There were three sets of controls: (i) persons with previously treated pulmonary TB, (ii) persons with latent M. tuberculosis infection, and (iii) persons who had been exposed to culture-positive pulmonary TB but were not infected (i.e., tuberculin skin test [TST] negative). Inclusion criteria consisted of the following: age of >18 years at the time of diagnosis of TB disease or infection; HIV-seronegative status; culture-confirmed disease, with therapy either near completion (within 1 month) or completed (for extrapulmonary cases and pulmonary controls); and TST induration of >10 mm (for latent M. tuberculosis infection controls). We did not require persons to complete therapy for latent infection to be enrolled. Only contacts of persons with culture-positive pulmonary TB or known TST converters were included as controls. Contacts of persons with culture-positive pulmonary TB were tested for latent infection at the beginning of the contact investigation and after 8 to 12 weeks (9). Exclusion criteria consisted of the following: serum creatinine level of >2 mg/dl, use of corticosteroids or other immunosuppressive agents at the time of diagnosis or study entry, and malignancy. All laboratory procedures were performed blinded to case-control status.
Extrapulmonary TB cases and pulmonary TB controls were identified by review of the Tennessee Department of Health TB registry. Ongoing contact investigations at local and regional TB clinics were reviewed to identify patients in the remaining control groups. Demographic and clinical characteristics were collected from the patient or the Tennessee TB registry.
The institutional review boards of Vanderbilt University, the Nashville Davidson Metro Public Health Department, and the Tennessee Department of Health approved the study. Study participants provided written informed consent.
Sample preparation.
Sixty-milliliter blood samples were collected in EDTA tubes, and plasmas were separated and frozen. PBMC were isolated under sterile conditions by Ficoll-Paque (GE Healthcare Bio-Science) density gradient centrifugation. Cells were washed twice in 1× Dulbecco's phosphate-buffered saline (DPBS) (Cellgro-Mediatech Inc.), and viability was estimated by trypan blue dye exclusion. PBMC were then cryopreserved in fetal bovine serum (FBS) (GemCell-Gemini Bioproducts). Blood samples were submitted to a commercial laboratory for HIV serology and complete blood counts. To determine CD4+ T cell numbers, the total lymphocyte counts were measured with an automated hematology analyzer and multiplied by the percentage of CD4+ T lymphocytes determined by flow cytometry.
Flow cytometry.
Aliquots of cryopreserved PBMC were thawed, washed in DPBS (Cellgro-Mediatech) twice, and stained for viability at room temperature with a Live/Dead fixable aqua dead cell staining kit (Invitrogen). Cells were surface stained with the following directly conjugated human antibodies: phycoerythrin (PE)–Cy5.5–anti-CD127 (Beckman Coulter), PE–Cy7–anti-CD38 (eBioscience), PE–Texas Red–anti-CD4 (Invitrogen Corp.), AF-700–anti-CD3, Pacific Blue (PB)–anti-CD8, fluorescein isothiocyanate (FITC)–anti-HLA-DR, and PE–anti-CD25 (Becton Dickinson). Cells were washed, fixed, and permeabilized for intracellular staining with anti-FoxP3–allophycocyanin (APC) (eBioscience) and were analyzed on a FACSAria flow cytometer. Fluorescence-minus-one controls for anti-CD38 and anti-CD127 were used to optimize compensation. Treg cells were defined as CD4+ CD25hi CD127low FoxP3+ cells. The absolute Treg cell frequency was determined by multiplying the percentage of CD4+ Treg cells by the absolute CD4+ T lymphocyte number.
Statistical analysis.
The primary study outcome was the difference in the frequency of Treg cells between subject groups. Based on a previous study, we determined that 10 subjects in each study group would provide 80% power to detect a difference in Treg cell frequency of 7% at a two-sided 5% significance level, using the two-sample t test (43). Comparisons of continuous variables between groups were performed using the Kruskal-Wallis test. Categorical variables were compared using the chi-square test. The correlation between Treg cell frequency and CD4+ immune activation was calculated using Spearman's rank correlation. Differences were considered statistically significant if the P value was <0.05. Statistical analyses were performed using GraphPad Prism software, version 5.0, for Windows and STATA, version 10.0 (College Station, TX).
RESULTS
There were 60 individuals enrolled in the study: 7 with previously treated extrapulmonary tuberculosis, 18 with previously treated pulmonary tuberculosis, 17 with latent M. tuberculosis infection (TST positive), and 18 without M. tuberculosis infection (TST negative). Demographic and clinical characteristics and laboratory data are summarized in Table 1. Five persons had received M. bovis BCG vaccination in the past (2 extrapulmonary cases, 2 pulmonary controls, and 1 latent infection control). There was no difference in median age or sex between groups. Persons with previous extrapulmonary or pulmonary tuberculosis were more likely to be black than persons with or without M. tuberculosis infection (P = 0.01). Pulmonary tuberculosis controls were more likely to have a history of smoking than the other groups, but the difference was not statistically significant. Persons with previous pulmonary tuberculosis were enrolled sooner after completion of therapy than the other groups. Persons with previous extrapulmonary tuberculosis had the lowest median white blood cell (WBC) count. They also had the lowest median number of CD4+ T lymphocytes and the highest median number of CD8+ T lymphocytes, but these differences were not statistically significant. There was no difference in the CD4+/CD8+ T lymphocyte ratio among groups (data not shown). Persons with previous extrapulmonary tuberculosis had a significantly lower median absolute number of neutrophils than the other groups. There were no differences between the groups in the median number of monocytes, eosinophils, or basophils (data not shown).
Table 1.
Demographic, clinical, and laboratory characteristics of the study population
| Characteristic | Value |
P valuea | |||
|---|---|---|---|---|---|
| Extrapulmonary TB (n = 7) | Pulmonary TB (n = 18) | Latent M. tuberculosis infection (n = 17) | No latent M. tuberculosis infection (n = 18) | ||
| Age (yr)b | 53 (26–70) | 47 (40–59) | 43 (36–48) | 42 (32–50) | 0.44 |
| No. (%) of male subjects | 3 (43) | 11 (61) | 6 (35) | 8 (44) | 0.48 |
| No. (%) of Hispanic subjects | 1 (14) | 2 (11) | 0 (0) | 1 (5) | 0.48 |
| No. (%) of black subjects | 3 (43) | 8 (44) | 2 (12) | 3 (17) | 0.01 |
| No. (%) of subjects who smoked | 1 (14.3) | 10 (55) | 5 (29) | 5 (28) | 0.12 |
| Time from end of treatment to blood draw (mo)b | 12 (6–25) | 1 (0–8) | 15 (1.5–26) | NA | 0.02 |
| Cell count (per mm3)b | |||||
| WBC | 5,900 (4,900–7,500) | 6,000 (5,300–7,700) | 7,200 (6,400–7,800) | 8,400 (7,300–9,200) | 0.008 |
| Monocytes | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.5 (0.4–0.6) | 0.4 (0.3–0.6) | 0.38 |
| CD4+ lymphocytes | 975 (962–1,078) | 1,060 (708–1,206) | 1,200 (863–1,495) | 1,136 (933–1,326) | 0.36 |
| CD8+ lymphocytes | 643 (340–945) | 443 (368–556) | 507 (440–673) | 452 (335–783) | 0.45 |
| Neutrophils | 3,100 (2,400–4,600) | 3,700 (2,700–4,700) | 3,900 (3,600–4,200) | 5,300 (4,700–6,200) | 0.013 |
By the Kruskal-Wallis test and Pearson's chi-square test.
Data are medians (interquartile ranges). NA, not applicable.
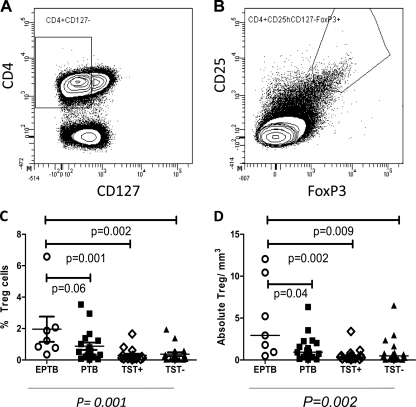
Figure 1A and B show the flow cytometry gating for Treg cells in the study population. Persons with previous extrapulmonary tuberculosis had the largest proportion of Treg cells (1.23%) compared to subjects with previous pulmonary tuberculosis (0.56%), TST-positive individuals (0.14%), and TST-negative individuals (0.20%) (P = 0.001) (Fig. 1C) and the largest absolute number of Treg cells among CD4+ T lymphocytes compared to controls (P = 0.002) (Fig. 1D).
Fig 1.
Frequencies of regulatory T cells among study groups. (A and B) Gating strategy for Treg cells. Treg cells were defined as CD4+ CD25high CD127low FoxP3+ cells. (C) Frequencies of Treg cells as percentages of CD4+ T cells among four study groups. (D) Absolute numbers of Treg cells/mm3 of blood among four study groups. Statistical comparisons of global differences between the study groups were performed with the Kruskal-Wallis test. Individual comparisons between the extrapulmonary group and each control group were calculated using the Wilcoxon rank sum test and were not adjusted for multiple comparisons. Horizontal bars represent the median values. EPTB, extrapulmonary TB; PTB, pulmonary TB; TST+, latent M. tuberculosis infection; TST−, no latent M. tuberculosis infection.
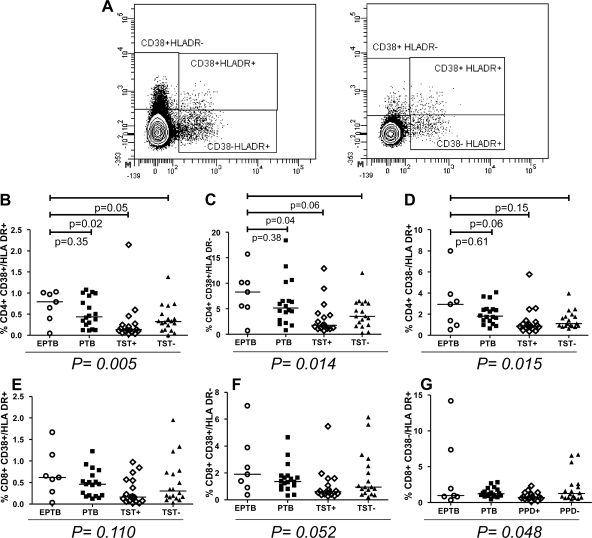
We measured the degree of generalized immune activation in study subjects by measuring expression of the activation markers CD38 and HLA-DR on CD4+ and CD8+ T lymphocytes (Fig. 2A). Treg cells express HLA-DR and therefore were excluded from the activation marker analysis (12). The frequency of CD4+ T lymphocytes that expressed the activation markers CD38 and HLA-DR was highest in persons with previous extrapulmonary tuberculosis (0.79%) compared to subjects with previous pulmonary tuberculosis (0.44%), TST-positive individuals (0.14%), and TST-negative individuals (0.32%) (P = 0.005) (Fig. 2B). Likewise, the proportions of CD4+ CD38+ HLA-DR− (P = 0.014) and CD4+ CD38− HLA-DR+ (P = 0.015) cells were highest in subjects with resolved extrapulmonary tuberculosis, as shown in Fig. 2C and D, respectively. We observed a trend of increased activated CD8+ T lymphocytes in persons with prior extrapulmonary tuberculosis. This trend was of borderline significance with either CD38 or HLA-DR (CD8+ CD38+ HLA-DR− cells [P = 0.052] [Fig. 2F] or CD8+ CD38− HLA-DR+ cells [P = 0.048] [Fig. 2G]) but was not significant when both activation markers on CD8+ T lymphocytes were compared between the four groups (CD8+ CD38+ HLA-DR+ cells [P = 0.11]) (Fig. 2E).
Fig 2.
Proportions of cells with activation markers on CD4+ and CD8+ T lymphocytes among study groups. (A) Gating strategy for measuring HLA-DR and CD38 expression on CD4+ and CD8+ T lymphocytes. (B to D) Distributions of activation markers on CD4+ T lymphocytes among study groups. (E to G) Distributions of activation markers on CD8+ T lymphocytes among study groups. Statistical comparisons were performed with the Kruskal-Wallis test. Individual comparisons between the extrapulmonary group and each control group were calculated using the Wilcoxon rank sum test and were not adjusted for multiple comparisons. Horizontal bars represent the median values. EPTB, extrapulmonary TB; PTB, pulmonary TB; TST+, latent M. tuberculosis infection; TST−, no latent M. tuberculosis infection.
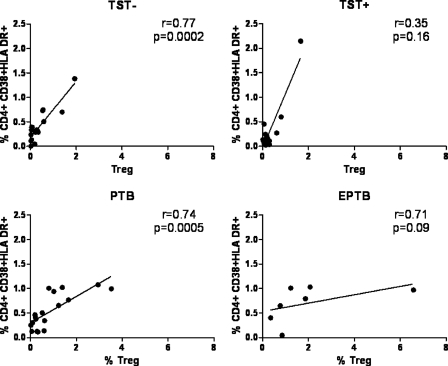
We found strong correlations between Treg cell frequency and CD4+ immune activation in TST-negative subjects (r = 0.77; P = 0.0002) and subjects with prior pulmonary tuberculosis (r = 0.74; P = 0.0005). We found a similar trend among subjects with prior extrapulmonary tuberculosis (r = 0.70; P = 0.08). We saw no association between Treg cell frequency and immune activation in TST-positive individuals (r = 0.35; P = 0.16) (Fig. 3).
Fig 3.
Correlation between CD4+ T cell activation and Treg cell frequency among study groups. Statistical calculations of correlation were performed with Pearson's correlation test. EPTB, extrapulmonary TB; PTB, pulmonary TB; TST+, latent M. tuberculosis infection; TST−, no latent M. tuberculosis infection.
DISCUSSION
Extrapulmonary tuberculosis is likely a marker of underlying immune suppression (31, 35) and has been associated with decreased in vitro secretion of gamma interferon (IFN-γ) in response to mycobacterial antigens compared to that of M. tuberculosis-infected controls (30). We found that subjects with prior extrapulmonary tuberculosis had both the highest frequency of Treg cells and the highest frequency of activated CD4+ T lymphocytes compared to those with prior pulmonary tuberculosis, individuals with latent M. tuberculosis infection, and uninfected controls. These findings suggest that immune dysregulation may be a feature of persons who develop extrapulmonary tuberculosis.
Previous studies have demonstrated an increase in Treg cell frequency and FoxP3+ mRNA both peripherally and at the site of disease in persons with active extrapulmonary TB (10, 22, 23, 46). Depletion of Treg cells from PBMC isolated from individuals with active tuberculosis has been shown to increase cytokine production in response to mycobacterial protein (10, 43); however, it is unclear whether blunted immune responses from Treg cell expansion are responsible for active disease or if this Treg cell expansion is simply part of the host inflammatory response to active infection. The evaluation of factors that predispose individuals to extrapulmonary tuberculosis may not be accurate in the setting of active disease, because active tuberculosis is associated with aberrations in the host immune response that resolve after therapy (25, 56).
We reasoned that evaluation of individuals after completion of therapy would more likely reflect their baseline immune status before exposure to M. tuberculosis. The results of a recent study of M. tuberculosis-infected cynomolgus macaques suggest that higher preinfection frequencies of Treg cells are associated with a higher likelihood of latent TB (21). While these results differ from the results of our study, the strategy for gating Treg cells by the investigators included all FoxP3+ cells and therefore may have included populations of recently activated (non-Treg) CD4+ T cells. Also, the animals were not evaluated after successful tuberculosis treatment to determine whether Treg cell frequencies after eradication of infectious organisms returned to preinfection levels. Further studies in a well-characterized animal infection model such as this will be quite useful. In humans, Ribeiro-Rodrigues et al. found that frequencies of Treg cells persisted at high levels in individuals with pulmonary tuberculosis compared to control subjects, even at the end of a treatment course (43). However, this group used only the CD25 marker to measure Treg cell frequencies, and this may simply reflect increased immune activation through the course of therapy. Chen et al. used a combination of the CD25 and FoxP3 markers to identify Treg cells, and they found that the frequencies of Treg cells in individuals 2 years after successful treatment for pulmonary tuberculosis were similar to those in controls (10). In addition to using a combination of markers thought to be highly specific for Treg cells, our study differs from prior work in that we studied persons with treated extrapulmonary tuberculosis. We evaluated individuals for up to several months after the completion of therapy and found that subjects with prior extrapulmonary tuberculosis had the highest frequency of Treg cells compared to controls with pulmonary tuberculosis, latent M. tuberculosis infection, or no M. tuberculosis infection. Our results suggest that an increased frequency of Treg cells is associated with and may predispose one to extrapulmonary tuberculosis.
Generalized immune activation is observed in chronic infections such as HIV infection and tuberculosis (11, 19, 29, 37, 44, 50). The perturbation of the immunologic environment during M. tuberculosis infection is felt to contribute to HIV disease severity and progression in coinfected persons (20). Previous studies have reported increased activation of CD4+ and CD8+ T lymphocytes in active tuberculosis and latent infection (4, 24, 43, 44, 54). These studies have either studied only persons with pulmonary tuberculosis or evaluated pulmonary and extrapulmonary tuberculosis together as one entity. Our study is unique in that we characterized immune activation in four distinct groups that represent the spectrum of tuberculosis disease. We found that among the four groups studied, persons with prior extrapulmonary tuberculosis had evidence of global activation of CD4+ T lymphocytes and, to a lesser extent, CD8+ T lymphocytes.
We found a strong correlation between immune activation and Treg cell frequency in uninfected persons, persons with previous pulmonary tuberculosis, and persons with previous extrapulmonary tuberculosis; the latter correlation was not statistically significant, likely because of fewer study subjects in this group. A significant correlation was not observed for persons with latent M. tuberculosis infection. Our findings differ from a previous study that recently found a positive correlation between Treg cell frequency and the frequency of CD4+ CD38+ HLA-DR+ T cells in healthy uninfected controls but not among subjects with active tuberculosis disease or latent M. tuberculosis infection (54). One reason that we may have observed different results could be because we compared immune activation between persons with pulmonary and extrapulmonary tuberculosis, whereas the referenced study combined these two patient groups. Also, the other study evaluated immune activation only in persons with active tuberculosis, whereas we studied persons after they had completed therapy.
The clinical significance of our findings is unclear but may reflect increased expansion of Treg cells as a response to generalized immune activation in persons with tuberculosis, which persists for up to several months after the completion of therapy. Another explanation for this finding is that increased immune activation in the presence of an increased frequency of Treg cells in persons with previous extrapulmonary tuberculosis could be a marker of subtle immune dysregulation that was present prior to infection and that may predispose individuals to this form of tuberculosis. We favor this explanation because these findings were observed several months after tuberculosis treatment. Also, these findings are consistent with our previous studies that found decreased numbers of CD4+ T lymphocytes and decreased in vitro cytokine production by peripheral blood mononuclear cells in persons with previous extrapulmonary tuberculosis (1, 47), indicating a possible subtle immune defect in these individuals.
Interestingly, we found that persons with previous extrapulmonary tuberculosis had a lower median absolute neutrophil count than the other study groups. A clear role for neutrophils in the immune response to tuberculosis has not been defined. Previous studies have reported variously that neutrophils are important in the early defense against M. tuberculosis infection (41), that they contribute to pathology rather than protection (15), and that they have no role in the immune response (45). In a previous study, we found that macrophages from persons with previous extrapulmonary tuberculosis produced lower levels of interleukin-8 (IL-8), an important chemoattractant for neutrophils, than did those from latently infected persons (16, 47). These findings suggest that the role of neutrophils in the immune response to M. tuberculosis may be complex and should be studied further.
Our study had some limitations. First, the sample size was small, which limited our ability to perform detailed subgroup analyses. Second, extrapulmonary tuberculosis is a complex disease, and different sites of disease manifestation may have different pathophysiologies (13). A larger study that assesses Treg cell frequency and activation according to the site of extrapulmonary disease is warranted. Also, immune activation in the periphery may differ from the immune response at the site of disease. Third, although we were unable to assess whether skin test positivity was affected by BCG vaccination or exposure to nontuberculous mycobacteria, only one person in the latent infection group had received BCG in the past. Furthermore, we used stringent criteria for latent infection and included subjects only if they had been exposed to a person with culture-positive pulmonary tuberculosis and were TST positive. We identified subjects who had completed therapy, and samples were not available from these individuals before and during active disease to document changes in immune activation and Treg cell frequency before, during, and after the course of treatment. For identification of Treg cells, we used the currently accepted combination of CD25high CD127low FOXP3+ staining (36, 40). Recent studies have identified the intracellular protein GARP as a potentially important marker for identification of activated T regulatory cells (49, 53). Future studies will be required to determine whether this will increase the specificity for Treg cells. We had insufficient cells to perform functional studies of Treg cells, but it would be interesting to evaluate whether Treg cells provide greater suppression of M. tuberculosis-specific T cell responses in individuals with prior extrapulmonary tuberculosis. Finally, we were unable to control for other factors, such as age (52) and nutrition (39), that may affect Treg cell frequency. However, there was no difference in median age between the study groups.
In conclusion, this study has several important findings. We found evidence of an increased frequency of regulatory T lymphocytes and generalized CD4+ and CD8+ T lymphocyte activation among persons with previously treated extrapulmonary tuberculosis. We also found a correlation between increased immune activation and an increased frequency of Treg cells in persons who had completed treatment for tuberculosis. The presence of persisting immune activation and correspondingly high frequencies of regulatory T lymphocytes may reflect immune dysregulation that predisposes individuals to clinical tuberculosis, specifically to extrapulmonary tuberculosis. Further prospective studies before infection and after therapy should be undertaken in order to better understand the regulation of the host immune response during M. tuberculosis infection.
ACKNOWLEDGMENTS
This work was supported by NIH grant K24 AI065298 (A.S.D.A., T.R.S.), the Vanderbilt Department of Medicine (A.S.D.A.), the Vanderbilt Institute for Clinical and Translational Research (A.S.D.A., S.A.K.), and the Vanderbilt Physician Scientist Development Program (C.T.F.). Flow cytometric cell acquisition and sorting were performed by the Vanderbilt-Meharry Center for AIDS Research (CFAR) Immunopathogenesis Core, an NIH-funded program (P30 AI 54999).
We thank those persons who assisted in identifying patients for study enrollment, namely, Celia Goodson, Diedra Freeman, Diane Pieterse, and Alisa Haushalter.
Footnotes
Published ahead of print 28 October 2011
REFERENCES
- 1. Antas PR, et al. 2006. Decreased CD4+ lymphocytes and innate immune responses in adults with previous extrapulmonary tuberculosis. J. Allergy Clin. Immunol. 117:916–923 [DOI] [PubMed] [Google Scholar]
- 2. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253 [DOI] [PubMed] [Google Scholar]
- 3. Baecher-Allan C, Wolf E, Hafler DA. 2005. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+ CD25+ T cells. Clin. Immunol. 115:10–18 [DOI] [PubMed] [Google Scholar]
- 4. Barcelos W, et al. 2006. Peripheral blood mononuclear cells immunophenotyping in pulmonary tuberculosis patients before and after treatment. Microbiol. Immunol. 50:597–605 [DOI] [PubMed] [Google Scholar]
- 5. Barnes PF, Samten B, Shams H, Vankayalapatib R. 2009. Progress in understanding the human immune responses to Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 89(Suppl 1):S5–S9 [DOI] [PubMed] [Google Scholar]
- 6. Belkaid Y, Rouse BT. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353–360 [DOI] [PubMed] [Google Scholar]
- 7. Cabrera R, et al. 2004. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology 40:1062–1071 [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention 2010. Decrease in reported tuberculosis cases—United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 59:289–294 [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention 2005. Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm. Rep. 54:1–47 [PubMed] [Google Scholar]
- 10. Chen X, et al. 2007. CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin. Immunol. 123:50–59 [DOI] [PubMed] [Google Scholar]
- 11. Deeks SG, et al. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942–947 [DOI] [PubMed] [Google Scholar]
- 12. Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. 2001. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J. Exp. Med. 193:1303–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Djoba Siawaya JF, et al. 2009. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine 47:132–136 [DOI] [PubMed] [Google Scholar]
- 14. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 282:677–686 [DOI] [PubMed] [Google Scholar]
- 15. Eruslanov EB, et al. 2005. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect. Immun. 73:1744–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fiske CT, et al. 2010. Abnormal immune responses in persons with previous extrapulmonary tuberculosis in an in vitro macrophage model that simulates in vivo infection with M. tuberculosis. Am. J. Respir. Crit. Care Med. 181:A3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fontenot JD, Gavin MA, Rudensky AY. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336 [DOI] [PubMed] [Google Scholar]
- 18. Gavin M, Rudensky A. 2003. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr. Opin. Immunol. 15:690–696 [DOI] [PubMed] [Google Scholar]
- 19. Giorgi JV, et al. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859–870 [DOI] [PubMed] [Google Scholar]
- 20. Goletti D, et al. 1996. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J. Immunol. 157:1271–1278 [PubMed] [Google Scholar]
- 21. Green AM, et al. 2010. CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J. Infect. Dis. 202:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. 2006. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 173:803–810 [DOI] [PubMed] [Google Scholar]
- 23. He XY, et al. 2010. T regulatory cells and Th1/Th2 cytokines in peripheral blood from tuberculosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 29:643–650 [DOI] [PubMed] [Google Scholar]
- 24. Hertoghe T, et al. 2000. T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB). Clin. Exp. Immunol. 122:350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hirsch CS, et al. 1999. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J. Infect. Dis. 180:2069–2073 [DOI] [PubMed] [Google Scholar]
- 26. Hirsch CS, et al. 1999. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J. Infect. Dis. 179:945–953 [DOI] [PubMed] [Google Scholar]
- 27. Hori S, Nomura T, Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061 [DOI] [PubMed] [Google Scholar]
- 28. Horsburgh CR., Jr 2004. Priorities for the treatment of latent tuberculosis infection in the United States. N. Engl. J. Med. 350:2060–2067 [DOI] [PubMed] [Google Scholar]
- 29. Hunt PW, et al. 2008. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 197:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hussain R, et al. 2002. Cytokine profiles using whole-blood assays can discriminate between tuberculosis patients and healthy endemic controls in a BCG-vaccinated population. J. Immunol. Methods 264:95–108 [DOI] [PubMed] [Google Scholar]
- 31. Jones BE, et al. 1993. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am. Rev. Respir. Dis. 148:1292–1297 [DOI] [PubMed] [Google Scholar]
- 32. Keane J, et al. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098–1104 [DOI] [PubMed] [Google Scholar]
- 33. Kinter A, et al. 2007. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc. Natl. Acad. Sci. U. S. A. 104:3390–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klucar P, et al. 2008. Characterization of effector functions of human peptide-specific CD4+ T-cell clones for an intracellular pathogen. Hum. Immunol. 69:475–483 [DOI] [PubMed] [Google Scholar]
- 35. Lewinsohn DA, Gennaro ML, Scholvinck L, Lewinsohn DM. 2004. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int. J. Tuberc. Lung Dis. 8:658–674 [PubMed] [Google Scholar]
- 36. Liu W, et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu Z, et al. 1997. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:83–92 [DOI] [PubMed] [Google Scholar]
- 38. Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS. 2003. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 71:1755–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matarese G, Procaccini C, De Rosa V, Horvath TL, La Cava A. 2010. Regulatory T cells in obesity: the leptin connection. Trends Mol. Med. 16:247–256 [DOI] [PubMed] [Google Scholar]
- 40. Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM. 2008. FOXP3 expressing CD127lo CD4+ T cells inversely correlate with CD38+ CD8+ T cell activation levels in primary HIV-1 infection. J. Leukoc. Biol. 83:254–262 [DOI] [PubMed] [Google Scholar]
- 41. Pedrosa J, et al. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Powrie F, Read S, Mottet C, Uhlig H, Maloy K. 2003. Control of immune pathology by regulatory T cells. Novartis Found. Symp. 252:92–98 [PubMed] [Google Scholar]
- 43. Ribeiro-Rodrigues R, et al. 2006. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin. Exp. Immunol. 144:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodrigues DS, et al. 2002. Immunophenotypic characterization of peripheral T lymphocytes in Mycobacterium tuberculosis infection and disease. Clin. Exp. Immunol. 128:149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seiler P, et al. 2000. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. J. Infect. Dis. 181:671–680 [DOI] [PubMed] [Google Scholar]
- 46. Sharma PK, et al. 2009. FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am. J. Respir. Crit. Care Med. 179:1061–1070 [DOI] [PubMed] [Google Scholar]
- 47. Sterling TR, et al. 2001. Human immunodeficiency virus-seronegative adults with extrapulmonary tuberculosis have abnormal innate immune responses. Clin. Infect. Dis. 33:976–982 [DOI] [PubMed] [Google Scholar]
- 48. Sugimoto K, et al. 2003. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology 38:1437–1448 [DOI] [PubMed] [Google Scholar]
- 49. Tran DQ, et al. 2009. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 106:13445–13450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vanham G, et al. 1996. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin. Exp. Immunol. 103:30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. von Boehmer H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6:338–344 [DOI] [PubMed] [Google Scholar]
- 52. Wang L, et al. 2010. An association between immunosenescence and CD4(+)CD25(+) regulatory T cells: a systematic review. Biomed. Environ. Sci. 23:327–332 [DOI] [PubMed] [Google Scholar]
- 53. Wang R, et al. 2009. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 106:13439–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wergeland I, Assmus J, Dyrhol-Riise AM. 2011. T regulatory cells and immune activation in Mycobacterium tuberculosis infection and the effect of preventive therapy. Scand. J. Immunol. 73:234–242 [DOI] [PubMed] [Google Scholar]
- 55. Wood KJ, Sakaguchi S. 2003. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 3:199–210 [DOI] [PubMed] [Google Scholar]
- 56. Young JM, Adetifa IM, Ota MO, Sutherland JS. 2010. Expanded polyfunctional T cell response to mycobacterial antigens in TB disease and contraction post-treatment. PLoS One 5:e11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ziegler SF. 2006. FOXP3: of mice and men. Annu. Rev. Immunol. 24:209–226 [DOI] [PubMed] [Google Scholar]