Abstract
Summary: Myiasis is defined as the infestation of live vertebrates (humans and/or animals) with dipterous larvae. In mammals (including humans), dipterous larvae can feed on the host's living or dead tissue, liquid body substance, or ingested food and cause a broad range of infestations depending on the body location and the relationship of the larvae with the host. In this review, we deeply discuss myiasis as a worldwide infestation with different agents and with its broad scenario of clinical manifestations as well as diagnosis techniques and treatment.
INTRODUCTION
Myiasis, a noun derived from Greek (mya, or fly), was first proposed by Hope to define diseases of humans caused by dipterous larvae, as opposed to those caused by insect larvae in general (161). Myiasis has since been defined as the infestation of live vertebrates (humans and/or animals) with dipterous larvae (371). Recognized in ancient times, flies causing myiasis are still some of the world's most devastating insects, responsible for severe losses in animal husbandry, with significant economic losses, including reduced milk production, weight and fertility issues, and reduced hide quality (371). In mammals (including humans), dipterous larvae can feed on the host's living or dead tissue, liquid body substance, or ingested food and can cause a broad range of infestations, depending on the body location and the relationship of the larvae with the host (250). The distribution of human myiasis is worldwide, with more species and greater abundance in poor socioeconomic regions of tropical and subtropical countries. In countries where it is not endemic, myiasis is an important condition, where it can represent the fourth most common travel-associated skin disease (59).
MYIASIS CLASSIFICATION
There are two main systems for categorizing myiasis: anatomical and ecological classifications. The anatomical system of classification, first proposed by Bishopp, is considered useful for practical diagnosis and to classify the infestation in relation to the location on the host (172, 269, 371). Since a single species can be assigned to more than one anatomical location, and the same location can be infested by different species, a classification system based on the degree of parasitism shown by the fly is also used.
Anatomical Classification
The anatomical classification system is based on the one proposed by Bishopp (172), later modified by James (172) and by Zumpt (371). Each of those authors used different terms with the same meaning, as shown in Table 1.
Table 1.
Anatomical classification of myiasis
| Classification by Zumpt | Classification by Bishopp | Classification by James |
|---|---|---|
| Sanguinivorous | Bloodsucking | Bloodsucking |
| Dermal/subdermal | Tissue-destroying | Furuncular |
| Subdermal migratory | Creeping | |
| Traumatic/wound | ||
| Anal/vaginal | ||
| Nasopharyngeal | Infestation of the head passages | Nose, mouth, sinuses |
| Aural | ||
| Ocular | ||
| Intestinal | Intestinal/urogenital | Enteric |
| Anal/vaginal | ||
| Urogenital | Intestinal/urogenital | Bladder, urinary passages |
| Anal/vaginal |
To avoid confusion, we will use the following classification, which is based in Bishopp's, James', and Zumpt's proposed classifications:
Sanguinivorous or bloodsucking
Cutaneous myiasis, furuncular and migratory
Wound myiasis
Cavitary myiasis, where the infestation receives the name of the affected organ, e.g., cerebral myiasis, aural myiasis, nasal myiasis, and ophthalmomyiasis.
Ecological Classification
Ecological classification (Table 2) takes into account the level of parasitism of the parasite and the host. When designing plague eradication programs for hospitals or nursery homes or in veterinary medicine, it is necessary to consider the ecological classification together with the specie life cycle.
Table 2.
Ecological classification of myiasis
| Ecological classification | Description |
|---|---|
| Specific/obligatory | Parasite dependent on host for part of its life cycle |
| Semispecific/facultative | |
| Primary | Free living and may initiate myiasis |
| Secondary | Free living and unable to initiate myiasis; may be involved once animal is infested by other species |
| Tertiary | Free living and unable to initiate myiasis; may be involved when host is near death |
| Accidental/pseudomyiasis | Free-living larva and not able to complete its life cycle; causes pathological reaction when accidentally in contact with the host |
TAXONOMIC CLASSIFICATION OF THE DIPTERA (TRUE FLIES)
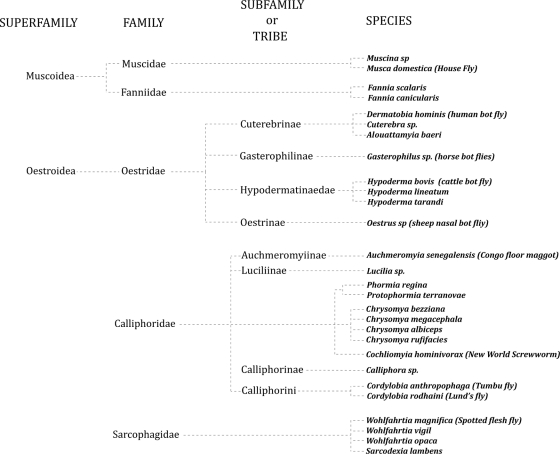
The order Diptera is a large order of insects that are commonly known as true flies. The presence of a single pair of functional wings with a reduced hind wing, termed halteres, distinguishes true flies from other insects (226, 227). Flies are ubiquitous and abundant, with approximately 150,000 species in 10,000 genera and 150 families. This order contains most of the insects vectoring diseases in humans.
The order Diptera is divided into two suborders, the Nematocera and the Brachycera. The Nematocera contain most families of blood-feeding flies that serve as vectors for a variety of viral, protozoan, and helminthic diseases, especially the Culicidae (115). Rarely, agents in this suborder can cause accidental myiasis (Table 3). The Brachycera are composed of infraorders. The infraorder Muscomorpha or “Cyclorrhapha” (term used in nonphylogenetic classifications) contains all species that cause specific myiasis and most of the species responsible for facultative myiasis, especially the species within the Calyptratae.
Table 3.
Agents reported to cause human myiasis outside the Calyptratae
| Species | Taxonomy | Clinical picture | Reference(s) |
|---|---|---|---|
| Psychoda albipennis | Diptera: Psychodidae | Urogenital myiasis | 2, 149, 159 |
| Telmatoscopus albipunctatus | Diptera: Psychodidae | Intestinal myiasis | 346 |
| Hermetia sp. | Diptera: Stratiomydae | Intestinal myiasis | 55, 133, 201, 308 |
| Hermetia sp. | Diptera: Stratiomydae | Furuncular myiasis | 3 |
| Scenopinus sp. | Diptera: Scenopinidae | Urogenital myiasis | 340 |
| Eristalis tenax | Diptera: Syrphidae | Urogenital myiasis | 5, 85, 96, 191, 241, 362 |
| Intestinal myiasis | 242, 330 | ||
| Megaselia scalaris | Diptera: Phoridae | Intestinal myiasis | 225, 314, 344 |
| Urogenital myiasis | 57, 93, 315, 359 | ||
| Nosocomial myiasis | 157 | ||
| Wound Myiasis | 164, 308 | ||
| Drosophila melanogaster | Diptera: Drosophilidae | Nasal myiasis | 21 |
| Ocular myiasis | 69 | ||
| Piophila casei | Diptera: Piophilidae | Urogenital myiasis | 101, 295 |
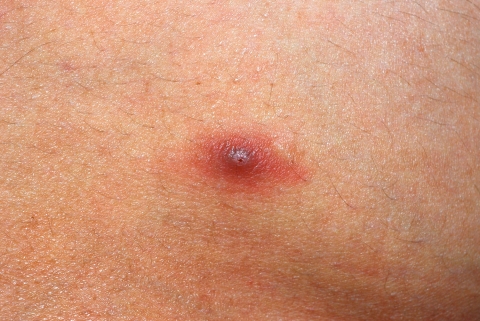
Figure 1 summarizes the taxonomy of the Diptera, and Fig. 2 presents the taxonomic division of the Calyptratae. Both were designed to include the agents related to human myiasis. Table 3 shows the species outside the Calyptratae reported to cause human myiasis. Tables 4 to 8 summarize the most important aspects of the species within the Muscoidea, Oestroidea, Calliphoridae, and Sarcophagidae that cause human myiasis.
Fig 1.
Taxonomy of the Diptera. Species within the dashed lines are reported to cause human myiasis and are included in the corresponding group. See Table 3 for references.
Fig 2.
Taxonomic division of the Calyptratae.
Table 4.
Superfamily Muscoidea
| Family, species, and parameter | Description (reference[s]) |
|---|---|
| Muscidae | |
| Muscina sp. | |
| Distribution | Worldwide |
| Classification | Facultative myiasis |
| Hosts | Decaying organic matter |
| Human myiasis | For Muscina stabulans, intestinal and urogenital myiases (18, 19, 37, 87, 249, 311); for Muscina sp., cutaneous myiasis (18, 19, 33, 37, 87, 249, 311) |
| Musca domestica (house fly) | |
| Distribution | Worldwide |
| Classification | Facultative myiasis |
| Hosts | Decaying organic matter |
| Human myiasis | Reported to cause intestinal (280, 302, 371), wound (112, 165, 370), and cavitary (209) myiases; related to allergic diseases (114, 336, 337, 357) |
| Fanniidae | |
| Fannia sp. (lesser house fly) | |
| Distribution | Holarctic and temperate neotropical regions |
| Classification | Facultative myiasis |
| Hosts | Decaying organic matter |
| Human myiasis | For Fannia scalaris, genitourinary myiasis (361); for Fannia canicularis, nasopharyngeal myiasis (17), intestinal myiasis (18, 37, 42, 366), and rectal myiasis (199) |
Table 8.
Agents responsible for furuncular myiasis
| Species | Frequency | Geographic location | No. of lesions | No. of maggots per lesion | Location(s) of lesions | Clinical characteristic(s) |
|---|---|---|---|---|---|---|
| Dermatobia hominis | Common | Latin America | Usually 1 | 1 | Exposed sites | Pain more common; history of insect bite |
| Cordylobia anthropophaga | Common | Africa | Multiple | 1 | Covered sites | Intense inflammatory reaction; more common in children; rainy season |
| Cordylobia rodhaini | Rare | Africa | Multiple | 1 | Covered sites; lower limbs | Similar to C. anthropophaga |
| Cuterebra spp. | Rare | North America | Usually 1 | 1 | Face, scalp, neck, shoulder | Pruriginous papule, 0.2–2 cm; migratory component; more common in children; summer months |
| Wohlfahrtia vigil | Rare | North America | Multiple, 12–24 | 1-5 | Head, neck, and skin folds | Patch may precede furuncle; more common in infants less than 6 mo old; between June and September |
| Wohlfahrtia magnifica | Rare | Old World | 1-5 | Up to 25 | Paroxysmal episodes of sharp pain; eosinophilia may occur | |
| Hypoderma bovis and H. lineatum | Rare | Northern hemisphere | 1 | 1 | Head, neck, and upper chest or back | Winter months; before or after migration |
Table 5.
Family Oestridae
| Subfamily, species, and parameter | Description (reference[s]) |
|---|---|
| Cuterebrinae | |
| Dermatobia hominis (human bot fly) | |
| Distribution | Between latitudes 25oN and 32oS (296), extending from Mexico through Central to South America (128, 196) |
| Classification | Obligatory myiasis |
| Hosts | Humans, cattle, swine, cats, dogs, horses, sheep, other mammals, and a few birds (143, 172) |
| Mechanism of infestation | Active delivery of the maggot by an intermediary mosquito (usually Diptera: Psorophora); this method of delivery is called phoresis |
| Human myiasis | Furuncular myiasis |
| Cuterebra sp. (rodent bot flies) | |
| Distribution | North America during summer in August, September, and October (24), with higher prevalence rates in northeastern and southeastern states (90) |
| Classification | Obligatory myiasis |
| Hosts | Rodents; humans are occasional hosts |
| Mechanism of infestation | Eggs are laid on leaves, grass, and stems of underbrush during the spring and early summer; first-stage larva can enter its host through mucous membranes of nose, eyes, mouth, or anus, or it can penetrate the skin directly (72) |
| Human myiasis | 85% of cases have cutaneous disease, and 15% have visceral infestation (70% eye and 30% upper respiratory tract) (90) |
| Alouattamyia baeri | |
| Distribution | Brazilian Amazon region |
| Classification | Obligatory cutaneous myiasis |
| Hosts | Primates |
| Human myiasis | Only 2 reported cases in humans; 1 case was pulmonary, and the other one affected the throat (142) |
| Gasterophilinae | |
| Gasterophilus sp. (horse bot flies) | |
| Distribution | Old World species, with three cosmopolitan species, G. haemorrhoidalis, G. intestinalis, and G. nasalis, distributed worldwide |
| Classification | Obligatory myiasis |
| Hosts | Horses, zebras, elephants and rhinoceroses; may affect humans who deal with horses |
| Mechanism of infestation | Direct contact with eggs on a horse's coat or by direct oviposition onto human skin, especially in warmer climates |
| Human myiasis | Mainly migratory myiasis; rarely external ophthalmomyiasis (230), oral myiasis (343), pulmonary myiasis (7) |
| Hypodermatinae | |
| Hypoderma bovis (cattle bot fly) | |
| Distribution | Distributed between latitudes 25° and 60°, in the northern hemisphere, in more than 50 countries of North America, Europe, Africa, and Asia |
| Classification | Obligatory myiasis |
| Hosts | Wild and domestic ruminants; human infestation occurs in those who are in close proximity to cattle (258) |
| Mechanism of infestation | In late spring and early summer, in periods of sunshine on warm days, the female flies attach their eggs onto the hair of the cattle |
| Human myiasis | Migratory myiasis, with reports of oral myiasis (103) and skin allergies with eosinophilia (244) |
| Hypoderma lineatum | |
| Distribution | Distributed between latitudes 25° and 60°, in the northern hemisphere, in more than 50 countries of North America, Europe, Africa, and Asia |
| Classification | Obligatory myiasis |
| Hosts | Wild and domestic ruminants; human infestation occurs in those who are in close proximity to cattle (258) |
| Human myiasis | Migratory myiasis, with a report of meningitis (77) |
| Hypoderma tarandi | |
| Distribution | Holarctic distribution (371) |
| Classification | Obligatory myiasis |
| Hosts | Reindeer and caribou |
| Human myiasis | Principal manifestation is ocular disease (181, 195, 333), with reports of cutaneous and oral myiases (105); cases usually occur in August to December in subjects who have visited areas in which reindeer and H. tarandi are found |
| Oestrinae | |
| O. ovis (sheep nasal bot fly) | |
| Distribution | Worldwide where sheep are tended |
| Classification | Obligatory cavitary myiasis |
| Hosts | Sheep and goats; there is no documentation that O. ovis is able to complete its life cycle in the human eye |
| Mechanism of infestation | The female fly is active during summer and early fall; the gravid adult female ejects the first-instar larvae, which have previously hatched; flies may deposit as many as 500 first-instar larvae; direct contact between the fly and its host is not necessary for infestation |
| Human myiasis | The most common cause of external ophthalmomyiasis in men (140, 174, 282, 324, 352); Oestrus ovis may cause allergic manifestations, especially when in the throat (223) |
Table 6.
Family Calliphoridae
| Subfamily or tribe, species, and parameter | Description (reference[s]) |
|---|---|
| Cochliomyia hominivorax | |
| Distribution | Once found in the tropics and warm temperate zones of the Southern United States, the Caribbean, and most of Latin America, its distribution is nowadays limited to South and Central America |
| Classification | Obligatory wound myiasis |
| Hosts | True obligate parasite of mammals |
| Mechanism of infestation | Eggs are laid on the wound or nearby (300 eggs in a few minutes) |
| Human myiasis | Typical infestation in humans is wound myiasis, where it can be very severe, with penetration and destruction of the underlying tissue; when in the nose or ears, the fatality rate may reach 8% (250) |
| Chrysomya bezziana (Old World screwworm) | |
| Distribution | India, Arabian Peninsula, Indonesia, Philippines, and New Guinea (not Australia) |
| Classification | Obligatory wound myiasis |
| Hosts | Sheep and humans |
| Mechanism of infestation | Eggs are laid on the wound or nearby (150 eggs) |
| Human myiasis | Wound myiasis |
| Chrysomya megacephala | |
| Distribution | Worldwide, with more prevalence in Oriental and Australasian regions |
| Classification | Facultative wound myiasis |
| Hosts | Decomposing flesh and feces |
| Human myiasis | Wound myiasis (111) and aural myiasis (203); important in forensic medicine (63) |
| Chrysomya albiceps and Chrysomya rufifacies | |
| Distribution | Native to Australia; is found worldwide |
| Classification | Facultative parasite of wound myiasis |
| Secondary myiasis in sheep | |
| Hosts | Feeding habits vary from excrement, urban garbage, and decomposing meat to fresh food; primary and secondary myiases in humans as well as other animals (371) |
| Human myiasis | Wound myiasis (112, 329) and nasal myiasis (172); important in forensic medicine |
| Auchmeromyiinae | |
| Auchmeromyia senegalensis (Congo floor maggot) | |
| Distribution | Sub-Saharan Africa and Cape Verde Islands |
| Classification | Sanguinivorous myiasis |
| Hosts | Humans |
| Mechanism of infestation | Eggs are laid in the soil or sand floors of traditional huts; the larvae hatch in 1 to 3 days, stay buried during the day, and emerge at night; maggot feeds on sleeping individuals for 20 min once, every 24 h, up to 16 times to complete its development; all three larval stages are bloodsucking |
| Human myiasis | No infestation |
| Luciliinae | |
| Lucilia sp. | |
| Distribution | South Africa and Australia (L. cuprina), Europe and North America (L. sericata) |
| Classification | Facultative wound myiasis |
| Hosts | These species prefer to feed on dead tissue, although they are able to invade living tissue when there are few options (355) |
| Mechanism of infestation | Females lay their eggs on carcasses, in neglected and suppurating wounds, and, in particular, on the wool of sheep soiled with urine, feces, or blood |
| Human myiasis | Facultative wound myiasis (355) |
| Calliphorinae | |
| Calliphora sp. | |
| Distribution | Females are attracted for oviposition to any decomposing flesh, of which carrion is most suitable; usually involved in myiasis as secondary species |
| Classification | Facultative myiasis |
| Hosts | Decomposing flesh |
| Mechanism of infestation | C. vicina in particular may be a primary invader (318, 371) |
| Human myiasis | For C. vicina, aural (172), intestinal (45), nasal (316), oral (147), wound (89), and urogenital (371) myiases; for C. hilli, ocular myiasis (200) |
| Calliphorini | |
| Cordylobia anthropophaga (tumbu fly) | |
| Distribution | Sub-Saharan Africa; was recently described in Portugal (73) |
| Classification | Obligatory cutaneous myiasis |
| Hosts | Wild rats are the main hosts, but other wild mammals are infested, including mice, monkeys, squirrels, antelopes, boars, leopards, and mongooses; dogs, cats, rabbits, guinea pigs, goats, and chickens are among the domestic animals infested; humans |
| Mechanism of infestation | Female flies deposit eggs on shaded soil, preferably contaminated with urine or feces, or drying clothes (especially improperly washed diapers and damp clothing laid on the ground) |
| Human myiasis | Furuncular myiasis |
| Cordylobia rodhaini (Lund's fly) | |
| Distribution | African tropical humid forest areas |
| Classification | Obligatory cutaneous myiasis |
| Hosts | Various thin-skinned forest mammals (particularly rodents); occasionally causes human myiasis |
| Mechanism of infestation | Females lay about 500 eggs in sand or soil containing feces or urine or on sun-exposed laundry |
| Human myiasis | Furuncular myiasis |
| Phormia regina and Protophormia terranovae | |
| Distribution | Confined to areas north of the Tropic of Cancer |
| Classification | Facultative wound myiasis |
| Hosts | Decomposing flesh; Protophormia terraenovae in particular may be a serious parasite of cattle, sheep, and reindeer (172, 318) |
| Mechanism of infestation | Females lay about 500 eggs in sand or soil containing feces or urine or on sun-exposed laundry |
| Human myiasis | P. regina has been found in wound myiasis in men (9, 154, 236, 301) and is an important agent in forensic entomology (52) |
Table 7.
Family Sarcophagidae
| Species and parameter | Description (reference[s]) |
|---|---|
| Wohlfahrtia magnifica (spotted flesh fly) | |
| Distribution | Mediterranean basin of Europe and North Africa and eastwards into China, including the steppe region of continental Europe and southern and Asiatic Russia (323, 371); it is a thermophilic fly; it likes warmth and light and is active mostly during summer days |
| Classification | Obligatory wound myiasis |
| Hosts | Humans and warm-blooded animals and infants are most commonly infested; sheep are a major host, but other livestock, including poultry, as well as wildlife in nature or zoos can be affected (108) |
| Mechanism of infestation | The female flies, which are larviparous (deposit their living larvae), are attracted to natural body orifices of their hosts (107) or to wounds, where they deposit their first-instar larvae (up to 150), occasionally within their eggshells |
| Human myiasis | Commonly reported in wound myiasis, although this parasite has also been reported in furuncular myiasis (348, 368), oral myiasis (95), and otomyiasis (100, 180, 264, 350, 369); fatal cases have been reported (172) |
| Wohlfahrtiavigil and Wohlfahrtia opaca | |
| Distribution | W. vigil is found in the United States from Maine to New York and west to North Dakota, in Central and Southern Europe, in Russia, and in Pakistan (327); W. opaca is found only in western and southwestern North America (319); Most cases occur in June to September |
| Classification | Obligatory furuncular myiasis |
| Hosts | Cats, dogs, rabbits, ferrets, mink, foxes, and humans (250) |
| Mechanism of infestation | In nearly all hosts, including humans, infestation occurs only in the very young, as the larval mouth hooks typically are not strong enough to penetrate adult skin |
| Human myiasis | Furuncular, with multiple lesions being common, with the avg no. being 12 to 14 (172) |
| Sarcodexia lambens | |
| Distribution | Widely distributed in the Americas, raging from the southern United States to Paraguay and Argentina |
| Classification | Obligatory wound myiasis |
| Hosts | Humans |
| Human myiasis | This agent infested 12.1% of 66 wound myiasis cases in Brazil (111) |
LARVAL PRESERVATION AND IDENTIFICATION
When treating a patient with myiasis, it is critical to make the correct identification of the larva. It helps not only to understand how the infestation was acquired but also to plan the treatment and to promote preventive measures.
After removal, the maggot should be killed by immersion for 30 s in very hot (enough to produce vapor) but not boiling water, which prevents decay and maintains the natural color. Larvae should then be preserved in a solution of 70% to 95% ethanol. The above-described method best preserves larval length and morphology. Killing of the larvae directly in a preservative will putrefy and shrink them. If a solution of 70% ethanol is not available, 70% isopropyl alcohol can be used instead. Formalin solutions should not be used, because they cause an excessive hardening of the larval tissues, making them difficult to process (229).
Identification can be very challenging and demanding. Specific knowledge of the morphological aspects of the larva is necessary for the identification of the maggot's species, a task usually done by a trained entomologist. Several aspects of the larva must be analyzed before the final species is determined, which is not possible in some cases. Body shape, aspects of the papillae, the posterior spiracles (position, shape, openings, and structures), pigmentation of the dorsal tracheal trunks, the body surface (spines), the anterior spiracles, the cephalopharyngeal skeleton, and clinical behavior are all taken into account when the offending maggot is analyzed. An excellent Internet resource to help identify the species when a trained expert is not present is the Natural History Museum (London, United Kingdom) website (http://www.nhm.ac.uk/research-curation/research/projects/myiasis-larvae/intro-myiasis/index.html), using the option, “launch the key to myiasis-causing larvae.”
MYIASIS AND TRAVEL MEDICINE
Increasing international travel, both for tourism and for business, raises the need for physicians to cover a large spectrum of diseases, especially those caused by infectious agents. Skin diseases, together with systemic febrile illness and acute diarrhea, are the leading causes of health problems in travelers and account for 8% to 12% of all tourists medical problems (160). Myiasis is usually among the five most common dermatologic conditions, representing 7.3% to 11% of cases (212, 290).
Travel clearly raised the importance of myiasis knowledge, especially in countries where the infestation is unusual, rare, and, in some cases, reported as being exotic (120, 124, 150). Even physicians unfamiliar with this condition can easily diagnose cases in which maggots are visible. On the other hand, furuncular, migratory, and cavitary cases and pseudomyiasis pose a diagnostic challenge, especially to those doctors unacquainted with myiasis and its possibilities. For the correct diagnosis, several aspects should be determined: the region where the patient visited, climate conditions, and the species habits of the region visited.
An accurate and timely diagnosis is important not only to alleviate the patient's symptoms but also to prevent the establishment of myiasis-causing flies in regions where they are not endemic, a phenomenon that is already happening with the movement of farm animals. In many cases, the patient receives unnecessary oral antibiotics, increasing the development of bacterial resistance (150).
CLINICAL MANIFESTATIONS
Myiasis is considered, in most cases, an embarrassing and repugnant disease to patients and to health care professionals. Poor hygiene and low socioeconomic status are the most important risk factors for acquiring myiasis (111, 220). Another important factor is an abundance of exposed preexisting suppurative lesions that attract and stimulate the deposit of eggs by the female insect. For specific species, habits of the population, such as sitting or lying on the ground and some religious rites, and climatic conditions influence the occurrence of myiasis (117).
The real importance of human myiasis is unknown, and identification of the species responsible for a case of myiasis is rarely done. Epidemiological data on human myiasis are scant, and registration of the cases is not usually obligatory. Health care professionals judge myiasis to be a disease of minor importance, leading to an inadequate registration of the case: the larva and dressings are normally discarded without further examination. In some countries, domestic and empirical treatments of the patients are made by family members, reducing the number of the cases seen in medical facilities. Access to entomologists with expertise in dipteran classification is usually difficult, especially in developing regions, where myiasis can be a real problem of public health.
Cutaneous Myiasis
Cutaneous myiasis, together with wound myiasis, is the most frequently encountered clinical form (92). Furuncular and migratory forms are included in this group.
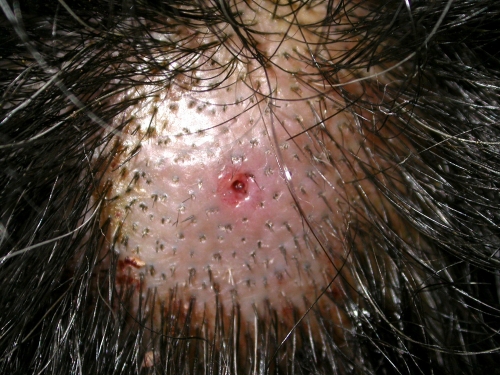
Furuncular myiasis.
Furuncular myiasis (warble) occurs after the penetration of the dipteran larva into healthy skin (Fig. 3), where an erythematous, furuncle-like nodule develops, with one or more maggots within it. Dermatobia hominis and Cordylobia anthropophaga are the most common causative agents of furuncular myiasis.
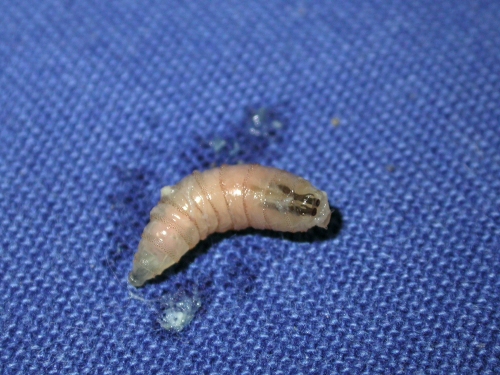
Fig 3.
Dermatobia hominis larva. Shown is a picture of the larva itself in a green sheet.
The typical furuncular lesion is a papule or nodule with a central punctum that exudes serosanguinous or purulent fluid. In the central pore, the presence of the parasite may be evidenced by the direct visualization of the posterior part of the larva—the respiratory spiracle (usually confused with tiny black eyes)—or indirectly by bubbles in the discharge (Fig. 4 and 5). The number of larvae within the lesion varies with the offending species.
Fig 4.
Furuncular myiasis. Shown is an initial lesion on the face.
Fig 5.
Furuncular myiasis on the scalp due to Dermatobia hominis. Note the bald area on the scalp with a small ulcer.
Pruritus, pain, and movement sensation are the most reported symptoms, and they usually happen suddenly at night, preceding the fluid leak (216). The difference in the numbers of lesions and its distribution patterns can be explained by the natural habits of each species.
Although a furuncle-like lesion is the most common presentation of furuncular myiasis, clinical variants have been described, such as vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions (117).
The lesion almost always heals completely, without leaving any trace. Sometimes, hyperpigmentation and scarring can occur. Clinical variants and severe cicatricial outcomes are more commonly seen in malnourished children. The most commonly reported complication of furuncular lesions is secondary bacterial infection (117).
(i) Agents of furuncular myiasis.
(a) Dermatobia hominis.
Dermatobia hominis is the most economically important cuterebrid fly and the most common cause of myiasis in the Americas. D. hominis infestation is also referred to as tórsalo, American warble fly, berne, tropical bot fly, colmoyote, moyocuil (both in Mexico, meaning “mosquito worm”), suglacuru (“from between breasts” in French Guyana), and berne (in Brazil) (163).
The unique and complex life cycle of human bot flies helps to explain the typical distribution of the lesions on exposed sites. The eggs are attached to the abdomen of a bloodsucking intermediary mosquito, a method of egg delivery called phoresis (137, 148). The eggs may also be laid onto foliage. After 1 week, when the insect approaches a warm-blooded animal, the heat induces the larva to hatch, and it must grab a host within 20 days. If the larva succeeds, it leaves the egg, and the first-instar, or stage I, larva (Fig. 3) penetrates painlessly into the skin (some patients may experiment a sensation of pruritus).
Afterwards, the maggot penetrates and gains access to the dermis, forming a typical furunculoid lesion with only one parasite in each papule. Within 5 to 10 weeks, the parasite goes to the second instar (stage II) and eventually to a third instar (stage III), when it leaves the host, during the night or early morning, to pupate in the soil (296).
Patients infested by D. hominis usually present with a single furuncular lesion on an exposed site (scalp, face, or extremities) and report pain (characteristically nocturnal) (198, 216). Some patients experience sudden paroxysmal episodes of lancinating pain. The presence of larval hooklets, allied with larval rotational movement around its axis, may explain the pain sensation (163). The sensation of “something crawling” or movement is a common symptom as well. The history of an insect bite may precede the lesion. In children, itching, distress, and disturbance to sleep have all been reported (351).
Secondary bacterial infection is a possible complication. The presence of local enlarged lymph nodes and/or systemic symptoms should raise this possibility (238). Staphylococcus aureus and group B streptococcus have been isolated from some lesions (137, 162). A fatal case where the larva penetrated the brain through the incompletely ossified bone of the skull of a very young child was reported (292).
D. hominis is the most commonly identified agent of myiasis in travelers who have gone to tropical America, and it should be considered for the differential diagnosis of furuncular lesions in these populations.
(b) Cordylobia anthropophaga.
The penetration of Cordylobia anthropophaga into the skin is usually asymptomatic, although the area of penetration can be slightly itchy for up to 2 days after the infestation. In a few days, a reddish papule develops and takes on a boil-like appearance when fully developed. An intense inflammatory reaction in the surrounding tissue of the lesions develops over a period of 6 days. Some lesions may develop a central pustule, similar to that of pyoderma (307). After 8 to 12 days, the mature third-instar maggot leaves the host.
Typically, symptoms develop within the first 2 days of infestation and can range from a mild or a “prickly heat” sensation to severe pain. Agitation and insomnia can also occur. Inflammatory changes around furuncle-like lesions are commonly seen in cases of tumbu fly infestations; in some cases, these changes can be very intense, mimicking soft tissue bacterial infections such as cellulitis (150). The lesions can be so numerous that large plaques of coalescing furuncles may form (252). A clear fluid, occasionally stained with blood or larval feces, may ooze from the boil. Once developed into the third instar, the posterior spiracles can sometimes be seen in the central pore. The C. anthropophaga discharge may become crusted, odoriferous, and purulent or may be serosanguinous (210). Findings associated with other toxemic symptoms, such as regional lymphadenopathy or malaise, are particularly seen in the presence of multiple lesions (284).
People are most commonly parasitized during the rainy season, when the natural sylvan hosts approach human villages. Adult flies tend to oviposit on soiled clothing, which explains the distribution of the lesions on covered sites such as trunk, buttocks, and thighs and also the elevated number of lesions. Children are particularly prone to the infestation; this is probably related to the thinner skin of infants and the immunity developed by adults who live in regions of endemicity after repeated exposures (41, 144, 188).
The parasitism and mechanism of infestation of Cordylobia rodhaini are similar to those of C. anthropophaga. The lesions can be larger and more painful (117, 124, 261, 335).
(c) Cuterebra sp.
Cuterebra sp. is an autochthonous facultative cause of cutaneous and ocular myiasis in humans living in North America (24). Cutaneous lesions represent 85% of all Cuterebrid myiases; 20% of these lesions are capable of producing a migratory eruption (90). A typical furuncular lesion, ranging in size from 0.2 to 2 cm, with pruritus, which can be painful and tender, develops after infestation. Some patients report a sensation of movement within the lesion. Other signs or symptoms reported are central necrosis and a pulsating lesion (274). Erythema, swelling, and edema may surround the furuncular lesion (156). Most cases occur in children, affecting the face, scalp, neck, shoulders, or chest. Infestations tend to occur in summer months, August, September, and October (24).
(d) Wohlfahrtia vigil.
Wohlfahrtia vigil causes furuncular lesions in children, usually with multiple lesions, with an average number of 12 to 24, with one to five maggots within the lesion. An erythematous patch may precede the furuncle and represents the failure of the larva to penetrate the skin (172, 251).
(e) Wohlfahrtia magnifica.
Wohlfahrtia magnifica furuncular myiasis develops 24 h after larval infestation, with a pruritic papule of 2 to 3 mm in diameter, followed by an erythematous nodule with a central pore that leaks a secretion. Paroxysmal episodes of sharp pain, itching, and movement sensation may be felt. One larva or a few larvae are present in the lesion. In a case where 25 larvae were removed, fever and marked eosinophilia were present (368). Local inflammatory lymph node enlargement with eosinophilia may compose the clinical picture (348).
(ii) Differential diagnoses.
Differential diagnoses of furuncular myiasis are furuncle, insect bite, insect prurigo, pyoderma (307), inflamed cyst, and tungiasis. C. anthropophaga myiasis can simulate severe soft tissue infections (150). Some cases can be misdiagnosed as delusional parasitosis. Labial cases may be confused with labial cellulitis. Breast myiasis may be confused with periductal mastitis (106), benign mass with microcalcification (176), and inflammatory carcinoma (349). Clinical and radiologic confusion with arteriovenous (AV) malformation and hemangioma occurred in one case of preauricular myiasis (43). Pain, erythema, itchiness, small vesicles, and crusting may pose a differential diagnosis of herpes simplex (234).
(iii) Diagnosis.
The diagnosis of furuncular myiasis is easily done based solely on clinical grounds, especially in regions where the disease is endemic. Patient history may help identify a possible predisposing factor or travel history. Dermoscopy has been used to identify the posterior parts of a D. hominis maggot within a furuncular lesion and may be helpful if diagnostic doubt is present. The examination reveals a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines (27). Ultrasound can be very useful to confirm a case of furuncular myiasis and also for the complete removal of the larvae (299, 334). A well-defined highly echogenic area surrounded by a hypoechoic area, the presence of segmentations on longitudinal sections, and distal shadowing are features of D. hominis in an ultrasound. The strong posterior acoustic shadowing may reflect the external coating of the larva. A definitive diagnosis of myiasis can be achieved if larval movement is detected during the ultrasound. High-frequency probes are more reliable for the diagnosis of furuncular myiasis. Color Doppler sonography, which is able to visualize the continuous movement of internal fluids of the larva, has proven to be useful for the detection of D. hominis and C. anthropophaga larvae when ultrasound is not able to detect the parasite (279, 286). Mammography of breast myiasis may not be able to exclude malignancy (81). Magnetic resonance and computed tomography (CT) are able to identify a subcutaneous segmented nodule; however, its morphology does not aid in the diagnosis (38, 43).
Laboratory examination is usually normal. In cases of chronic infestation or multiple infestations, laboratory signs of systemic inflammation, peripheral eosinophilia, and elevated immunoglobulin E levels may be found (351).
Molecular diagnosis has been successfully used to identify the offending larva and may be a future method for the identification of cutaneous myiasis in hospitals with no expertise in tropical medicine (50).
(iv) Pathology.
Biopsies are not necessary for the diagnosis of furuncular myiasis and should be restricted for academic purposes. Fine-needle aspiration cytology is not indicated for myiasis, although it can be diagnostic (275). Histopathological findings include an ulcerated epidermis with or without hyperkeratosis; the dermis contains a polymorphous inflammatory infiltrate that is composed mainly of lymphocytes and neutrophils, with an admixture of eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells. The dipteran larva is located in the dermis, within a fibrous cystic sinus tract (141).
A cuticle of variable thickness that is covered with spines characterizes the bot fly larva. Striated muscle is found directly under the entire cuticle. When the larva is well conserved, a large central tubular cavity; the digestive tube; the respiratory tract, composed of smaller tubular structures; a circulatory system; and the pigmented posterior respiratory spiracle can all be identified (26, 56). Polarized light microscopy may show a bright retractile material (328).
(v) Treatment.
Therapy consists of three general techniques: (i) the application of a toxic substance to the larva and egg, (ii) the production of localized hypoxia to force the emergence of the larva, and (iii) the mechanical or surgical removal of the maggots (117).
Although it is a possible option, leaving the parasite to perform its natural cycle and controlling the symptoms should not be done, especially in cases where the parasite is imported. The goal of treatment is the complete removal of the larva from the skin, with the prevention and control of secondary infection. Secondary infection may result if the larva is ruptured or killed within its cavity and not removed.
Unlike D. hominis, the larvae of C. anthropophaga do not migrate deeper into tissue and are easier to remove. The expression of C. anthropophaga may be adequate, preferably with two wooden spatulas, which reduces the chance of rupturing the larva (255). The obstruction of the hole with liquid paraffin may be useful; it forces the maggot to wriggle a little further and lubricates the pocket, helping subsequent extraction. Note that the immature maggot is usually reluctant to emerge.
Expression should be avoided when D. hominis is the offending agent, because it is usually fruitless and painful, with a risk of rupturing the maggot. Reports have noted the successful eradication of D. hominis infestation by occluding the punctum (breathing hole in the skin) with a substance to prevent gas exchange (47, 162). To avoid asphyxiation, the organism emerges far enough to be grasped by the forceps of a vigilant patient or physician. Occluding substances that may be used are petrolatum, bacon (48), fingernail, adhesive tape, and others. Some authors do not recommended adhesive tape, because frequently, this technique leaves behind fragments (14). Polymyxin B was reported to be a sterile option (285). Occlusion may have to be maintained for 24 h or more to have the desired effect. The risk of attempted occlusion is that the organism may asphyxiate without emerging, and the dead larva may elicit an inflammatory response, with the formation of a foreign-body granuloma and, eventually, progression to calcification. The injection of 1% lidocaine (2 ml per nodule) is sometimes used to paralyze the larva, making the extraction easier (208). Liquid nitrogen used before extraction stiffens the larva and helps aid in its removal (307). Topical 1% ivermectin may be used for a furuncular lesion caused by D. hominis (70), although there is a possibility that the dead larva may be trapped within the skin.
Surgical excision is usually unnecessary for treatment, although it may be needed to remove the larva (46). Some researchers advocate a cruciate incision to remove D. hominis, which prevents damage to the larva and allows an easier extraction without leaving remnants in the wound. In some cases, debridement of necrotic tissue surrounding the lesion inside the pocked may be indicated (189).
Furuncular lesions caused by Cuterebra are also treated with occlusion and the posterior expression of the larva. Direct extraction may be difficult in some cases because of both its depth in the skin and the presence of surface spines which anchor it. Spontaneous extrusion may occur (312).
The expression of a W. vigil furuncular lesion is usually easily performed (128).
Oral treatment is not recommended for furuncular myiasis. Ivermectin may kill the larva inside the lesion, with a consequent inflammatory reaction (279). Antibiotics should be used only if there is a bacterial infection.
Migratory myiasis.
Migratory myiasis, or creeping myiasis, occurs when a dipteran maggot starts to migrate, aimlessly, through burrows in the skin, producing the migratory pattern of the lesions. The deepness of the tunnel and the migration speed are the factors responsible for the clinical picture. Larvae of the genera Gasterophilus (horse bot fly) and Hypoderma (cattle bot fly) cause almost all cases of creeping myiasis in humans. Humans are accidental hosts of these agents, and these agents are unable to complete their life cycle within human skin. Hypoderma spp. could simulate their larval development (although without reaching the fully mature third instar). G. intestinalis rarely develops past the first instar, although a second-instar larva was removed from a newborn infant (294).
(i) Agents of migratory myiasis.
(a) Gasterophilus. Gasterophilus intestinalis is the most common cause of human migratory myiasis. Infestation is related to the handling of animals, particularly after putting hands on the tips of a horse (371), and occurs throughout the year (24). Direct ovipositing by the female fly may occasionally occur (294).
The larva of Gasterophilus initially produces a papule similar to that found for furuncular myiasis. Next, the larva burrows into the lower layers of the epidermis, causing an intensely pruritic inflammatory lesion. The erythematous tortuous lesion with raised borders telltales the presence of the maggot and indicates the advancing border, while at the other end, the lesion gradually fades as the larva wanders about in search of a proper place to molt, leaving a path of migration (229). The larva may live for months in human skin and may migrate 1 to 30 cm per day. Infestation may present with pustules, nodules, or recurrent swelling (109). Rarely, the larva may appear in the lungs and cause a nodular parenchymal lesion. The placement of a small amount of mineral oil over the advancing lesion, where the black transverse bands of the spines of the larva can be detected, can confirm the diagnosis of the subcutaneous lesion. The infestation may end spontaneously, with or without suppuration (172).
(b) Hypoderma.
Hypoderma myiases are reported in areas where the disease is endemic in cattle and yaks and tends to occur in farm children and in people who handle cattle. Most human cases occur during the winter months, and the seasonal occurrence of the disease is characteristic (24). Imported cases of human disease have been reported (278). The highest known prevalence of human hypodermosis (0.4% to 7%, in farmers) has been reported in China (278). In the United Kingdom, Ireland, and Denmark, where cattle hypodermosis has been eradicated, the number of reports of human infestation has been greatly reduced (258).
Hypoderma bovis and H. lineatum are the most common offending agents of human hypodermosis. The disease might occur when eggs are laid onto the hairs of the human body or when newly hatched larvae on the coat of infected animals come into contact with the hands or bare arms.
After penetration, a furuncular lesion develops, where the larva maturates and penetrates deeply into the skin. In sequence, the maggot starts burrowing subcutaneously and produces an inflamed and painful swelling (migratory swelling) resembling a boil. During larval migration, a slightly erythematous, tender, ill-defined, 1- to 5-cm, slightly raised area is often seen, which marks the subcutaneous location of the larva. If nodules form, they are usually first noticed on the chest and neck in the winter months (207). A “prickly” sensation and, less frequently, burning and pruritus have been reported. Erythema persists for several hours to several days and then subsides, leaving a yellow-pigmented patch as the larva wanders to another location. The course of migration is generally much straighter and less bizarre than that of Gasterophilus myiasis (229). The palpable tract is scarcely a faint irregular line connecting the old area of erythema with the newer one (342). In the migration tract, multiple erythematous pruriginous papules may appear instead (207).
The larva has been observed to migrate 2 to 30 cm in a 24-h period. When located superficially, the larva may travel vary fast, 125 to 150 cm per 12 h. In one series, in only 1 out of 15 human cases did the subcutaneous larva eventually penetrate the dermis and form a slowly enlarging tender erythematous nodule (warble) (109). At this stage, a central pore develops, from which the respiratory spiracle can be observed, and this central pore intermittently drains a serosanguinous discharge that later becomes purulent. Throbbing and spontaneous pain usually are absent at this stage, but the pruritus becomes more intense, and movement may be felt (207). The larva next undergoes a period of rapid growth and then usually exits through the skin of a proximal extremity, the scalp, the face, or the neck, and falls to the ground to pupate. Most often, however, the larva dies in human tissue beneath the skin.
Hypoderma human myiasis is usually self-limiting; sometimes, the larvae migrate considerable distances and have been reported to cause ascites, pleuropericardium, hemopericardium, high fever, myalgias, arthralgias, scrotal edema, meningitis, intracerebral invasion (177, 276), temporary limb paralysis, and hypereosinophilia (326). They can invade the nervous system, eye, and ear, with possible blindness, paralysis, or death.
Hypoderma sinense, H. tarandi, and H. diana have also been related with human migratory diseases (278, 342). H. sinense can cause a migratory lesion with painful swelling, adenopathy, and eosinophilia (278).
(c) Cuterebra.
Few cases of Cuterebra migratory myiasis have been described (24, 90, 127, 128). In 20% of furuncular lesions caused by Cuterebra, a migratory eruption may be associated (90).
(ii) Differential diagnosis.
Three clinical features distinguish migratory myiasis from helminthic cutaneous larva migrans. First, migratory myiasis extends more slowly, and its cutaneous presentation is generally less widespread. Second, fly larvae can survive for months in human skin, much longer than helminths. Finally, fly larvae are generally larger than the helminths and, particularly in the case of Gasterophilus, can be visualized by applying mineral oil and using magnification (117, 231). Cutaneous larva migrans, migratory myiasis, gnathostomiasis, and sparganosis should be remembered for the differential diagnosis of cases with cutaneous migratory lesions with eosinophilia. Hypereosinophilic syndrome can occasionally be caused by creeping myiasis (326).
(iii) Diagnosis.
A definitive diagnosis is made with the identification of a dipteran larva in the migratory lesion. The parasite can be visualized by applying mineral oil and using magnification. A drop or two of mineral oil applied just in advance of the visible line of inflammation will generally reveal the parasite in Gasterophilus cases. The furuncle-like lesion in Hypoderma infestation is where the maggot will probably be found. As with other parasites too large to be phagocytosed, peripheral eosinophilia may occur and can reach notable values. An ultrasound scan can reveal the larva in a Hypoderma furuncular lesion (278). Hypoderma infestation produces hypodermin C, a circulating antigen that appears to reflect periods of larval activity in cattle (71). The episodic release of hypodermin C limits the usefulness of antigen capture enzyme-linked immunosorbent assay (ELISA) as a tool for the diagnosis of hypodermosis in cattle (263). In humans, serology has been useful for confirming the diagnosis of a suspected case of hypodermosis (278). However, serology is not able to identify the species due to cross-reactivity between members of the subfamily Hypodermatinae (262). PCR-restriction fragment length polymorphism (PCR-RFLP) analysis targeting cytochrome oxidase I (COI) of mitochondrial DNA can be used for the molecular identification of the parasite and differentiating the most common Hypoderma species (259, 260, 278). No immunological techniques are currently used to diagnose gasterophilosis, although studies are being undertaken in this field (288).
(iv) Treatment.
The treatment of a lesion caused by horse bot fly can be performed by the identification of the position of the larvae and its removal with a needle.
Hypoderma larvae are best removed through a cruciform incision or may be expressed if a furuncle-like lesion is formed. Surgical excision is almost always required for migratory myiasis. When the larva is migrating deep into tissue, extraction may not be possible. The use of oral albendazole or ivermectin was previously reported to mobilize the parasites toward the body surface, allowing the identification and surgical removal of the maggot in one case caused by H. sinense larvae (278). This case was successfully treated after three rounds of oral ivermectin treatment.
Wound myiasis.
Wound myiasis occurs when fly larvae infest open wounds of a mammalian host. This kind of infestation may be the result of facultative or obligatory parasites. Cochliomyia hominivorax, Chrysomya bezziana, and W. magnifica are the most common flies, worldwide, that cause obligatory human wound myiasis. Numerous species of Muscidae, Calliphoridae, and Sarcophagidae (also known as filth flies) have been implicated in facultative wound myiasis. In one series, 87% of cases of human wound myiasis found in the United States were caused by flies of the family Calliphoridae, which include Lucilia sericata (the green bottle blow fly) and Phormia regina (the black blow fly) (308). C. hominivorax was the most common agent in 66 cases of myiasis in Brazil (62.1%) (111) and in 17 cases in Argentina (355).
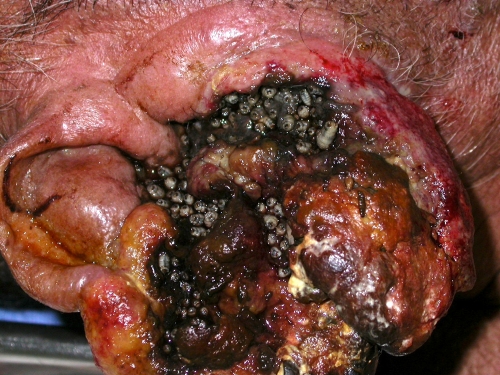
Wound myiasis is most often initiated when flies oviposit in necrotic, hemorrhaging, or pus-filled lesions (Fig. 6 and 7). Wounds with alkaline discharges (pH 7.1 to 7.5) have been reported to be especially attractive to blow flies (129). The presence of necrosis is also an important factor (111). In human cases, there is usually only one offending species in the lesion, although mixed infestation can occur, reaching rates of 3% in one series (111).
Fig 6.
Myiasis due to C. hominivorax in a B lymphoma patient. Shown is a huge ulcer filled with larvae.
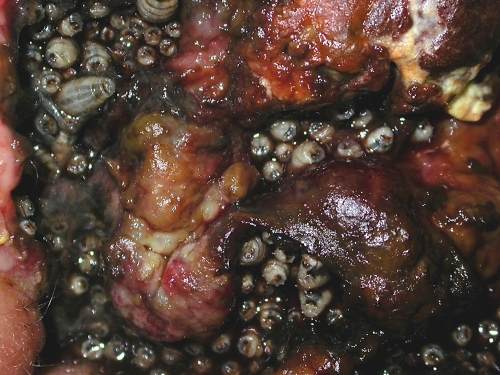
Fig 7.
C. hominivorax myiasis. Shown is a closer view of the huge ulcer in Fig. 6 showing many larvae in detail.
A lack of hygiene and poor socioeconomic status, in the presence of an open wound, are the most important predisposing factors for human wound myiasis (111). A lack of adequate medical and nursery care of the elderly, psychiatric patients, alcoholics, and other helpless patients, especially those with the inability to discourage flies from depositing eggs or larvae, also makes humans prone to wound infestation (185). Poor visual acuity may limit the detection of myiasis. Human natural disasters may be another predisposing factor for wound myiasis (206).
Dermatological conditions have also been described to be a predisposing factor for myiasis-causing flies, more importantly lesions with ulcers and secondly the presence of hyperkeratosis (111). Dermatologic conditions associated with myiasis are neuropathic ulcers (111), psoriasis (38), seborrheic keratosis (126), onychomycosis (28), vascular insufficiency ulcer, cutaneous B lymphoma (Fig. 6 and 7), basal cell carcinoma (Fig. 8), lipedema (187), herpes zoster virus infection (243), noma (4), filarial lymphoedema, condyloma acuminatum (268), hemorrhoid (145), leprosy (111), pediculosis, and impetigo (111, 220).
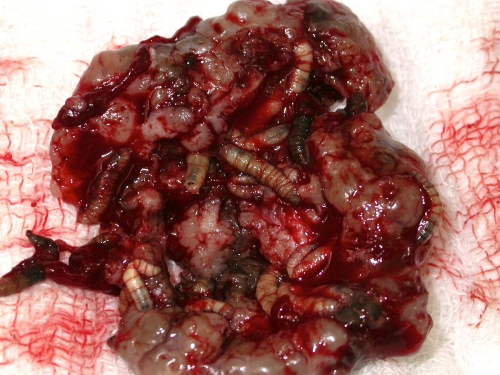
Fig 8.
C. hominivorax myiasis in a basal cell carcinoma case. Shown are many large myiasis larvae.
Local destruction, invasion into deep tissues, and secondary infection are possible complications of myiasis, especially where obligatory parasites are concerned. In some cases, a facultative agent may prevent or even help treat an infection by cleaning the necrotic tissue, producing substances with bactericidal properties, and stimulating granulation (111, 118).
(i) Agents of wound myiasis.
C. hominivorax and C. bezziana myiases are typically very painful. Cavernous lesions are formed, so it is difficult to extract the larvae in a single session, and this delay makes the situation more dangerous. As long as larvae are present, a foul-smelling, bloody discharge is observed (308).
Both New World and Old World screwworms cause multiple infestations, with 100 to 500 eggs. The females often oviposit on or near a wound. Upon hatching, the larvae begin feeding, causing an extensive destruction of tissue and a bloody discharge (355). Tissues around the lesion become swollen, and pockets may be eaten out beneath the skin (Fig. 6 to 8). Tissue invasion and local destruction caused by the larvae when leaving the necrotic tissue—more mature larvae are often more invasive—lead to significant local pain and secondary bacterial infection (83). Other clinical manifestations include fever, chills, bleeding, and fistula formation. Neutrophil leukocytosis and hypereosinophilia can occur. An infested person may die from tissue destruction.
Wohlfahrtia magnifica infestation begins with the depositing of first-instar maggots, which immediately start to feed on the host's cutaneous or underlying tissue, causing serious damage. These wounds become increasingly attractive to females, and therefore, more flies arrive and deposit their larvae (153). This results in severe infestations populated by larvae from more than one female. In contrast with the facultative species, W. magnifica may infest individuals without any identifiable predisposing conditions (152). Parasitological infestation occurs preferentially during the summer, a favorable period for the biological evolution of the flies.
Other agents of wound myiasis include D. hominis (291), Musca domestica (187), Chrysomya megacephala (111), Calliphora vicina (89), L. sericata (355), Chrysomya albiceps (111), Phormia regina (236), Parasarcophaga argyrostoma (51), Lucilia cuprina (111, 192), and Sarcodexia lambens (111).
(ii) Differential diagnosis.
Dipteran larvae are the major cause of wound infestation of humans by macroscopic parasites.
(iii) Diagnosis.
Usually, wound myiasis is readily diagnosed by a clinical inspection of the wound. Sizable wounds are most commonly affected; minor injuries may be affected, such as scratch, imposing a clinical challenge. The possibility of the presence of the maggots should be raised when pain, movement sensation, or the presence of a malodorous suppurating sore is noted. Biopsy or imaging techniques are rarely indicated or even necessary.
(iv) Treatment.
Treatment requires the removal of all visible larvae, followed by debridement in cases where necrotic tissue still remains. Irrigation may be particularly useful for lesions with holes and cavities. Fifteen percent chloroform in olive oil or another oil or ether may help to immobilize the larvae and facilitate maggot removal. The use of a thick layer of petrolatum, with its removal every 3 h until the complete removal of the larvae is achieved, is a practical treatment option (219). The current treatment concept in the case of myiasis in malignant wounds comprises the mechanical removal of maggots, surgical debridement of the infested wound bed, intensive rinsing with antiseptic solutions, and consistent dressing changes on a daily basis (304). Topical treatment with 1% ivermectin in a propylene glycol solution, a maximum of 400 μg per kg of body weight, applied directly to the affected area for 2 h and washed with saline solution (354), is another option. Extensive wound exploration may be indicated, depending on the degree of involvement.
Oral treatment is not the consensus for the treatment of human myiasis, and studies must be done to consolidate this modality of treatment. Ivermectin is the most commonly used drug for human infestation. Much of the experience with ivermectin came from the veterinary use of this drug. Its use may cause a migration of the larva out of the skin. One study reported a case of complete resolution with a dose of 200 μg per kg. There has been a report of the use of oral ivermectin in a case caused by H. lineatum, with a spontaneous emigration of the maggots (173).
Cavitary Myiasis
Cavitary myiasis corresponds to the infestation of natural body cavities. The infestation usually receives the name of the anatomic region affected. Internal organs affected by dipteran larvae are also included in this group.
Ophthalmomyiasis.
Ophthalmomyiasis, or oculomyiasis, is the infestation of any anatomic structure of the eye. This group is further subclassified into ophthalmomyiasis externa (or superficial) and ophthalmomyiasis interna. Orbital myiasis, or “ophtalmomyiase profonde” (French term meaning profound or deep), is used to bring together palpebral or periocular infestation with intraocular myiasis.
(i) Ophthalmomyiasis externa.
Ophthalmomyiasis externa refers to the superficial infestation of ocular tissue. Conjunctival myiasis is the most common form of ophthalmomyiasis, and it is a relatively mild, self-limited, and benign disease. Patients commonly complain of acute foreign-body sensation with lacrimation, characteristically with an abrupt onset (13). Upon examination, unilateral disease is the rule. The movement of the larva may be felt by the patient (257). In response to the movement of the larva across the external surface of the eyeball (12), any of the following symptoms may be found upon an ophthalmologic examination: red eye, photophobia, conjunctival hyperemia, lid edema, punctate conjunctival hemorrhages, pseudomembrane formation, and superficial punctate keratopathy (62, 174, 282, 352).
Lachrymal gland myiasis may complicate conjunctival infestation, and a canalicular lesion may also follow external ocular myiasis (297). Migration through the lacrimal canal to the nose cavity is a possibility (104). Mild pain and inflammation usually last for 10 days.
Ocular myiasis should be considered in any case of unilateral foreign body sensation with a marked onset. Other differential diagnoses of this clinical picture include catarrhal conjunctivitis, keratitis (324), periorbital or preseptal cellulitis (102, 297, 364), keratouveitis (174), and chalazion (363).
Oestrus ovis is the main agent causing external ocular myiasis, and as expected, the cases are usually described to occur in the autumn months in cooler latitudes of the northern and southern hemispheres, especially in rural areas. The majority of cases have been described in the Mediterranean basin and Middle East (49, 78, 94, 158, 174, 331). Other agents implicated in this form of disease are Rhinoestrus purpureus (97), D. hominis (30, 91, 135, 277, 297), C. bezziana (53), Lucilia spp. (167), and Cuterebra (110).
External manifestations are managed by the mechanical removal of larvae from the surface of the anesthetized eyeball, using fine, nontoothed forceps. Slit-lamp examination facilitates the process, but viable larvae have the tendency to avoid bright light. The use of lidocaine or cocaine as an anesthetic has the additional benefit of maggot immobilization, which facilitates removal. Occlusion with a thick ointment may assist in removal by encouraging the egress of organisms from the conjunctival sac, if it is involved. The average number of extracted parasites varies from 9 to 18 (13). One case was successfully treated with ivermectin (358).
(ii) Ophthalmomyiasis interna.
Ophthalmomyiasis interna is a term used when the infestation involves the anterior or posterior segment of the eyeball. The fly larva may be seen in the anterior segment and the vitreous and subretinal space (171). This clinical picture may be a complication of ophthalmomyiasis externa (365). However, the entry site is usually not apparent; these larvae probably penetrate the sclera and migrate into the eye. Usually, there is only one larva inside the eye; however, two to three larvae in the same eye and bilateral involvement have also been reported (222).
Anterior ophthalmomyiasis interna is less common and appears clinically as anterior uveitis, sometimes accompanied by posterior segment inflammation, which may be severe (298).
In its classical clinical presentation, posterior ophthalmomyiasis interna is characterized by pigmented and atrophic retinal pigment epithelium (RPE) tracts in a crisscrossing pattern seen in conjunction with hemorrhages, fibrovascular proliferation, exudative detachment of the retina, and even fibrovascular scarring (121, 122, 317). Subretinal myiasis can cause exudative and fibrovascular detachments and even focal hemorrhages, multifocal fibrous scarring, total detachment, and blindness (74, 332). However, its typical clinical manifestation is that of multiple-crisscross RPE with atrophic pigmentary tracts (204). Only a few other agents have been reported to produce a subretinal tract (40, 221, 228).
Red eye, vision loss, floaters, eye pain, and scotomas are the symptoms that have been described for ophthalmomyiasis interna. Vision loss can be severe and is more commonly associated with Hypoderma tarandi. Ophthalmomyiasis interna should be considered for the differential diagnosis of retinal detachment, panuveitis (183), orbital cellulitis (125), cavernous sinus thrombosis (10), chorioretinitis (254), and endophthalmitis (138).
Accordingly, the diagnosis of subretinal myiasis is made on the basis of sub-RPE tracts and the typical clinical morphological changes known to be characteristic of ophthalmomyiasis interna. The fly larva, which is 10 to 20 mm in size, has a cigar-shaped appearance, with multiple skeletal braces. Hemorrhage may occur as the fly larva erodes through vascular tissue; choroidal neovascularization may also occur, with exudative hemorrhagic detachment and eventual fibrovascular scarring.
The epidemiologic data concerning ophthalmomyiasis interna are not precise, because in many cases, the larva is destroyed by laser photocoagulation. The reindeer or caribou warble fly Hypoderma species are considered the most common cause, with Hypoderma tarandi being the most frequent cause in northern European countries such as Norway (50, 181).
(iii) Orbital myiasis.
Orbital myiasis has a severe clinical picture characterized by the intraocular invasion of maggots from eyelid myiasis, a peculiar kind of wound myiasis. Eyelid tumors are the most common predisposing factor associated with this clinical picture (29, 54, 60, 170, 367), although it may affect a previously healthy individual. Causative agents are the same as those that cause wound myiasis and vary with the geographical distribution of the flies.
The management of internal infestation is more variable and highly dependent on the clinical situation. Dead larvae, without significant inflammation, may be left in place and eventually regress. Inflammation requires management with topical steroids and mydriatics, with close monitoring. The presence of persistently viable larvae may require surgical removal, particularly when critical structures are at risk. Living organisms in the appropriate location, such as the subretinal space, may be amenable to destruction by laser photocoagulation (125). In extensive orbital myiasis, exenteration is needed to prevent the intracranial extension of tissue destruction (367). Ivermectin is a therapeutic option to prevent the further extension of the necrotizing process into deeper structures and, therefore, to decrease the risk of lethal outcomes and the need for enucleation (256).
ENT myiasis.
Ear-nose-throat (ENT) myiasis is a term used to group myiases affecting the nose, ears, oral cavity, larynx, and trachea.
(i) Oral myiasis.
Oral myiasis, first described in the literature in 1909, is a kind of wound myiasis associated with poor oral hygiene (44, 197), alcoholism (116), senility (123), severe halitosis (32), socket orifice (306), suppurating lesions, gingival disease, trauma, and mental debility (123, 293) and with people who maintain their mouths open for a long period of time (306). Infants who were breastfed by mothers with breasts infected by C. anthropophaga presented with larvae in the upper and lower lips and in other parts of the body (253). Poor hygiene is the most important risk factor and is present in almost all cases (95).
The infestation of the oral cavity may occur through direct infestation in cases where the lesions are at the anterior mandibular or maxillary region (32). Infestation into the buccal gingiva of the mandibular molar region supports the possibility by which the ingestion of contaminated food may be the way of transmission (95).
Pain and swelling of the mouth, the teeth, the lips, or the palates and a sensation of movement are some of the reported symptoms related to oral myiasis. In one case, the larva died in the submucosa and manifested clinically like a salivary gland adenoma (178).
The diagnosis of oral myiasis is usually easy and should be made at an early stage so that an involvement of deeper tissues can be prevented. This is especially important for individuals with a low socioeconomic status, who may be unaware of the oral lesions (273). Moreover, a lack of regular oral care in these patients may cause the lesions to go unnoticed until extensive tissue involvement occurs. Destructive complications are possible, and a CT scan should be performed when those complications are suspected (246, 310).
Species reported to cause this clinical picture are C. hominivorax (32, 80, 123, 130, 283, 310), W. magnifica (95), M. domestica (39, 197), C. bezziana (246), O. ovis (151), H. bovis (103), H. tarandi (105), Musca nebulo (306), G. intestinalis (343), and C. vicina (147).
Extensive tissue destruction may follow infection, and palatal perforation is a possible complication (16, 123). The treatment of oral myiasis is ideally done by the surgical removal of the maggots (95). An alternative treatment for myiasis is the creation of an anaerobic environment inside the wound to kill or expulse the maggots. Turpentine solution may help the extraction of maggots (306). The use of drugs to treat oral myiasis is incipient, and few reports can be found (309, 310). Nitrofurazone (0.2%; 20 ml) topically applied over the infested wound 3 times per day during 3 days proved to be successful in two cases (205). Treatment with ivermectin with a partial or complete response was reported (123). Prompt and correct management may lower the complication rate of oral myiasis (16).
(ii) Aural myiasis.
Aural myiasis, or otomyiasis, involves the infestation of the external ear and/or middle ear. The eggs or larvae are oviposited around the aural cavity. A widened outer ear canal may facilitate the process (350). Although considered rare, auditory myiasis represented 86.16% of 94 cases of ENT myiasis in one study of pediatric patients (313). It is usually seen in children younger than 10 years of age or in debilitated individuals (6, 58, 322, 360, 369). Chronic otorrhea has been implicated as a risk factor for aural myiasis in healthy, mobile patients (16, 224, 369). Bilateral disease is an exception (34, 369).
The clinical presentation of aural infestation is variable. Signs and symptoms include foreign-body sensation, otalgia, otorrhea, bleeding (360), itching, aural malodor, tinnitus, vertigo, restlessness, impaired hearing, and perforation of the tympanic membrane (65, 180, 214, 289). Although most patients with aural myiasis have an uncomplicated treatment course, early intervention is key to avoiding complications involving adjacent structures. Upon examination, diffuse inflammation of the skin of the external auditory canal may be found.
Aural myiasis should be considered, especially in children, in cases where a foreign body is suspected. Otoscopy may reveal maggots (Fig. 9); an inflamed and swollen auditory canal is a common finding, and in some cases, tympanic membrane perforation can be visualized (369). Imaging studies, such as computed tomography, are indicated to evaluate possible complications other than tympanic membrane perforation, such as the invasion and destruction of the mastoid cavity (8, 180, 350). Other reported complications are deafness and penetration within the central nervous system with meningitis, where death may occur (350). Together with nasal myiasis, the fatality rate may reach 8% (369).
Fig 9.
Otoscopy in a leprosy patient with otomyiasis. Otoscopy shows a larva.
The most important species causing aural myiasis are C. hominivorax (232, 245, 355), W. magnifica (34, 100, 180, 264, 341, 350), C. bezziana (1, 8, 134, 289), C. megacephala (203), Sarcophaga (6, 214, 347, 353, 360), and Parasarcophaga crassipalpis (240).
Aural myiasis must be treated by the manual extraction of the larvae (Fig. 10); irrigation of the ear with saline, 70% ethanol, 10% chloroform, normal saline, oil drops, urea, dextrose, creatine, topical ivermectin, or iodine saline has been used to help remove the maggots (214, 369). Suctioning has been used to aid in the removal of the maggots (369). The presence of tympanic membrane perforation and middle ear involvement may require multiple procedures for the complete extraction of the larvae (155).
Fig 10.
Otomyiasis due to C. hominivorax. Shown is a picture of the larva itself in a blue sheet.
(iii) Nasal myiasis.
Nasal myiasis is the infestation of the nasal cavity by flies ovipositing either directly within the nasal cavity or in the vicinity while the patient is sleeping. Direct inspiration of the maggot was reported (21). In one study, this manifestation represented 70% to 75% of ENT myiasis cases (16), and in another study of pediatric patients, this type of infestation represented 11.7% of cases (313). In a review of 252 patients with nasal myiasis, 41.26% of the patients were older than 50 years old. Beside old age, low socioeconomic status and poor nutritional status were also risk factors.
Cases more commonly occur in people who suffer from atrophic rhinitis, a condition that reduces the sneezing reflex and widens the nasal cavity; this predisposing factor was found for 97% of patients in a series of 252 patients (305), and epistaxis and rhinorrhea are nowadays rarely associated with myiasis of the nasal cavity (16). Leprosy patients are more prone to this kind of infestation, due to the lack of the sneezing reflex, painless ulceration, atrophic rhinitis, and the inability to clean the nose properly, on account of hand deformities (166, 339). Tuberculosis and rhinoscleroma were also related to nasal myiasis (305).
Nasal myiasis signs and symptoms are usually related to the presence and movement of the larvae, which include foreign-body sensation, with or without movement sensation; nasal pain; facial pain; blood-stained or mucopurulent nasal discharge; epistaxis; foul smell; and anosmia (131, 213, 345). All but 20% of 252 patients in one study reported a history of maggots coming out of the nose. Rarely, symptoms may be allergic in origin. This happens when the maggots fall into the throat, which manifests as cough, laryngospasm, dyspnea, and stridor (21, 223).
Several complications may occur during the infestation of the nose cavity. These complications may be infectious, such as orbit or facial cellulites, or destructive in nature, with the ulceration of the posterior pharyngeal wall, septal perforation with saddle nose, palatal perforation (16, 305), and, in extreme cases, penetration into the central nervous system, with meningitis, pneumocephalus (193), or death, with a fatality rate of up to 1.19% (305). The invasion of the sinus cavities may impose a differential diagnosis of sinusitis. Round hypolucent images by computed tomography may suggest a diagnosis of myiasis (99).
Rhinoscopy examination may not only confirm the diagnosis but also be used to treat the patient, aiding in the removal of the maggots with a forceps. Upon examination, mucosal edema, congestion, and ulcers are possible findings (345). Sometimes, the larvae are not noticed because they are photophobic and tend to hide in the deepest parts of the nasal cavity.
The agents reported to cause nasal myiasis are C. hominivorax (131), C. bezziana (202), Oestrus ovis (22, 211, 218), W. magnifica, Lucilia sericata (184), Drosophila melanogaster (21), and C. vicina (316).
The goal of the treatment is the prompt removal of the parasites and limiting tissue destruction. The endoscopic use of forceps to remove the agents is usually sufficient and is considered superior to manual extraction (321). Some authors used a turpentine solution to facilitate the extraction by killing the maggots. The treatment of atrophic rhinitis is also advisable; in one study, 80% of patients who refused treatment had recurrent nasal myiasis (305).
(iv) Throat myiasis.
Throat myiasis is caused mainly by O. ovis, affecting people in close contact with sheep and goats, and should be considered an occupational disease in countries where the disease is endemic, such as Iran and Italy. As expected, cases occur between April and September. Symptoms begin abruptly in individuals working outdoors. The first symptom is the sense of a foreign body in the throat, followed by a burning sensation and itching and then followed by cough, in some cases very severe. These symptoms, which are caused by physical irritation caused by the presence of the larvae, are followed by other symptoms, suspected to be allergic in nature. Rhinorrhea; sneezing; ear, nose, and throat itching; wheezing; and lacrimation are all included as allergic symptoms. Treatment is done by spraying the throat with lidocaine and then washing out the larvae with normal saline (simply by asking the patients to gargle and spit). The lidocaine not only anesthetizes the throat mucosa but also appears to anesthetize the O. ovis larvae, causing them to release their hold on the mucosa (223).
(v) Tracheostomy myiasis.
Tracheostomy myiasis is not common, with few reported cases (119). Some cases in hospital settings have been reported (175).
Urogenital myiasis.
Urogenital myiasis is the infestation of the genitourinary tract of males and females by dipteran larvae. Depending on the anatomical location, this condition could be subclassified as external or internal urogenital myiasis.
(i) External urogenital myiasis.
External urogenital myiasis is clinically, epidemiologically, and entomologically similar to furuncular myiasis or wound myiasis. It affects women more commonly. A lack of underwear, cervix carcinoma (356), the presence of a urethral stent, urethral discharge, and sexually transmitted diseases (268) are all peculiar predisposing factors of external urogenital myiasis.
In females, external urogenital myiasis cases have been described to affect clitoris (190), vulva, urethra (356), vaginal cavity (75), and uterus, a rare event that should be classified as external genitourinary myiasis, since all reported cases have been associated with uterine prolapse.
In males, cases have been described to affect glans penis, usually the furuncular type caused by D. hominis and C. anthropophaga (270); scrotum; and urethra (manifested with pruritus and pain in the periurethral region) (101, 295). Some authors suggested the inclusion of penile myiasis for the differential diagnosis of genital ulcers (267).
(ii) Internal urogenital myiasis.
Internal urogenital myiasis is a rare event that occurs when the maggot reaches an internal genitourinary organ. All described cases are considered cases of accidental myiasis. Dysuria, lumbar pain, and ureteric obstruction are the symptoms related to this kind of infestation. Microhematuria, albuminuria, and leucocyturia may be found upon laboratory examination. Symptoms subside after the expelling or removal of the maggots. Uncommon myiasis agents are associated with this type of accidental myiasis, for example, Megaselia scalaris (93), Psychoda albipennis (149), Eristalis tenax (132, 242), Piophila casei (101, 295), Fannia scalaris (361), Fannia canicularis (20, 272, 325), and Muscina stabulans (19).
The rationale for the treatment of genitourinary myiasis is to remove the offending larvae. In many cases, when the diagnosis is done, the maggot has already been expelled. Several substances, such as several antiseptics and mercury, have been used in the form of lavage and instillations. Endoscopic imaging should be performed in cases where urogenital internal myiasis is suspected, not only to diagnose but also to treat. In select cases, broad antibiotic coverage might be recommended to prevent secondary infections (356).
Intestinal myiasis.
Intestinal myiasis is a kind of pseudomyiasis or accidental myiasis that is probably related to the ingestion of contaminated food or water with dipteran fly larvae (113). Poor socioeconomic status and poor hygiene are risk factors. The water supply is a possible contamination source (191). The female flies may oviposit the eggs around the mouth of comatose and psychiatric patients, and any patient who sleeps or keeps the mouth open is also prone to gastrointestinal infestation.
The clinical presentation is variable and depends on the number of maggots, the species of the parasite, and their location within the digestive tract. Symptoms range from asymptomatic cases, where the larvae are identified in the stools, to abdominal pain, nausea, vomiting, anal pruritus, or rectal bleeding (179). The presence of the maggots may cause an inflammation of the intestinal mucosa and justify the symptoms. In some cases, the origin of the symptoms may be confusing, because patients may have a concomitant parasitic enteric infection, such as Ascaris lumbricoides (55, 133), or antiparasitic drugs (albendazole [179] or mebendazole [346]) may have been used before the diagnosis of pseudomyiasis. On the other hand, the relief of symptoms after the elimination of the maggots is incontestable proof that intestinal myiasis may be responsible for these symptoms (85).
The presence of numerous larvae in one or more consecutive stool specimens is diagnostic. Ideally, the sample should be collected in the laboratory so that a posterior infestation of the stool can be ruled out. Maggot identification from specimens collected by sigmoidoscopy is more reliable if external contamination is suspected (302).
Myiasis agents associated with this type of accidental myiasis are Fannia canicularis (366), Sarcophaga spp., Hermetia illucens (55, 133), Muscina stabulans, Megaselia scalaris (225), Eristalis tenax (96), Musca domestica, Phormia regina (182), Lucilia cuprina (239), and Stomoxys calcitrans (113).
Even though no specific treatment is valid for the treatment of intestinal myiasis, purgatives, albendazole, mebendazole, and levamizole were reported to cure the disease in some patients. Mesalazine has been used as an anti-inflammatory agent. Colonoscopy may have diagnostic and therapeutic functions (179).
Cerebral myiasis.
Cerebral myiasis is exceptionally rare, with nine confirmed cases and two additional cases with clinical and radiological suspicions (64, 84, 177, 218, 276, 292, 338). The evolution is usually fatal, with two surviving cases. The offending species were Hypoderma bovis in three of the cases; Phaenicia sericata, Hypoderma lineatum, Hypoderma spp., and Dermatobia hominis in one case each; and undetermined in two cases. The location of the infestation seems to have a predilection for the frontal lobes, as seen for seven of the nine cases. Although potentially fatal, in one case, the presence of the maggots in a head trauma case might have prevented a fatal outcome by the beneficial effects of the myiasis flies in wounds, protecting the patient from bacterial invasion and consequent meningitis and encephalitis. Intracranial hypertension with hydrocephalus (118), reduced mental status, motor deficits, seizures, and extrapyramidal symptoms were all reported for cerebral myiasis.
Tracheopulmonary myiasis.
Tracheopulmonary myiasis is a rare manifestation of myiasis, with few published cases. It is not clear if this type of myiasis is accidental in nature, since a fully developed third-instar cuterebrid maggot was implicated in one case (72). A history of whooping cough with occasional bloody sputum, without fever, and with normal chest auscultation and chest X ray may be the clinical findings. Tracheopulmonary myiasis in humans may be caused by the horse bot fly (7), Alouattamyia baeri (142), Megaselia spicularis (186), cuterebrids (23, 72, 300), and Gasterophilus sp. (7).
Accidental Myiasis or Pseudomyiasis
Accidental myiasis or pseudomyiasis is not an anatomical classification; rather, it is an ecological classification. This term represents all situations where the dipteran species is not able to fully develop when parasitizing a host. In humans, pseudomyiasis should be used as a complement of an anatomical classification, e.g., accidental intestinal myiasis. Some confusion regarding the definition occurs when some authors consider pseudomyiasis to be the presence of any symptom caused by a nonparasitizing dipteran maggot. For example, in intestinal myiasis, the symptoms are caused by the passage of the larvae through the gastrointestinal tract, with consequent inflammation in a reaction to the physical presence of the parasite, leading to symptoms. In migratory myiasis, however, the agent is able to develop in human skin, although it is not able to complete its life cycle, and the symptoms are caused by the ability of the maggot to parasitize the human skin. In our point of view, migratory myiasis should also be considered a case of pseudomyiasis, since the larvae are not able to complete their life cycle.
Myiasis in Special Clinical Settings
Leprosy.
Leprosy is endemic in countries where myiasis-causing flies are highly prevalent. The geographical coincidence of these two diseases and the typical handicaps inherent to advanced leprosy disease favor myiasis in this group of patients. Nasal myiasis and wound myiasis are the most common presentations of the dipteran infestation, although the real incidence of myiasis in leprosy patients is still unknown. The nerve damage and the eye damage typical of leprosy infection may postpone the diagnosis of myiasis, raising the possibilities of complication and even death caused by the larvae. A loss of the capability to perceive sensory stimuli, atrophic rhinitis, ulcers of nasal mucosa, and suppurative wounds of the nasal cavity predispose leprosy patients to nasal myiasis. Open wounds and ulcers have been seen in 15 to 20% of cases of all types leprosy (217). Suppuration, secondary infection, and open ulcers attract the flies to lay their eggs/larvae nearby. The localization of the lesions in the lower limbs and/or plantar region allied with the loss of sensation and loss of visual acuity and even blindness impede the prompt diagnosis of the infestation.
Any species known to cause myiasis can affect leprosy patients, and the epidemiology varies with the region, although atypical agents have been identified, including Sarcophaga ruficornis, Cochliomyia americana, and M. domestica. Treatment is the same for this group of patients. Since leprosy patients are also exposed to the most important risk factors for the development of myiasis, preventive and educative measures should both be applied to preclude infestation.
Umbilical cord myiasis.
Umbilical cord myiasis is a rare type of wound myiasis in humans, although it is a well-recognized type of myiasis in animals. The natural mummification of fetal tissue during the umbilical separation process is not well described as a risk factor for myiasis in the literature, and similarly, the best means to protect this process are still unclear (36). C. hominivorax is the offending agent related to umbilical cord myiasis. Sepsis secondary to omphalitis is a possible complication.
Malignant wounds.
Although malignant wounds are well recognized as a predisposing factor for wound myiasis, only recently have malignant wound myiasis cases started to be reported (15). The infestation of parts of one's body by fly larvae, especially by patients suffering from end-stage cancer, may bring to mind the impending prostration and decomposition of the whole body and cause sustainable psychological effects on patients' minds, compromising the patient's quality of life and treatment outcome.
Myiasis is more commonly related to open-skin malignancies such as basal cell carcinoma and squamous cell carcinoma. Other malignancies related to myiasis are eccrine adnexal neoplasm (168), angiosarcoma (64), larynx carcinoma (136), breast cancer (194), Kaposi's sarcoma (233), melanoblastoma (237), non-Hodgkin's lymphoma (79), and cervical cancer (356). The symptoms are similar to those of all reported wound myiasis cases, and the presence of unpleasant odorous secretions may be a clue to the presence of maggots. None of the cases reported so far have reported permanent health damage to the infested individual.
Members of the families Calliphoridae and Sarcophagidae cause almost all cases myiasis in malignancies, with L. sericata being the most commonly identified agent.
When feasible, surgical excision of the malignancy should be the ideal treatment. However, in some cases, especially with head and neck malignancies, advanced disease may be reached without any effective treatment. In this case, wound care is of the utmost importance, to prevent the occurrence of myiasis. The mechanical removal of the larvae with or without irrigation is the treatment of choice for these patients. Oral ivermectin treatment is a possibility when mechanical removal is not indicated.
Pin-site myiasis.
Pin-site myiasis is a rare complication of metallic pins used for the management of open fractures. Recently, there has been an increasing number of reports of myiasis as a complication of fracture treatment by means of external fixation. Of six published case reports, three cases were from Greece, two were from Venezuela, and one was from the United States. The species was identified in only two cases, both being C. hominivorax. Risk factors for wound myiasis were present in all cases. For treatment, the pin had to be removed in half of the cases (61, 265, 266).
Nosocomial myiasis.
Nosocomial myiasis, first reported in 1980 (235), is a term used when the infestation affects subjects in hospital settings. Although this form of infestation is considered rare in rich countries, it may be underreported in developing and poor countries. Its presence reflects a lack of adequate care, especially for patients who need close attention, such as patients with motility and consciousness losses, puncture wounds, draining of abscesses, assisted-breathing and tracheal tubes, and other injuries resulting from the continuous exposure of mucosae to secretions.
Comatose and handicapped patients are particularly prone to myiasis, with reported cases of wound myiasis (169), nasal and nasopharyngeal myiases (98, 139, 157, 175, 184, 215, 281, 318), orotracheal myiasis (68), myiasis of trachea (139), ocular myiasis (66), pin-site myiasis (157), urogenital myiasis (146), myiasis of nasogastric tube (67), and intestinal myiasis (320).
When considering nosocomial myiasis, the identification of the myiasis-causing species is crucial to recognize the dipteran habits and to schedule the pest eradication program. The following species have been recognized as causative agents of nosocomial myiasis: L. sericata (35, 76, 139, 157, 169, 215), Megaselia scalaris (157), Sarcophaga spp. (235), C. hominivorax (82, 98, 281), Cochliomyia macellaria (66, 175, 318), and M. domestica (146).
Nosocomial myiasis may alert one to the presence of other infestations, as exemplified by the case of a hospital with a mouse infestation (mouse carcasses could have attracted flies) and nasal myiasis cases in the intensive care unit (ICU) (35). The presence of nosocomial myiasis also reflects the quality of the staff responsible for the patients. Nets for windows and other ventilation ducts are simple measures that could help prevent nosocomial myiasis. Storage and manipulation of food should also be reviewed.
ORAL TREATMENT AND MYIASIS
Oral treatment of human myiasis is based on anecdotal reports, and most of the experience comes from veterinary medicine. Ivermectin, a semisynthetic agent of the macrolide family (derived from a natural substance, avermectin, which is obtained from actinomycetes) is the most common agent used for the treatment of myiasis. Introduced for medical use in the 1980s as a broad-spectrum antiparasitic drug, ivermectin proved to be effective against most intestinal parasites, most arthropods, and some nematodes. Medical use in humans started as a prophylactic treatment of filariosis caused by Onchocerca volvulus, Wuchereria bancrofti, Brugia malayi, and Brugia timori. Lately, it has also been used for the treatment of scabies, with excellent results.
Different therapeutic schemes have been adopted for ivermectin use in the treatment of myiasis: just one oral dose of 150 to 200 μg/kg of body weight is the most commonly prescribed dose. The possible undesirable effects of ivermectin consist of dermal eruptions, fever, dizziness, migraines, muscular pains, and joint and lymphatic pain.
Although promising, the use of ivermectin for the treatment of myiasis is an off-label treatment in many countries and should be reserved for selected cases. There are no controlled double-blind studies measuring the impact of ivermectin use on myiasis. It is very difficult to design good controlled studies, because myiasis represents a large group of pathological processes caused by a diverse number of agents. Even cases of wound myiasis caused by the same agent may not be completely comparable: numbers of larvae, kinds of lesions, and the presence of necrosis may vary between cases. Table 9 describes some reports of ivermectin use and its outcome. Another agent for the oral treatment of myiasis is tiabendazole. It has been used for the treatment of immature O. ovis maggots in sheep, and in one case of human myiasis caused by A. baeri, the treatment was successful (142).
Table 9.
Clinical pictures, treatments, and outcomes of cases of oral myiasis
| Reference | Species | Clinical picture | Ivermectin dose (μg/kg) (no. of doses) | Outcome |
|---|---|---|---|---|
| 271 | Lucilia sericata | Wound myiasis | 200 (1) | Complete cure |
| 358 | D. hominis | External ophthalmomyiasis | 200 (1) | Larva emigration |
| 256 | C. hominivorax | Orbital myiasis | 200 (1) | Larva death |
| 256 | C. hominivorax | Orbital myiasis | 200 (1) | Larva death |
| 86 | C. hominivorax | Orbital myiasis | 200 (1) | Larva death |
| 287 | Unknown | Orbital myiasis | 200 (1) | Larva death |
| C. hominivorax | Oral myiasis | 200 (2) | Incomplete response | |
| 123 | C. hominivorax | Oral myiasis | 200 (2) | Larva death |
| 310 | C. hominivorax | Oral myiasis | 200 (2) | Larva death |
PREVENTION
Poor sanitation is probably the most important risk factor for human myiasis. Low socioeconomic status, especially in poor countries, has an intimate relationship with the lack of basic sanitation and inadequate garbage disposal, leaving organic material exposed, which attracts insects and small animals, creating a sustainable cycle of filth. Adequate sanitation can be reached only when government, population, and education programs work together.
Individual actions should also be implemented and include emptying and steam cleaning dumpsters on a regular schedule, washing food and making a visual inspection of the food before consumption, storing food in adequate receptacles, making sure wounds are cleaned and dressed regularly, and more. Good sanitation can avert many myiasis cases. In regions of endemicity, sleeping nude, outdoors, and on the floor should be avoided. Appropriate precautions will help avoid infestations. The use of screens and mosquito nets is essential to prevent flies from reaching the skin. D. hominis infestation may be thwarted by the application of insect repellents containing diethyltoluamide (DEET). Drying clothes in bright sunlight and ironing them are effective methods of destroying occult eggs laid in clothing, especially by C. anthropophaga (41). Other general precautions include wearing long-sleeved clothing, covering wounds, and avoiding falling asleep outdoors.
Field control of flies is extremely important. All available methods should be used, including aerial sprays, destruction of animal carcasses, elementary sanitary and hygiene practices, and clearing of debris and rubbish near houses. The inactivation of females by the release of large numbers of males previously sterilized by ionizing radiation has been highly successful. Reports on the control of Cochliomyia infestation in sheep with the use of ivermectin, which has been reported to be 100% effective in controlling existing infestations and as a prophylaxis, suggest that this may be the route of the future.
MAGGOT THERAPY
Maggot therapy is essentially an artificially induced myiasis performed in a controlled environment by experienced medical practitioners. The selection of a suitable fly species for use in maggot therapy is of paramount importance, as it determines both the safety and success of the treatment. It is imperative to select a species that feeds almost exclusively on necrotic tissue. L. sericata is considered the most suitable species for maggot therapy. The larvae must be prepared and maintained sterile before clinical use (247).
Maggot therapy has the following three core beneficial effects on a wound: debridement, disinfection, and enhanced healing. Debridement is the removal of cellular debris and nonviable necrotic tissue from the wound bed. The removal of necrotic tissue, which acts as a microbial substrate, may also reduce the risk of infection. Maggots debride wounds quickly and effectively, without damage to viable tissue. Maggots are photophobic and will naturally move into the deep crevices that may be beyond the reach of a surgeon's scalpel. Reports have been published marveling at the benefits of maggot debridement therapy (MDT) in all sorts of wounds.
Several mechanisms have been suggested for disinfection, including the simple mechanical irrigation of the wound by increased secretions/excretions produced by larvae, the action of the midgut commensal Proteus mirabilis on digested bacteria, and the elimination of antibacterial products from living maggots.
Enhanced healing is started by the proteolytic digestion of necrotic tissue and disinfection promoted by the maggots. Second, researchers have suggested that maggots exhibit other, more direct, mechanisms that contribute to the enhanced healing of wounds, although these mechanisms are not completely understood. Maggots encourage wound healing, centered on their ability to physically stimulate viable tissue and to enhance tissue oxygenation in chronic wounds. Allantoin (2,5-dioxo-4-imadazolidinyl urea) or ammonia bicarbonate could be responsible for the abundant growth of granulation tissue. The growth-stimulating effects of alimentary secretions and hemolymph from the blow fly P. sericata on human fibroblast tissue may also contribute to enhanced healing (247, 248).
Biographies

Fabio Francesconi, M.D., M.Sc., graduated in medicine at the Federal University of Rio de Janeiro (UFRJ) and performed his medical residence in dermatology at the State University of Rio de Janeiro (UERJ), Brazil. He is now serving as assistant professor at Amazon Federal University, and he is also a researcher at the Tropical Medicine Foundation-Heitor Vieira Dourado in Manaus, Brazil.

Omar Lupi, M.D., M.Sc., Ph.D., received his undergraduate and medical degrees from UERJ (1985 to 1990), followed by residency training in Dermatology at UFRJ (1991 to 1993). He received his M.Sc. (1995) and Ph.D. (1998) and completed 1 year of postdoctoral training at the University of Texas Medical Branch (UTMB) in Galveston, TX (2001), with special training in molecular biology and immunology. He is Chairman and Titular Professor of Dermatology at Policlinica Geral do Rio de Janeiro and Adjunct Professor of Dermatology at the Federal University of Rio de Janeiro (UniRio). He has served on the Medical Faculty of the Federal University of Rio de Janeiro since 1998, as a physician and postgraduate professor of Dermatology (1998 to 2007), and as an immunologist (since 2007). He served as the president of the Brazilian Society of Dermatology (2009 to 2010). He has published more than 180 scientific papers and 45 book chapters, with special interest in dermatovirology, sexually transmitted diseases, and skin cancer. He is also the editor of 5 textbooks in Dermatology.
REFERENCES
- 1. Abed-Benamara M, Achir I, Rodhain F, Perez-Eid C. 1997. First Algerian case of human otomyiasis due to Chrysomya bezziana. Bull. Soc. Pathol. Exot. 90: 172–175 (In French) [PubMed] [Google Scholar]
- 2. Abul-Hab JK, Salman YG. 1999. Human urinogenital myiasis by Psychoda. Saudi Med. J. 20: 635–636 [PubMed] [Google Scholar]
- 3. Adler AI, Brancato FP. 1995. Human furuncular myiasis caused by Hermetia illucens (Diptera: Stratiomyidae). J. Med. Entomol. 32: 745–746 [DOI] [PubMed] [Google Scholar]
- 4. Aguiar AMM, Enwonwu CO, Pires FR. 2003. Noma (cancrum oris) associated with oral myiasis in an adult. Oral Dis. 9: 158–159 [DOI] [PubMed] [Google Scholar]
- 5. Aguilera A, Cid A, Regueiro BJ, Prieto JM, Noya M. 1999. Intestinal myiasis caused by Eristalis tenax. J. Clin. Microbiol. 37: 3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmad NW, et al. 2009. Aural myiasis in a neonate in peninsular Malaysia. Parasit. Vectors 2: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed MJ, Miller A. 1969. Pulmonary coin lesion containing a horse bot, Gasterophilus—a report of a case of myiasis. Am. J. Clin. Pathol. 52: 414–419 [DOI] [PubMed] [Google Scholar]
- 8. Al-Abidi AA, Bello C, Al-Ahmari M, Fawehinmi Y. 2003. Mastoid cells myiasis in a Saudi man: a case report. West Afr. J. Med. 22: 366–368 [DOI] [PubMed] [Google Scholar]
- 9. Alexis JB, Mittleman RE. 1988. An unusual case of Phormia regina myiasis of the scalp. Am. J. Clin. Pathol. 90: 734–737 [DOI] [PubMed] [Google Scholar]
- 10. Alves CA, Ribeiro O, Jr, Borba AM, Ribeiro AN, Guimaraes J., Jr 2010. Cavernous sinus thrombosis in a patient with facial myiasis. Quintessence Int. 41: e72–e74 [PubMed] [Google Scholar]
- 11. Reference deleted.
- 12. Amr ZS, Amr BA, Abo-Shehada MN. 1993. Ophthalmomyiasis externa caused by Oestrus ovis L. in the Ajloun area of northern Jordan. Ann. Trop. Med. Parasitol. 87: 259–262 [DOI] [PubMed] [Google Scholar]
- 13. Anane S, Hssine LB. 2010. Conjonctival [sic] human myiasis by Oestrus ovis in southern Tunisia. Bull. Soc. Pathol. Exot. 103: 299–304 (In French) [DOI] [PubMed] [Google Scholar]
- 14. Anderson RN. 1996. An adult female with furuncles and formication. J. Emerg. Nurs. 22: 83–84 [DOI] [PubMed] [Google Scholar]
- 15. Arbit E, Varon RE, Brem SS. 1986. Myiatic scalp and skull infection with diptera Sarcophaga: case report. Neurosurgery 18: 361–362 [DOI] [PubMed] [Google Scholar]
- 16. Arora S, Sharma JK, Pippal SK, Sethi Y, Yadav A. 2009. Clinical etiology of myiasis in ENT: a reterograde period—interval study. Braz. J. Otorhinolaryngol. 75: 356–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arsalane L, Agoumi A, Behjawi A, Elhoum A, Bouazzaoui N. 2001. Bilateral ear myiasis due to Fannia canicularis (Linne 1761). Med. Trop. (Mars.) 61: 548–549 [PubMed] [Google Scholar]
- 18. Aspock H. 1972. Rectal myiasis due to Fannia canicularis (Linne) and Muscina stabulans (Fallen). Dtsch. Med. Wochenschr. 97: 1174–1175 [DOI] [PubMed] [Google Scholar]
- 19. Aspock H. 1972. Urethral myiasis caused by Muscina stabulans (Fallen). Zentralbl. Bakteriol. Orig. A221: 352–356 [PubMed] [Google Scholar]
- 20. Aspock H, Burkert S, Reichmann A. 1972. Urethral myiasis caused by Fannia canicularis. Wien. Klin. Wochenschr. 84: 280–281 [PubMed] [Google Scholar]
- 21. Aydin E, Uysal S, Akkuzu B, Can F. 2006. Nasal myiasis by fruit fly larvae: a case report. Eur. Arch. Otorhinolaryngol. 263: 1142–1143 [DOI] [PubMed] [Google Scholar]
- 22. Badia L, Lund VJ. 1994. Vile bodies: an endoscopic approach to nasal myiasis. J. Laryngol. Otol. 108: 1083–1085 [DOI] [PubMed] [Google Scholar]
- 23. Baird CR, Podgore JK, Sabrosky CW. 1982. Cuterebra myiasis in humans—six new case-reports from the United States with summary of known cases (Diptera: Cuterebridae). J. Med. Entomol. 19: 263–267 [DOI] [PubMed] [Google Scholar]
- 24. Baird JK, Baird CR, Sabrosky CW. 1989. North American cuterebrid myiasis. Report of seventeen new infections of human beings and review of the disease. J. Am. Acad. Dermatol. 21: 763–772 [PubMed] [Google Scholar]
- 25. Reference deleted.
- 26. Baker DJ, Kantor GR, Stierstorfer MB, Brady G. 1995. Furuncular myiasis from Dermatobia hominis infestation. Diagnosis by light microscopy. Am. J. Dermatopathol. 17: 389–394 [DOI] [PubMed] [Google Scholar]
- 27. Bakos RM, Bakos L. 2007. Dermoscopic diagnosis of furuncular myiasis. Arch. Dermatol. 143: 123–124 [DOI] [PubMed] [Google Scholar]
- 28. Balcioglu IC, Ecemis T, Ayer A, Ozbel Y. 2008. Subungual myiasis in a woman with psychiatric disturbance. Parasitol. Int. 57: 509–511 [DOI] [PubMed] [Google Scholar]
- 29. Baliga MJ, Davis P, Rai P, Rajasekhar V. 2001. Orbital myiasis: a case report. Int. J. Oral Maxillofac. Surg. 30: 83–84 [DOI] [PubMed] [Google Scholar]
- 30. Bangsgaard R, Holst B, Krogh E, Heegaard S. 2000. Palpebral myiasis in a Danish traveler caused by the human bot-fly (Dermatobia hominis). Acta Ophthalmol. Scand. 78: 487–489 [DOI] [PubMed] [Google Scholar]
- 31. Reference deleted.
- 32. Barbosa TD, Rocha R, Guirado CG, Rocha FJ, Gaviao MBD. 2008. Oral infection by Diptera larvae in children: a case report. Int. J. Dermatol. 47: 696–699 [DOI] [PubMed] [Google Scholar]
- 33. Barr RA, Thompson JH., Jr 1955. A case of cutaneous myiasis in a child caused by Muscina sp. J. Parasitol. 41: 552–553 [Google Scholar]
- 34. Bayindir T, Miman O, Miman MC, Atambay M, Saki CE. 2010. Bilateral aural myiasis (Wohlfahrtia magnifica): a case with chronic suppurative otitis media. Turkiye Parazitol. Derg. 34: 65–67 [PubMed] [Google Scholar]
- 35. Beckendorf R, Klotz SA, Hinkle N, Bartholomew W. 2002. Nasal myiasis in an intensive care unit linked to hospital-wide mouse infestation. Arch. Intern. Med. 162: 638–640 [DOI] [PubMed] [Google Scholar]
- 36. Beeregowda YC, Kiran B, Gowda NY. 2010. Neonatal umbilical myiasis with sepsis. Indian J. Pediatr. 77: 1443–1445 [DOI] [PubMed] [Google Scholar]
- 37. Bernhard K. 1987. Demonstration of rectal myiasis in humans. Angew. Parasitol. 28: 59–61. (In German.) [PubMed] [Google Scholar]
- 38. Bhandari R, Janos DP, Sinnis P. 2007. Furuncular myiasis caused by Dermatobia hominis in a returning traveler. Am. J. Trop. Med. Hyg. 76: 598–599 [PMC free article] [PubMed] [Google Scholar]
- 39. Bhatt AP, Jayakrishnan A. 2000. Oral myiasis: a case report. Int. J. Paediatr. Dent. 10: 67–70 [DOI] [PubMed] [Google Scholar]
- 40. Biswas J, Gopal L, Sharma T, Badrinath SS. 1994. Intraocular Gnathostoma spinigerum. Clinicopathologic study of two cases with review of literature. Retina 14: 438–444 [DOI] [PubMed] [Google Scholar]
- 41. Blacklock B, Thompson MG. 1923. A study of the tumbu-fly Cordylobia anthropophaga Grunberg in Sierra Leone. Trop. Med. Parasitol. 17: 443–502 [Google Scholar]
- 42. Blondeau JM, Haldane DJ, Murray U, Buchholz KP. 1993. Gastric myiasis. Role of the lesser housefly. Can. Fam. Physician 39: 646–648 [PMC free article] [PubMed] [Google Scholar]
- 43. Boruk M, Rosenfeld RM, Alexis R. 2006. Human botfly infestation presenting as peri-auricular mass. Int. J. Pediatr. Otorhinolaryngol. 70: 335–338 [DOI] [PubMed] [Google Scholar]
- 44. Bozzo L, Lima IA, de Almeida OP, Scully C. 1992. Oral myiasis caused by sarcophagidae in an extraction wound. Oral Surg. Oral Med. Oral Pathol. 74: 733–735 [DOI] [PubMed] [Google Scholar]
- 45. Brand AF. 1931. Gastrointestinal myiasis: report of a case. Arch. Intern. Med. 47: 149–154 [Google Scholar]
- 46. Brent AJ, Hay D, Conlon CP. 2008. Souvenirs to make your skin crawl. Lancet Infect. Dis. 8: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brewer TF, Felsenstein D, Wilson ME. 1994. Furuncular myiasis—alternatives to bacon therapy. JAMA 271: 901–902 [DOI] [PubMed] [Google Scholar]
- 48. Brewer TF, Wilson ME, Gonzalez E, Felsenstein D. 1993. Bacon therapy and furuncular myiasis. JAMA 270: 2087–2088 [PubMed] [Google Scholar]
- 49. Brisou P, Menard G. 2000. External ophthalmomyiasis by Oestrus ovis from a beach in Var. Med. Trop. (Mars.) 60: 64–66(In French) [PubMed] [Google Scholar]
- 50. Buettner H. 2002. Ophthalmomyiasis interna. Arch. Ophthalmol. 120: 1598–1599 [DOI] [PubMed] [Google Scholar]
- 51. Burgess I, Spraggs PDR. 1992. Myiasis due to Parasarcophaga argyrostoma—first recorded case in Britain. Clin. Exp. Dermatol. 17: 261–263 [DOI] [PubMed] [Google Scholar]
- 52. Byrd JH, Allen JC. 2001. The development of the black blow fly, Phormia regina (Meigen). Forensic Sci. Int. 120: 79–88 [DOI] [PubMed] [Google Scholar]
- 53. Caca I, Satar A, Unlu K, Sakalar YB, Ari S. 2006. External ophthalmomyiasis infestation. Jpn. J. Ophthalmol. 50: 176–177 [DOI] [PubMed] [Google Scholar]
- 54. Caca I, et al. 2003. Orbital myiasis: case report. Jpn. J. Ophthalmol. 47: 412–414 [DOI] [PubMed] [Google Scholar]
- 55. Calderón-Arguedas O, Murillo Barrantes J, Solano ME. 2005. Enteric myiasis by Hermetia illucens (Diptera: Stratiomyidae) in a geriatric patient of Costa Rica. Parasitol. Latinoam. 60: 162–164 [Google Scholar]
- 56. Cardot-Leccia N, et al. 2008. An unusual furuncule. Presse Med. 37: 1342–1345 [DOI] [PubMed] [Google Scholar]
- 57. Carpenter TL, Chastain DO. 1992. Facultative myiasis by Megaselia sp. (Diptera: Phoridae) in Texas: a case report. J. Med. Entomol. 29: 561–563 [DOI] [PubMed] [Google Scholar]
- 58. Casanova-Roman M, Sanchez-Legaza E, Sanchez-Porto A, Murga C. 2010. Aural myiasis in an infant. Infez. Med. 18: 175–176 [PubMed] [Google Scholar]
- 59. Caumes E, et al. 1995. Dermatoses associated with travel to tropical countries—a prospective study of the diagnosis and management of 269 patients presenting to a tropical disease unit. Clin. Infect. Dis. 20: 542–548 [DOI] [PubMed] [Google Scholar]
- 60. Cavusoglu T, Apan T, Eker E, Vargel I, Saray A. 2009. Massive oculofacial myiasis infestation with Lucilia sericata. J. Am. Acad. Dermatol. 61: 169–170 [DOI] [PubMed] [Google Scholar]
- 61. Cazorla-Perfetti DJ, Acosta-Quintero ME, Morales P, Bermudez SE, Rodriguez-Morales AJ. 2009. Pin-site myiasis: an emerging infectious complication of external bone fixation? Int. J. Infect. Dis. 13: e514–e516 [DOI] [PubMed] [Google Scholar]
- 62. Chandra DB, Agrawal TN. 1981. Ocular myiasis caused by oestrus ovis. (A case report.) Indian J. Ophthalmol. 29: 199–200 [PubMed] [Google Scholar]
- 63. Chen WY, Hung TH, Shiao SF. 2004. Molecular identification of forensically important blow fly species (Diptera: Calliphoridae) in Taiwan. J. Med. Entomol. 41: 47–57 [DOI] [PubMed] [Google Scholar]
- 64. Cheshier SH, Bababeygy SR, Higgins D, Parsonnet J, Huhn SL. 2007. Cerebral myiasis associated with angiosarcoma of the scalp: case report. Neurosurgery 61: E167. [DOI] [PubMed] [Google Scholar]
- 65. Cho JH, Kim HB, Cho CS, Huh S, Ree HI. 1999. An aural myiasis case in a 54-year-old male farmer in Korea. Korean J. Parasitol. 37: 51–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chodosh J, Clarridge JE, Matoba A. 1991. Nosocomial conjunctival ophthalmomyiasis with Cochliomyia macellaria. Am. J. Ophthalmol. 111: 520–521 [DOI] [PubMed] [Google Scholar]
- 67. Chung PR, et al. 1996. A human case of internal myiasis in Korea. Korean J. Parasitol. 34: 151–154 (In Korean) [DOI] [PubMed] [Google Scholar]
- 68. Ciftcioglu N, Altintas K, Haberal M. 1997. A case of human orotracheal myiasis caused by Wohlfahrtia magnifica. Parasitol. Res. 83: 34–36 [DOI] [PubMed] [Google Scholar]
- 69. Clark JM, Weeks WR, Tatton J. 1982. Drosophila myiasis mimicking sepsis in a newborn. West. J. Med. 136: 443–444 [PMC free article] [PubMed] [Google Scholar]
- 70. Clyti E, et al. 2007. Myiasis owing to Dermatobia hominis in a HIV-infected subject: treatment by topical ivermectin. Int. J. Dermatol. 46: 52–54 [DOI] [PubMed] [Google Scholar]
- 71. Colwell DD, Otranto D. 2006. Cross-transmission studies with Hypoderma lineatum de Vill. (Diptera: Oestridae): attempted infestation of goats (Capra hircus). Vet. Parasitol. 141: 302–306 [DOI] [PubMed] [Google Scholar]
- 72. Cornet M, et al. 2003. Tracheopulmonary myiasis caused by a mature third-instar Cuterebra larva: case report and review. J. Clin. Microbiol. 41: 5810–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Curtis SJ, Edwards C, Athulathmuda C, Paul J. 2006. Cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg. Med. J. 23: 236–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Custis PH, Pakalnis VA, Klintworth GK, Anderson WB, Jr, Machemer R. 1983. Posterior internal ophthalmomyiasis. Identification of a surgically removed Cuterebra larva by scanning electron microscopy. Ophthalmology 90: 1583–1590 [DOI] [PubMed] [Google Scholar]
- 75. Daniel M, Sramova H, Zalabska E. 1994. Lucilia sericata (Diptera: Calliphoridae) causing hospital-acquired myiasis of a traumatic wound. J. Hosp. Infect. 28: 149–152 [DOI] [PubMed] [Google Scholar]
- 76. Danjou R, et al. 1975. Eosinophilic meningitis: a new case of hypodermosis from Hypoderma lineatum. Acta Trop. 32: 389–391 [PubMed] [Google Scholar]
- 77. Dar MS, Amer MB, Dar FK, Papazotos V. 1980. Ophthalmomyiasis caused by the sheep nasal bot, Oestrus ovis (Oestridae) larvae, in the Benghazi area of Eastern Libya. Trans. R. Soc. Trop. Med. Hyg. 74: 303–306 [DOI] [PubMed] [Google Scholar]
- 78. da Silva BB, Borges US, Pimentel ICC. 2005. Human vaginal myiasis caused by Cochliomyia hominivorax. Int. J. Gynecol. Obstet. 89: 152–153 [DOI] [PubMed] [Google Scholar]
- 79. David S, Rupa V, Mathai E, Nair S. 1996. Non Hodgkins lymphoma of ethmoidal sinus with rhinoorbital myiasis. Indian J. Cancer 33: 171–172 [PubMed] [Google Scholar]
- 80. De Araujo RJG, Correa AM, Araujo Santos WR, Moreira MT., Jr 2008. Advanced stage of oral myiasis in children: a clinical case report. Quintessence Int. 39: 39–43 [PubMed] [Google Scholar]
- 81. de Barros N, et al. 2001. Cutaneous myiasis of the breast: mammographic and US features—report of five cases. Radiology 218: 517–520 [DOI] [PubMed] [Google Scholar]
- 82. Defoliart GR, Pelton EC. 1955. A case of human intestinal myiasis caused by Muscina stabulans (Fallen). Am. J. Trop. Med. Hyg. 4: 953–955 [DOI] [PubMed] [Google Scholar]
- 83. de Kaminsky RG. 1993. Nosocomial myiasis by Cochliomyia hominivorax in Honduras. Trans. R. Soc. Trop. Med. Hyg. 87: 199–200 [DOI] [PubMed] [Google Scholar]
- 84. de la Ossa N, et al. 2009. Cutaneous myiasis by Cochliomyia hominivorax (Coquerel) (Diptera Calliphoridae) in Hospital Universidad del Norte, Soledad, Atlantico. Biomedica 29: 12–17(In Spanish) [PubMed] [Google Scholar]
- 85. Delhaes L, et al. 2001. Human nasal myiasis due to Oestrus ovis. Parasite 8: 289–296 (In French) [DOI] [PubMed] [Google Scholar]
- 86. Delhaes L, et al. 2001. Recovery of Calliphora vicina first-instar larvae from a human traumatic wound associated with a progressive necrotizing bacterial infection. Am. J. Trop. Med. Hyg. 64: 159–161 [DOI] [PubMed] [Google Scholar]
- 87. Delshad E, Rubin AI, Almeida L, Niedt GW. 2008. Cuterebra cutaneous myiasis: case report and world literature review. Int. J. Dermatol. 47: 363–366 [DOI] [PubMed] [Google Scholar]
- 88. Reference deleted.
- 89. Denion E, et al. 2004. External ophthalmomyiasis caused by Dermatobia hominis. a retrospective study of nine cases and a review of the literature. Acta Ophthalmol. Scand. 82: 576–584 [DOI] [PubMed] [Google Scholar]
- 90. de Rodriguez ZR, Anciani ID, Villalobos R. 2007. The importance of epidemiological study in the diagnosis of human intestinal miasis: a case study. Kasmera 35: 65–69 [Google Scholar]
- 91. De Tarso P, Pierre P, Minguini N, Pierre LM, Pierre AM. 2004. Use of ivermectin in the treatment of orbital myiasis caused by Cochliomyia hominivorax. Scand. J. Infect. Dis. 36: 503–505 [DOI] [PubMed] [Google Scholar]
- 92. Diaz JH. 2009. Myiasis and tungiasis, chapter 295. In Mandell GL, Bennett JE, Dolin R. (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, vol 2 Churchill, Livingstone, Elsevier, Philadelphia, PA: [Google Scholar]
- 93. Disney RHL, Kurahashi H. 1978. Case of urogential myiasis caused by a species of Megaselia scalaris (Diptera-Phoridae). J. Med. Entomol. 14: 717. [DOI] [PubMed] [Google Scholar]
- 94. Dono M, et al. 2005. Three cases of ophthalmomyiasis externa by sheep botfly Oestrus ovis in Italy. New Microbiol. 28: 365–368 [PubMed] [Google Scholar]
- 95. Droma EB, et al. 2007. Oral myiasis: a case report and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 103: 92–96 [DOI] [PubMed] [Google Scholar]
- 96. Dubois E, Durieux M, Franchimont MM, Hermant P. 2004. An unusual case in Belgium of intestinal myiasis due to Eristalis tenax. Acta Clin. Belg. 59: 168–170 (In French) [DOI] [PubMed] [Google Scholar]
- 97. Ducournau D, Bonnet M. 1982. Posterior internal ophthalmomyiasis. Bull. Soc. Ophtalmol. Fr. 82: 1529–1530(In French) [PubMed] [Google Scholar]
- 98. Duque CS, Marrugo G, Valderrama R. 1990. Otolaryngic manifestations of myiasis. Ear Nose Throat J. 69: 619–622 [PubMed] [Google Scholar]
- 99. Duque CS, Mosquera CA, Casiano RR, Abreu CE. 2006. Radiologic findings in sinonasal myiasis. Otolaryngol. Head Neck Surg. 135: 638–639 [DOI] [PubMed] [Google Scholar]
- 100. el Kadery AA, el-Begermy MA. 1989. Aural myiasis caused by Wohlfahrtia magnifica. J. Egypt. Soc. Parasitol. 19: 751–753 [PubMed] [Google Scholar]
- 101. el-Serougi AO. 1991. A case of urinary myiasis due to Piophila casei. J. Egypt. Soc. Parasitol. 21: 595–596 [PubMed] [Google Scholar]
- 102. Engelbrecht NE, Yeatts RP, Slansky F. 1998. Palpebral myiasis causing preseptal cellulitis. Arch. Ophthalmol. 116: 684. [PubMed] [Google Scholar]
- 103. Erol B, Unlu G, Balci K, Tanrikulu R. 2000. Oral myiasis caused by Hypoderma bovis larvae in a child: a case report. J. Oral Sci. 42: 247–249 [DOI] [PubMed] [Google Scholar]
- 104. Eyigor H, Dost T, Dayanir V, Basak S, Eren H. 2008. A case of naso-ophthalmic myiasis. Kulak Burun Bogaz Ihtis. Derg. 18: 371–373 [PubMed] [Google Scholar]
- 105. Faber TE, Hendrikx WML. 2006. Oral myiasis in a child by the reindeer warble fly larva Hypoderma tarandi. Med. Vet. Entomol. 20: 345–346 [DOI] [PubMed] [Google Scholar]
- 106. Facina G, Nazario ACP, Kemp C, Gebrim LH, Lima GR. 1999. Myiasis by Dermatobia hominis mimicking periductal mastitis. Rev. Bras. Mastol. 9: 84–85 [Google Scholar]
- 107. Farkas R. 1996. Myiasis caused by Wohlfahrtia magnifica. Magyar Allatorvosok Lapja 51: 349–352 [Google Scholar]
- 108. Farkas R, Kepes G. 2001. Traumatic myiasis of horses caused by Wohlfahrtia magnifica. Acta Vet. Hung. 49: 311–318 [DOI] [PubMed] [Google Scholar]
- 109. Fassler C, et al. 2000. Subcutaneous myiasis: a case report. Arch. Pediatr. 7: 840–843 [DOI] [PubMed] [Google Scholar]
- 110. Feder HM, Mitchell PR, Seeley MZ. 2003. A warble in Connecticut. Lancet 361: 1952. [DOI] [PubMed] [Google Scholar]
- 111. Fernandes LF, Pimenta FC, Fernandes FF. 2009. First report of human myiasis in GoiaS state, Brazil: frequency of different types of myiasis, their various etiological agents, and associated factors. J. Parasitol. 95: 32–38 [DOI] [PubMed] [Google Scholar]
- 112. Ferraz AC, et al. 2010. First record of human myiasis caused by association of the species Chrysomya megacephala (Diptera: Calliplioridae), Sarcophaga (Liopygia) ruficornis (Diptera: Sarcophagidae), and Musca domestica (Diptera: Muscidae). J. Med. Entomol. 47: 487–490 [DOI] [PubMed] [Google Scholar]
- 113. Ferreira MF, et al. 1990. Intestinal myiasis in Macao. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 8: 214–216 (In Chinese) [PubMed] [Google Scholar]
- 114. Focke M, et al. 2003. Specific sensitization to the common housefly (Musca domestica) not related to insect panallergy. Allergy 58: 448–451 [DOI] [PubMed] [Google Scholar]
- 115. Foley DH, Rueda LM, Wilkerson RC. 2007. Insight into global mosquito biogeography from country species records. J. Med. Entomol. 44: 554–567 [DOI] [PubMed] [Google Scholar]
- 116. Fonseca DR, et al. 2007. Miasis buco-maxilo-facial: reporte de un caso. Acta Odontol. Venez. 45: 565–567 [Google Scholar]
- 117. Francesconi F, Lupi O. 2006. Myiasis, p 232–239 In Tyring SK, Lupi O, Hengge UR. (ed), Tropical dermatology. Elsevier, Philadelphia, PA [Google Scholar]
- 118. Francois P, Martin G, Goullier A, Plasse M, Beaudoing A. 1987. Neuromeningeal hypodermyiasis complicated by hydrocephaly. Value of nuclear magnetic resonance imaging. Presse Med. 16: 1231–1233 [PubMed] [Google Scholar]
- 119. Franza R, Leo L, Minerva T, Sanapo F. 2006. Myiasis of the tracheostomy wound: case report. Acta Otorhinolaryngol. Ital. 26: 222–224 [PMC free article] [PubMed] [Google Scholar]
- 120. Fujisaki R, et al. 2008. Exotic myiasis caused by 19 larvae of Cordylobia anthropophaga in Namibia and identified using molecular methods in Japan. Trans. R. Soc. Trop. Med. Hyg. 102: 599–601 [DOI] [PubMed] [Google Scholar]
- 121. Gass JD, Lewis RA. 1976. Subretinal tracks in ophthalmomyiasis. Arch. Ophthalmol. 94: 1500–1505 [DOI] [PubMed] [Google Scholar]
- 122. Gass JD, Lewis RA. 1976. Subretinal tracts in ophthalmomyiasis. Trans. Sect. Ophthalmol. Am. Acad. Ophthalmol. Otolaryngol. 81: 483–490 [PubMed] [Google Scholar]
- 123. Gealh WC, Ferreira GM, Farah GJ, Teodoro U, Camarini ET. 2009. Treatment of oral myiasis caused by Cochliomyia hominivorax: two cases treated with ivermectin. Br. J. Oral Maxillofac. Surg. 47: 23–26 [DOI] [PubMed] [Google Scholar]
- 124. Geary MJ, Hudson BJ, Russell RC, Hardy A. 1999. Exotic myiasis with Lund's fly (Cordylobia rodhaini). Med. J. Aust. 171: 654–655 [DOI] [PubMed] [Google Scholar]
- 125. Georgalas I, Ladas I, Maselos S, Lymperopoulos K, Marcomichelakis N. 2011. Intraocular safari: ophthalmomyiasis interna. Clin. Exp. Ophthalmol. 39: 84–85 [DOI] [PubMed] [Google Scholar]
- 126. Ghosh SK, Bandyopadhyay D, Sarkar S. 2010. Myiasis in a large perigenital seborrheic keratosis. Indian J. Dermatol. 55: 305–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Goddard J. 1997. Human infestation with rodent botfly larvae: a new route of entry? South. Med. J. 90: 254–255 [DOI] [PubMed] [Google Scholar]
- 128. Goddard J. 2003. Physician's guide to arthropods of medical importance, 4th ed, p 195–214 CRC Press, Boca Raton, FL [Google Scholar]
- 129. Goddard J. 2003. Physician's guide to arthropods of medical importance, 4th ed, p 61–65 CRC Press, Boca Raton, FL [Google Scholar]
- 130. Gomez RS, et al. 2003. Oral myiasis by screwworm Cochliomyia hominivorax. Br. J. Oral Maxillofac. Surg. 41: 115–116 [DOI] [PubMed] [Google Scholar]
- 131. González AC, Salamanca GJC, Olano VM, Pérez CE. 2008. Cavitary myiasis: case report. Rev. Fac. Med. 16: 95–98 [Google Scholar]
- 132. Gonzalez MM, Comte MG, Monardez PJ, Diaz de Valdes LM, Matamala CI. 2009. Accidental genital myiasis by Eristalis tenax. Rev. Chilena Infectol. 26: 270–272 (In Spanish) [PubMed] [Google Scholar]
- 133. González OF, Oliva GR. 2009. First report of intestinal myiasis caused by Hermetia illucens (Diptera: Stratiomyidae). Rev. Cubana Med. Trop. 61: 97–99 [Google Scholar]
- 134. Gonzalez Poggioli N, Vazquez Barro JC. 2009. Otic myiasis. Case report. Acta Otorrinolaringol. Esp. 60: 213–214(In Spanish) [PubMed] [Google Scholar]
- 135. Goodman RL, et al. 2000. Anterior orbital myiasis caused by human botfly (Dermatobia hominis). Arch. Ophthalmol. 118: 1002–1003 [PubMed] [Google Scholar]
- 136. Gopalakrishnan S, Srinivasan R, Saxena SK, Shanmugapriya J. 2008. Myiasis in different types of carcinoma cases in southern India. Indian J. Med. Microbiol. 26: 189–192 [DOI] [PubMed] [Google Scholar]
- 137. Gordon PM, Hepburn NC, Williams AE, Bunney MH. 1995. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br. J. Dermatol. 132: 811–814 [DOI] [PubMed] [Google Scholar]
- 138. Gozum N, Kir N, Ovali T. 2003. Internal ophthalmomyiasis presenting as endophthalmitis associated with an intraocular foreign body. Ophthalmic Surg. Lasers Imaging 34: 472–474 [PubMed] [Google Scholar]
- 139. Greenberg B. 1984. Two cases of human myiasis caused by Phaenicia sericata (Diptera: Calliphoridae) in Chicago area hospitals. J. Med. Entomol. 21: 615. [DOI] [PubMed] [Google Scholar]
- 140. Gregory AR, Schatz S, Laubach H. 2004. Ophthalmomyiasis caused by the sheep bot fly Oestrus ovis in northern Iraq. Optom. Vis. Sci. 81: 586–590 [DOI] [PubMed] [Google Scholar]
- 141. Grogan TM, et al. 1987. Cutaneous myiasis. Immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am. J. Dermatopathol. 9: 232–239 [PubMed] [Google Scholar]
- 142. Guimarães JH, Coimbra CEA., Jr 1982. Miíase humana por Alouattamyia baeri (Shannon & Greene) (Diptera, Cuterebridae). Comunicação de dois casos na região norte do Brasil. Rev. Bras. Zool. 1: 35–39 [Google Scholar]
- 143. Guimaraes JH, Papavero NA. 1966. A tentative annotated bibliography of Dermatobia hominis. Arq. Zool. 14: 223–294 [Google Scholar]
- 144. Gunther S. 1971. Clinical and epidemiological aspects of dermal tumbu-fly myiasis in Equatorial Africa. Br. J. Dermatol. 85: 226–231 [DOI] [PubMed] [Google Scholar]
- 145. Gupta PJ. 2006. Myiasis: a perianal wound infection caused by fly larvae in gangrenous hemorrhoid. Tech. Coloproctol. 10: 254–255 [PubMed] [Google Scholar]
- 146. Gupta SC, Kumar S, Srivastava A. 1983. Urethral myiasis. Trop. Geogr. Med. 35: 73–74 [PubMed] [Google Scholar]
- 147. Gursel M, Aldemir OS, Ozgur Z, Ataoglu T. 2002. A rare case of gingival myiasis caused by Diptera (Calliphoridae). J. Clin. Periodontol. 29: 777–780 [DOI] [PubMed] [Google Scholar]
- 148. Guse ST, Tieszen ME. 1997. Cutaneous myiasis from Dermatobia hominis. Wilderness Environ. Med. 8: 156–160 [DOI] [PubMed] [Google Scholar]
- 149. Guven E, Kar S, Dogan N, Karaer Z. 2008. Urogenital myiasis caused by Psychoda albipennis in a woman. Turkiye Parazitol. Derg. 32: 174–176 (In Turkish) [PubMed] [Google Scholar]
- 150. Hakeem MJ, Bhattacharyya DN. 2009. Exotic human myiasis. Travel Med. Infect. Dis. 7: 198–202 [DOI] [PubMed] [Google Scholar]
- 151. Hakimi R, Yazdi I. 2002. Oral mucosa myiasis caused by Oestrus ovis. Arch. Iran. Med. 5: 194–196 [Google Scholar]
- 152. Hall MJ. 1995. Trapping the flies that cause myiasis: their responses to host-stimuli. Ann. Trop. Med. Parasitol. 89: 333–357 [DOI] [PubMed] [Google Scholar]
- 153. Hall MJ, Farkas R, Kelemen F, Hosier MJ, el-Khoga JM. 1995. Orientation of agents of wound myiasis to hosts and artificial stimuli in Hungary. Med. Vet. Entomol. 9: 77–84 [DOI] [PubMed] [Google Scholar]
- 154. Hall RD, Anderson PC, Clark DP. 1986. A case of human myiasis caused by Phormia regina (Diptera: Calliphoridae) in Missouri, USA. J. Med. Entomol. 23: 578–579 [DOI] [PubMed] [Google Scholar]
- 155. Hatten K, Gulleth Y, Meyer T, Eisenman DJ. 2010. Myiasis of the external and middle ear. Ann. Otol. Rhinol. Laryngol. 119: 436–438 [DOI] [PubMed] [Google Scholar]
- 156. Helm TN, Augenblick K, Gall W. 2010. Furuncular myiasis: a case report. Cutis 86: 85–86 [PubMed] [Google Scholar]
- 157. Hira PR, et al. 2004. Myiasis in Kuwait: nosocomial infections caused by Lucilia sericata and Megaselia scalaris. Am. J. Trop. Med. Hyg. 70: 386–389 [PubMed] [Google Scholar]
- 158. Hira PR, Hajj B, al-Ali F, Hall MJ. 1993. Ophthalmomyiasis in Kuwait: first report of infections due to the larvae of Oestrus ovis before and after the Gulf conflict. J. Trop. Med. Hyg. 96: 241–244 [PubMed] [Google Scholar]
- 159. Hira PR, et al. 1997. Human myiasis in Kuwait due to Oestrus ovis, Psychoda species and Megaselia species. Med. Princ. Pract. 6: 129–136 [Google Scholar]
- 160. Hochedez P, Caumes E. 2008. Common skin infections in travelers. J. Travel Med. 15: 252–262 [DOI] [PubMed] [Google Scholar]
- 161. Hope FW. 1840. On insects and their larvae occasionally found in the human body. Trans. R. Entomol. Soc. Lond. 1840: 256–271 [Google Scholar]
- 162. Hubler WR, Rudolph AH, Doughert EF. 1974. Dermal myiasis. Arch. Dermatol. 110: 109–110 [PubMed] [Google Scholar]
- 163. Hunter JM. 1990. Bot-fly maggot infestation in Latin America. Geogr. Rev. 80: 382–389 [Google Scholar]
- 164. Huntington TE, Voict DW, Higley LG. 2008. Not the usual suspects: human wound myiasis by phorids. J. Med. Entomol. 45: 157–159 [DOI] [PubMed] [Google Scholar]
- 165. Husain A, Husain S, Malaviya GN, Bahadur RR. 1993. Myiasis in leprosy. Acta Leprol. 8: 137–141 [PubMed] [Google Scholar]
- 166. Husain S, Malaviya GN, Girdhar A, Sreevatsa , Girdhar BK. 1991. Nasal myiasis in leprosy. Lepr. Rev. 62: 389–394 [DOI] [PubMed] [Google Scholar]
- 167. Huynh N, Dolan B, Lee S, Whitcher JP, Stanley J. 2005. Management of phaeniciatic ophthalmomyiasis externa. Br. J. Ophthalmol. 89: 1377–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ibrahim SF, Pryor J, Merritt RW, Scott G, Beck LA. 2008. Cutaneous myiasis arising in an eccrine adnexal neoplasm. Dermatol. Online J. 14: 6. [PubMed] [Google Scholar]
- 169. Jacobson JA, Kolts RL, Conti M, Burke JP. 1980. Hospital-aquired myiasis. Infect. Control Hosp. Epidemiol. 1: 319–320 [DOI] [PubMed] [Google Scholar]
- 170. Jain A, Desai RU, Ehrlich J. 2007. Fulminant orbital myiasis in the developed world. Br. J. Ophthalmol. 91: 1565–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Jakobs EM, Adelberg DA, Lewis JM, Trpis M, Green WR. 1997. Ophthalmomyiasis interna posterior. Report of a case with optic atrophy. Retina 17: 310–314 [DOI] [PubMed] [Google Scholar]
- 172. James MT. 1947. The flies that cause myiasis in man, p 1–175 U.S. Department of Agriculture miscellaneous publication no. 631. USDA, Washington, DC [Google Scholar]
- 173. Jelinek T, Nothdurft HD, Rieder N, Loscher T. 1995. Cutaneous myiasis: review of 13 cases in travelers returning from tropical countries. Int. J. Dermatol 34: 624–626 [DOI] [PubMed] [Google Scholar]
- 174. Jenzeri S, et al. 2008. External ophthalmomyiasis manifesting with keratouveitis. Int. Ophthalmol. 29:533–535 [DOI] [PubMed] [Google Scholar]
- 175. Josephson RL, Krajden S. 1993. An unusual nosocomial infection: nasotracheal myiasis. J. Otolaryngol. 22: 46–47 [PubMed] [Google Scholar]
- 176. Kahn DG. 1999. Myiasis secondary to Dermatobia hominis (human botfly) presenting as a long-standing breast mass. Arch. Pathol. Lab. Med. 123: 829–831 [DOI] [PubMed] [Google Scholar]
- 177. Kalelioglu M, et al. 1989. Intracerebral myiasis from Hypoderma bovis larva in a child. Case report. J. Neurosurg. 71: 929–931 [DOI] [PubMed] [Google Scholar]
- 178. Kamboj M, Mahajan S, Boaz K. 2007. Oral myiasis misinterpreted as salivary gland adenoma. J. Clin. Pathol. 60: 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Karabiber H, Oguzkurt DG, Dogan DG, Aktas M, Selimoglu MA. 2010. An unusual cause of rectal bleeding: intestinal myiasis. J. Pediatr. Gastroenterol. Nutr. 51: 530–531 [DOI] [PubMed] [Google Scholar]
- 180. Karaman E, et al. 2009. Otomyiasis by Wohlfahrtia magnifica. J. Craniofac. Surg. 20: 2123–2124 [DOI] [PubMed] [Google Scholar]
- 181. Kearney MS, Nilssen AC, Lyslo A, Syrdalen P, Dannevig L. 1991. Ophthalmomyiasis caused by the reindeer warble fly larva. J. Clin. Pathol. 44: 276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Kenney M, Eveland LK, Yermakov V, Kassouny DY. 1976. Two cases of enteric myiasis in man—pseudomyiasis and true intestinal myiasis. Am. J. Clin. Pathol. 66: 786–791 [DOI] [PubMed] [Google Scholar]
- 183. Khoumiri R, et al. 2008. Ophthalmomyiasis interna: two case studies. J. Fr. Ophtalmol. 31: 299–302. (In French.) [DOI] [PubMed] [Google Scholar]
- 184. Kim JS, et al. 2009. A nasal myiasis in a 76-year-old female in Korea. Korean J. Parasitol. 47: 405–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kokcam I, Saki CE. 2005. A case of cutaneous myiasis caused by Wohlfahrtia magnifica. J. Dermatol. 32: 459–463 [DOI] [PubMed] [Google Scholar]
- 186. Komori K, Hara K, Smith KGV, Oda T, Karamine D. 1978. Case of lung myiasis caused by the larvae of Megaselia spiracularis Schimitz (Diptera: Phoridae). Trans. R. Soc. Trop. Med. Hyg. 72: 467–470 [DOI] [PubMed] [Google Scholar]
- 187. Koss T, Lanatra N, Stiller MJ, Grossman ME. 2004. An unusual combination: lipedema with myiasis. J. Am. Acad. Dermatol. 50: 969–972 [DOI] [PubMed] [Google Scholar]
- 188. Kozminska-Kubarska A. 1981. Cordylobia anthropophaga infestation. Int. J. Dermatol. 20: 495–496 [DOI] [PubMed] [Google Scholar]
- 189. Krajewski A, Allen B, Hoss D, Patel C, Chandawarkar RY. 2009. Cutaneous myiasis. J. Plast. Reconstr. Aesthet. Surg. 62: e383–e386 [DOI] [PubMed] [Google Scholar]
- 190. Kudur MH, Pooja M, Nayak S. 2010. Unusual presentation of cutaneous myiasis. Indian J. Dermatol. Venereol. Leprol. 76: 712–714 [DOI] [PubMed] [Google Scholar]
- 191. Kun M, Kreiter A, Semenas L. 1998. Gastrointestinal human myiasis for Eristalis tenax. Rev. Saude Publica 32: 367–369 [DOI] [PubMed] [Google Scholar]
- 192. Kuria SK, et al. 2010. New fly species causing human myiasis identified in Eastern Cape, South Africa. S. Afr. Med. J. 100: 580–581 [DOI] [PubMed] [Google Scholar]
- 193. Kuruvilla G, Albert RRA, Job A, Ranjith VT, Selvakumar P. 2006. Pneumocephalus: a rare complication of nasal myiasis. Am. J. Otolaryngol. 27: 133–135 [DOI] [PubMed] [Google Scholar]
- 194. Kwong A, Yiu WK, Chow LWC, Wong S. 2007. Chrysomya bezziana: a rare infestation of the breast. Breast J. 13: 297–301 [DOI] [PubMed] [Google Scholar]
- 195. Lagace-Wiens PRS, et al. 2008. Human ophthalmomyiasis interna caused by Hyoiderma tarandi, northern Canada. Emerg. Infect. Dis. 14: 64–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lane RP, Lowell CR, Griffiths WA, Sonnex TS. 1987. Human cutaneous myiasis—a review and report of three cases due to Dermatobia hominis. Clin. Exp. Dermatol. 12: 40–45 [DOI] [PubMed] [Google Scholar]
- 197. Lata J, Kapila BK, Aggarwal P. 1996. Oral myiasis—a case report. Int. J. Oral Maxillofac. Surg. 25: 455–456 [DOI] [PubMed] [Google Scholar]
- 198. Latorre M, Ullate JV, Sanchez J, Calvo F, Cisterna R. 1993. A case of myiasis due to Dermatobia hominis. Eur. J. Clin. Microbiol. Infect. Dis. 12: 968–969 [DOI] [PubMed] [Google Scholar]
- 199. Leclercq M, Laurent P. 1973. Rectal myiasis with larvae of Fannia scalaris FAB (Diptera muscidae) in Belgium. Rev. Med. Liege 28: 27–28 (In French) [PubMed] [Google Scholar]
- 200. Lee DJ. 1968. Human myiasis in Australia. Med. J. Aust. 1: 170–173 [DOI] [PubMed] [Google Scholar]
- 201. Lee HL, Chandrawathani P, Wong WY, Tharam S, Lim WY. 1995. A case of human enteric myiasis due to larvae of Hermetia illucens (family: Stratiomyiadae): first report in Malaysia. Malays. J. Pathol. 17: 109–111 [PubMed] [Google Scholar]
- 202. Lee HL, Krishnasamy M, Jeffery J. 2005. A case of human nasopharyngeal myiasis caused by Chrysomya bezziana Villeneuve, 1914 (Diptera: Calliphoridae) in Malaysia. Trop. Biomed. 22: 87–88 [PubMed] [Google Scholar]
- 203. Lee HL, Yong YK. 1991. Human aural myiasis. Southeast Asian J. Trop. Med. Public Health 22: 274–275 [PubMed] [Google Scholar]
- 204. Lima LH, et al. 2010. Ophthalmomyiasis with a singular subretinal track. Am. J. Ophthalmol. 150: 731.e1–736.e1. [DOI] [PubMed] [Google Scholar]
- 205. Lima Junior SM, Asprino L, Prado AP, Moreira RW, de Moraes M. 2010. Oral myiasis caused by Cochliomyia hominivorax treated nonsurgically with nitrofurazone: report of 2 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109: e70–e73 [DOI] [PubMed] [Google Scholar]
- 206. Lindsay R, Stancil J, Ray JM. 2010. Myiasis of facial wounds by Cochliomyia hominivorax sustained in a natural disaster in Haiti. Otolaryngol. Head Neck Surg. 143: 595–596 [DOI] [PubMed] [Google Scholar]
- 207. Logar J, Marinic-Fiser N. 2008. Cutaneous myiasis caused by Hypoderma lineatum. Wien. Klin. Wochenschr. 120: 619–621 [DOI] [PubMed] [Google Scholar]
- 208. Loong PTL, Lui H, Buck HW. 1992. Cutaneous myiasis: a simple and effective technique for extraction of Dermatobia hominis larvae. Int. J. Dermatol. 31: 657–659 [DOI] [PubMed] [Google Scholar]
- 209. Lopes-Costa PV, dos Santos AR, Pereira-Filho JD, da Silva BB. 2008. Myiasis in the uterine cavity of an elderly woman with a complete uterine prolapse. Trans. R. Soc. Trop. Med. Hyg. 102: 1058–1060 [DOI] [PubMed] [Google Scholar]
- 210. Lucchina LC, Wilson ME, Drake LA. 1997. Dermatology and the recently returned traveler: infectious diseases with dermatologic manifestations. Int. J. Dermatol. 36: 167–181 [DOI] [PubMed] [Google Scholar]
- 211. Lucientes J, et al. 1997. One case of nasal human myiasis caused by third stage instar larvae of Oestrus ovis. Am. J. Trop. Med. Hyg. 56: 608–609 [DOI] [PubMed] [Google Scholar]
- 212. Lukin LG. 1989. Human cutaneous myiasis in Brisbane: a prospective study. Med. J. Aust. 150: 237–240 [DOI] [PubMed] [Google Scholar]
- 213. Madana J, et al. 2009. Hypohidrotic ectodermal dysplasia with atrophic rhinitis and nasal myiasis. Int. J. Pediatr. Otorhinolaryngol. 73: 1467–1469 [DOI] [PubMed] [Google Scholar]
- 214. Magliulo G, Gagliardi M, D'Amico R. 2000. Human aural myiasis. Otolaryngol. Head Neck Surg. 122: 777. [DOI] [PubMed] [Google Scholar]
- 215. Magnarelli LA, Andreadis TG. 1981. Human cases of furuncular, traumatic, and nasal myiasis in Connecticut. Am. J. Trop. Med. Hyg. 30: 894–896 [DOI] [PubMed] [Google Scholar]
- 216. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J. Emerg. Med., in press [DOI] [PubMed] [Google Scholar]
- 217. Malaviya GN. 2005. Myiasis in leprosy. Int. J. Lepr. Other Mycobact. Dis. 73: 277–279 [PubMed] [Google Scholar]
- 218. Marco de Lucas E, et al. 2008. Unusual MRI findings in a patient with history of frontal fracture and skin infestation by fly larvae, as a possible sign of intracerebral myiasis. Clin. Neurol. Neurosurg. 110: 725–728 [DOI] [PubMed] [Google Scholar]
- 219. Mariwalla K, Langhan M, Welch KA, Kaplan DH. 2007. Cutaneous myiasis associated with scalp psoriasis. J. Am. Acad. Dermatol. 57: S51–S52 [DOI] [PubMed] [Google Scholar]
- 220. Marquez AT, Mattos MD, Nascimento SB. 2007. Myiasis associated with some socioeconomic factors in five urban areas of the State of Rio de Janeiro. Rev. Soc. Bras. Med. Trop. 40: 175–180 [DOI] [PubMed] [Google Scholar]
- 221. Martidis A, Greenberg PB, Rogers AH, Velazquez-Estades LJ, Baumal CR. 2002. Multifocal electroretinography response after laser photocoagulation of a subretinal nematode. Am. J. Ophthalmol. 133: 417–419 [DOI] [PubMed] [Google Scholar]
- 222. Mason GI. 1981. Bilateral ophthalmomyiasis interna. Am. J. Ophthalmol. 91: 65–70 [DOI] [PubMed] [Google Scholar]
- 223. Masoodi M, Hosseini K. 2003. The respiratory and allergic manifestations of human myiasis caused by larvae of the sheep bot fly (Oestrus ovis): a report of 33 pharyngeal cases from southern Iran. Ann. Trop. Med. Parasitol. 97: 75–81 [DOI] [PubMed] [Google Scholar]
- 224. Maturo S, Michaelson PG, Horlbeck D, Brennan J. 2007. Auricular myiasis. Otolaryngol. Head Neck Surg. 136: 668–669 [DOI] [PubMed] [Google Scholar]
- 225. Mazayad SA, Rifaat MM. 2005. Megaselia scalaris causing human intestinal myiasis in Egypt. J. Egypt. Soc. Parasitol. 35: 331–340 [PubMed] [Google Scholar]
- 226. McAlpine JF. 1981. Morphology and terminology: adults, p 9–63, monograph 27. In McAlpine JF, et al. (ed), Manual of neartic diptera, monograph 32. Agriculture Canada Research Branch, Ottawa, Ontario, Canada [Google Scholar]
- 227. McAlpine JF. 1989. Phylogeny and classification of the Muscopmorpha, p 1397–1502 In McAlpine JF, Wood DM. (ed), Manual of neartic diptera, monograph 32. Agriculture Canada Research Branch, Ottawa, Ontario, Canada [Google Scholar]
- 228. McDonald HR, Kazacos KR, Schatz H, Johnson RN. 1994. Two cases of intraocular infection with Alaria mesocercaria (Trematoda). Am. J. Ophthalmol. 117: 447–455 [DOI] [PubMed] [Google Scholar]
- 229. McGraw TA, Turiansky GW. 2008. Cutaneous myiasis. J. Am. Acad. Dermatol. 58: 907–926 [DOI] [PubMed] [Google Scholar]
- 230. Medownick M, Lazarus M, Finkelstein E, Weiner JM. 1985. Human external ophthalmomyiasis caused by the horse bot fly larva (Gasterophilus spp.). Aust. N. Z. J. Ophthalmol. 13: 387–390 [DOI] [PubMed] [Google Scholar]
- 231. Meinking TL, Burkhart CN, Burkhart CG. 2003. Changing paradigms in parasitic infections: common dermatological helminthic infections and cutaneous myiasis. Clin. Dermatol. 21: 407–416 [DOI] [PubMed] [Google Scholar]
- 232. Menghi CI, Gatta CL, Oliva A. 2010. Otomyiasis by Cochliomyia hominivorax in two children from the outskirts of Buenos Aires, Argentina. Rev. Argent. Microbiol. 42: 176–178 (In Spanish) [PubMed] [Google Scholar]
- 233. Merino FJ, Campos A, Nebreda T, Canovas C, Cuezva F. 2000. Cutaneous myiasis by Sarcophaga sp. Enferm. Infecc. Microbiol. Clin. 18: 19–21(In Spanish) [PubMed] [Google Scholar]
- 234. Messahel A, Sen P, Wilson A, Patel M. 2010. An unusual case of myiasis. J. Infect. Public Health 3: 43–45 [DOI] [PubMed] [Google Scholar]
- 235. Mielke U, Schlote A. 1980. Hospitalerkrankung durch Fliegenmaden. Z. Arztl. Fortbild. (Jena) 74: 556–558 [PubMed] [Google Scholar]
- 236. Miller KB, Hribar LJ, Sanders LJ. 1990. Human myiasis caused by Phormia regina in Pennsylvania. J. Am. Podiatr. Med. Assoc. 80: 600–602 [DOI] [PubMed] [Google Scholar]
- 237. Minar J, Valkoun A. 1998. Myiasis in a skin tumor. Epidemiol. Mikrobiol. Imunol. 47: 32–34 (In Czech) [PubMed] [Google Scholar]
- 238. Mohrenschlager M, et al. 2007. Scanning electron microscopy of Dermatobia hominis reveals cutaneous anchoring features. J. Am. Acad. Dermatol. 57: 716–718 [DOI] [PubMed] [Google Scholar]
- 239. Morris AJ, Berry MA. 1996. Intestinal myiasis with Phaenicia cuprina. Am. J. Gastroenterol. 91: 1290. [PubMed] [Google Scholar]
- 240. Morris B. 1987. First reported case of human aural myiasis caused by the flesh fly Parasarcophaga crassipalpis (Diptera: Sarcophagidae). J. Parasitol. 73:1068–1069 [PubMed] [Google Scholar]
- 241. Moutaj R, et al. 2000. A case of gastric myiasis due to Eristalis tenax larvae (Linneus, 1758) (Insecta: Diptera). Parasite 7: 56–57(In French) [PubMed] [Google Scholar]
- 242. Mumcuoglu I, Akarsu GA, Balaban N, Keles I. 2005. Eristalis tenax as a cause of urinary myiasis. Scand. J. Infect. Dis. 37: 942–943 [DOI] [PubMed] [Google Scholar]
- 243. Murthy SC, Udagari MM. 2003. Myiasis complicating herpes zoster in an immunocompromised patient. Indian J. Dermatol. Venereol. Leprol. 69: 194. [PubMed] [Google Scholar]
- 244. Navajas A, et al. 1998. Hypereosinophilia due to myiasis. Acta Haematol. 99: 27–30 [DOI] [PubMed] [Google Scholar]
- 245. Neira P, Munoz N, Cantero D. 2002. Auricular myiasis caused by Cochliomyia hominivorax (Diptera: Calliphoridae). Rev. Med. Chile 130: 907–909 [PubMed] [Google Scholar]
- 246. Ng KH, et al. 2003. A case of oral myiasis due to Chrysomya bezziana. Hong Kong Med. J. 9: 454–456 [PubMed] [Google Scholar]
- 247. Nigam Y, Bexfield A, Thomas S, Ratcliffe NA. 2006. Maggot therapy: the science and implication for CAM. Part I—history and bacterial resistance. Evid. Based Complement. Alternat. Med. 3: 223–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Nigam Y, Bexfield A, Thomas S, Ratcliffe NA. 2006. Maggot therapy: the science and implication for CAM. Part II—maggots combat infection. Evid. Based Complement. Alternat. Med. 3: 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. North DE, et al. 1987. Intestinal myiasis in a baby attending a public health clinic. Nurse Pract. 12: 60–63 [PubMed] [Google Scholar]
- 250. Noutsis C, Millikan LE. 1994. Myiasis. Dermatol. Clin. 12: 729–736 [PubMed] [Google Scholar]
- 251. Ockenhouse CF, et al. 1990. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch. Dermatol. 126: 199–202 [PubMed] [Google Scholar]
- 252. Ogbalu OK, Achufusi TGO, Adibe C. 2006. Incidence of multiple myiases in breasts of rural women and oral infection in infants from the human warble fly larvae in the humid Tropic-Niger Delta. Int. J. Dermatol. 45: 1069–1070 [DOI] [PubMed] [Google Scholar]
- 253. Ollivier FJ, et al. 2006. Pars plana vitrectomy for the treatment of ophthalmomyiasis interna posterior in a dog. Vet. Ophthalmol. 9: 259–264 [DOI] [PubMed] [Google Scholar]
- 254. Olumide YM. 1994. Cutaneous myiasis: a simple and effective technique for extraction of Dermatobia hominis larvae. Int. J. Dermatol. 33: 148–149 [DOI] [PubMed] [Google Scholar]
- 255. O'Rourke FJ. 1954. Furuncular myiasis due to Wohlfahrtia vigil (Walker). CMAJ 71: 146–149 [PMC free article] [PubMed] [Google Scholar]
- 256. Osorio J, et al. 2006. Role of ivermectin in the treatment of severe orbital myiasis due to Cochliomyia hominivorax. Clin. Infect. Dis. 43: e57–e59 [DOI] [PubMed] [Google Scholar]
- 257. Otranto D, Cantacessi C, Santantonio M, Rizzo G. 2009. Oestrus ovis causing human ocular myiasis: from countryside to town centre. Clin. Exp. Ophthalmol. 37: 327–328 [DOI] [PubMed] [Google Scholar]
- 258. Otranto D, Colwell DD. 2008. Biodiversity and extinction versus control of oestrid causing myiasis in Mediterranean area. Parasite 15: 257–260 [DOI] [PubMed] [Google Scholar]
- 259. Otranto D, Colwell DD, Traversa D, Stevens JR. 2003. Species identification of Hypoderma affecting domestic and wild ruminants by morphological and molecular characterization. Med. Vet. Entomol. 17: 316–325 [DOI] [PubMed] [Google Scholar]
- 260. Otranto D, Traversa D, Tarsitano E, Stevens J. 2003. Molecular differentiation of Hypoderma bovis and Hypoderma lineatum (Diptera, Oestridae) by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). Vet. Parasitol. 112: 197–201 [DOI] [PubMed] [Google Scholar]
- 261. Pampiglione S, Schiavon S, Candiani G, Fioravanti ML. 1991. Clinical and parasitological observations on a case of disseminated furuncular myiasis caused by Cordylobia rodhaini in a man in Ethiopia. Parassitologia 33: 159–167 (In Italian) [PubMed] [Google Scholar]
- 262. Panadero R, et al. 2000. Influence of internal and environmental factors on the distribution and occurrence of Hypoderma (Diptera: Oestridae) in cattle in Galicia (Northwest of Spain). J. Med. Entomol. 37: 27–28 [DOI] [PubMed] [Google Scholar]
- 263. Panadero R, et al. 2007. Evaluation of an antigen capture ELISA for the early diagnosis of Hypoderma lineatum in cattle under field conditions. Vet. Parasitol. 147: 297–302 [DOI] [PubMed] [Google Scholar]
- 264. Panu F, Cabras G, Contini C, Onnis D. 2000. Human auricolar myiasis caused by Wohlfartia magnifica (Schiner) (Diptera: Sarcophagidae): first case found in Sardinia. J. Laryngol. Otol. 114: 450–452 [DOI] [PubMed] [Google Scholar]
- 265. Paris LA, Viscarret M, Uban C, Vargas J, Rodriguez-Morales AJ. 2008. Pin-site myiasis: a rare complication of a treated open fracture of tibia. Surg. Infect. (Larchmt.) 9: 403–406 [DOI] [PubMed] [Google Scholar]
- 266. Park P, Lodhia KR, Eden SV, Lewandrowski KU, McGillicuddy JE. 2005. Pin-site myiasis: a rare complication of halo orthosis. Spinal Cord 43: 684–686 [DOI] [PubMed] [Google Scholar]
- 267. Passos MR, et al. 2008. Penile myiasis as a differential diagnosis for genital ulcer: a case report. Braz. J. Infect. Dis. 12: 155–157 [DOI] [PubMed] [Google Scholar]
- 268. Passos MR, et al. 2002. Vulvar myiasis during pregnancy. Infect. Dis. Obstet. Gynecol. 10: 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Patton WS. 1922. Notes on myiasis producing Diptera of man and animals. Bull. Entomol. Res. 12: 239–261 [Google Scholar]
- 270. Pepper WC, Benaragama SK, Kalsi JS, Karim O. 2008. Cutaneous myiasis of Cordylobia anthropophaga. Urology 72: 65. [DOI] [PubMed] [Google Scholar]
- 271. Pereyra-Rodriguez JJ, Bernabeu-Wittel J, Conejo-Mir MD, Ruiz-Perez de Pipaon M, Conejo-Mir J. 2010. Treatment of cutaneous myiasis associated with scalp psoriasis in a 13-year-old girl with oral ivermectin. J. Am. Acad. Dermatol. 63: 908–909 [DOI] [PubMed] [Google Scholar]
- 272. Perez-Eid C, Mouffok N. 1999. Human urinary myiasis due to Fannia canicularis (Diptera, Muscidae) larvae in Algeria. Presse Med. 28: 580–581 [PubMed] [Google Scholar]
- 273. Philip J, Matthews R, Scipio JE. 2007. Larvae in the mouth. Br. Dent. J. 203: 174–175 [DOI] [PubMed] [Google Scholar]
- 274. Plotinsky RN, Talbot EA, Davis H. 2007. Cuterebra cutaneous myiasis, New Hampshire, 2004. Am. J. Trop. Med. Hyg. 76: 596–597 [PubMed] [Google Scholar]
- 275. Pogany P, Szucs E, Lichtenberger G, Vass L. 2008. Diagnosis of myiasis by fine needle aspiration cytology: a case report. Acta Cytol. 52: 228–230 [DOI] [PubMed] [Google Scholar]
- 276. Pouillaude JM, Dupont J, Gilly R, Lapras C. 1980. Intra-cerebral myiasis in a child. Pediatr. Radiol. 10: 121–123 [DOI] [PubMed] [Google Scholar]
- 277. Price KM, Murchison AP, Bernardino CR, Kang SJ, Grossniklaus HE. 2007. Ophthalmomyiasis externa caused by Dermatobia hominis in Florida. Br. J. Ophthalmol. 91: 6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Puente S, et al. 2010. First diagnosis of an imported human myiasis caused by Hypoderma sinense (Diptera: Oestridae), detected in a European traveler returning from India. J. Travel Med. 17: 419–423 [DOI] [PubMed] [Google Scholar]
- 279. Quintanilla-Cedillo MR, Leon-Urena H, Contreras-Ruiz J, Arenas R. 2005. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int. J. Dermatol. 44: 34–37 [DOI] [PubMed] [Google Scholar]
- 280. Ramsy JM. 1952. A case of intestinal myiasis by Musca domestica in a laboratory assistant. Ohio J. Sci. 52: 62 [Google Scholar]
- 281. Rawlins SC, Barnett DB. 1983. Internal human myiasis. West Indian Med. J. 32: 184–186 [PubMed] [Google Scholar]
- 282. Reingold WJ, et al. 1984. Oestrus ovis ophthalmomyiasis externa. Am. J. Ophthalmol. 97: 7–10 [DOI] [PubMed] [Google Scholar]
- 283. Ribeiro MC, et al. Oral myiasis in an elderly patient. Gerodontology, in press [DOI] [PubMed] [Google Scholar]
- 284. Rice PL, Gleason N. 1972. Two cases of myiasis in the U.S. by the African tumbu fly Cordylobia anthropophaga (Diptera, Calliphoridae). Am. J. Trop. Med. Hyg. 21: 62–65 [DOI] [PubMed] [Google Scholar]
- 285. Richards KA, Brieva J. 2000. Myiasis in a pregnant woman and an effective, sterile method of surgical extraction. Dermatol. Surg. 26: 955–957 [DOI] [PubMed] [Google Scholar]
- 286. Richter J, Schmitt M, Muller-Stover I, Gobels K, Haussinger D. 2008. Sonographic detection of subcutaneous fly larvae in human myiasis. J. Clin. Ultrasound 36: 169–173 [DOI] [PubMed] [Google Scholar]
- 287. Rodriguez MEL, Aoki L, Nicoletti AGB, Matayoshi S, Fernandes JBVD. 2003. Ivermectina no tratamento de miíase orbitária: relato de caso. Arq. Bras. Oftalmol. 66: 519–521 [Google Scholar]
- 288. Roelfstra L, et al. 2009. Protein expression profile of Gasterophilus intestinalis larvae causing horse gastric myiasis and characterization of horse immune reaction. Parasit. Vectors 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Rohela M, Jamaiah I, Amir L, Nissapatorn V. 2006. A case of auricular myiasis in Malaysia. Southeast Asian J. Trop. Med. Public Health 37(Suppl. 3): 91–94 [PubMed] [Google Scholar]
- 290. Romano C, Albanese G, Gianni C. 2004. Emerging imported parasitoses in Italy. Eur. J. Dermatol. 14: 58–60 [PubMed] [Google Scholar]
- 291. Romero-Cabello R, Sánchez-Vega JT, Tay-Zavala J, Ruiz-Sanchez D, Calderon-Romero L. 2004. Miasis asociada a síndrome de complejo vascular periférico. Parasitol. Latinoam. 59: 159–161 [Google Scholar]
- 292. Rossi MA, Zucoloto S. 1973. Fatal cerebral myiasis caused by tropical warble fly, Dermatobia hominis. Am. J. Trop. Med. Hyg. 22: 267–269 [DOI] [PubMed] [Google Scholar]
- 293. Rossi-Schneider T, Cherubini K, Yurgel LS, Salum F, Figueiredo MA. 2007. Oral myiasis: a case report. J. Oral Sci. 49: 85–88 [DOI] [PubMed] [Google Scholar]
- 294. Royce LA, Rossignol PA, Kubitz ML, Burton FR. 1999. Recovery of a second instar Gasterophilus larva in a human infant: a case report. Am. J. Trop. Med. Hyg. 60: 403–404 [DOI] [PubMed] [Google Scholar]
- 295. Saleh MS, el Sibae MM. 1993. Urino-genital myiasis due to Piophila casei. J. Egypt. Soc. Parasitol. 23: 737–739 [PubMed] [Google Scholar]
- 296. Sancho E. 1988. Dermatobia, the neotropical warble fly. Parasitol. Today 4: 242–246 [DOI] [PubMed] [Google Scholar]
- 297. Saraiva FP, Fernandes JBVD, Tomikawa VO, Costa PG, Matayoshi S. 2005. Oftalmomiiase como causa de lesaão canalicular. J. Pediatr. 81: 85–87 [PubMed] [Google Scholar]
- 298. Saraiva VS, Amaro MH, Belfort R, Jr, Burnier MN., Jr 2006. A case of anterior internal ophthalmomyiasis: case report. Arq. Bras. Oftalmol. 69: 741–743 [DOI] [PubMed] [Google Scholar]
- 299. Schechter E, Lazar J, Nix ME, Mallon WK, Moore CL. 2008. Identification of subcutaneous myiasis using bedside emergency physician performed ultrasound. J. Emerg. Med. 40: e1–e3 [DOI] [PubMed] [Google Scholar]
- 300. Scholten T, Chrom VH. 1979. Myiasis due to Cuterebra in humans. CMAJ 120: 1392–1393 [PMC free article] [PubMed] [Google Scholar]
- 301. Seaquist ER, Henry TR, Cheong E, Theologides A. 1983. Phormia regina myiasis in a malignant wound. Minn. Med. 66: 409–410 [PubMed] [Google Scholar]
- 302. Sehgal R, et al. 2002. Intestinal myiasis due to Musca domestica: a report of two cases. Jpn. J. Infect. Dis. 55: 191–193 [PubMed] [Google Scholar]
- 303. Reference deleted.
- 304. Sesterhenn AM, et al. 2009. Cutaneous manifestation of myiasis in malignant wounds of the head and neck. Eur. J. Dermatol. 19: 64–68 [DOI] [PubMed] [Google Scholar]
- 305. Sharma H, Dayal D, Agrawal SP. 1989. Nasal myiasis: review of 10 years experience. J. Laryngol. Otol. 103: 489–491 [DOI] [PubMed] [Google Scholar]
- 306. Sharma J, Mamatha GP, Acharya R. 2008. Primary oral myiasis: a case report. Med. Oral Patol. Oral Cir. Bucal. 13: E714–E716 [PubMed] [Google Scholar]
- 307. Sharma P, Pai HS, Pai GS. 2008. Furuncular myiasis mimicking pyoderma. Indian J. Dermatol. Venereol. Leprol. 74: 679–681 [DOI] [PubMed] [Google Scholar]
- 308. Sherman RA. 2000. Wound myiasis in urban and suburban United States. Arch. Intern. Med. 160: 2004–2014 [DOI] [PubMed] [Google Scholar]
- 309. Shinohara EH. 2003. Treatment of oral myiasis with ivermectin. Br. J. Oral Maxillofac. Surg. 41: 421. [DOI] [PubMed] [Google Scholar]
- 310. Shinohara EH, Martini MZ, de Oliveira Neto HG, Takahashi A. 2004. Oral myiasis treated with ivermectin: case report. Braz. Dent. J. 15: 79–81 [DOI] [PubMed] [Google Scholar]
- 311. Shivekar S, et al. 2008. Intestinal myiasis caused by Muscina stabulans. Indian J. Med. Microbiol. 26: 83–85 [DOI] [PubMed] [Google Scholar]
- 312. Shorter N, Werninghaus K, Mooney D, Graham A. 1997. Furuncular cuterebrid myiasis. J. Pediatr. Surg. 32: 1511–1513 [DOI] [PubMed] [Google Scholar]
- 313. Singh I, Gathwala G, Yadav SP, Wig U, Jakhar KK. 1993. Myiasis in children: the Indian perspective. Int. J. Pediatr. Otorhinolaryngol. 25: 127–131 [DOI] [PubMed] [Google Scholar]
- 314. Singh NB, Singh TK, Singh YI, Razaque MA. 1988. Intestinal myiasis caused by Megaselia scalaris (Diptera: Phoridae): a case report. J. Commun. Dis. 20: 163. [PubMed] [Google Scholar]
- 315. Singh TS, Rana D. 1989. Urogenital myiasis caused by Megaselia scalaris (Diptera: Phoridae): a case report. J. Med. Entomol. 26: 228–229 [DOI] [PubMed] [Google Scholar]
- 316. Skibsted R, Larsen M, Gomme G. 1995. Nasal myiasis. Ugeskr. Laeger 157: 2158–2160 (In Danish) [PubMed] [Google Scholar]
- 317. Slusher MM, Holland WD, Weaver RG, Tyler ME. 1979. Ophthalmomyiasis interna posterior. Subretinal tracks and intraocular larvae. Arch. Ophthalmol. 97: 885–887 [DOI] [PubMed] [Google Scholar]
- 318. Smith DR, Clevenger RR. 1986. Nosocomial nasal myiasis. Arch. Pathol. Lab. Med. 110: 439–440 [PubMed] [Google Scholar]
- 319. Smith FD, Shaffer KL, Gasseling PA, McFadden HW. 1981. Furuncular myiasis caused by Wohlfahrtia vigil (Walker). First case reported in Nebraska. Arch. Dermatol. 117: 119–120 [DOI] [PubMed] [Google Scholar]
- 320. Soliman RS, Phillips G, Spence G. 2003. ‘I know an old lady who swallowed a fly’: a case of (hospital-acquired) human intestinal myiasis. J. Hosp. Infect. 53: 157–158 [DOI] [PubMed] [Google Scholar]
- 321. Soni NK. 2000. Endoscopy in nasal myiasis. Trop. Doct. 30: 225–227 [DOI] [PubMed] [Google Scholar]
- 322. Sood VP, Kakar PK, Wattal BL. 1976. Myiasis in otorhinolaryngology with entomological aspects. J. Laryngol. Otol. 90: 393–399 [DOI] [PubMed] [Google Scholar]
- 323. Sotiraki S, Farkas R, Hall MJ. 2010. Fleshflies in the flesh: epidemiology, population genetics and control of outbreaks of traumatic myiasis in the Mediterranean Basin. Vet. Parasitol. 174: 12–18 [DOI] [PubMed] [Google Scholar]
- 324. Sreejith RS, Reddy AK, Ganeshpuri SS, Garg P. 2010. Oestrus ovis ophthalmomyiasis with keratitis. Indian J. Med. Microbiol. 28: 399–402 [DOI] [PubMed] [Google Scholar]
- 325. Starkoff O. 1957. A case of myiasis of the urinary tract caused by Fannia canicularis (L.). Nuovi Ann. Ig. Microbiol. 8: 406–409 (In Italian) [PubMed] [Google Scholar]
- 326. Starr J, Pruett JH, Yunginger JW, Gleich GJ. 2000. Myiasis due to Hypoderma lineatum infection mimicking the hypereosinophilic syndrome. Mayo Clin. Proc. 75: 755–759 [DOI] [PubMed] [Google Scholar]
- 327. Staub HP, Kimmel GC, Sholler LJ, Berglund EB. 1964. Cutaneous myiasis due to Wohlfahrtia vigil. Pediatrics 34: 880–884 [PubMed] [Google Scholar]
- 328. Stetsenko GY, Zlotoff BJ, Padilla RS. 2007. Polarizable material as a clue. Am. J. Dermatopathol. 29: 414-415, 419-420 [DOI] [PubMed] [Google Scholar]
- 329. Sukontason KL, et al. 2005. First report of human myiasis caused by Chrysomya megacephala and Chrysomya rufifacies (Diptera: Calliphoridae) in Thailand, and its implication in forensic entomology. J. Med. Entomol. 42: 702–704 [DOI] [PubMed] [Google Scholar]
- 330. Supple CJ. 1958. Genitourinary myiasis due to Eristalis tenax—report of a case. JAMA 167: 1838–1839 [DOI] [PubMed] [Google Scholar]
- 331. Suzzoni-Blatger J, Villeneuve L, Morassin B, Chevallier J. 2000. A case of external human ophthalmomyiasis by Oestrus ovis in Toulouse (France). J. Fr. Ophtalmol. 23: 1020–1022 [PubMed] [Google Scholar]
- 332. Syrdalen P, Nitter T, Mehl R. 1982. Ophthalmomyiasis interna posterior: report of case caused by the reindeer warble fly larva and review of previous reported cases. Br. J. Ophthalmol. 66: 589–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Syrdalen P, Stenkula S. 1987. Ophthalmomyiasis interna posterior. Graefes Arch. Clin. Exp. Ophthalmol. 225: 103–106 [DOI] [PubMed] [Google Scholar]
- 334. Szczurko C, et al. 1994. Ultrasonography of furuncular cutaneous myiasis: detection of Dermatobia hominis larvae and treatment. Int. J. Dermatol. 33: 282–283 [DOI] [PubMed] [Google Scholar]
- 335. Tamir J, Haik J, Schwartz E. 2003. Mylasis with Lund's fly (Cordylobia rodhaini) in travelers. J. Travel Med. 10: 293–295 [DOI] [PubMed] [Google Scholar]
- 336. Tas E, Jappe U, Beltraminelli H, Bircher A. 2007. Occupational inhalant allergy to the common housefly (Musca domestica). Hautarzt 58: 156–160 (In German) [DOI] [PubMed] [Google Scholar]
- 337. Tee RD, et al. 1985. Occupational allergy to the common house fly (Musca domestica): use of immunologic response to identify atmospheric allergen. J. Allergy Clin. Immunol. 76: 826–831 [DOI] [PubMed] [Google Scholar]
- 338. Terterov S, Taghva A, MacDougall M, Giannotta S. 2010. Posttraumatic human cerebral myiasis. World Neurosurg. 73: 557–559 [DOI] [PubMed] [Google Scholar]
- 339. Thami GP, Baruah MC, Sharmce SC, Behera NK. 1995. Nasal myiasis in leprosy leading to unusual tissue destruction. J. Dermatol. 22: 348–350 [DOI] [PubMed] [Google Scholar]
- 340. Thompson JH, Knutson LV. 1970. Larva of Scenopinus sp (Diptera: Scenopinidae) causing human urogenital myiasis. Mayo Clin. Proc. 45: 597–601 [PubMed] [Google Scholar]
- 341. Tligui H, Bouazzaoui A, Agoumi A. 2007. Human auricular myiasis caused by Wohlfahrtia magnifica (Diptera: Sarcophagidae): about three observations in Morocco. Bull. Soc. Pathol. Exot. 100: 61–64 (In French) [PubMed] [Google Scholar]
- 342. Toomey N. 1922. Hypodermiasis (ox-warble disease). Br. J. Dermatol. 34: 31–42 [Google Scholar]
- 343. Townsend LH, Hall RD, Turner EC. 1978. Human oral myiasis in Virginia caused by Gasterophilus intestinalis (Diptera: Gasterophilidae). Proc. Entomol. Soc. Wash. 80: 129–130 [Google Scholar]
- 344. Trape JF, Vattierbernard G, Trouillet J. 1982. A case of intestinal myiasis caused by larvae of Megaselia scalaris (Dipterra, Phoridae) in the Congo. Bull. Soc. Pathol. Exot. 75: 443–446 [PubMed] [Google Scholar]
- 345. Tsang WS, Lee DL. 2009. Nasal myiasis: the role of endoscopy. Ear Nose Throat J. 88: 1250–1251 [PubMed] [Google Scholar]
- 346. Tu WC, Chen HC, Chen KM, Tang LC, Lai SC. 2007. Intestinal myiasis caused by larvae of Telmatoscopus albipunctatus in a Taiwanese man. J. Clin. Gastroenterol. 41: 400–402 [DOI] [PubMed] [Google Scholar]
- 347. Turk M, et al. 2006. A case of nasomyiasis whose agent was Sarcophaga sp.. Turkiye Parazitol. Derg. 30: 330–332 [PubMed] [Google Scholar]
- 348. Tuygun N, Taylan-Ozkan A, Tanir G, Mumcuoglu KY. 2009. Furuncular myiasis in a child caused by Wohlfahrtia magnifica (Diptera: Sarcophagidae) associated with eosinophilia. Turk. J. Pediatr. 51: 279–281 [PubMed] [Google Scholar]
- 349. Ugwu BT, Nwadiaro PO. 1999. Cordylobia anthropophaga mastitis mimicking breast cancer: case report. East Afr. Med. J. 76: 115–116 [PubMed] [Google Scholar]
- 350. Uzun L, Cinar F, Beder LB, Aslan T, Altintas K. 2004. Radical mastoidectomy cavity myiasis caused by Wohlfahrtia magnifica. J. Laryngol. Otol. 118: 54–56 [DOI] [PubMed] [Google Scholar]
- 351. Varani S, et al. 2007. A case of furuncular myiasis associated with systemic inflammation. Parasitol. Int. 56: 330–333 [DOI] [PubMed] [Google Scholar]
- 352. Verstrynge K, Foets B. 2004. External ophthalmomyiasis: a case report. Bull. Soc. Belge Ophtalmol. 2004: 67–71 [PubMed] [Google Scholar]
- 353. Vetrella M, Tremblay E, Sanguigno E, Mancini D. 1996. A case of aural myiasis in a newborn. Riv. Ital. Pediatr. 22: 106–107 [Google Scholar]
- 354. Victoria J, Trujillo R, Barreto M. 1999. Myiasis: a successful treatment with topical ivermectin. Int. J. Dermatol. 38: 142–144 [DOI] [PubMed] [Google Scholar]
- 355. Visciarelli E, Costamagna S, Lucchi L, Basabe N. 2007. Human myiasis in Bahía Blanca, Argentina: period 2000/2005. Neotrop. Entomol. 36: 605–611 (In Spanish) [DOI] [PubMed] [Google Scholar]
- 356. Wadhwa V, Kharbanda P, Rai S, Uppal B. 2006. Urogenital myiasis due to Chrysomyia bezziana. Indian J. Med. Microbiol. 24: 70–71 [DOI] [PubMed] [Google Scholar]
- 357. Wahl R, Fraedrich J. 1997. Occupational allergy to the housefly (Musca domestica). Allergy 52: 236–238 [DOI] [PubMed] [Google Scholar]
- 358. Wakamatsu TH, Pierre-Filho PTP. 2006. Ophthalmomyiasis externa caused by Dermatobia hominis: a successful treatment with oral ivermectin. Eye 20: 1088–1090 [DOI] [PubMed] [Google Scholar]
- 359. Wakid MH. 2008. A laboratory-based study for first documented case of urinary myiasis caused by larvae of Megaselia scalaris (Dipteria: Phoridae) in Saudi Arabia. Korean J. Parasitol. 46: 33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Werminghaus P, Hoffmann TK, Mehlhorn H, Bas M. 2008. Aural myiasis in a patient with Alzheimer's disease. Eur. Arch. Otorhinolaryngol. 265: 851–853 [DOI] [PubMed] [Google Scholar]
- 361. Werner H, Rall E, Hendrischk A. 1975. Urogenital myiasis with Fannia scalaris. Dtsch. Med. Wochenschr. 100: 1397–1398 (In German) [DOI] [PubMed] [Google Scholar]
- 362. Whish-Wilson PB. 2000. A possible case of intestinal myiasis due to Eristalis tenax. Med. J. Aust. 173: 652. [DOI] [PubMed] [Google Scholar]
- 363. Wilhelmus KR. 1986. Myiasis palpebrarum. Am. J. Ophthalmol. 101:496–498 [DOI] [PubMed] [Google Scholar]
- 364. Williams DJ, Wharton S, Ravandi A, Achong M. 2006. Cutaneous myiasis of the eyelid masquerading as periorbital cellulitis. Emerg. Med. J. 23: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Wolfelschneider P, Wiedemann P. 1996. External ophthalmic myiasis cause by Oestrus ovis (sheep and goat botfly). Klin. Monbl. Augenheilkd. 209: 256–258 (In German) [DOI] [PubMed] [Google Scholar]
- 366. Yang ZR, Bao DW, Liang XY. 2005. Gastric myiasis caused by the larvae of Fannia canicularis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 23: 216. [PubMed] [Google Scholar]
- 367. Yeung JC, Chung CF, Lai JS. 2010. Orbital myiasis complicating squamous cell carcinoma of eyelid. Hong Kong Med. J. 16: 63–65 [PubMed] [Google Scholar]
- 368. Yildirim I, et al. 2008. What's eating you? Cutaneous myiasis (Wohlfahrtia magnifica). Cutis 82: 396–398 [PubMed] [Google Scholar]
- 369. Yuca K, et al. 2005. Aural myiasis in children and literature review. Tohoku J. Exp. Med. 206: 125–130 [DOI] [PubMed] [Google Scholar]
- 370. Zhu L, Lin R. 1999. A case of myiasis of the knee joint caused by Musca domestica vicina. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17: 349 (In Chinese) [PubMed] [Google Scholar]
- 371. Zumpt F. 1965. Myiasis in man and animals in the Old World. Butterworths, London, United Kingdom [Google Scholar]