Abstract
Summary: Since the late 1990s, bed bugs of the species Cimex lectularius and Cimex hemipterus have undergone a worldwide resurgence. These bed bugs are blood-sucking insects that readily bite humans. Cutaneous reactions may occur and can start out as small macular lesions that can develop into distinctive wheals of around 5 cm in diameter, which are accompanied by intense itching. Occasionally, bullous eruptions may result. If bed bugs are numerous, the patient can present with widespread urticaria or eythematous rashes. Often, bites occur in lines along the limbs. Over 40 pathogens have been detected in bed bugs, but there is no definitive evidence that they transmit any disease-causing organisms to humans. Anemia may result when bed bugs are numerous, and their allergens can trigger asthmatic reactions. The misuse of chemicals and other technologies for controlling bed bugs has the potential to have a deleterious impact on human health, while the insect itself can be the cause of significant psychological trauma. The control of bed bugs is challenging and should encompass a multidisciplinary approach utilizing nonchemical means of control and the judicious use of insecticides. For accommodation providers, risk management procedures should be implemented to reduce the potential of bed bug infestations.
INTRODUCTION
In recent years, bed bugs have undergone a major resurgence in the number of infestations, leading to clinical and control problems. This rise in activity is truly a global event, with increases in numbers of infestations reported for the Americas (153, 238, 246), Australia (99, 102, 104), Europe (37, 44, 170, 196, 232, 254), Asia (145, 149, 177, 282, 284), and Africa (227).
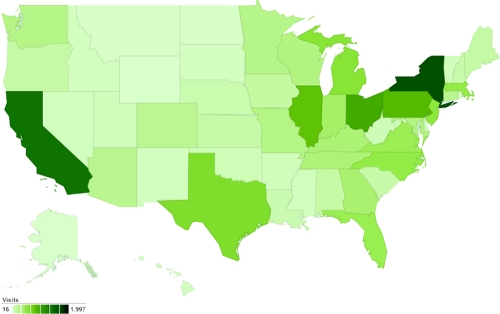
Although bed bugs have a long association with humans, for a period from the 1950s to almost the start of the 21st century, this pest had become relatively uncommon, particularly in the more economically advantaged nations. Not only was the reappearance of this pest unexpected, but the degree of the resurgence has almost been met with awe by many in the pest management industry. A survey of Australian professional pest managers in 2006 indicated that numbers of infestations rose by over 4,500% in the early years of the 21st century (102), comparable to what has been seen in other parts of the world (189, 246, 254). Bed bugs have become especially problematic in the United States, being reported in all 50 states; now, an estimated 1 out of 5 Americans either has had a bed bug infestation in their home or knows someone who has encountered them (220). Currently, there is no peer-reviewed published data on the actual prevalence of bed bug infestations across the United States. However, two of the largest pest control firms in the United States have released information on the most affected cities, based on the numbers of bed bug treatments undertaken by their respective companies, and this information corresponds well to hits on a dedicated bed bug website (http://www.bedbug.org.au). Despite limitations and potential biases in these data (Table 1), the information probably represents a moderately accurate indication of the current situation, with the most problematic cities being Chicago, New York, Detroit, Cincinnati, and Philadelphia (Table 1 and Fig. 1). Data from New York City showed that the number of bed bug complaints to the city council rose from 537 in 2004 to 10,985 in 2009 (43). Thus, bed bugs have rapidly become a widespread societal pest, and the risk of exposure through normal daily life appears to be increasing. In addition to homes and hotels, infestations are appearing in the office and retail environment, in the health and transport sectors, and in cinemas; in fact, they can be found in almost any location where people sleep or sit (103).
Table 1.
The top 15 bed bug cities in the United States according to two large U.S.-based pest control companies and hits to a dedicated bed bug website
| Ranking | Most affected city, determined by: |
||
|---|---|---|---|
| Terminixa | Orkinb | bedbug.org.auc | |
| 1 | New York | Cincinnati | New York |
| 2 | Cincinnati | Columbus, OH | Chicago |
| 3 | Detroit | Chicago | Los Angeles |
| 4 | Chicago | Denver | Washington, DC |
| 5 | Philadelphia | Detroit | Philadelphia |
| 6 | Denver | Washington, DC | San Francisco |
| 7 | Washington, DC | New York | Houston |
| 8 | Los Angeles | Philadelphia | Columbus |
| 9 | Boston | Dayton, OH | Denver |
| 10 | San Francisco | Baltimore | Seattle |
| 11 | Columbus, OH | Lexington, KY | Cincinnati |
| 12 | Dayton, OH | Minneapolis-St. Paul, MN | Minneapolis |
| 13 | Baltimore | Hartford-New Haven, CT | Indianapolis |
| 14 | Louisville, KY | Boston-Manchester, MA | Phoenix |
| 15 | Dallas | Los Angeles | Raleigh |
In order of the numbers of bed bug treatments by Terminix pest control during 2011; the period of recording was not stated (286).
In order of the numbers of bed bug treatments by Orkin pest control between January 2008 and July 2010 (29). Note that both rankings 1 and 2 will be biased based on the respective companies' client bases.
In order of the number of hits to http://www.bedbug.org.au as determined by Google Analytics from 6 June 2011 to 2 November 2011, based on 18,672 hits (134). These data may be biased against the socially disadvantaged, who may not have Internet access.
Fig 1.
Relative hits from the United States by state to the website http://www.bedbug.org.au as determined by Google Analytics, 6 June 2011 to 2 November 2011, based on 18,672 hits (134).
What is particularly confounding about the resurgence is that it involves two species: the common bed bug, Cimex lectularius L., and the tropical bed bug, Cimex hemipterus F. The involvement of these two species makes the ascertainment of the reasons for this global resurgence more challenging.
Various factors have been postulated to be responsible for the resurgence. Resistance to carbamate and pyrethroid insecticides (45, 167, 171, 185, 209, 261, 284), and, more recently, to the organophosphates (171, 284), has been well documented for both species. Resistance to the organochlorines and pyrethrin has been known since the 1950s (57), and resistance to the organochlorines infers cross-resistance to pyrethroids due to their similar modes of action. The difference today, compared with the past, is that most recent bed bug populations carry pyrethroid resistance (261, 303, 325), and the vast majority of insecticide products currently in use belong to the pyrethroid group. Insecticide resistance is probably the key initiator of the bed bug resurgence, and resistant bed bugs have been disseminated worldwide through increased international travel. The latter has been borne out by genetic investigations using microsatellite markers and mitochondrial DNA sequence data, where bed bug populations from the eastern United States have heterogeneous origins suggestive of multiple introductions (303). In contrast, that same study found that within a single multiple-residential-unit complex, the level of bed bug genetic diversity from all infested apartments was low. This finding suggests that the entire building's infestation started from the introduction of a few bed bugs or, possibly, even a single female. Poor pest control has been implicated in the spread of an infestation from a single point source to more than 68 of 320 rooms within a staff accommodation block (104). Thus, it appears that imperfect pest-management-related practices have probably contributed to the magnitude of the bed bug resurgence. This includes the lack of training of pest control technicians in the eradication of insecticide-resistant bed bugs, the slow response of pest management industry associations to develop bed bug management standards for control, the failure of regulatory authorities to ensure that marketed insecticide products are efficacious against current field strains, and the difficulty in obtaining quality information on the efficacy of bed bug control products (102, 103). Clearly, the reasons behind the resurgence are multifactorial, and many other possible contributors were reviewed previously (239).
The implication of the huge upturn in the number of bed bug infestations is that people are increasingly being exposed to the insect and, as a consequence, the various associated health risks. This has prompted a number of recent clinical reviews (67, 72, 78, 106, 126, 130, 143, 144, 175, 287, 289), while contemporary clinical investigations have only just begun to emerge (241, 250). Despite numerous reviews, articles continue to present unsubstantiated scientific “facts” on bed bugs. This paper reviews the health impacts of bed bugs and both the direct clinical effects (notably the cutaneous reactions produced from the bite) and the indirect but far-reaching impacts while attempting to dispel some of the long-standing urban myths about bed bugs and their bites and clinical effects. This is preceded by a brief discourse on bed bug identification and biology, which is essential knowledge for the understanding of control and the reasons why the species is a public health pest. An overview of the control of this pest is also provided, given the challenges of eliminating bed bugs in the human environment.
BED BUG IDENTIFICATION AND BIOLOGY
Bed bugs are hematophagous arthropods of the family Cimicidae within the order Hemiptera. The Hemiptera include the “true bugs,” namely, those insects with specialized elongated mouthparts, with most of them being phytophagous (i.e., feed on plant sap), and include common garden insects such as aphids and cicadas. Members of the Cimicidae can be distinguished from other hemipterans by being flightless (although they appear wingless, in fact, the wings are reduced to short transverse scales), are ovoid and flattened in shape, and are all obligatory blood feeders on vertebrates (60). The adult males have a pointed abdomen, while the female abdomen is much rounder (Fig. 2).
Fig 2.
The various life stages of the common bed bug, Cimex lectularius. Bar, 5 mm. Depicted are the egg stage, the five instars, and both adults. All stages were identified according to the key of Usinger (297). (Reprinted from references 95 and 105.)
Of the 90 or so species within the family Cimicidae, only a handful bite humans, with the two main species being the common bed bug, C. lectularius, and the tropical bed bug, C. hemipterus. The common bed bug is aptly named; the Latin for the genus is “bug,” while for lectularius, it is “bed” (255). The two species are superficially similar, and samples should be referred to an experienced entomologist for identification. The species can be distinguishing by the presence of an upturned lateral flange on the margin of the pronotum on the thorax of C. lectularius, making the thorax relatively much wider than that of the tropical species (95, 297), although this feature is less obvious in the juvenile stages. In regard to their relative distributions, the tropical bed bug is confined mainly to approximately within the 30° latitudes (98, 102, 104, 216, 217), and the common bed bug is usually found outside this range (102). However, both species may appear beyond their normal ranges (104, 119).
The natural history of bed bugs has been reviewed in Usinger's seminal work, Monograph of Cimicidae (297), and more recently by Reinhardt and Siva-Jothy (252). The following section provides a brief overview of the biology of bed bugs.
In both bed bug species, there are five juvenile stages, called “instars,” which are miniature versions of the adults in general appearance albeit different in coloration (Fig. 2). The first instar is around 1 mm in length and off-white in color when unfed, becoming a deep red-brown and 5 to 6 mm long when unfed as fully grown adults (297). All nymphal stages and adults of both sexes require blood for nutrition and development, and it takes 3 to 10 min for complete engorgement to occur. The insects are attracted to the host by the carbon dioxide exhaled, body heat, and various compounds emitted across the skin, and they walk to feed off the host (bed bugs do not fly or jump). The preferred host is humans, but bed bugs will feed on other warm-blooded animals, including pets (71). In the United States, bed bugs (notably C. lectularius) can heavily infest poultry sheds, resulting in anemia and decreased egg production (63). Bed bugs tend not to live on the human body, and the only contact is for a blood meal, which occurs every few days if a sleeping host is available. Being a cryptic species (bed bugs are photophobic and thus quite “secretive”), blood feeding typically occurs at night, with peak feeding occurring between 1 and 5 a.m., when people are in their deepest sleep. During the day, bed bugs seek shelter in a variety of cracks and crevices and become inactive while digesting the blood meal. Bed bugs stay in close contact with each other and release aggregation pheromones to help relocate their harborage after a blood meal; this grouping also aids in water conservation (40). The presence of a harborage is indicated by fecal spotting (Fig. 3). Bed bugs also release alarm pheromones, which become most evident during the course of a treatment when the infestation is disturbed. The smell is very typically “buggy” in odor, which some authors often describe as being “sickly sweet.”
Fig 3.
Bed bug infestation on a mattress. People most commonly encounter bed bugs in infested beds. The insects typically harbor along the mattress piping. Various stages can be observed, along with dark fecal spotting.
If a blood meal is continually available, the female C. lectularius bed bug will lay 5 to 8 eggs per week for 18 weeks at 23°C and at 90% relative humidity (158), while C. hemipterus bed bugs will lay up to 50 eggs in their lifetime (150). It is often quoted that bed bugs can lay up to 500 eggs in their lifetime; however, this figure was based on one particularly highly fecund female C. lectularius bed bug that laid 541 eggs (297) and is atypical. The cream-colored eggs, which are elongated and around 1 mm in length (Fig. 2), are cemented onto rough surfaces of hiding places and will hatch within approximately 9 to 12 days at a room temperature of around 22°C, but hatching will take longer under cooler conditions.
The length of the life cycle is extremely variable and is dependent on ambient temperatures. Under cool conditions of 10°C, once-fed adults of C. lectularius can live for up to almost 485 days, while C. hemipterus can live up to around 300 days (226). These lengthy periods would generally not normally be observed, as under average home and hotel living conditions at temperatures of around 22°C, the life cycle of both species takes around 2 months to complete, and the adult lives for up to a maximum of 4.5 months (58).
Encounters with bed bugs occur mostly commonly when people sleep in infested beds (Fig. 3), and often, the furniture and furnishings in the same room will also be harboring the insect. However, bed bugs can infest almost any site that people frequent (95).
CLINICAL RELEVANCE
Clinical Overview
The most common clinical consequences of bed bugs are the direct cutaneous reactions from the bite. The possibility of bed bugs acting as vectors for various infectious agents has been mentioned in the literature, although there is often little supporting evidence. There are other health impacts of bed bugs: the challenge and costs of pest control often lead people to desperate and dangerous acts; infestations have closed down hospitals, threatening the provision of health services; and bed bugs present various social issues. The mental health consequences of having an infestation are potentially serious (and may encompass delusions of parasitosis) but are poorly understood.
Bed Bug Bite Reactions
Bed bug mouthparts are adapted for piercing the skin and sucking blood and have extremely fine needlelike stylets that are inserted into the skin and are withdrawn after feeding. During feeding, the bug injects saliva that contains various protein fractions, some of which have anticoagulant properties. In C. lectularius, this includes nitrophorin, which is a vasodilator inducer (301); apyrase, which inhibits platelet activation and aggregation (299); and an inhibitor of factor X, which delays blood clot formation (300). A recent investigation on the sialome (saliva) of C. lectularius revealed 46 different protein components (116): many play a role in overcoming host hemostasis, some function in host protection (including possibly as antimicrobial agents), and others have a role that is as yet unrecognized. It has been stated that bed bugs inject an anesthetic, although none has yet been identified.
The salivary components of C. hemipterus have undergone only limited study. It has been found that this species contains a small amount of hemeproteins and has reduced anticlotting activity compared with C. lectularius, although the total protein contents of the saliva were similar between the two species (34). It is unknown if there are differences in salivary components between the instars or the sexes of either species.
After the removal of the stylets, some oozing at the bite site may occur, seen on bed sheets as small flecks of blood (276). Bites are often reported as occurring along the arms and legs but will occur on any area of exposed skin (241), although clothing can inhibit bites (241, 279).
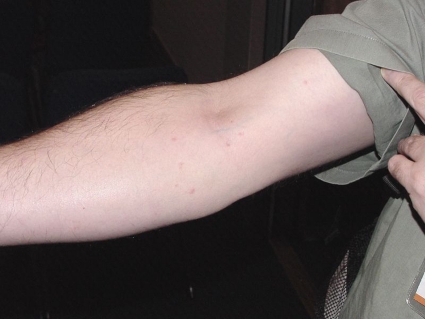
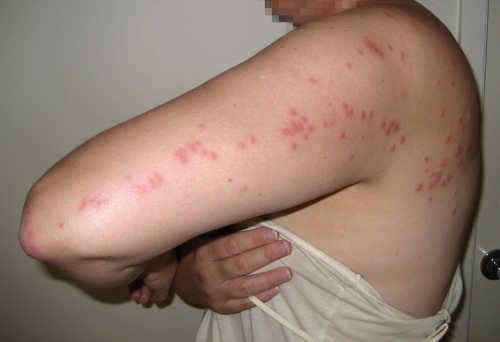
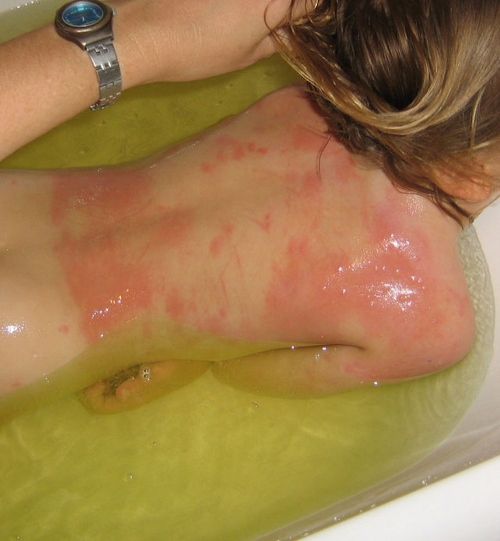
The severity of cutaneous reactions from a bed bug bite varies between individuals. It is important that published bed bug bite descriptions have been based on a single observation as a one-off clinical presentation, and the source of the bite (as in bed bug instar, stage, or species) is usually not known, not identified, or not stated. Some individuals will develop no reaction, although this may depend on previous exposures (129, 250). The reaction may start out as small indistinct red macular lesions less than 5 mm in diameter (130) (Fig. 4), which may later progress into large circular or ovoid wheals (77, 79, 280), usually described as papular urticaria (although they may last for more than 24 h and should not be regarded as urticaria), and may be as big as 2 to 6 cm in diameter, which represents the more classic bed bug “wheal.” These lesions tend to be intensely itchy (Fig. 5 and 6). Although the wheals have been reported to be up to 20 cm in diameter (72), those authors did not cite their original source, and it is possible that such reactions could have resulted from multiple bites or from trauma at the bite site through scratching, thereby increasing the size of the lesions. Pseudopodium-like extensions of erythema around the periphery have also been noted (70), which may correspond to livedo-like patterns. It has often been stated that a small hemorrhagic punctum can be at the center of the bite mark at the time of the bite (70, 79, 266), but this has not been observed in our experience. If there are large numbers of bed bugs (Fig. 7), the individual lesions can coalesce to give the appearance of a more generalized rash, possibly enhanced by trauma, such as scratching, to the affected areas (Fig. 8), complicating the differential diagnosis (49, 70, 194, 267, 287). If the bed bug infestation is unrecognized or not treated, the cutaneous reactions can become chronic (46, 73), and dermatitis “outbreaks” from bed bug bites have been reported in health care facilities (81). Bite reactions can take some weeks to resolve (130), depending on the severity of the reaction. Patients with multiple bites or a severe cutaneous reaction may develop systemic symptoms, including fever and malaise (182), although this appears to be rare (42).
Fig 4.
Bite reactions the morning after being bitten by bed bugs. The bites are faint erythematous macules and papules 2 to 3 mm in diameter. The bed bug species was not identified; the bites occurred in a region where both C. lectularius and C. hemipterus occur. (Reprinted from reference 105.)
Fig 5.
Same patient shown in Fig. 4 but 4 days later. Lines of bites that run along the body can be observed, along with the classic bed bug wheal, measuring 2 to 3 cm in diameter. (Reprinted from reference 105 [courtesy of Nigel Hill].)
Fig 6.
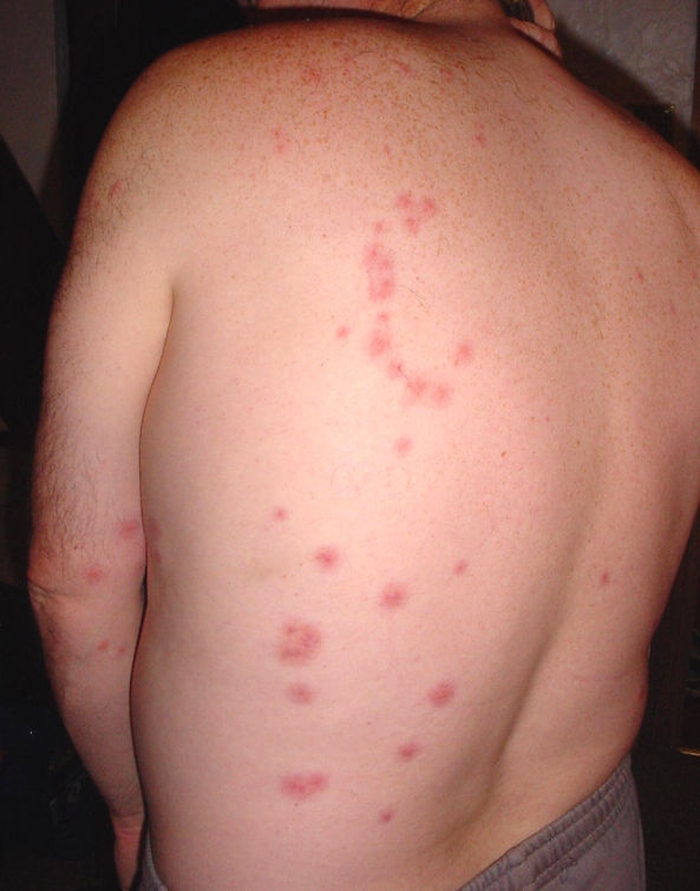
Lines of bites along the arm. The bed bug species was not identified.
Fig 7.
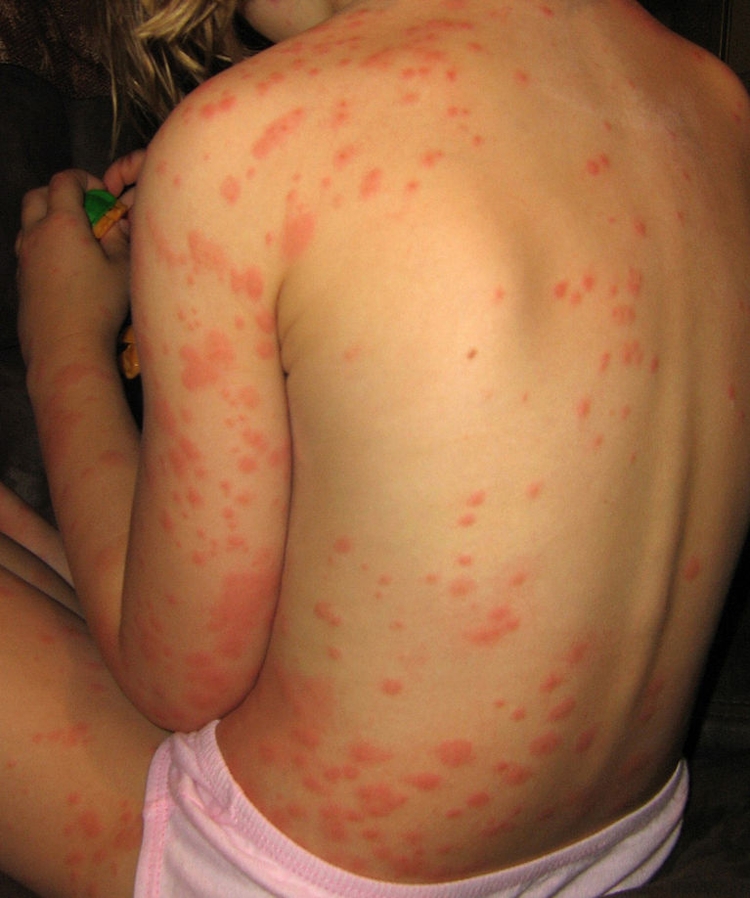
A 4-year-old girl bitten by hundreds of C. lectularius bed bugs (identified by the authors). Multiple discrete bed bug wheals, some with purpuric centers, cover much of the body. (Reprinted from reference 105.)
Fig 8.
Same patient shown in Fig. 7. A diffused erythema has developed in the more severely bitten areas. This could be the result of trauma (e.g., scratching) to the affected areas.
Vesicles and bullae containing clear or bloody exudate that appear some days following a bite have been reported (75, 115, 172, 181, 182, 266, 279, 288) (Fig. 9). The frequency of bullous eruptions is unknown; it has been stated that they are uncommon (182), but an investigation of bed bug bites on passengers on a tram found eight patients who all developed such eruptions on the legs (172).
Fig 9.
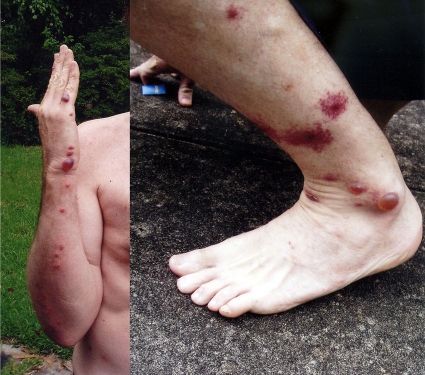
Bullae with hemorrhagic serum on the hands and ankles from the bite of C. lectularius (identified by the authors). These appeared between 24 and 36 h following the bites. The purpuric/necrotic lesions on the ankle indicate the severity of the reaction. (Reprinted from reference 105.)
The immune basis of the clinical reaction is largely uncertain. In one study of 15 patients with papular urticaria, all had IgG antibodies to C. lectularius antigens, although as whole ground dried salivary glands were used as the antigen, the antibody responses to the individual antigenic proteins were not identified (1). Nitrophorin has been shown to induce allergen-specific IgE antibodies in one patient hypersensitive to the bite (181). Similarly, the pathological changes in the skin from a bed bug bite have been poorly studied. Biopsy specimens of the bites show edema present between the collagen bundles in the dermis, with lymphocytes and numerous eosinophils being present around the blood vessels (73). Epidermal spongiosis and inflammatory infiltrate in the upper and lower dermis around vessels and epidermal adnexal structures, a perivascular inflammatory infiltrate in the papillary dermis, and lymphomononuclear cells and numerous eosinophils around the vessels and between the collagen fiber bundles have all been observed (78). Intra- and subepidermal bullae with an inflammatory infiltrate composed mainly of lymphocytes, histiocytes, and some neutrophils and eosinophils have also been noted (288). However, the correlation of specific histopathological findings with the timing of the initial bites is limited, and further investigation is needed.
There are limited data on the percentage of individuals reacting to bed bug bites. Most reports were of isolated observations rather than prospective clinical studies of the change in reactions when patients are exposed to bed bugs repeatedly over time. Reinhardt and colleagues (250) reviewed the literature on human reactions to bed bug bites, and when data from all the studies were combined, 249 out of 331 (75%) patients developed a reaction. However, those investigations were not directly comparable; some involved placing bed bugs on volunteers to record the reaction, some undertook repeated exposures to measure changes in reactions over time, and other studies noted cutaneous reactions in people within infested premises (i.e., isolated patient observations).
The largest of the early studies was undertaken at an internally displaced-person camp in Freetown, Sierra Leone (119). Of 221 individuals living with bed bugs, 196 (86%) had wheals from the bites, although it was not stated what percentage of the individuals had previous exposure to bed bugs. The study was further complicated in that both C. hemipterus and C. lectularius coexisted in the camp, and no attempt was made to distinguish the relative clinical reactions from the two species. To date, no study has compared the differences in clinical reactions from the bites of the two species.
Most contemporary studies have reported only the effect of C. lectularius bites. In a prospective clinical study of volunteers bitten by C. lectularius bed bugs, it was found that 18 of 19 patients (94.7%) developed a skin reaction albeit often only after repeated exposures (250). With the first bites, 13 of 24 patients (54.2%) had no reaction. The period for the bite reaction to appear decreased from around 10 days to a few seconds following repeated bites (250).
The most comprehensive study of reactions to bed bug bites involved 474 individuals in the United States, all with an independently confirmed infestation (241). The species of bed bug was not stated, although it is assumed to be C. lectularius, as there have been no reports of C. hemipterus from the U.S. mainland (as stated above, C. hemipterus occurs mainly within the 30° latitudes). Seventy percent of respondents (raw data not given) reported skin reactions, although none were confirmed through cutaneous examination by a medical practitioner. Respondents over the age of 65 years reported a lower reaction rate (42% claimed no reaction). While the sample size was not considered significant, a similar level of no reaction (41%) was recorded for children between 1 and 10 years of age. Around half of those surveyed stated that they had the infestation for 1 month or less, and so it is probable that some had yet to develop bite sensitivity.
Some individuals may not develop a reaction, even with repeated exposures. Goddard reported feeding 15 C. lectularius on himself every 3 weeks over a 6-month period, yet no cutaneous or clinical reaction developed (129).
Bed bug bites may appear in a linear fashion, either individually or as groups. A number of authors have stated that the bites appear in groups of three, colloquially referred to as “breakfast, lunch, and dinner” (67, 73, 78, 143, 175, 289, 326). To date, the distribution of bites on patients has not been quantified or analyzed. Even when authors have pointed to a group of three bites on a photograph of a patient within a publication, this pattern is not often obvious and certainly not evident for all bite patterns (78).
We have reviewed images sent to our laboratory of 30 patients with clinical bite reactions to bed bugs, and a pattern of three was difficult to discern. Bites were often singular and somewhat random, in groups of two or more, and often in lines, or there were so many bites that a widespread rash was hiding individual bite reactions. At least one other author has questioned the often-quoted “three-bite” relationship and similarly observed that bites are often arranged in rows or clusters (79). The concept of breakfast, lunch, and dinner bite clustering may be just one of the many myths surrounding bed bugs.
In reviewing the same 30 patients, a linear pattern of bed bug bites was often obvious when there were multiple bites and frequently on a line of 30 cm or more in length (Fig. 5 and 6). It should be noted, however, that many of the bites seemed to be randomly situated. There are at least three possible explanations for the lines of bites:
The insects are “test” biting and do not always locate a readily flowing vein or capillary on the first probe
The feeding is interrupted by the movement of the host, and the bed bug resumes feeding after the sleeping victim settles (38)
Many bed bugs are biting along the one line.
In the case of explanations 1 and 2, these would not necessarily explain a line of bites, unless the biting occurred along a blood vessel line. Also, as the line of bites can cover some distance, it is not intuitive that an insect moves away some distance before refeeding or test feeding. Once a bed bug starts feeding and the stylets are implanted, it takes quite some physical disturbance for the insect to dislocate and move. From data from the 30 patients mentioned above and from other published images of bed bug bites (5, 73, 78, 178, 267, 280), it is evident that there tends to be a general direction to the lines of bites; they tend to be along the limbs rather than across and vertically along the torso rather than across. On the shoulder, lines of bites often run along the collarbone region. Thus, the direction of the bite lines appears not to be random.
As stated above, bed bugs release aggregation pheromones such that they tend to harbor in groups. Presumably, when blood feeding, a number of bed bugs from the same harborage would move in a general direction toward the recumbent sleeping host. Bed bugs, while feeding, sometimes keep the body in contact with the bedding and project the mouthparts forward into the skin (H. J. Harlan, unpublished data). A line of bites could then be the result of a number of bugs feeding on the patient during sleep over an area, with the line being coincidental and merely a result of the limitation of where the bugs can reach from the bedding. This hypothesis would explain the directional nature of the bites along the limbs and torso.
Another controversial issue is the times often cited in the literature for a bed bug reaction to appear. Historically, a delayed reaction of up to 9 to 10 days has been reported (266), although most patients tend to show observable clinical signs within 24 to 48 h or even within minutes in hypersensitive individuals (250). Bed bugs are highly secretive insects that are most active at night, and many patients would not notice the presence of the insect, especially if the infestation is relatively light. As the insect can be transferred in luggage, furniture, and other belongings, the first sight of the insect may not represent the initial encounter, especially while people are traveling. Patients may not develop an allergic response upon the first exposure to the bite but often do so upon a subsequent exposure. A new bite, while the sensitivity reaction to an earlier bite is developing, may be confused with the appearance of a delayed reaction. Thus, there could be doubts about some of the earlier times cited in the literature for the appearance of reactions. In the prospective clinical study mentioned above by Reinhardt and colleagues (250), it took up to 11 days for many patients to develop their first reaction, which does coincide with data from earlier reports.
There are no reports relating to patients known to be sensitive to the bite of either C. lectularius or C. hemipterus regarding whether they would develop a similar lesion when bitten by the alternative Cimex species. Usinger (297) commented on his own cutaneous reactions when feeding various species. After feeding C. lectularius on himself for 3 years, a bite from Cimex pilosellus (Horvath), a bat-feeding species, produced an immediate reaction similar to that of C. lectularius. Hesperocimex sonorensis Ryckman (a bird-associated species) also produced an immediate reaction but of a different clinical appearance, while Leptocimex duplicatus Usinger (another bat feeder) produced no clinical reaction with the first bite and a mild reaction upon subsequent bites. Usinger concluded that the degree of clinical cross-reactivity was dependent on the relationship of the various species; the two Cimex species produced evidence of a cross-reaction, whereas the more distantly related Hesperocimex and Leptocimex had either a different or no reaction.
Cimex lectularius and C. hemipterus are very closely related species. In both the laboratory and the field, the two species will undergo interspecific mating (215, 307), even occasionally producing a species hybrid (214). Thus, it could be presumed that the antigenic compounds within the saliva would be similar and produce comparable clinical cross-reactions in patients sensitive to the bite of either species. However, as discussed above, there are some differences in salivary compounds between the species, with C. hemipterus having fewer hemeproteins (34). The senior author (S.L.D.) has a known sensitivity to C. lectularius bites, yet when he fed an adult female C. hemipterus to repletion (i.e., full engorgement) for the first time, no clinical reaction was observed (S. L. Doggett, unpublished data). Of course, this represents only a single observation, and further investigations are required to confirm if this is a general phenomenon. However, perhaps C. hemipterus is lacking the salivary proteins of C. lectularius that induce the antigenic response, but the reverse may not hold true, and a sensitivity to C. hemipterus may result in a reaction to C. lectularius bites. Further research is required in this area.
Diagnosis and Misdiagnosis
Insect bites are generally difficult to definitively identify and characterize, and the bite reaction can vary tremendously between individuals, even for the same insect species. This makes accurate diagnosis and management challenging (137). A single bite from a bed bug, particularly soon or some time after the bite, is not easily identifiable as being caused by that insect. The development of a wheal may suggest that bed bugs are present, and a line of bites along the limb may provide further circumstantial evidence. Ultimately, a positive insect identification is the only sure way of definitively diagnosing the cause. A thorough inspection of the home, particularly around sleeping areas or in the locations suspected to be where the bites were acquired, by an experienced pest manager should be undertaken to confirm the presence of bed bugs and to exclude other possible biting arthropod pests (91). One report suggested that bed bugs should be considered for the differential diagnosis of patients who present with “mysterious skin rashes” (128).
It is not uncommon to misdiagnose the bite of bed bugs as scabies (278) (which should always be confirmed by a skin scraping), antibiotic reactions, food and other allergies, mosquito or spider bites, chicken pox (105), Staphylococcus infections (133), allergic skin reactions (4), and prurigo (111). In one case of an anaphylactic reaction following a bed bug bite, the patient was initially diagnosed with a coronary occlusion (230). Misdiagnosis may result in inappropriate medical treatments, such as the use of scabicides (278), lesion biopsy specimens, and various other investigations (133).
If misdiagnosed, the bed bug infestation may continue and become firmly entrenched, with the risk of further spread. For example, in one instance, a child was suspected to have been suffering for 3 months with “hives” before the pest was identified, with the bugs being found only when the mother checked the child during the night and saw the bugs feasting (131). In another example, a 10-year-old girl went through a continuing nightmare of biopsies, blood tests, and ointments for over 6 months (133). The parents suspected bed bugs only following an Internet search, which led to the child's room being inspected at night and the cause of that misery being identified. One woman who was suffering ongoing skin reactions discovered the cause only after she heard media discussion of bed bugs; suspecting the worst, she lifted the mattress cover and was horrified to find literally thousands of bed bugs residing on her mattress (92). Another report recounted the tale of a woman who was being treated for scabies by her dermatologist and found out about bed bugs only after a conversation with a neighbor (278). In one unusual case, eight women developed a zone of raised spots across the back of their calves in almost identical positions. Investigations found two things in common: all traveled on the same tram, and all wore skirts. Upon inspection, the seat cushions were found to harbor bed bugs. The bugs could access only the exposed legs of women in skirts, which coincided with the position of the bite marks (172).
On the other hand, there are dermatologic diseases that may be misdiagnosed as bed bug infestations, such as Grover's disease, miliaria, prurigo, dermatitis herpetiformis, or acral papular or papulovesicular dermatitis of Gianotti-Crosti (73, 289). The differential diagnosis of bed bug bites depends on the morphology of the skin lesions and can be quite extensive. In delusions of parasitosis, the patient has the conviction that the skin is infected by parasites, and bed bugs can be one of the most commonly blamed arthropods. In cases where the clinical diagnosis is difficult, a skin biopsy is usually helpful to differentiate most dermatologic diagnoses from insect bites (73, 289).
Bite Treatment
Treatment regimens and outcomes for dermatological reactions to bed bug bites have been recently reviewed (130). There are no specific treatments (144), and the clinical reactions tend to be treated empirically as per other insect bites. For cutaneous reactions, topical steroids are used to control inflammation, and systemic antihistamines can provide relief from itching (130, 147, 262). Antibiotics or topical antiseptic lotions may be prescribed if secondary bacterial infections are present (276) or when the risk of secondary bacterial infections is high, e.g., those with numerous bites and skin excoriation due to scratching (142). Generally, however, antibiotic therapy is not required for otherwise healthy individuals, although good dermal hygienic practices are recommended (276). For patients who develop severe systemic reactions and anaphylaxis, intramuscular epinephrine, corticosteroids, and antihistamines may be required (130).
Even without treatment, symptoms tend to disappear within 1 to 2 weeks once the bed bug infestation is eradicated, as the bites are self-limiting (78), and the insect does not infest the skin. Scarring appearing as a deeper-colored skin tone may remain for some weeks to months (Doggett, unpublished). For individuals with severe anxiety or a secondary delusional infestation-like syndrome (117) associated with bed bugs, psychological therapy may be needed.
No bed bug allergens are available for desensitization programs for people who have severe bite reactions, although recent research into the bed bug sialome may identify appropriate allergens (62). Ultimate relief from bed bugs can be achieved only through the eradication of the active infestation.
The Bite: Clinical Complications
Sleep deprivation is commonly associated with bed bugs (105, 130, 241). In a survey of 474 individuals with a bed bug infestation, 29% claimed insomnia or sleeplessness (241). Patients can awake during the night because of the bite's itch, and scratching can exacerbate the itch sensation, leading to greater sleep disturbance (known as the “itch-scratch cycle”) (291). Some patients have disturbed sleep from just the knowledge of having an active or past infestation in their own bed. Vandam (302) cited an example of a woman who would wake up in the night and place her pillow into the freezer. Sleep deprivation is a serious medical problem that can affect neurocognitive functioning, emotional status, and various physiological factors and may contribute to long-term health problems such as coronary heart disease (272, 314). Sleep loss is considered to have a major economic impact, and some of the most serious human-caused disasters (e.g., Three Mile Island, Chernobyl, and the grounding of the Exxon Valdez) have been attributed to a lack of sleep (74). No study has quantified the impact of bed bug infestations on sleep outcomes and its associated economic impacts; this needs investigation in light of the increasing frequency of infestations.
Various secondary bacterial infections as a consequence of the scratching of the bed bug bite site have been recorded, including cellulitis, impetigo, ecthyma, lymphangitis (54), and folliculitis (130). How widespread and common these secondary infections are is presently unknown.
There is a single report from 1922 reporting that liquid excreted from bed bugs during feeding can induce a urticarial reaction (237). It was also stated that chronic bed bug infestations can cause “nervousness, lethargy, pallor and diarrhea” (287); however, it is not clear if these symptoms are due to the bed bug bites or the mental trauma associated with having an infestation. It was also reported that repeated bed bug bites may produce a severe reaction with serum sickness (287), but, again, the frequency of this reaction is unknown.
Infectious Diseases
It is not surprising that bed bugs are often lumped together with other hematophagous arthropods and suspected of being vectors of various pathogens. In the preantibiotic era, numerous investigations were undertaken to find a possible link between various diseases and bed bugs. The race to find such associations may even have influenced research outcomes, particularly when reviewed in the context of modern medical knowledge. Studies up to the early 1960s (around 75% were from between 1911 and 1940) were reviewed by Burton (55, 56), who described some 43 human diseases suspected of being transmitted by bed bugs. Of these, all but 6 were pathogen related and included examples of bacteria, rickettsia, viruses, protozoans, and nematodes. With the current understanding of vector-pathogen dynamics, as discussed below, some of the implicated organisms must be viewed with a high degree of suspicion. The nonpathogen disease associations included beriberi, pellagra, and cancer and must be treated with some skepticism. Burton did admit that several of the claimed associations were based on “inference, deductive reasoning, or conjecture” and were therefore not based on statistical epidemiology, microbial or vector investigations, or experimentation. Many of the reports cited were the result of the detection of a pathogen within bed bugs, but this does not mean that the insect is capable of transmitting the agent. In fact, there is no current evidence to suggest that bed bugs transmit any pathogen (124–126).
Subsequent to the reviews by Burton (55, 56), there was a decline in the research interest in bed bugs as potential disease vectors, presumably due to reduced infestations following the widespread use of the highly effective insecticide dichlorodiphenyltrichloroethane (DDT).
Later projects relating to the potential vector status of bed bugs often coincided with research trends on certain human pathogens. In an era of intense investigation on filariasis in the 1960s, a number of papers examined the potential of bed bugs to transmit the causative filaria Wuchereria and Brugia. Despite wild-caught bed bugs being found to be infected with filarial worms, experiments failed to demonstrate transmission (55).
From the mid-1970s to the early 1990s, interest in blood-borne viruses, notably hepatitis B virus (HBV) and human immunodeficiency virus (HIV), sparked a number of investigations examining their possible transmission by bed bugs. As this was an area of intense research, the investigations are reviewed herein with some detail. Initially, it was noted in parts of Africa that there were high rates of HBV coincident with large numbers of bed bugs, and the rates of transmission within the community could not, at the time, be readily explained (165). The first suggestion that bed bugs may have a role in HBV transmission occurred during the early 1970s, when HBV surface antigen (HBsAg) was detected by radioimmunoassay (RIA) in 1 out of 18 pools of engorged bed bugs (species not stated) from the Ivory Coast (51). Collections of C. hemipterus on four separate occasions from villages in Senegal found HBsAg in nonengorged nymphs and adults in all four collections, with a total of 15 bugs positive by RIA out of 143 tested (321). At one of these sites, the occupant was known to be HBsAg positive. In a field investigation from the Northern Transvaal in Southern Africa (165), some 1,368 C. lectularius bed bugs were collected and tested in pools by HBsAg RIA. Thirty-two out of 140 pools contained HBsAg, with an average infection rate of 30.6/1,000 bugs. Both engorged and nonengorged bugs were HBsAg positive, with the latter suggesting possible transstadial transmission (i.e., from one nymphal instar to the next stage).
In laboratory investigations, Newkirk et al. (218) found that HBsAg could be retained in C. lectularius for 5 weeks and was maintained transstadially. Those authors also noted a change in HBsAg levels, suggesting the possible replication of the virus. However, they were unable to detect HBsAg in feces or eggs, with the latter suggesting a lack of vertical transmission. No attempt was undertaken to determine if HBV was still infectious or if bed bugs could transmit the virus via feeding on an HBV-negative host. In an almost identical experiment, the potential of HBV to be maintained in tropical bed bugs was examined, and HBsAg was found to persist for up to 6 weeks postfeeding (225). Again, no transmission studies were undertaken. Some years later, using molecular techniques, HBV DNA was detected in C. lectularius and feces for up to 6 weeks after feeding on HBV-infected blood (273). The authors of that study also tested the possible persistence of hepatitis C virus (HCV), but HCV could not be maintained within the bed bug. Other researchers employing molecular techniques found that HBV DNA persisted for up to 35 days in bed bug bodies and could be detected in feces for the same period and that the virus could be maintained transstadially through only one molt.
The first experiment testing the ability of C. lectularius to transmit HBV was undertaken via artificial blood-feeding devices (membrane feeders) and on laboratory animals (162). Transmission did occur albeit at very low rates. For the membrane feeders, HBsAg was detected in 3 of 35 bed bugs tested, while for the laboratory animals, HBsAg antibodies were detected in 2 of 10 guinea pigs and l rabbit. Those authors concluded that biological transmission was unlikely and that transmission was probably mechanical in origin. That study did show that HBsAg could persist for at least 7.5 weeks but that transstadial transmission was inefficient, with antigen being maintained for only one molt. Similarly, a transmission study with C. lectularius involving artificial feeding via membranes also found a very low rate of transmission, with only 1 of 7 serum samples in the membrane feeder testing HBsAg positive (285). In comparison with the above-mentioned study, the authors of the latter study concluded that HBV replicated within the bed bug. Those authors also reported a much longer period of HBsAg detection: up to 122 days postfeeding. Again, there was no evidence for the vertical transmission of HBV. It was subsequently demonstrated that HBsAg could be excreted in the feces by C. hemipterus (224). This finding suggested that, perhaps, the transmission of HBV through contact with infected bed bug feces may occur, although virion infectiousness was not examined.
In examining possible modes of transmission, Jupp and colleagues (164) concluded that the biological multiplication of HBV in bed bugs was not occurring, because virus loads decreased over time in bed bugs fed on HBV-infected blood. This finding was further strengthened by the lack of HBV in the salivary glands, as examined via electron microscopy in C. lectularius bed bugs that had been infected orally with HBV (163). The conclusion was that transmission was most likely to be mechanical and to occur through the crushing of live HBV-infected bed bugs, via contact with contaminated feces, or through interrupted feeding and/or regurgitation. Those authors also reported finding hepatitis E virus (HEV) antigen in wild-captured C. lectularius bed bugs collected from the Northern Transvaal, but transmission rates were much lower than those found for HBV. In India, HEV was also detected in bed bugs (292).
Arguably, the most compelling transmission research to date involved the feeding of HBV-infected C. lectularius bed bugs on three susceptible chimpanzees (166). Despite the monitoring of the chimpanzees for HBV antibodies for almost 1 year, none indicated infection, suggesting that transmission did not occur.
Epidemiological investigations examining possible associations between HBV-infected individuals and bed bugs are scant. For Gambian children under 5 years of age, there was a significant association between bed bugs in the beds and hepatitis B e antigen (HBe) antigenemia (198). Another report noted that there were high rates of HBV infections in a group of former prisoners of war held by the Japanese during World War II, and many had been heavily exposed to biting insects such as bed bugs and mosquitoes (123). The epidemiological link between bed bugs and HBV in these cases is by inference only. Following the initial epidemiological investigation in the Gambia, an intervention program to manage the bed bugs was implemented (199). While bed bug control was successful, there was no change in the incidence of childhood HBV rates, suggesting that bed bugs were not the major route of childhood HBV transmission.
In summary, the potential of HBV transmission via bed bugs is probably minimal. Perhaps, the risk of infection was always more perceived rather than real, since despite a considerable amount of research, there is no direct evidence to suggest that individuals have ever become infected with HBV through contact with bed bugs.
As for HBV, it was postulated that bed bugs may be involved in HIV transmission. It was observed that high rates of HIV infection occurred in children in parts of Africa where bed bugs were prevalent (190), and other explanations for the high rates had yet to be understood. Initial tests examining viral survival in bed bugs demonstrated that HIV could persist for up to 4 h in C. lectularius (161, 190). Attempts to transmit the virus via interrupted feeding did not occur, and it was concluded that HIV transmission was unlikely to happen in the normal human environment. A similar conclusion was found in a later study, whereby while HIV could be detected in bed bugs up to 8 days after oral exposure, HIV could not be detected in the feces, and no virus replication was found (315).
Within the insect order Hemiptera, to which bed bugs belong, the triatomine bugs from the family Reduviidae are known to transmit Trypanosoma cruzi, the etiological agent of Chagas' disease (169). The developmental cycle of this protozoan is relatively simple and occurs only within the gut of the insect. Transmission to humans results through contact with infected feces. In regions of South America where Chagas' disease is endemic, both bed bugs and triatomine bugs occur in the domestic environment. Thus, in light of the coexistence and relatedness of the bugs and the simple parasite developmental pathway, a number of field and laboratory investigations on the relationship between T. cruzi and bed bugs have been undertaken. Research up until the early 1960s was reviewed by Burton (55), and indications were that both C. lectularius and C. hemipterus were capable of acquiring and maintaining the parasite, and infectious stages could be transmitted in the feces. Since the review by Burton, several related articles have been published. An examination of beds in a region of Venezuela where Chagas' disease is endemic collected 138 C. hemipterus bed bugs, none of which had T. cruzi detected (the test methodology was not stated) (293). Those authors concluded that C. hemipterus would have a minor role, if any, in the transmission of T. cruzi in the domestic environment. In Argentina, C. lectularius bed bugs fed on wild-infected rodents were capable of transmitting T. cruzi and at an efficiency equivalent to that of triatomine bugs (159, 160). In those studies, T. cruzi was found to persist for more than 320 days in the bed bugs, and the complete developmental stages in the gut were observed via microscopy. Similar findings were recorded in another study for in another study C. lectularius infected with T. cruzi; there was complete parasite development within the gut, including the growth of the metacyclic infective stages, which were subsequently successfully transmitted to mice (69). Solid epidemiological or other evidence linking bed bugs with T. cruzi transmission within the domestic environment is currently lacking (127). However, it may be exceedingly difficult to differentiate the relative contribution to T. cruzi transmission in localities where both bed bugs and triatomine bugs coexist.
The current bed bug pandemic has again put bed bugs in the spotlight in terms of the potential to transmit human pathogens. A recent review of bed bugs and infectious diseases was published in 2011 by Delaunay and colleagues (82), who described some 45 pathogens as being potentially transmissible by bed bugs. Those authors also reviewed the vectorial potential of these pathogens, and thus, this information is largely not repeated herein.
Beyond HBV and T. cruzi, as described above, Delaunay and colleagues also reviewed the literature in relation to the possible transmission of Coxiella burnetii (the agent of Q fever) and fungi. For Q fever, data are very limited, with just one study suggesting that bed bugs may be capable of transmitting C. burnetii. Clearly, this information needs validation by other researchers, especially as C. burnetii is so readily transmitted by aerosols and other means (169). The fungi (and bacteria) detected on C. lectularius were all common environmental contaminants (251, 252), and there is no real suggestion that bed bugs may be transmitting these infections to humans. In fact, the risk may be to the female insect. Bed bugs undergo a complex mating behavior known as “traumatic insemination,” whereby the male's reproductive organ is modified to pierce the cuticle of the female abdomen to transfer sperm, thereby potentially introducing microbes (251).
One of the genera of bacteria listed by Delauny and colleagues, Wolbachia, includes symbiotic intracellular parasites of insects that are transmitted vertically. They are not passed by bed bugs through blood feeding and do not infect humans. The majority of bed bugs are naturally infected with Wolbachia as well as other endosymbionts (68, 154, 265). Considerable research has recently focused on various Wolbachia species, notably those that infect the dengue virus mosquito vector, Aedes aegypti (322). Infection in the mosquito can lead to a shortened life span or incompetence, ensuring that dengue virus is unable to complete development, and mass releases and the establishment of Wolbachia-infected mosquitoes into local populations may lead to a reduction in the incidence of human dengue virus infection. Releasing bed bugs into premises would not be popularly received, and thus, a similar approach could not be undertaken. In contrast to mosquitoes, studies have shown that the removal of Wolbachia has a negative impact on bed bugs; exposure to elevated temperatures kills the endosymbionts, leading to reduced fecundity (68). Whether such information could be practically employed to control infestations of bed bugs is yet to be ascertained.
Regarding other viruses, a reovirus from the ventriculus within the gut of the common bed bug, C. lectularius, was identified by electron microscopy (109). The origins of the bed bugs tested showing the presence of the virus were not stated in that study; the virus may have been acquired in a previous blood meal or could be an invertebrate virus. No further work on this virus has subsequently been reported. One group of researchers speculated that a virus may be present in the saliva of C. lectularius (110). Those researchers allowed bed bugs that had been gamma irradiated to repeatedly feed on rabbits, which induced the formation of skin papillomas. Those authors speculated that a virus was being transmitted, which was the cause of the clinical reaction in the rabbits. To date, this proposed virus has not been identified, nor have the results of the experiments been confirmed by others.
A recent letter to the editor attracted considerable media interest, as bed bugs were implicated as possible vectors of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE) (188). In that study, a sample of only five bed bugs was collected in Vancouver from a room infested with bed bugs (it was not stated if the residents had MRSA or VRE colonization or infections) and was tested for both bacterial strains. MRSA was isolated from three “bed bugs” (the species was not stated; it is possible that they could have been bat bugs), and VRE was isolated from two. Those authors surmised that as bed bugs feed and break the skin surface, there is a risk that such bacteria could be introduced. They concluded that this could be especially problematic in low-income housing, where hygiene standards are often lower and the levels of MRSA tend to be higher.
Perhaps, such findings should come as no surprise, considering the rates of antibiotic-resistant bacteria in the general community. In the United States, one study estimated that 1.5% of the population (or 4.1 million people) have MRSA nasal colonization (135). In an investigation of inpatients admitted to the hospital at a tertiary care center in Texas, 10.8% were found to be MRSA-positive nasal carriers (231). In Europe, it was estimated that 150,000 patients acquire MRSA infections annually (174). One study found that MRSA could readily survive for up to 8 weeks on a variety of fomites, indicating that many objects could serve as potential bacterial sources (83). MRSA is a very common organism, and the risk of potential exposure to the general community is high.
It was stated in the above-described letter that “S. aureus…has been reported to colonize the salivary glands of bedbugs for as long as 15 days” (188), and those authors quoted a paper from the mid-1930s to strengthen their argument of the possible bed bug transmission of MRSA. To be exact, the original paper actually stated that, “staphylococci were quite frequently discovered in smears of the salivary glands and of the contents of the intestine” (112). Histological examination using specific markers to identify Staphylococcus within salivary cells was not undertaken (nor, presumably, were such techniques then available), and so the possibility of an environmental contamination of the smears cannot be discounted. There was no attempt to dissect out the salivary glands in the Vancouver study, but rather, whole-bug homogenates were tested, and so the presence of the bacteria externally on the bed bugs cannot be excluded. In light of modern knowledge on pathogen development in vectors, it would seem unlikely that Staphylococcus would progress to the salivary glands through the various inherent dissemination barriers within the insect (Staphylococcus bacteria are not known to undergo biological transmission in any vector). Thus, the discovery of a fairly ubiquitous bacterial strain on an urban pest should come as no real surprise. Again, as per most of the other pathogens mentioned above, a link between MRSA/VRE transmission and bed bugs is arguably possible but probably tenuous.
As inferred as described above, pathogens can be passed by arthropod vectors via either mechanical or biological transmission. In mechanical transmission, there is no development of the pathogen within the arthropod, whereas with the biological form, the pathogen matures and undergoes some amplification (295). Mechanically transmitted pathogens may be acquired during feeding and remain on the mouthparts to be passed onto another host in subsequent feeds or may be shed in the feces. It would appear that mechanical transmission is less efficient, principally because the pathogen must remain stable through a variety of environments. As there is no pathogen development, there may be little vector specificity (295).
In contrast, with biological transmission, a pathogen has to overcome certain dissemination barriers in order to undergo development within a vector; it has to be able to recognize each barrier, circumvent the barrier in order to pass through (i.e., have the right key for the right door), and then make it to the appropriate organ for replication. As a result, there tends to be high vector specificity with biological transmission. For example, for an arthropod-borne virus (“arbovirus”) to be transmitted through a bite, after the initial blood meal, the virus has to penetrate the midgut, escape into the hemocele (the arthropod's blood), infect the salivary gland, replicate, and then finally pass into the saliva during subsequent feeds (295). Arboviruses have evolved specific envelope proteins which recognize and overcome these barriers that otherwise limit the potential biological transmission of a pathogen.
Some pathogens undergo a simpler form of biological transmission. Trypanosomes develop in the gut of the insect and are passed in the feces. Similarly, the agent of murine typhus (Rickettsia typhi) also develops in the midgut and is shed in the feces (169). Even with this basic form of biological transmission, there are still barriers to infection that have to be overcome for the arthropod to be a competent pathogen vector.
Beyond these barriers, the environments within a warm-blooded host and that within an arthropod are extremely different. Human blood has a stable temperature and pH, whereas an arthropod's temperature is ostensibly the same as the ambient temperature. Any ingested pathogen has to be able to survive the different pHs of the bug's gut. Also, the pattern of blood cell digestion within hematophagous arthropods puts constraints on the ability of a vector to transmit a pathogen (304). Thus, vector-borne organisms have evolved to cope with these environmental changes.
As indicated above, there is no solid evidence yet to suggest that either C. lectularius or C. hemipterus is a competent vector of any pathogen. However, this is not the case for other members of the family Cimicidae. Swallow bugs (Oeciacus vicarious Horvath) are known to biologically transmit the arboviruses Fort Morgan virus (263), Buggy Creek virus (52), and Stone Lake virus (50) to swallow birds (Petrochelidon spp.). There is evidence to suggest that the arbovirus Kaeng Khoi virus can be transmitted to bats by the bat bugs Stricticimex parvus Ueshima and Cimex insuetus Ueshima (320). As other Cimicidae are capable of transmitting pathogens, then why not bed bugs?
The dissemination barriers and environmental differences, as discussed above, mean that many pathogens are simply unable to survive within the arthropod or to be transmitted. Recently, it was discovered that components of C. lectularius saliva include lysozyme and other peptides that are presumed to have antimicrobial activities (116). This may further reduce the ability of bed bugs to act as a vector. However, beyond the intrinsic factors within the vector, there are also various extrinsic variables that influence the possibility of an arthropod being able to transmit infectious agents of disease. Some of these variables may help to explain why bed bugs do not seem to be involved in pathogen transmission. Extrinsic factors include environmental factors that influence vector feeding behavior, host preferences by the vector, and the availability of appropriate vertebrate hosts. For the pathogen cycle to be completed, the vertebrate host must be able to become infected with the pathogen and then maintain it within the blood at a sufficient level and duration to infect other vectors (82, 295). If there are insufficient numbers of vertebrate hosts or insufficient numbers of immunologically naïve individuals within the population, then pathogen transmission may not occur.
It is only in the relatively recent period of human history that individuals (and populations) have become highly mobile, largely through developments in transportation. Prior to the 1900s, most bed bug infestations would have been supported largely by a limited number of individuals, such as a single family group within a home. Many vector-borne pathogens involve a native vertebrate that acts as either a reservoir, an amplification host, or both. The lack of alternative vertebrate hosts and minimal host numbers may mean that it is difficult to sustain any vector-borne disease. While this is just speculation, perhaps computer modeling may give some credence to this hypothesis. In contrast, swallow and bat bugs would have the opportunity to feed on multiple individuals, and thus, there is a greater likelihood that a pathogen could be maintained within a population.
In reviewing the potential of bed bugs to transmit human disease agents, Goddard came to the conclusion, “Even though bed bugs have been found naturally infected with many disease agents, they have never been proved to transmit even 1” (124, 125). It was correctly pointed out that the lack of evidence is “not equivalent to the assumptions that bed bugs do not transmit disease” (108). Of course, it is impossible to prove a negative, and, as such, this comment arguably has little scientific merit. Various authors suggested that further research is required to identify new pathogens and to examine the role of bed bugs in the transmission of infectious agents (82). Given that there is no evidence as yet that bed bugs have definitively transmitted any pathogen, what, then, is the risk?
Very few authors have attempted to quantify the overall number of infestations within a nation or the number of bed bugs within a single infestation, yet such information has direct relevance for the analysis of the risk of bed bugs potentially spreading infectious agents. From a survey of Australian professional pest managers in 2006, it was concluded that the overall number of bed bug infestations across the nation between the years 2000 and 2006 was conservatively estimated to be 100,000, with the year 2000 being around the time when the resurgence began to be evident (104). As the survey indicated that numbers of bed bug infestations were the highest in the year of the survey, presumably, by 2011, this number would have easily doubled (i.e., 200,000). In an apartment complex in Indianapolis, IN, the average numbers of bed bugs per infestation in two treatment groups were 103 and 507, while the median counts were 73.5 and 77 (309). As that study was undertaken in low-income housing, it could be argued that such numbers are higher than those at other sites. However, the cryptic nature of bed bugs means that it would not be possible to identify every individual, especially the juvenile stages, and the estimated numbers do not seem excessive in our experience.
Combining the estimated number of infestations in Australia in 2011 (200,000) with the lowest infestation count of 73.5 would give a total of 14.7 million individual bed bugs involved in the resurgence in Australia since the year 2000. If these figures are extrapolated to the United States, based on the United States having a human population of 300 million (about 15 times that of Australia, with around 20 million [318, 319]), a figure of 220.5 million bed bugs is obtained for the number of bed bugs involved in the resurgence in the United States since 2000. Of course, these figures are a very rough estimate, but they do provide an indication of the magnitude of the numbers of bed bugs since the resurgence began, and the overall worldwide figure obviously would be expected to be much higher still. It is not possible to prove that bed bugs cannot transmit any infectious agent; however, with over 200 million bed bugs biting (and biting multiple times), and without any evidence of any disease resulting, the indications are that the risk of contracting an infectious disease through the bite of a bed bug is almost nonexistent.
Miscellaneous Health Impacts
Arthropods that live in close association with humans produce a variety of allergens that can induce reactions and act as triggers for asthmatic attacks. Common domestic pests, including cockroaches (298), carpet beetles (155), and various acarines (notably the house dust mite [264]), have all been implicated in asthma, although exposure to any arthropod allergen could potentially lead to hypersensitivity (248). As bed bugs live in and around sleeping areas, the level of contact with the insects is high, and similar allergic and respiratory reactions have been recorded around the world (2, 157, 277, 312, 313). The control of bed bugs led to a subsequent reduction in the number of asthma attacks for an individual patient (277). Pest managers who undertake regular bed management have a risk of occupational exposure to bed bug allergens, with the potential development of hypersensitivity. This may be exacerbated by certain control activities, such as vacuuming, that could increase the amount of airborne allergens. The use of personal protective equipment (e.g., face masks) and vacuums with HEPA filters would reduce this risk.
In comparison to other hematophagous arthropods, bed bugs take a large blood meal, up to 13.9 mg (or 13.2 ml, with blood having a specific gravity of 1.0506 at 37°C [294]), with an average of 7.81 mg (7.4 ml) for an adult female (297). As a consequence, when large numbers of bed bugs are present, anemia may occur. Excessive biting by bed bugs was implicated as the cause of iron deficiency in infants and children in Hyderabad, India (305), while severe anemia was reported in a 60-year-old patient, again with bed bugs being implicated as the cause (247). Robert Usinger, the author of the seminal work Monograph of Cimicidae, reported on his own declining hemoglobin levels as a result of maintaining a bed bug colony on himself (297). His hemoglobin levels declined from 14.5 g/10 ml of blood to 11.5 g over 5 years of feeding bed bugs and remained below normal levels despite supplementary iron intake. His hemoglobin levels rose to 13.2 g after several months following ceasing the feeding of the bugs.
It has been stated that bed bugs can produce an ill-defined syndrome that involves “nervous disorders in sensitive people, and may contribute to the ill health of both children and adults” (270). The original report was not cited, and thus, the etiology of the condition is uncertain. Despite this, the condition is often quoted.
Since the start of the bed bug resurgence, a number of indirect health impacts have become apparent. With the difficulty in controlling modern strains of bed bugs resistant to both pyrethroids and carbamates, people are undertaking extreme and desperate measures to control infestations, thereby putting their own and others' health at risk. For example, the use of rubbing alcohol to control bed bugs led to severe burns in one individual (120), and pest managers employing propane gas heaters have set homes and apartments on fire (20, 146), with one incident resulting in damages of Can$4.5 million (146). Fire fighters in New York have even found several incidences of people using highly volatile and inflammable liquids, such as gasoline, to control bed bugs (132).
The overuse and misuse of insecticides for bed bug control are becoming more common. Historically, extremely hazardous chemicals were used, including arsenic and mercury compounds, which were used as contact sprays, and hydrogen cyanide, which was employed as a fumigant, and human death did result (240). Products that currently are registered as safe for household use have much lower levels of mammalian toxicity; however, use not in accordance with label directions can pose a threat to health. Insecticide overuse is symptomatic of the high degree of resistance of bed bugs to most of the currently available insecticidal products. Some pest managers are now using illegal pesticides not registered for use against bed bugs (12), some of which are known to adversely affect the cognitive development of children (47), while a widely used but largely ineffectual pyrethroid (permethrin) can be highly toxic to cats (33). In mid-2011, a series of unexplained deaths in Chiang Mai, Thailand, were suggested to be the result of the overapplication of chorpyrifos (an organophosphate insecticide) to control bed bugs (21, 22, 59); however, the link has yet to be conclusively established. A CDC publication, which examined reports of acute illnesses associated with insecticide application against bed bugs from seven states between 2003 and 2010, identified 111 patients with adverse reactions and 1 fatality and a growing incidence over the period investigated (65). However, only 16% of these cases were categorized as being definitely or probably related to insecticide exposure (66), and the one fatality was a patient who had several predisposing serious medical conditions. Most of the adverse reactions were associated with pyrethroid or pyrethrin use (65).
Even in routine bed bug control, multiple applications of insecticides are usually required, which may expose the public to a greater risk of adverse reactions. There have been attempts in the United States to reregister compounds such as propoxur, which has a higher risk of toxicity to humans than currently available products. As yet, the U.S. Environmental Protection Agency (EPA), the government authority responsible for insecticide registration, has refused the registration application (41), and interestingly, insecticide resistance to this compound was recently reported for both the common and tropical bed bug species in Thailand (284). It is important that the hazard of a product relates to both its inherent toxicity as well as the risk of exposure (121). Perhaps, the targeted use of an effective product, albeit with a higher level of mammalian toxicity, may pose less of a risk than a less toxic product applied in much larger doses and on multiple occasions. This needs to be considered by insecticide registration authorities. “Home-brew” cocktails of various chemicals are now appearing on the Internet to control bed bugs (24), and the safety (and effectiveness) of such products is unknown. With the fears of insecticide misuse, the U.S. EPA and CDC released a joint statement in 2010 warning against the inappropriate application of products (64). Despite this warning, most respondents (93%) to a recent survey had little anxiety about insecticide applications to control bed bugs (246). It seems that the concern for most people is the eradication of the insect rather than the processes of achieving it.
Bed bugs have come to pose a threat to human health through other means. One of the busiest fire stations in Salt Lake City, UT, had to be temporarily closed for treatment as a result of staff being bitten by bed bugs (8). A shelter for the homeless in Franklin County, OH, had to close for 4 weeks due to bed bugs (205), forcing residents onto the street.
Bed bugs are even coming to threaten the provision of health services. For example, in Aurora, CO, a woman was refused medical treatment because her home was infested by bed bugs (23). The facility rejected the patient for fears that she may introduce bed bugs into the treatment rooms. On the basis of one patient thinking that she saw a single bed bug, a New York medical facility shut down an entire treatment floor (113). This response was somewhat premature, as none were found. In Kerry, Ireland, bed bugs led to a partial closure of a hospital for 2 weeks (253). In Australia, a whole wing of an adolescent ward of a major Sydney hospital in 2008 had to close down due to a severe bed bug infestation, with some 18 rooms being affected (104). In parts of the United States, the presence of even one bed bug in an intensive care unit will result in the unit being taken out of service for pest treatments (179). Bed bug infestations in hospitals are now becoming common (14), such that procedural guidelines to reduce the risk of bed bugs (13) and management protocols (152) are being developed. It is now clear that bed bugs are impinging upon human health in multiple ways as the insect is increasingly becoming a societal pest.
Mental Health Impacts
There are numerous reports of bed bugs impacting the mental health of individuals; however, these are largely anecdotal. Mental health impacts that are reported may include fatigue, distress, shame, anxiety, social isolation and stigma (235), exasperation, and irritation (18). A survey of 474 individuals with confirmed bed bug infestations in the United States during 2009 found that 22% of the respondents reported “emotional stress,” 20% stated that they had “anxiety,” and 14% had “stress” (241). In mid-May 2011, a presentation at the annual meeting of the American Psychiatric Association indicated that the mental health impacts of bed bugs can be far reaching (61), including “a wide variety of affective, anxiety, and psychotic spectrum illnesses causing significant impairment, including suicidality and psychiatric hospitalization.” Patients with previous mental issues can be further destabilized, while new patients can develop psychoses (61). Clearly, the mental trauma surrounding this pest is very real and cannot be ignored.
There is a social stigma associated with bed bugs, particularly as older articles published before the current resurgence painted a gloomy picture of the insect, typically associating it with poor housekeeping and hygiene (290). This public perception continues (61, 221, 239), and papers published even today often erroneously continue to associate poor hygiene with bed bugs (179). As a result, when people learn that there is an insect in their bed which is biting them at night, they are horrified and disgusted. Some people state that they feel “dirty and unclean” (92). For most, the bedroom is an inner sanctum for people to rest and unwind from the daily stresses of life, and to feel insecure in this highly intimate area of the home is understandably detestable. It is thus not surprising that in a recent survey, most people (99%) who had experienced bed bugs reported being “upset and concerned” (241). One pest manager who had to pacify many traumatized clients even quipped, “rats, even V.D., is [sic] more socially acceptable than bedbugs” (156). The psychological impacts of an infestation within the home has been investigated for other vermin and include “depression, phobic anxiety, somatization [the conversion of anxiety into physical symptoms], hostility, and anomia [the inability to name objects]” (324). There is no reason to suggest that bed bugs would not produce a similar spectrum of psychological responses.
Bed bugs can produce bite marks that can be very obvious and disfiguring if on the face and neck. This can interfere with peoples' employment and self-esteem. For example, in relation to impacting one's career, a nurse who was badly bitten in the staff accommodation block of a major Sydney teaching hospital was unable to attend patients, as it was feared that she had an infectious disease (92). An international athlete was sent home, as it was incorrectly assumed that she had chicken pox and could be infectious to other players (92). An actress was not given a movie role that required large parts of the body to be exposed, as she was heavily scarred from bed bug bites (92). A former guest of the Waldorf Astoria hotel in New York is seeking $10 million in compensation, claiming that plastic surgery is required to treat permanent scarring on the face after being heavily bitten by bed bugs (25).
This is an insect that is changing human behaviors; there is a report of people not using gym lockers in case they take bed bugs home (26). Charity workers are being prevented from their social efforts; volunteers who make quilts for veterans in Minneapolis are no longer having their donations accepted, again due to fears of bed bug transmission (53). A survey in early 2011 by the National Pest Management Association (NPMA), the peak industry body for pest managers in the United States, found that many Americans have modified their behavior due to the bed bug resurgence (220). Around one-quarter of respondents inspected or laundered their clothing after returning from travel, and a similar percentage inspected the hotel room. Some 17% examined their luggage upon return, while 12% cancelled or altered their travel plans. Of those who knew someone who had an infestation, 40% stated that they avoided entering the infested premise, and 33% discouraged those infested from entering their own homes.
People infested with bed bugs may become socially isolated. In Denver, CO, a patron of a local library was banned after bringing bed bugs into the building (10). The library even felt compelled to add further shame to the individual by publicly naming him. In a Facebook survey, 56% of people would abandon their date if bed bug bites were noticed on the skin, while 47% said that they would ask if their date had bed bugs before going back to their home (210). There are even instances where people have abandoned their dwelling to live in cars or tents to avoid nuisance bites (148). People with bed bugs often undertake fewer social interactions by not having friends and family to their home while the infestation is present (R. Cooper and S. L. Doggett, unpublished data). In the United Kingdom, one man lost his job as a result of sleeping at work, as he was unable to do so in his infested home, and his ex-wife refused to allow access to the children in case they acquired bed bugs (O. Madge, unpublished data).
Bed bugs are coming to disrupt everyday life, with all the associated inherent social and economic consequences. While they tend to be most often associated with homes and hospitality groups, bed bugs are turning up almost everywhere. Hospitals have been affected, resulting in ward closures and untreated patients; fire stations have closed; and the homeless have been forced back onto the street. Shopping stores have had to shut their doors (16, 180, 271), bugs have invaded office buildings (269), and iconic landmarks such as the Empire State building (268) and cinemas (104) are not exempt. Students' educations have been impacted: preschools have closed (15), and college dormitories have been evacuated for treatment (17). Aircraft have had to be grounded due to seats being infested (28). Bed bugs can be found virtually anywhere where people gather, from churches to brothels (104, 246). The widespread nature of infestations must be adding to community paranoia.
People can undertake irrational behavior when bed bugs are present or even if an infestation is suspected. Various authors have noted that people will discard furniture even if it is only suspected of being infested (36, 239). In a recent case of an enquiry to the senior author of this article (S.L.D.), one caller stated that she had discarded her bed and mattress and undertaken various control attempts on the basis of seeing “something bed bug-like” run under the bed (Doggett, unpublished). Despite specimens being sent to our department that proved to be nymphal cockroaches, and despite that advice was given that an inspection by an expertly trained pest manager should be undertaken to establish if bed bugs were actually infesting the home, the client still believed that bed bugs were present despite a lack of evidence. In one case of a delusional patient with no evidence of bed bugs, his symptoms improved through the daily regimen of scrubbing with bleach (61). This particular patient became socially withdrawn for fear of exposing others to his perceived infestation.
With some individuals, even when the problem is solved, the psychological trauma can develop into a delusionary state, whereby the patients feel bites and insects crawling on them, even if the bed bugs have been eliminated. Patients suffering delusions of parasitosis may be linked back to an initial bed bug infestation. Such states of paranoia are being enhanced by the media, whose love affair with this pest in recent years has often led to the creation of irrational fears within the populace (6, 201). Statements such as “I am super paranoid that I have bedbugs” are not uncommon (173). As noted above, pest managers are finding cases of individuals convinced that they have bed bugs despite a lack of evidence (239). At this point, the patient would require specialized psychological intervention (61).
The other aspect of how bed bugs impact the mental health of people relates to the financial consequences of an infestation, with control costs amounting to tens of thousands to even hundreds of thousands of dollars for some larger hotels (92, 104). The threat of litigation is a concern for those in the hospitality industry, particularly as some claimants are now lodging multimillion-dollar lawsuits (27). A survey released in July 2011 of its affiliates by the U.S.-based National Apartment Association revealed that “bed bugs” were considered to be the most important issue, even ahead of taxes and fees and general landlord and tenant issues (136). A number of politicians in the United States want to develop a public registry of bed bug infestations (187, 274). This has the potential to severely tarnish a facility's reputation and business, even if only one room was infested. The high actual costs (such as eradication expenses and risk management strategies) and potential costs (including a loss of patronage with damaged reputation and litigation threats) mean that profit margins of companies in the hospitality industries may be impacted. It is thus easy to imagine just how such a little pest can increase the daily stresses of individuals' lives. Even for the resident, the cost of the eradication of the pest from the home is high and can lead to severe anxiety (61). A survey in New York during 2010 found that the average bed bug eradication cost was US$1,310 (268), while across the United States, average costs were around US$800 to US$1,200 for the treatment of a single infestation (210). However, for many with low disposable incomes, paying the high price for control is simply not an option. The result is that some people are forced to endure the presence of the insect and attempt control themselves. As explained above, this can lead to desperate and dangerous eradication practices.
People who are unfortunate enough to experience a bed bug infestation are not the only ones traumatized by this pest. Pest managers face an enormous challenge in light of the high degree of insecticide resistance, the poor performance or failure of so many bed bug products (97, 103, 183, 184), and the lack of quality information on product efficacy, particularly in pest management magazines, where pseudoscientific advertorials often masquerade as impartial research (103). Government insecticide-regulatory authorities have registered products with inappropriate efficacy evaluations for bed bugs. There are also examples of pest management associations being sponsored by companies that have products demonstrated to be ineffectual by independent testing (103). As a consequence, pest managers can be quite confused as to what constitutes quality information, and treatment failures are common (103, 104). Treatment failures within the hospitality industry can lead to the loss of other general pest control contracts, which may involve tens of thousands of dollars and a damaged reputation for the pest management company concerned (186). Pest managers also have to deal with clients who are reluctant to use insecticides, with clients' fears often being fuelled by illogical and emotion-based articles that use terms like “toxic pesticides” or “dangerous chemical insecticides” (80). Often, such terms are employed by companies as a marketing strategy to promote their own non-chemical-based products or by self-interest groups, such as antipesticide lobbies. If insecticides are registered, then in most countries, the product has been assessed for human toxicity and is deemed safe for use when applied according to the label instructions.
In 2010, according to AOL, the term “bed bugs” was the most searched health-related topic, ahead of potentially fatal conditions such as breast cancer and diabetes (32). One recent report suggested that many people in the United States have transferred their anxiety from bioterrorism attacks or socialism to potential attacks by bed bugs (6). This level of anxiety is out of touch with reality, as the direct health impacts of bed bugs are fairly minimal. However, this insect that has come to worry many in the world and is a pest that can cause mental anguish to those affected by its presence. The mental health impacts are varied but considerable and are probably contributing substantially to the overall economic costs associated with the emergence of this pest. Research in this area is urgently required.
Health Benefits?
The constant contact between humans and bed bugs over time has led to a number of tribal groups employing these insects in traditional medicine (35, 84, 176, 229, 297). An example is the treatment of ringworm with Cimex crushed in holy basil, Ocimum sanctum, in India (176). In other parts of India, bed bugs were used for the treatment of epilepsy, “piles,” alopecia, urinary disorders, and snake bites (229). Greek army surgeons around 50 AD claimed that bed bugs could neutralize snake venom (297). Others throughout history claimed that bed bugs, if taken with meat and beans, could cure fevers; could remove leeches if drunk with wine or vinegar; and, if put into a “urinaria fistula,” could cure dysuria (297).
Such curious procedures have no scientific basis and are unlikely to offer any real cure; however, bed bugs may yet prove beneficial in modern medicine. The insects themselves are thigmotactic, meaning that they prefer to be in direct contact with other surfaces, and will aggregate in groups within harborages (38). In the process, they defecate on each other; presumably, the digested blood could act as an ideal medium for the growth of fungi and other pathogens that may prove detrimental to the insect. Bed bugs release secretions from metathoracic scent glands that can inhibit the growth of bacteria such as Staphylococcus albus (281) and prevent the germination of spores from the fungi Curvularia lunata and Fusarium oxysporum (249). Perhaps, in the future, synthesized antibacterial components from the bed bug may be used against human pathogens. Additionally, the saliva of bed bugs is considered to be a “rich source of pharmacologically active molecules,” with possibly antimicrobial proteins, and could prove to be a potential resource for the discovery of new drugs (62).
Clinical Relevance Summary
With the bed bug resurgence in recent years, there have been calls to encourage research into the role that bed bugs play in the transmission of infectious disease (82, 108). In light of the facts that there has not been one proven case of bed bugs transmitting any infectious agent and that the estimated risk appears to be very low, it seems that these calls are hardly justified. However, despite this, to refute that bed bugs are a public health pest would be to deny the intense physical discomfort and mental distress experienced by affected individuals.
The vast majority of reports on the bed bug resurgence are from the developed world, as in the less economically advantaged countries where vector-borne diseases are a major issue, bed bugs are probably seen as a low priority. However, in developed nations, it is the poor that have been most affected by bed bugs, and there is a social inequity with this pest (108). A report from New York City in 2009 stated that 10% of adults reported bed bugs in their home in high-poverty neighborhoods, whereas for low-poverty areas, this figure was down to 2.9% (223). It is the socially disadvantaged who often do not have the economic resources to pay for control and are forced to live with this pest or to take desperate measures to eradicate an infestation, with all the associated risks. While it is hard to justify research on bed bugs as vectors of disease, investigations of the other health impacts are warranted, especially as there is much anecdotal information but little science in the areas of the direct clinical impacts and the indirect impacts (notably the mental heath effects). As there is a global resurgence, it means that bed bugs will increasingly come into contact with people, and the overall impacts can only be expected to become worse.
BED BUG CONTROL
Control Overview
Bed bugs are considered to be one of the most challenging of all insects to control. This is due to widespread insecticide resistance, the current lack of effective insecticidal products, and the biology of the pest (the cryptic nature is such that bed bugs tend to hide in tiny cracks and crevices, making detection and control difficult). For homes that are heavily cluttered (particularly if the resident has hoarding tendencies), numerous bed bug harborages will be available, making control even more difficult. For pest managers to be successful in bed bug eradication, they need to have specialized training in the management of the insect. They need to have knowledge of the pest's ecology, they need to be extremely thorough in their eradication attempts, they must undertake ongoing surveillance throughout the control program, and, most importantly, they must not rely on a single management option. Successful pest managers embrace the concept of integrated pest management (IPM), whereby nonchemical means of control are employed in conjunction with the judicious use of insecticides (208). Pest managers must also work in close association with the client, which is essential if eradication is to be achieved (95). For those in the accommodation sector, risk management measures should be undertaken by staff to reduce the potential of bed bugs and the more serious financial consequences associated with this pest.
To assist in the management of bed bugs, recent key industry standards have been developed to encourage “best practice” in bed bug eradication. These standards include A Code of Practice for the Control of Bed Bug Infestations in Australia (there have been seven versions, with the eighth presently in development [85–89, 93, 95]), the European Code of Practice, Bed Bug Management (with two versions to date [191, 192]), and the U.S. NPMA BMP Bed Bugs Best Management Practices (221). In the United States, a number of groups have also developed procedural guidelines for bed bug control (9, 11, 43, 118, 138, 168, 206, 283). However, if best practice is defined as the promotion of management technologies where there is evidence of efficacy through independent scientific evaluations (and preferably in peer-reviewed publications) or where there is evidence of efficacy through common practice (95, 102), many of the above-mentioned guidelines do not promote best practice. Most include technologies where evidence of efficacy is lacking. As bed bug management is extremely complex, the information presented herein is an overview, and the above-mentioned industry standards or key texts (239) should be consulted for greater detail.
The control process is broadly as follows: positive identification of the pest, inspection of the site to determine which areas require treatment, nonchemical control options, insecticide application, evaluation of the success of the treatment program, and risk management procedures. In hotels, student dormitories, apartment complexes, and other multiple-occupancy dwellings, the inspection process should include the examination of all rooms adjoining the room with the infestation, and ideally, risk management should be ongoing and be implemented even prior to infestations occurring.
The complete eradication of an infestation is usually the only acceptable outcome expected by the client (95). The failure to achieve this in an apartment complex can result in bed bugs spreading to adjoining units and a subsequent increase in costs before eradication is finally achieved. For example, in a staff accommodation facility attached to one of the major teaching hospitals in Sydney, Australia, one infested unit that was poorly treated resulted in bed bugs spreading to 68 of the 320 rooms (∼21%) (104). The final expenditure was approximately Aus$42,000, when the cost for the first treatment should have been around Aus$400 (based on contract pricing), a cost increase of 100-fold.
As indicated above, bed bug treatments are expensive. Beyond the facility mentioned above, we are aware of hotels spending over Aus$300,000 to achieve complete eradication after the initial infestation had spread throughout the facility (Doggett, unpublished). The dilemma of who pays for bed bug control in socially disadvantaged situations is leading some academics to suggest that bed bug suppression may be the only achievable outcome (210). However, this comes with the cost of continuing human suffering and the risk of spreading the infestation to other parts of society. Governments should provide financial support to those without the means if society wishes to reverse the current bed bug resurgence.
For the accommodation sector, bed bugs are especially problematic, as they expose the facility to expensive litigation and unwanted publicity, thereby damaging brand reputations. In 2003, in a high-profile case of a brother and sister who stayed in a Chicago motel and were badly bitten by bed bugs, the court awarded damages of US$382,000 (295a). The judge's decision was based on the fact that the motel failed to take steps to eradicate the bed bug infestations or to warn clients of the presence of the insect. Thus, they failed in their “duty of care” to protect the health of their guests. To reduce the risk of potentially successful litigation, it is important for accommodation providers to show “due diligence,” namely, to demonstrate beyond a reasonable doubt that they have done everything possible to minimize the risk of bed bugs (192). To this end, it is essential that accommodation providers have a bed bug action plan (202, 203) or a policy and procedural guide (94) to direct the processes of bed bug management. A quality bed bug policy should include defining staff responsibilities, education and training (of staff, tenants, and contractors), documentation (especially in relation to documenting the processes of the eradication of active infestations), occupational health and safety, the eradication processes, preventative measures (i.e., risk management), and communication with the media (94).
Bed Bug Prevention (Risk Management)
It is impossible to prevent bed bugs; however, there is the potential to minimize their impacts through risk management. For those in the accommodation sector, the key to reducing bed bug impacts is early detection. This minimizes the risk of the infestation spreading, control is more easily achieved, and the potential for clients to be bitten (with subsequent litigation and a damaged reputation for the organization) is reduced. Thus, early detection is about saving money and protecting the company brand. The early detection of bed bugs can be undertaken by various means, including the use of trained canines (now in widespread use across the United States and elsewhere). Regular and thorough inspections by housekeeping staff during routine room maintenance, or via pest managers, are important components of an early detection program. The use of bed bug monitors or traps may assist in early detection.
Canines have been evaluated to detect bed bugs in a controlled experiment within a hotel. They were found to have a 98% success rate in detecting bed bugs (236). The dogs did not give false-positive indications (i.e., indicating when no bed bugs were present) when tested against a range of other domestic pests, including an ant, a cockroach, and a termite species. However, in actual field trials, this success rate appears to be much lower. Dogs from seven canine firms in the eastern United States were evaluated for their ability to detect bed bugs, and the successful detection rate ranged from 11 to 83%, with an average of 43%, while the false-positive rate ranged from 0 to 38% (308). This demonstrates that canines are not as accurate as first thought but also highlights issues in the appropriate training of dogs for bed bug detection.
Bed bug traps, particularly of the “pit-fall” type, have been used for many years in research programs for the monitoring of populations. Mellanby (200) employed the Demon trap, a commercially available unit for cockroach trapping, which consisted of a hemispherical construction that insects could climb upon and drop into. Johnson (158) used a homemade trap that consisted of two petri dishes, one large and one smaller, with the latter being inverted and supported by a cork stopper. Paper bridges allowed the bugs to access the inner petri dish, where they would fall off and be trapped in the larger dish. More recently, the marketplace has been flooded with bed bug-monitoring devices. Broadly, these fit into two categories: those that are “active” and have various attractants, such as heat, carbon dioxide, or various semiochemicals, and the “passive” type, which have no attractants and act as simple harborages (101). For the active type, those traps which utilize carbon dioxide as an attractant are more effective at detecting bed bugs than those that employ heat alone (7, 310). The rate of carbon dioxide flow will influence the success of the trap; generally, the greater the flow rate, the more bugs attracted. A cheap homemade monitor employing solid CO2 as dry ice producing a flow rate of 731 to 800 ml/min collected around three times the number of bed bugs compared to that collected by two commercial units (both costing several hundred dollars) that have small CO2 cylinders producing flow rates of 42 and 161 ml/min (311). The downside of many of the baited detection traps is that they are more expensive and may not be economically viable to operate on a daily basis in all rooms of a hotel (97). To date, none of the passive-harborage-type traps have been demonstrated to be effective in independent scientific trials for the early monitoring of infestations, and there are few studies comparing the efficacies of commercially available active monitors. Similarly, no comparison between canine, trap, and human monitoring has been undertaken to determine the relative cost-effectivenesses and detection sensitivities of these early-monitoring methodologies. Another device, the ClimbUp Insect Interceptor, acts as both a barrier to bed bugs and a monitor. This device consists of an ultrasmooth plastic bowl with an outer bowl and has been shown to be more effective at detecting bed bugs than visual inspection, thereby contributing to the efficacy of IPM bed bug programs (309).
As early detection is crucial, technology in this area is rapidly evolving, and several new devices have been produced. These include “sniffer” technologies that are claimed to detect various emissions from the bed bug, such as carbon dioxide or a combination of carbon dioxide and various pheromones (31, 96). These devices have yet to undergo independent scientific evaluations. Research using acoustic indicators has demonstrated that such devices can successfully detect bed bugs (195), but as of July 2011, these have yet to appear in the marketplace.
While it is impossible to definitely prevent bed bugs, risk management is about undertaking various measures to minimize the potential of an infestation. There are four broad phases in the dynamics of a bed bug infestation: (i) the introduction of the pest, (ii) the establishment of the infestation, (iii) the growth of the pest population, and (iv) the spread of the insect (95). Strategies against all four phases can be undertaken. To minimize the introduction of the pest, the homeowner can learn how to recognize the signs of bed bugs while traveling, to determine if a room is potentially infested, and to know how to avoid bed bugs and how to treat luggage suspected of being contaminated. For student accommodations and other lodging groups, the banning of second-hand furniture and external bedding and linen can aid in reducing the likelihood of the introduction of bed bugs.
In order to reduce the risk of the establishment of an infestation, rooms in accommodation lodgings can be made less susceptible to bed bugs via reducing potential harborages. This can be achieved by ensuring that cracks and crevices are minimized in the room, that furniture and beds are constructed of materials such as smooth metals and plastics rather than timber, and that mattress encasements are installed on the bed (95). Mattress encasements, as well as providing fewer hiding areas for bed bugs, have the additional benefits of being white, making bed bug detection easier; furthermore, some are bite-proof and encase infested mattresses to prevent the escape of bed bugs, which means that the mattress does not have to be discarded (76). Barriers can be fitted to minimize the risk of bed bugs accessing the bed (107, 309, 311).
Strategies against the growth phase involve mainly early detection, as discussed above, and include the training of housekeepers in bed bug detection and the educating of tenants on bed bug recognition to encourage early reporting. Limiting the spread of bed bugs can be achieved through the immediate implementation of control measures upon bed bug detection, the quarantining of infested rooms, and ensuring that infested items are bagged within the room before removal and are treated before relocation (95). It should be noted that many of the above-described recommendations are based on the knowledge of the pest's biology rather than the scientific evaluation of such procedures.
Nonchemical Control
Various nonchemical means of control can be undertaken to either reduce the biomass of the bed bug infestation or achieve complete control. Nonchemical technologies tend to have a more immediate effect on reducing bed bug numbers and have the added advantage of being generally less hazardous than insecticides (244). Usually, some level of insecticide application will be needed, although an integrated program utilizing nonchemical means of control will reduce the amount of insecticidal product required.
The simplest form of nonchemical control is the disposal of infested items. These items need to be sealed in plastic before removal to prevent them from becoming a contamination risk. Furniture earmarked for disposal should be either destroyed or rendered unusable to prevent others from taking the items and subsequently acquiring the infestation. Disposal is not always necessary, as many items can be treated, but the disposal of infested items may be the only economically viable option for heavily cluttered premises.
Vacuuming can very rapidly reduce the bed bug biomass in an infestation and even remove many eggs (90, 106). Vacuum machines are cheap, require little training or operator licensing, and present a minimal risk of spreading an infestation. It is important that the vacuum machine has a disposable bag, which is immediately removed and sealed in plastic after use. Vacuum machines may not remove bed bugs in deep harborages.
Heat is a very practical and effective means of nonchemical bed bug control. The exposure of C. lectularius to 45°C for 1 h will kill all stages (158), and at temperatures over 60°C, all bed bugs are rapidly killed (213). Heat can be applied via the use of steam, through the laundering of infested clothing and bedding, via hot washing and drying (213), and through the use of contained or circulated heat treatments (122, 233, 234). The heating of whole rooms comes with the risk of spreading the infestation, as bed bugs will seek cooler areas above temperatures of 30°C to 35°C (140), and there can be thermally protected areas which do not reach the required temperatures to kill all bed bugs, especially in cluttered rooms (245). The application of heat after insecticide application was found to increase bed bug mortality, as the heat draws the insecticide out of porous surfaces (211). In contrast, the wrapping of infested mattresses in black plastic and exposing them to the sun for thermal control was found to be unsuitable for bed bug management (100).
Conversely, cold temperatures can also be lethal to bed bugs. Infested items can be placed into the freezer; temperatures of −17°C for at least 2 h are required to kill C. lectularius (213). There are various systems that employ gases to instantly freeze bed bugs; however, these can operate only under high pressure, and it is known that small air currents can disperse bed bugs (114). Such devices have been excluded from the Australian bed bug code of practice for their propensity to blow bed bugs about nonlethally and thereby potentially spread an infestation (95).
Keeping a room vacant to starve bed bugs is not a practical option, as bed bugs are long-lived insects. For example, at 18°C, a once-fed bed bug can live for up to 277.1 days (297), while for a typical hotel room set to a constant temperature of 22°C, once-fed bed bugs can survive for around 135 days without a blood meal (91).
Insecticidal Control
Insecticides are usually employed in bed bug management, excepting small infestations. The right type of product and the right formulation are critical for achieving a successful eradication. In light of the insecticide resistance seen in recent bed bug strains and the relatively few products available today, the following discussion will focus on the more recent literature pertaining to insecticide efficacy. Other texts should be consulted for historical information on insecticide use and efficacy (57, 240, 297).
The main groups of insecticides in use worldwide today against bed bugs includes pyrethroids, silicates, and insect growth regulators (IGRs). In some parts of the world, the carbamates and some organophosphates are still in use, while more recently, neonicatinoids and arylpyrroles have begun to be employed.
The pyrethroids are the most common insecticide products in the marketplace and constitute, for example, around 95% of the products registered for bed bug control in Australia (95). However, resistance to these products is well documented (45, 171, 185, 209, 261, 282, 284, 303, 323). The pyrethroids are classified according to when they were discovered and are placed into four generations (48); generally, the older the generation, the less effective against bed bugs. For example, in resistance testing comparing a modern resistant strain of C. lectularius with an old susceptible strain, the lethal dose to kill 50% of the test bugs (i.e., LD50) was 1.4 million times different with permethrin (a third-generation pyrethroid) and around 430,000 times different with deltamethrin (fourth generation) (106, 185). When fourth-generation pyrethroid products were applied directly onto adults of a resistant C. lectularius strain at label rates, after 10 days, only around 60% mortality was achieved (with the control mortality rate being 20%). When the same bed bug strain was placed onto dried residuals of pyrethroids treated at label rates, the rate of mortality was reduced to around 30% after 10 days (106, 183, 184). The addition of a synergist, such as piperonyl butoxide (PBO), can increase topical mortality by overcoming the resistance mechanism in some (but not all) strains of C. lectularius and C. hemipterus; likewise, the addition of PBO does not always enhance residual efficacy (151, 184, 258). Thus, the pyrethroids generally have poor efficacy, particularly when applied as a residual, against modern resistant bed bug strains. The other disadvantage is that when exposed to sublethal doses of pyrethroids, resistant bed bugs can become excited (259). The implication is that sublethal doses may lead to the dispersal of an infestation in poorly treated premises. In contrast, a susceptible strain was not found repellent (207). Natural pyrethrins have also been found to be ineffective against a modern resistant C. lectularius strain (183, 184).
A number of groups are now marketing permethrin-impregnated fabrics such as mattress ticking and mattress covers, with the claim that they are able to control bed bugs. As indicated above, permethrin has very poor efficacy against resistant bed bug strains, and it is almost expected that when such products have been evaluated in independent studies, they have proven to be ineffectual against modern strains C. lectularius (103). Thus, there appears to be no benefit in the use of permethrin-impregnated fabrics in a bed bug management program.
There are a number of silicate products available around the world in an aerosol or dust formulation, with the most common being diatomaceous earth dust (DED). The silicates have a very different mode of action from those of the other insecticides. Most products disrupt the insect's physiology, but the silicates have a physical action: they absorb lipids on the waxy surface of the epicuticle such that the insect can no longer maintain moisture and dies from dehydration (275). The silicates offer a number of benefits; they have a very long shelf life, very low mammalian toxicity, a long residual life, and a low possibility of resistance developing due to the physical action of the product and are one of the few products that could be used as a prophylactic insecticide (104). Their main disadvantage is that they are slow acting. One study found that DED took up 6 days to achieve 100% mortality in adult bed bugs (the species was not stated but was presumably C. lectularius from the images of dusted bed bugs) (257). Against an Australian strain of adult C. lectularius, DED took up to 15 days to yield 100% mortality, although the rate was dose dependent; higher doses produced a faster kill (104). First-instar bed bugs succumb more quickly to DED, and most die within 3 days of exposure (104). Another advantage of the slow action of DED is that dusted bed bugs can transfer the insecticide to untreated bugs, thereby inducing a high rate of secondary mortality. By placing dusted adult bed bugs with first-instar C. lectularius insects, 80% nymphal mortality was achieved within 4 days, and all had died by day 12 (Doggett, unpublished).
IGRs function to disrupt the physiology of the insect, and the pest tends to die during subsequent molts after being dosed. One IGR, (S)-methoprene, was found to be effective in laboratory trials at killing both susceptible and resistant strains of C. lectularius in the United Kingdom (212). When another IGR, hydropene, underwent field evaluations, being used in conjunction with pyrethroids, a 95% reduction in bed bug populations was achieved, although it was impossible to determine the relative contribution of the IGR to the suppression of the bed bug populations (207). There are, however, ethical issues surrounding the use of IGRs. When the product is applied to the nymphal stages, there are few direct adverse effects; rather, the insect needs to obtain a blood meal for the insecticide to work. Thus, the product relies on people being bitten (97). Potentially, this product could present a litigation risk to pest managers; a litigious customer may not appreciate the use of a product that is reliant on their being fed upon.
There is also resistance to the carbamate group of insecticides (45, 185), although the degree of resistance is much lower than with the pyrethroids. In one of the resistance studies mentioned above, bendiocarb had an LD50 that was only 238 times different between the resistant and susceptible strains of C. lectularius (185). In topical and residual trials against a resistant C. lectularius strain, bendiocarb applied at label rates performed similarly to the pyrethroids; however, when applied at half the label rate (termed a “maintenance” dose in Australia), the level of efficacy did not noticeably decrease, while the pyrethroids failed to provide any level of control (183, 184). As noted above, there have been attempts in the United States to have propoxur reregistered for bed bug control. However, propoxur is a carbamate, and therefore, some level of resistance must be expected, and this has been observed in our laboratory trials (Doggett, unpublished). Bed bugs from Thailand were also found to be resistant to propoxur (284). Propoxur also has an unpleasant odor, meaning that many clients would not want products with this active ingredient applied in their facility or home.
In Europe and the United States, organophosphates (OPs) are no longer available for bed bug management, except in impregnated strips, although they are employed in many other countries (246). One OP, pirimiphos methyl, has been assayed against a pyrethroid-resistant strain of C. lectularius, and no resistance was detected (185). When the insecticide was applied directly to this strain at label rates, all bed bugs died within 5 h (184). The pyrethroid-resistant strain was also exposed to aged deposits of pirimiphos methyl with the surface treated at label rates; even 52 weeks after the initial application, 100% mortality was achieved within 24 h of exposure (106; Doggett, unpublished). The major downside of these OP products is that although they are still registered, they have staining and odor issues such that they tend not to be widely used by pest managers in Australia. Dichlorvos (2,2-dichlorovinyl dimethyl phosphate [DDVP]) is another OP that is used as a vapor toxicant in many countries, whereby the product is impregnated into plastic strips. It is used for the small-scale fumigation of infested items; items with bed bugs, such as luggage or small electronic devices, can be placed into sealed plastic bags with the strips, and high levels of control can be achieved over some days (242). The speed of efficacy of dichlorvos can be increased substantially through the application of heat, which increases the volatility of the insecticide (233).
In late 2011, resistance to the OPs was reported for both C. lectularius and C. hemipterus bed bugs from Thailand (284) and for C. lectularius bed bugs collected from Denmark (171). Those investigations appear to be the first modern reports of resistance to OPs. In the Danish study, the frequency of resistance in bed bug populations was found to be low, and when the resistant populations were tested against a microencapsulated formulation of the OP chlorpyrifos, high morality rates ensued. This indicates that the degree of resistance is presently not high. Molecular evidence shows that bed bug populations are genetically heterogeneous across different locations (303), with the implication that they are continually being spread across different nations. The translocation of OP-resistant strains elsewhere in the world to locations where resistance to this insecticide group is not currently present may make future bed bug control even more challenging.
Of the arylpyrrole insecticides, the active ingredient chlorfenapyr is registered in a number of countries for the control of bed bugs. This product has a very different mode of action from that of the pyrethroids, and therefore, resistance is unlikely. The published efficacy data have demonstrated variable findings, although they consistently show that the insecticide is very slow acting. In the first laboratory trial published, Phantom Insecticide (the commercial product name of chlorfenapyr), when tested as a contact insecticide against C. lectularius, was so ineffective that the treated bed bugs mated and laid eggs, and many of the hatching nymphs survived (207). The authors of that study also evaluated Phantom Insecticide in conjunction with other insecticides in field trials, whereby the bed bug population was reduced by 86% (208); however, again, it was impossible to determine the relative contribution of each insecticide to the reduction of the bed bug population. In a field trial in Cincinnati, OH, 15 bed-bug-infested apartments (presumably C. lectularius bed bugs) were treated with Phantom Insecticide on a monthly basis. Additionally, some nonchemical means of management were undertaken, along with a limited application of silicaceous products to nine of the apartments (243). It took an extraordinary 5 months before bed bugs could no longer be detected in 12 of the apartments, and 3 remained infested. Such a prolonged time to achieve only 80% control suggests that this product has limited practical value, particularly as other control methodologies were coemployed. However, laboratory trials from that same research group found that the product could control both pyrethroid-susceptible and -resistant strains of C. lectularius albeit slowly: the calculated lethal time to achieve 90% mortality was up to around 9 days (260). Those authors also observed that the bed bugs did not avoid treated surfaces, suggesting that the product is nonrepellent, and that aged deposits of insecticides of up to 4 months were as efficacious as freshly dried deposits (260). Similarly, a trial from Thailand found that chlorfenapyr was effective against multiple-insecticide-resistant C. lectularius and C. hemipterus bed bugs (284). In contrast, another laboratory trial evaluating Phantom Insecticide against C. lectularius observed almost no efficacy (103). The product was applied to susceptible and resistant strains of C. lectularius via direct spray at label rates, and the mortality was monitored for up to 22 days postapplication. At that time, both test and control mortality rates were 70% and not appreciably different with either strain. When applied as a residual, mortality was again poor; after 22 days, the test mortality rate was only around 20% greater than that of the controls. Chlorfenapyr is the only insecticide tested by our laboratory to date that has failed to control an insecticide-susceptible strain of C. lectularius. The reasons for the reported variations in efficacy with chlorfenapyr are unknown and could relate to the different experimental protocols, variations in strain efficacy, or, possibly, batch variation with the insecticide. Presently, it is not known if such variations in efficacy have been widely translated to the treatment of field infestations.
Within the neonicatinoid insecticides, imidacloprid has been evaluated against resistant strains of C. lectularius and C. hemipterus, and no resistance to this insecticide was found (185, 284). In one trial, for the direct topical application of formulations of this product at the label rate, 100% mortality was achieved within 2 h against pyrethroid-susceptible and -resistant C. lectularius strains (185). When applied as a residual treatment, the product was less effective, producing a mortality rate of around 50% after 12 days of exposure to the pyrethroid-resistant C. lectularius strain (Doggett, unpublished). Another research group tested the insecticide against C. hemipterus and found that imidacloprid was less efficacious than some pyrethroids (151), but the susceptibility of the bed bug strain used was not stated, as resistance in colonized strains can be lost over time. Despite the poor residual effect, imidacloprid should prove beneficial to the pest manager for the control of bed bugs, and this insecticide is starting to appear on the market in commercial formulations.
In the United States, there are a number of chemicals with insecticidal properties being marketed for bed bug control that are exempt from EPA registration. These include “enzymes” (95) and cedar oil. The modes of action of these chemicals are not known, and published efficacy data are lacking. A preliminary report from an independent group indicated that Best Yet cedar oil can kill all bed bugs (species not stated) within 1 min and has a strong ovicidal effect, with no nymphs emerging from treated eggs, although residual control is poor (19). Further efficacy work is required to determine if these products have a real benefit for the control of field infestations.
The type of insecticide formulation can influence treatment success, as the product needs to be applied directly onto the insects. Insecticide “bombs” (which apply insecticides to open spaces via aerosols), space sprays, and incendiary smoke generators tend not to place the insecticide into cracks and crevices where bed bugs harbor. These products tend to have pyrethroids as the active insecticide and may induce a flushing effect, thereby potentially spreading the infestation.
Insecticide dusts are often more effective than their liquid counterparts. One study evaluated the pyrethroid cyfluthrin as a dust, with all pyrethroid-resistant bed bugs (presumably C. lectularius) being killed within 24 h (257). Why a dust formulation would be more effective is unknown; the authors of that study concluded that the carriers in the dust may facilitate insecticide uptake or have insecticidal properties themselves. Similarly, aerosol formulations tend to be more effective than nonaerosolized liquids, often producing a complete kill within 2 h, but tend to perform poorly as dried residuals (Doggett, unpublished). This finding suggests that either the carriers or the propellants are increasing insecticide absorption or have an insecticidal action.
Fumigation is the process of employing gaseous insecticides to control insects and can be undertaken on whole structures or smaller contained areas. The great advantage of fumigants is their ability to penetrate into all areas. Fumigation with sulfuryl fluoride was successfully undertaken on an 80-room apartment building in Pennsylvania (204). The decision to use this process was based on the high number of premises (40 out of 80) that became infested despite repeated spray treatments. Generally, however, for bed bug management, whole-structure fumigation is rarely undertaken, as it is expensive and presents logistical problems when treating whole apartment complexes, as all residents must be relocated during the treatment. Fumigants are highly toxic to humans and require specialized training for their application. It is not appropriate to treat single rooms within apartment complexes, as the gas cannot be tightly contained. Thus, there is a high risk of injury to others in the same building, and there have been deaths due to the inappropriate use of fumigants (103). Off-site containment fumigation for controlling bed bugs in infested furnishings and other transportable items has been found to be effective and poses less of a human health risk, as the application can be undertaken away from residences (306).
The Future of Insecticides
The cost to develop and market new insecticidal agents is prohibitively expensive. It was estimated in 2006 that the introduction of a new active insecticide would cost over US$180 million (317). This means that it is highly unlikely that new active insecticides will be developed specifically for bed bug control, as the financial returns may not cover this expenditure, and thus, no magical “silver bullet” will be forthcoming. Rather, insecticide manufacturers will be forced to look into existing active agents registered for other insect applications or to examine currently registered compounds and develop “smarter” formulations that can better deliver the insecticide to the pest or increase the contact of the pest with the insecticide. Bed bugs are extremely waxy insects and are very resilient to dehydration (40). We have observed water-based insecticides beading on the cuticle of bed bugs in laboratory trials (Doggett, unpublished), which means that in field applications, the products may bounce off the insects during spray operations. In laboratory trials, we have found that an insecticide placed directly onto the insect and allowed to dry produces a higher rate of efficacy than the same product applied via spray (103). Thus, a product that better adheres to the insect may provide improved efficacy.
The addition of bed bug alarm pheromone components to silicate desiccant dusts has found to increase efficacy against C. lectularius (39), although there can be odor issues with such compounds. Other researchers have suggested that pheromones may be used for the control of bed bugs (139, 141, 316), but such products may be some time off from entering the marketplace.
One of the major issues with insecticide is that many products presently on the market are ineffective. This stems from the fact that government insecticide registration authorities around the world fail to insist on appropriate efficacy evaluations of new products and old products when reregistration is required. In Australia, for example, the Australian Pesticides and Veterinary Medicines Authority often requires no data on efficacy against bed bugs to be included on insecticide product labels and does not insist that when data on efficacy against bed bugs are required, they are gathered from modern resistant strains, despite the many publications on the existence of resistance (103). This, unfortunately, is a common scenario and one which some companies have exploited to the full. There have been claims that permethrin-impregnated fabrics can kill bed bugs within 48 h (although they have shown not to do so in independent tests with a modern pyrethroid-resistant field strain [103]) and that various pyrethroid products can knock down “all” bed bugs within 25 min, which is highly improbable with modern resistant strains. Unfortunately, researchers may be in a difficult position and are reluctant to publicly contradict manufacturers of inefficacious products for fear of litigation or offending potential funding sources (193). The result of this misinformation is confusion for the pest management industry and repeated treatment failures.
Recently, in 2011, the NPMA released a public policy position statement on the registration of pesticides for bed bugs (222). The NPMA is encouraging the U.S. EPA to expedite the registration of new products for bed bug control, to consider the impacts on society of not registering a particular insecticide, and to ensure that efficacy data must be required for all insecticides claiming to control bed bugs. Of course, as stated above, the efficacy data must be obtained for modern resistant strains. This stance by the NPMA should be applauded, and all pest managers and researchers around the world should use this position statement as a model to encourage policy change with their own respective registration authorities.
BED BUGS: THE FUTURE
Indications are that bed bugs will continue be a societal pest for many years to come. In the near future, there is unlikely to be any magical silver-bullet technology developed for controlling this pest which might rapidly defeat this insect, as in the case of DDT during the 1950s. This means that people will continue to be exposed to bed bugs and all their various deleterious effects. Multidisciplinary strategies need to be implemented to combat this pest, and we believe that a long-term strategy should encompass the following four key areas (95):
Defining the cost of the resurgence
Developing industry standards that promote best practice in bed bug management
Educating stakeholders in best practice
Research
Despite the large number of publications that have reported the bed bug resurgence over recent years, the economic consequences are still largely ill defined. In 2011, it was very conservatively estimated that bed bugs have cost the Australian economy at least Aus$200 million since the start of the resurgence (102). Perhaps, these figures may be able to provide crude estimates of financial costs to other nations. For example, the United States has a population 15 times that of Australia, and thus, the crude calculation of the financial cost becomes US$3 billion. However, the United States has one particular risk factor that suggests that the real costs are probably much higher: the larger number of people living in apartment complexes. As discussed above, bed bugs can rapidly spread from one unit to others in an apartment complex, particularly if control is poorly undertaken. In the United States, a much greater proportion of the population lives in apartment and unit complexes: 45% of the population live in complexes with five or more separate units (219), compared to around 22% in Australia (3). The other compounding factor that may suggest that bed bug fiscal impacts have been much greater than the above-mentioned crude figure is that the key industry pest management association had up until this year failed to provide an industry standard on bed bug management.
These crude figures are conservative and do not factor-in many related costs, nor can they account for the associated human morbidity (102). The involvement of health economists is imperative to accurately determine the real costs of bed bugs on society, and calculations must encompass both the direct and indirect financial impacts. Doing so may provide the necessary justification for government and granting bodies to commit funds for bed bug control in those situations where residents do not have the fiscal resources to pay the eradication costs and for the progression of the other strategies listed above. Spending money to combat bed bugs now is an investment for the future and will be economic in the long term. In our experience, what has encouraged accommodation facilities to undertake bed bug risk management the most has been the calculation of the actual costs resulting from bed bug infestations within their facilities (Doggett, unpublished).
The development of quality industry standards for bed bug management is a vital strategy for the long-term combat against bed bugs. Such standards aim to encourage best practice in terms of the management of both of active infestations and potential infestations. The advantages of bed bug industry standards have been extensively reviewed (102), and one key benefit is customer protection. In recent years, as mentioned above, the marketplace has been flooded with management devices. Many of these devices appear to be conceptually flawed and perhaps are just an unscrupulous attempt to gain short-term profits, and others appear conceptually weak (such as the myriad of harborage-type traps that are little more than highly marketed pieces of cardboard), while some technologies actually appear conceptually sound and are based on aspects of the pest's biology (97). However, most do not come with quality efficacy data; an industry standard can review these technologies independently and make recommendations on their use. Industry standards, compared with the other strategies, are relatively cheap and quick to produce and can offer immediate benefits.
The bed bug management field is highly dynamic. Almost every week, a new management technology appears on the market or a scientific article is published. Thus, industry standards must be reviewed regularly and updated accordingly, especially to deliver new and complex scientific information to stakeholders in a readily understandable form. For these reasons, the Australian bed bug code of practice comes with a use-by date; it is stated within the document that it is considered valid for 18 months from the date that appears on the front cover (95). However, this standard has been updated annually since it first appeared.
Industry standards and their working parties need to maintain a high degree of independence. This is particularly challenging in the pest management world, as industry associations all receive considerable funding from insecticide manufacturers. Probity guidelines, such as those developed in Australia (228), need to be established and complied with by those involved in the development of industry standards. The process of producing and reviewing industry standards must be transparent and open to public scrutiny. Standards should also be available to all at nil cost to encourage all stakeholders to undertake best practice in bed bug management.
Currently, there are many threats to the provision of quality education in bed bug management (103). Many articles in pest management magazines are simply advertorials masquerading as science, while company presentations at meetings are simply about product promotion and tend to be at the expense of quality information. As industry standards aim to present best practice, these should form the basis of educational and training programs on bed bug management. For pest managers trained in best practice and for accommodation groups undertaking risk management, fewer treatment failures should result, there will be a reduced risk of bed bugs becoming established, and this should lead ultimately to a reversal of current resurgence trends.
Research on bed bugs is required in many fields but especially in the area of pest management and, in particular, in the development of technologies which can make bed bug control more affordable. The results of such research can be used to develop best-practice guidelines within industry standards.
In addition to the above-mentioned strategies, one added approach that is almost uniquely American is legal enforcement. In recent years, several states in the United States have introduced or proposed legislation to combat the rise in bed bugs (30). In New York City, landlords must disclose if bed bugs have been in the building within the preceding year. In Maine and Massachusetts, landlords are now responsible for paying for bed bug control, even if the tenants introduced the infestation. In New Jersey, landlords must provide educational materials on bed bugs to tenants and ensure that bed bug control is undertaken promptly when an infestation is recognized; failure to take action can result in severe financial penalties. Several other states are now considering similar legislation. One of the more positive proposed bills, which was introduced to the U.S. House of Representatives on 9 March 2011 and is presently pending, is HR 967, the Bed Bug Management, Prevention, and Research Act (296). This act, currently relevant to only three states, would establish a grant program to provide funding for bed bug research and for assistance in control. This is a positive move, as one of the greatest challenges faced today among the socially disadvantaged is determining who pays for bed bug management. Without funds to assist those who cannot pay the high price of control, bed bugs are set to endure among the lower socioeconomic groups, who will then come to act as a pest reservoir for the wider society (256). Government support for control in such segments of society is essential for achieving a long-term downturn in numbers of bed bug infestations.
As of late 2011, it is not possible to write a definitive conclusion to this story; the global fight against bed bugs has only just begun.
ACKNOWLEDGMENTS
We are grateful to Harold Harlan, Armed Forces Pest Management Board, who provided discussions on bed bug feeding behavior and the relevance of the recent findings of MRSA on bed bugs; Greg Whiteley, Whiteley Corporation, who provided papers relating to MRSA rates in the general community; and Richard Cooper, Coopers Pest Solution, and Oliver Madge, the Bed Bug Foundation, who provided input on the mental health impacts of bed bugs. We are also grateful to the various individuals who permitted the reproduction of their bed bug reactions who cannot be named, in order to maintain patient confidentiality.
Biographies

Stephen L. Doggett is a Senior Hospital Scientist with the Department of Medical Entomology at Westmead Hospital in Sydney, Australia. His research background is extremely broad, having worked on various arthropods of medical importance, notably ticks, mosquitoes, and bed bugs. Since the beginning of the modern bed bug resurgence, Doggett has been at the forefront of documenting the rise and impact of bed bugs within Australia and has produced almost 100 articles for industry and scientific journals and given over 85 presentations on bed bugs and their control. He is widely consulted by the pest management and accommodation industries and the media, both locally and internationally. Over the last six years, he has worked with a distinguished team of Australian experts in producing an industry standard on bed bug management, A Code of Practice for the Control of Bed Bugs in Australia, and is the principal author. The code is now in its draft 4th edition (and seventh version) and has been adopted by other pest control organizations around the world. More recently, to assist the Hospitality industry and other accommodation providers in achieving “best practice” in bed bug eradication, Doggett developed A Bed Bug Management Policy for Accommodation Providers. Both the Code of Practice and the Management Policy are available for free from http://www.bedbug.org.au.

Dominic E. Dwyer is an infectious diseases physician and Director of the Centre for Infectious Diseases and Microbiology Laboratory Services at Westmead Hospital in Sydney, Australia. He is a member of the Sydney Institute for Emerging Infectious Diseases and Biosecurity at Sydney University. He has a clinical and research interest in viral and other diseases of public health importance and runs a clinical trials unit in antiviral drugs and vaccines. He has a research interest in antiviral drug resistance. He assists State and National governments in planning for emerging infections and is actively involved in disease outbreak investigations.

Pablo F. Peñas is Associate Professor in Dermatology at the University of Sydney, Senior Medical Practitioner (Academic) at Westmead Hospital, and Head of Research at the Skin and Cancer Foundation Westmead. Dr. Peñas is an M.D. and Ph.D. of the Universidad Autonoma of Madrid, Spain. Previously Staff Specialist at Hospital Universitario de la Princesa (Madrid, 1995 to 2007), he moved to Sydney, Australia, in 2007. Although his Ph.D. was on the adhesion and motility of keratinocytes, and he has worked in photobiology with melanocytes and dendritic cells, he is focusing on translational and clinical research in Dermatology. He has been a Board Member of the Spanish and the European Academies of Dermatology and Venereology. He is a member of the Maintenance Team of the IEC/EN 60335-2-27 Standard, which deals with the safety of sunbeds, both in the International Electrotechnical Commission and in the European Committee for Electrotechnical Standardization. He has recently been invited to join the WHO ICD11 Dermatology Topic Advisory Group.

Richard C. Russell is Professor of Medical Entomology at the University of Sydney and Founding Director of the Department of Medical Entomology at Westmead Hospital in Sydney, Australia. He has experience with all aspects of medical and public health entomology. His research has been conducted primarily on mosquitoes and their role as pests and vectors of pathogens, and he established the NSW Mosquito and Arbovirus Surveillance Program in 1984. He is author and coauthor of several monographs, has published more than 200 scientific papers on medical entomology topics and more than 200 consultancy reports, and has presented more than 150 papers at scientific meetings in Australia and in many countries internationally. He is an advisor on vector-borne disease to the Commonwealth, NSW, and other State Health Departments, has been the Australian delegate to the International Federation for Tropical Medicine, and has worked as a consultant in all Australian states and internationally in 18 countries, principally for the World Health Organization and AusAID.
REFERENCES
- 1. Abdel-Nasar MB, Lotfy RA, Al-Sherbiny MM, Ali NMS. 2006. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol. Res. 98: 550–556 [DOI] [PubMed] [Google Scholar]
- 2. Abou Gamra ESM, El-Shayed FA, Morsy TA, Hussein HM, Shehata ESZ. 1991. The relation between Cimex lectularius antigen and bronchial asthma in Egypt. J. Egypt. Soc. Parasitol. 21: 735–746 [PubMed] [Google Scholar]
- 3. ABS 2008. Housing occupancy and costs, 2007–08. Australian Bureau of Statistics, Canberra, Australia: http://www.ausstats.abs.gov.au/Ausstats/subscriber.nsf/0/0182E400E70CD78BCA25766500159C4F/$File/41300do001_200708.xls [Google Scholar]
- 4. Amara S, Anuradha A, Silverman B, Schneider AT. 2007. Resurgence of bedbug bites misdiagnosed as allergic skin rashes in inner-city population. Ann. Allergy Asthma Immunol. 98(Suppl. 1): A86 [Google Scholar]
- 5. Anders D, Bröker E-B, Hamm H. 2010. Cimex lectularius—an unwelcome train attendant. Eur. J. Dermatol. 20: 239–240 [DOI] [PubMed] [Google Scholar]
- 6. Anderson A. 2011. The decade of bedbugs and fear. Environ. Health Insights 5: 53–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson JF, Ferrandino FJ, McKnight S, Nolen J, Miller J. 2009. A carbon dioxide, heat and chemical lure trap for the bedbug, Cimex lectularius. Med. Vet. Entomol. 23: 99–105 [DOI] [PubMed] [Google Scholar]
- 8. Anonymous. 25 January 2007. Bed bug problems heat up Salt Lake fire department. Pest Management Professional Online Headline News. http://www.mypmp.net/bed-bugs/bed-bug-problems-heat-up-salt-lake-fire-department
- 9. Anonymous. 2007. Guidelines for the control and prevention of bed bug infestations in California. State of California Health and Human Services Agency, Department of Health Services, Sacramento, CA: http://www.cdph.ca.gov/pubsforms/Guidelines/Documents/CaliforniaBedBugGuidelines.pdf [Google Scholar]
- 10. Anonymous. 2009. Bedbugs lead to PL ban. Library J. 134: 14 [Google Scholar]
- 11. Anonymous. 2009. Preventing and getting rid of bed bugs safely, a guide for property owners, managers and tenants. New York City Department of Health and Mental Hygiene and Department of Housing Preservation and Development, New York, NY: http://www.nyc.gov/html/doh/downloads/pdf/vector/bed-bug-guide.pdf [Google Scholar]
- 12. Anonymous. 2010. Unlicensed PCO charged with using banned pesticides to treat bed bugs. Pest Control Technologies Online Headline News. http://www.pctonline.com/pct-062210-unlicensed-pco-charged-banned-pesticides-bed-bugs.aspx
- 13. Anonymous. 2010. EDs trying not to let the bed bugs bites. ED Manag. 2010: 100–101 [PubMed] [Google Scholar]
- 14. Anonymous. 6 November 2010. PA hospital had bed bugs. Star-Phoenix, p A4 [Google Scholar]
- 15. Anonymous. 7 December 2010. Portsmouth preschool to reopen after bed bug infestation. NewsChannel3. http://www.wtkr.com/news/wtkr-bedbug-story-suri,0,1545343.story [Google Scholar]
- 16. Anonymous. 12 January 2010. Juicy Couture hit with case of bedbugs. MSNBC.com. http://www.msnbc.msn.com/id/40450948/ns/business-us_business [Google Scholar]
- 17. Anonymous. 16 September 2010. N. C. college closes half its dorms due to bed bug problem. Pest Control Technology Online News. http://www.pctonline.com/Catawba-College-bed-bug-closings.aspx
- 18. Anonymous. 2010. Bedbug bites becoming bigger battle. CMAJ 182: 1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anonymous. 2010. A review of “Best Yet” cedar oil product. BedBug Central. http://www.bedbugcentral.com/research-and-development/topic.cfm/a-review-of-best-yet-cedar-oil-product [Google Scholar]
- 20. Anonymous. 2011. Bed bug treatment sets house on fire. National Pest Management Association, Fairfax, VA: http://www.allthingsbedbugs.org/bed-bug-news/bed-bug-treatment-sets-house-on-fire.aspx [Google Scholar]
- 21. Anonymous. 9 May 2011. Toxin linked to hotel deaths. New Zealand Herald. http://www.nzherald.co.nz/nz/news/article.cfm?c_id=1&objectid=10724300 [Google Scholar]
- 22. Anonymous. 9 May 2011. Tourist may have died from insecticides. MSNNZ News. http://news.msn.co.nz/article/8246721/tourist-may-have-died-from-insecticides [Google Scholar]
- 23. Anonymous. 2011. Woman turned away from hospital because of bed bugs. National Pest Management Association, Fairfax, VA: http://allthingsbedbugs.pestworld.org/bed-bug-news/woman-turned-away-from-hospital-because-of-bed-bugs.aspx [Google Scholar]
- 24. Anonymous. 2011. Bed bug spray. Bed Bug Mundo. http://www.bedbugmundo.com/bed-bug-spray.html [Google Scholar]
- 25. Anonymous. 2011. Bugs biting back? Int. Pest Control 53: 9 [Google Scholar]
- 26. Anonymous. 9 April 2011. Bedbugs on the treadmill? New York Post. www.nypost.com/p/pagesix/bedbugs_on_the_treadmills_vwj856cUV86g504cvqFt5K [Google Scholar]
- 27. Anonymous. 14 March 2011. Retailers can squash bedbug-related lawsuits. PR Newswire. www.prnewswire.com/news-releases/retailers-can-squash-bedbug-related-lawsuits-attorney-says-117928974.html [Google Scholar]
- 28. Anonymous. 28 February 2011. British Airways jet grounded after bed bug infestation. Sydney Morning Herald Online. http://www.smh.com.au/travel/travel-news/british-airways-jet-grounded-after-bed-bug-infestation-20110228-1bar3.html [Google Scholar]
- 29. Anonymous. 2011. Orkin's top 50 bed bug cities. Pest Control Technol. 39: 147 [Google Scholar]
- 30. Anonymous. 2011. Bed bug laws. J. Prop. Manag. 2011(March-April): 16 [Google Scholar]
- 31. Anonymous. 2011. Invention awards: sniffing out bedbugs. National Pest Management Association, Fairfax, VA: http://allthingsbedbugs.pestworld.org/bed-bug-news/2011-invention-awards-sniffing-out-bedbugs.aspx [Google Scholar]
- 32. AOL 2010. 2010 year end hot searches. AOL. http://about-search.aol.com/hotsearches2010/index.html [Google Scholar]
- 33. APVMA 2011. What is the APVMA doing about permethrin toxicity in cats? Australian Pesticides and Veterinary Medicines Authority, Canberra, Australia: http://www.apvma.gov.au/news_media/community/2011-01_permethrin_cats.php [Google Scholar]
- 34. Araujo RN, Costa FS, Gontijo NF, Goncalves TCM, Pereira MH. 2009. The feeding process of Cimex lectularius (Linnaeus 1758) and Cimex hemipterus (Fabricius 1803) on different bloodmeal sources. J. Insect Physiol. 55: 1151–1157 [DOI] [PubMed] [Google Scholar]
- 35. Azmi HK, Ali SZ. 1998. Use of invertebrates as traditional drugs among the tribals of Chhatisgarh. J. Living World 5: 22–29 [Google Scholar]
- 36. Bello P. 2010. Bed bugs, the human side. Pest Manag. Prof. 2010(February): 43–45 [Google Scholar]
- 37. Bencheton AL, et al. 2010. Resurgence of bedbugs in southern France: a local problem or the tip of the iceberg? J. Eur. Acad. Dermatol. 25: 599–602 [DOI] [PubMed] [Google Scholar]
- 38. Benoit J. 2011. Stress tolerance of bed bugs: a review of factors that cause trauma to Cimex lectularius and C. hemipterus. Insects 2: 151–172 doi:10.3390/insects2020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benoit JB, et al. 2009. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. J. Med. Entomol. 46: 572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benoit JB, Del Grosso NA, Yoder JA, Denlinger DL. 2007. Resistance to dehydration between bouts of blood feeding in the bed bug, Cimex lectularius, is enhanced by water conservation, aggregation, and quiescence. Am. J. Trop. Med. Hyg. 76: 987–993 [PubMed] [Google Scholar]
- 41. Berg R. 2010. Bed bugs: the pesticides dilemma. J. Environ. Health 72: 32–49 [PubMed] [Google Scholar]
- 42. Bircher AJ. 2005. Systemic immediate allergic reactions to arthropod bites and stings. Dermatology 210: 119–127 [DOI] [PubMed] [Google Scholar]
- 43. Bloom GM, Cooper RA, Corea R, Gangloff-Kaufmann JL, Lopez R. 2010. Recommendations for the management of bed bugs in New York City. New York City Bed Bug Advisory Board, New York, NY: http://council.nyc.gov/downloads/pdf/bed_bugs_report_2010.pdf [Google Scholar]
- 44. Boase C. 2001. Bedbugs–back from the brink. Pestic. Outlook 2001(August): 159–162 [Google Scholar]
- 45. Boase C, Small G, Naylor R. 2006. Interim report of insecticides susceptibility status of UK bedbugs. Prof. Pest Control 2006(Summer): 12–13 [Google Scholar]
- 46. Borts MR. 2006. Cimex lectularius (bedbug) bites presenting as chronic urticaria. J. Allergy Clin. Immunol. 2: S310 [Google Scholar]
- 47. Bouchard MF, et al. 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environ. Health Perspect. 119: 1189–1195 doi:10.1289/ehp.1003185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Braness GA. 2004. Insecticides & pesticide safety, p 1098–1163 In Mallis A, Hedges SA, Moreland D. (ed), Handbook of pest control, 9th ed. GIE Media, Richfield, OH [Google Scholar]
- 49. Brasch J, Schwarz T. 2006. 26-year old male with urticarial papules. J. Dtsch. Dermatol. Ges. 4: 1077–1079 [DOI] [PubMed] [Google Scholar]
- 50. Brault AC, et al. 2009. Stone Lakes virus (family Togaviridae, genus Alphavirus), a variant of Fort Morgan virus isolated from swallow bugs (Hemiptera: Cimicidae) west of the Continental Divide. J. Med. Entomol. 46: 1203–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brotman B, Prince AM, Godfrey HR. 1973. Role of arthropods in transmission of hepatitis-B virus in the tropics. Lancet 301: 1305–1308 [DOI] [PubMed] [Google Scholar]
- 52. Brown CR, Moore AT, Young GR, Komar N. 2010. Persistence of Buggy Creek virus (Togaviridae, Alphavirus) for two years in unfed swallow bugs (Hemiptera: Cimicidae: Oeciacus vicarius). J. Med. Entomol. 47: 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brunswick M. 21 April 2011. VA to quilters: thanks but please quit. Star Tribune. www.startribune.com/local/minneapolis/120325859.html [Google Scholar]
- 54. Burnett JW, Calton GJ, Morgan RJ. 1986. Bedbugs. Cutis 38: 20. [PubMed] [Google Scholar]
- 55. Burton GJ. 1963. Bedbugs in relation to transmission of human diseases. Public Health Rep. 78: 513–524 [PMC free article] [PubMed] [Google Scholar]
- 56. Burton GJ. 1963. Bedbugs in relation to transmission of human diseases, addenda. Public Health Rep. 78: 953. [PMC free article] [PubMed] [Google Scholar]
- 57. Busvine JR. 1958. Insecticide-resistance in bed-bugs. Bull. World Health Organ. 19: 1041–1052 [PMC free article] [PubMed] [Google Scholar]
- 58. Busvine JR. 1980. Insects & hygiene. The biology and control of insect pests of medical and domestic importance, 3rd ed. Chapman & Hall, London, United Kingdom [Google Scholar]
- 59. Carter S. 11 May 2011. Debate over pesticide's role in tourists' mystery deaths. Sydney Morning Herald Online. http://www.smh.com.au/travel/travel-incidents/debate-over-pesticides-role-in-tourists-mystery-deaths-20110511-1ei2f.html [Google Scholar]
- 60. Carver M, Gross GF, Woodward TE. 1991. Hempitera, p 429–509 In Naumann ID. (ed), The insects of Australia, 2nd ed, vol 1 Melbourne University Press, Carlton, Australia [Google Scholar]
- 61. Cassels C. 14 May 2011. Impact of bed bugs much more than skin deep. Medscape Today News. http://www.medscape.com/viewarticle/742775 [Google Scholar]
- 62. Cassidy L. 2010. Taking a bite out of the bed bug sialome. J. Proteome Res. 9: 3765. [DOI] [PubMed] [Google Scholar]
- 63. Cater J, Magee D, Hubbard SA, Edwards KT, Goddard J. 2011. Severe infestation of bed bugs in a poultry breeder house. J. Am. Vet. Med. Assoc. 239: 919. [DOI] [PubMed] [Google Scholar]
- 64. Centers for Disease Control and Prevention and U. S. Environmental Protection Agency 2010. Joint statement on bed bug control in the United States from the U. S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (EPA) Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nceh/ehs/publications/bed_bugs_cdc-epa_statement.htm [Google Scholar]
- 65. Centers for Disease Control and Prevention 2011. Acute illnesses associated with insecticides used to control bed bugs—seven states, 2003–2010. MMWR Morb. Mortal. Wkly. Rep. 60: 1269–1273 [PubMed] [Google Scholar]
- 66. Centers for Disease Control Prevention 2011. Acute illnesses associated with insecticides used to control bed bugs—seven states, 2003-2010, editorial note. MMWR Morb. Mortal. Wkly. Rep. 60: 1273–1274 [PubMed] [Google Scholar]
- 67. Cestari TF, Martignago BF. 2005. Scabies, pediculosis, bedbugs, and stinkbugs: uncommon presentations. Clin. Dermatol. 23: 545–554 [DOI] [PubMed] [Google Scholar]
- 68. Chang KP. 1974. Effects of elevated temperature on mycetome and symbiotes of the bed bug, Cimex lectularius (Heteroptera). J. Invertebr. Pathol. 23: 333–340 [DOI] [PubMed] [Google Scholar]
- 69. Chang C, Chao D. 1999. Comparative study on the insect forms of a low virulence isolate of Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) developed in cimicid bugs and in its regular insect vector species. Chin. J. Entomol. 19: 145–152 [Google Scholar]
- 70. Churchill TP. 1930. Urticaria due to bedbug bites. JAMA 95: 1975 [Google Scholar]
- 71. Clark S, Gilleard JS, McGoldrick J. 2002. Human bedbug infestation of a domestic cat. Vet. Rec. 151: 336. [PubMed] [Google Scholar]
- 72. Cleary CJ, Buchanan D. 2004. Diagnosis and management of bedbugs. Nurse Practit. 29: 47–48 [DOI] [PubMed] [Google Scholar]
- 73. Cohen PR, Tschen JA, Robinson FW, Gray J. 2010. Recurrent episodes of painful and pruritic red skin lesions. Am. J. Clin. Dermatol. 11: 73–78 [DOI] [PubMed] [Google Scholar]
- 74. Colton HR, Altevogt BM. (ed). 2006. Functional and economic impact of sleep loss and sleep-related disorders, p 137–172 In Sleep disorders and sleep deprivation: an unmet public health problem. Committee on Sleep Medicine and Research Board on Health Sciences Policy, National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- 75. Cooper DL. 1948. Bedbug bites. JAMA 138: 1206 [Google Scholar]
- 76. Cooper R. 2007. Just encase: mattress and box-spring encasements can serve as an essential tool in effective bed bug management. Pest Control 75: 64–75 [Google Scholar]
- 77. Criado PR, Criado RFJ. 2011. Bedbugs (Heteroptera, Cimicidae): an etiology of pruritis to be remembered. An. Bras. Dermatol. 86: 163–164 http://www.scielo.br/pdf/abd/v86n1/en_v86n1a28.pdf [DOI] [PubMed] [Google Scholar]
- 78. Criado PR, Belda W, Jr, Jardim RF, Criado RFJ, Vasconcellos C. 2011. Bedbugs (Cimicidae infestation): the worldwide renaissance of an old partner of human kind. Braz. J. Infect. Dis. 15: 74–80 [DOI] [PubMed] [Google Scholar]
- 79. Crissy JT. 1981. Bedbugs. An old problem with a new dimension. Int. J. Dermatol. 20: 411–414 [DOI] [PubMed] [Google Scholar]
- 80. Davio S. 2010. Got bed bugs? Don't panic. Bed bugs do not transmit disease and can be controlled without toxic pesticides. Pestic. You 30: 13–17 http://www.beyondpesticides.org/infoservices/pesticidesandyou/Winter06-07/bedbugs.pdf [Google Scholar]
- 81. Delaunay P, et al. 2009. Bedbugs and healthcare-associated dermatitis, France. Emerg. Infect. Dis. 15: 989–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Delaunay P, et al. 2011. Bedbugs and infectious diseases. Clin. Infect. Dis. 52: 200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Desai R, et al. 2011. Survival and transmission of community-associated methicillin-resistant Staphylococcus aureus from fomites. Am. J. Infect. Control 39: 219–225 [DOI] [PubMed] [Google Scholar]
- 84. Doby JM. 1993. Les arthropods ectoparasites de l'homme utilises en therapeutique depuis l'antiquite jusqu'a nos jours. Bull. Soc. Fr. Parasitol. 11: 275–299 [Google Scholar]
- 85. Doggett SL. 2005. A code of practice for the control of bed bug infestations in Australia (draft). Department of Medical Entomology and Australian Environmental Pest Managers Association, Westmead Hospital, Sydney, Australia: http://medent.usyd.edu.au/bedbug/bed_bug_cop_draft_complete.pdf [Google Scholar]
- 86. Doggett SL. 2006. A code of practice for the control of bed bug infestations in Australia. Department of Medical Entomology and Australian Environmental Pest Managers Association, Westmead Hospital, Sydney, Australia: http://medent.usyd.edu.au/bedbug/bed_bug_cop_v1.pdf [Google Scholar]
- 87. Doggett SL. 2007. A code of practice for the control of bed bug infestations in Australia, 2nd ed (draft) Department of Medical Entomology and Australian Environmental Pest Managers Association, Westmead Hospital, Sydney, Australia: http://medent.usyd.edu.au/bedbug/cop_ed2_completed.pdf [Google Scholar]
- 88. Doggett SL. 2007. A code of practice for the control of bed bug infestations in Australia, 2nd ed. Department of Medical Entomology and Australian Environmental Pest Managers Association, Westmead Hospital, Sydney, Australia: http://medent.usyd.edu.au/bedbug/cop_ed2_final.pdf [Google Scholar]
- 89. Doggett SL. 2009. A code of practice for the control of bed bug infestations in Australia, 3rd ed (draft) Department of Medical Entomology and Australian Environmental Pest Managers Association, Westmead Hospital, Sydney, Australia: http://medent.usyd.edu.au/bedbug/cop_ed3_draft.pdf [Google Scholar]
- 90. Doggett SL. 2009. Non-chemical methods of bed bug control: a case study. Prof. Pest Manag. 13: 27–29 [Google Scholar]
- 91. Doggett SL. 2009. Identification & natural history, p 13–22 In Doggett SL. (ed), Bed bug workshop, Australian Environmental Pest Managers Association course notes. Australian Environmental Pest Managers Association and Department of Medical Entomology, Westmead Hospital, Sydney, Australia [Google Scholar]
- 92. Doggett SL. 2009. Bed bugs and human health, p 23–28 In Doggett SL. (ed), Bed bug workshop, Australian Environmental Pest Managers Association course notes. Australian Environmental Pest Managers Association and Department of Medical Entomology, Westmead Hospital, Sydney, Australia [Google Scholar]
- 93. Doggett SL. 2010. A code of practice for the control of bed bug infestations in Australia, 3rd ed. Department of Medical Entomology and Australian Environmental Pest Managers Association, Westmead Hospital, Sydney, Australia: http://medent.usyd.edu.au/bedbug/cop_3ed_final.pdf [Google Scholar]
- 94. Doggett SL. 2011. A bed bug management policy for accommodation providers. Department of Medical Entomology, Westmead Hospital, Sydney, Australia: http://medent.usyd.edu.au/bedbug/man_policy_ed1_final.pdf [Google Scholar]
- 95. Doggett SL. 2011. A code of practice for the control of bed bug infestations in Australia, 4th ed (draft) Department of Medical Entomology and Australian Environmental Pest Managers Association, Westmead Hospital, Sydney, Australia: http://medent.usyd.edu.au/bedbug/cop_ed4_draft.pdf [Google Scholar]
- 96. Doggett SL. 2011. Why all the stink about new technology? Prof. Pest Manag. 15: 5–6 [Google Scholar]
- 97. Doggett SL. 2011. Bed bug products not always what they're cracked up to be. Prof. Pest Manag. 15: 31–32 [Google Scholar]
- 98. Doggett SL, Geary MJ, Crowe WJ, Wilson P, Russell RC. 2003. Has the tropical bed bug, Cimex hemipterus (Hemiptera: Cimicidae), invaded Australia? Environ. Health 3: 80–82 [Google Scholar]
- 99. Doggett SL, Geary MJ, Russell RC. 2004. The resurgence of bed bugs in Australia, with notes on their ecology and control. Environ. Health 4: 30–38 [Google Scholar]
- 100. Doggett SL, Geary MJ, Russell RC. 2006. Encasing mattresses in black plastic will not provide thermal control of bed bugs, Cimex spp. (Hemiptera: Cimicidae). J. Econ. Entomol. 99: 2132–2135 [DOI] [PubMed] [Google Scholar]
- 101. Doggett S, Lilly D, Russell RC. 2011. Battling bed bugs; the latest in weaponry. Prof. Pest Manag. 15: 25–26, 31 [Google Scholar]
- 102. Doggett SL, Orton CJ, Lilly D, Russell RC. 2011. Bed bugs: the Australian response. Insects 2: 96–111 www.mdpi.com/2075-4450/2/2/96/pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Doggett SL, Orton CJ, Lilly DG, Russell RC. 2011. Bed bugs—a growing problem worldwide, Australian and international trends update and causes for concern, session 2A. Abstr. Aust. Environ. Pest Manag. Assoc. NSW Conf. 2011, Sydney, Australia, 2 June 2011 http://medent.usyd.edu.au/bedbug/papers/aepma_2011_doggett.pdf [Google Scholar]
- 104. Doggett SL, Russell RC. 2008. The resurgence of bed bugs, Cimex spp. (Hemiptera: Cimicidae) in Australia: experiences from down under, p 407–425 In Robinson WH, Bajomi D. (ed). Proceedings of the 6th International Conference on Urban Pests, Budapest, Hungary, 13 to 16 July 2008 OOK-Press, Pápai, Hungary [Google Scholar]
- 105. Doggett SL, Russell RC. 2009. Bed bugs for the general practitioner. Aust. Fam. Physician 38: 880–884 [PubMed] [Google Scholar]
- 106. Doggett SL, Russell RC. 2009. Emerging challenges in bed bug management, p 6.1–6.44 In Doggett SL. (ed), Emerging Pest Management Challenges Symposium course notes. Westmead Hospital, Sydney, Australia [Google Scholar]
- 107. Doggett SL, Russell RC. 2010. Laboratory investigations of the ‘BB Secure Ring’ and its ability to act as a barrier to the common bed bug, Cimex lectularius. Department of Medical Entomology, Westmead Hospital, Sydney, Australia: http://www.bedbugsecure.com/BB%20Secure%20Ring%20Laboratory%20Investigation%20Report.pdf [Google Scholar]
- 108. Eddy C, Jones SC. 2011. Bed bugs, public health, and social justice: part 1, a call to action. J. Environ. Health 73: 8–14 [PubMed] [Google Scholar]
- 109. Eley SM, Garder R, Molyneux DH, Moore NF. 1987. A reovirus from the bedbug, Cimex lectularius. J. Gen. Virol. 68: 195–199 [DOI] [PubMed] [Google Scholar]
- 110. El-Mofty MM, Sakr SA, Younis WF. 1989. Induction of skin papillomas in the rabbit, Oryctologus cuniculus, by bites of a blood-sucking insect, Cimex lectularius, irradiated by gamma rays. J. Invest. Dermatol. 93: 630–632 [DOI] [PubMed] [Google Scholar]
- 111. El Okbi LM, El-Okbi MM, Khaled ML. 1985. Insects bites as a possible aetiological factor of Prurigo of Hebra in Egypt. J. Egypt. Soc. Parasitol. 15: 57–60 [PubMed] [Google Scholar]
- 112. Epstein GV, Exemplarskaya EV, Silvers IL, Babekova ON. 1936. Bedbugs as transmitters of hemolytic staphylococci to experimental animals. G. Batteriol. Immunol. 17: 495–501 [Google Scholar]
- 113. Evans H. 2 December 2010. NYU's Hospital for Joint Diseases shuts 10th floor area down after patient claims she saw a bedbug. New York Daily News. http://articles.nydailynews.com/2010-12-02/local/27083006_1_bedbug-rheumatoid-arthritis-patient-claims [Google Scholar]
- 114. Feldlaufer MF, Loudon C. 2011. Undesirable dispersal of eggs and early-stage nymphs of the bed bug (Hemiptera: Cimicidae) by static electricity and air currents. J. Entomol. Sci. 46: 169–170 [Google Scholar]
- 115. Fletcher CL, Ardern-Jones MR, Hay RJ. 2002. Widespread bullous eruption due to multiple bed bug bites. Clin. Exp. Dermatol. 27: 74–75 [DOI] [PubMed] [Google Scholar]
- 116. Francischetti MB, et al. 2010. Insight into the sialoma of the bed bug, Cimex lectularius. J. Proteome Res. 9: 3820–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Freudenmann RW, Lepping P. 2009. Delusional infestation. Clin. Microbiol. Rev. 22: 690–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gangloff-Kaufmann JL, Pichler C. 2008. Guidelines for prevention and management of bed bugs in shelters and group living facilities. New York State IPM Program, Cornell University, New York, NY: http://www.nysipm.cornell.edu/publications/bb_guidelines/files/bb_guidelines_intro.pdf [Google Scholar]
- 119. Gbakima AA, et al. 2002. High prevalence of bedbugs Cimex hemipterus and Cimex lectularius in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr. J. Med. 21: 268–271 [DOI] [PubMed] [Google Scholar]
- 120. Geist-May K. 2011. Tenant tries to kill bed bugs, starts fire. Cincinnati News. http://cincinnati.com/blogs/considerthisclermont/2011/01/24/tenant-tries-to-kill-bed-bugs-starts-fire/ [Google Scholar]
- 121. Gerozisis J, Hadlington P, Staunton I. 2008. Urban pest management in Australia, 5th ed. UNSW Press, Sydney, Australia [Google Scholar]
- 122. Getty GM, Taylor RL, Lewis VR. 2008. Hot house. Pest Control Technol. 36: 96–100 [Google Scholar]
- 123. Gill GV, Bell DR, Vandervelde EM. 1991. Horizontal transmission of hepatitis B virus. Lancet 337: 247–248 [DOI] [PubMed] [Google Scholar]
- 124. Goddard J. 2003. Do bed bugs carry human diseases? A controversy. Pest Control Technol. 31: 38, 40 [Google Scholar]
- 125. Goddard J. 2003. Bed bugs bounce back—but do they transmit disease? Infect. Med. 20: 473–474 [Google Scholar]
- 126. Goddard J. 2009. Bed bugs: vectors of human disease? Pest Control Technol. 37: 44–45, 48, 50–52, 54–55 [Google Scholar]
- 127. Goddard J. 2011. Bedbugs and transmission of Trypanosoma cruzi. Clin. Infect. Dis. 53: 210. [DOI] [PubMed] [Google Scholar]
- 128. Goddard J, de Shazo R. 2008. Rapid rise in bed bug populations: the need to include them in the differential diagnosis of mysterious skin rashes. South. Med. Assoc. 101: 854–855 [DOI] [PubMed] [Google Scholar]
- 129. Goddard J, de Shazo R. 2009. Multiple feeding by the common bed bug, Cimex lectularius, without sensitization. Midsouth Entomol. 2: 90–92 [Google Scholar]
- 130. Goddard J, de Shazo R. 2009. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 301: 1358–1366 [DOI] [PubMed] [Google Scholar]
- 131. Goff L. 2004. Bedbugs are back! Good Housekeeping 238: 100–101 [Google Scholar]
- 132. Goff L. 2011. FDNY warns don't use gasoline products on bedbugs. The Queens Gazette. http://www.qgazette.com/news/2011-03-09/Front_Page/FDNY_Warns_Dont_Use_Gasoline_Products_On_Bedbugs.html [Google Scholar]
- 133. Gooch H. 9 August 2004. Bed bug issue might separate the professional from the not-so-professional. Pest Management Professional Buzz Online Newsletter. http://www.pestcontrolmag.com/pestcontrol/article/articleDetail.jsp?id=109597 [Google Scholar]
- 134.Google Analytics.2011. Web statistics for www.bedbug.org.au for 6 June 2011 to 29 October 2011. http://www.google.com/analytics/
- 135. Gorwitz RJ, et al. 2008. Changes in the prevalence of nasal colonisation with Staphylococcus aureus in the United States, 2001–2004. J. Infect. Dis. 197: 1226–1234 [DOI] [PubMed] [Google Scholar]
- 136. Haislip S. 2011. Survey reveals bed bugs are top concern for local affiliates. Units 2011(June): 91–92 [Google Scholar]
- 137. Hamann ID. 2004. Insect bites and skin infestations. Med. Today 5: 39–46 [Google Scholar]
- 138. Harlan HJ. 2006, 2010 Bed bugs—importance, biology, and control strategies. Technical guide no. 44 Armed Forces Pest Management Board, Washington, DC: http://www.afpmb.org/sites/default/files/pubs/techguides/tg44.pdf [Google Scholar]
- 139. Harraca V, Ryne C, Ignell R. 2010. Nymphs of the common bed bug (Cimex lectularius) produce anti-aphrodisiac defence against conspecific males. BMC Biol. 8: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hasenböhler A, Kassell A. 2011. Thermal treatment for bed bugs, p 261–265 In Robinson WH, Bajomi D. (ed), Proceedings of the 7th International Conference on Urban Pests, Ouro Preto, Brazil Instituto Biologico, Sao Paulo, Brazil [Google Scholar]
- 141. Haynes KF, Goodman MH, Potter MF. 2010. Bed bug deterrence. BMC Biol. 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Heukelbach J, Hengge UR. 2009. Bed bugs, leeches and hookworm larvae in the skin. Clin. Dermatol. 27: 285–290 [DOI] [PubMed] [Google Scholar]
- 143. Heymann WR. 2009. Bed bugs: a new morning for the nighttime pests. J. Am. Acad. Dermatol. 60: 482–483 [DOI] [PubMed] [Google Scholar]
- 144. Hildreth CJ, Burke AE, Glass RM. 2010. Bed bugs. JAMA 301: 1398. [DOI] [PubMed] [Google Scholar]
- 145. Hirao M. 2010. Recent resurgence of bedbug and its management. Med. Entomol. Zool. 61: 211–221 [Google Scholar]
- 146. Hoang L. 19 July 2011. Heater used to exterminated bed bugs ruled as cause for Royal Scot apartment fire. Global BC. http://www.globaltvbc.com/heater+used+to+exterminate+bed+bugs+ruled+as+cause+for+royal+scot+apartment+fire/308175/story.html [Google Scholar]
- 147. Honig PJ. 1986. Arthropod bites, stings, and infestations: their prevention and treatment. Pediatr. Dermatol. 3: 189–197 [DOI] [PubMed] [Google Scholar]
- 148. Hopes V. 18 August 2010. Unbearable bugs: Patricia Dupuis living out of her car. The News. [Google Scholar]
- 149. How Y-F, Lee C-Y. 2009. Survey of bed bugs in infested premises in Malaysia and Singapore. J. Vector Ecol. 35: 89–94 [DOI] [PubMed] [Google Scholar]
- 150. How Y-F, Lee C-Y. 2010. Fecundity, nymphal development and longevity of field-collected tropical bedbugs, Cimex hemipterus. Med. Vet. Entomol. 24: 108–116 [DOI] [PubMed] [Google Scholar]
- 151. How Y-F, Lee C-Y. 2011. Surface contact toxicity and synergism of several insecticides against different stages of the tropical bed bug, Cimex hemipterus (Hemiptera: Cimicidae). Pest Manag. Sci. 67: 734–740 [DOI] [PubMed] [Google Scholar]
- 152. Hurst S, Humphreys M. 2011. Bedbugs not back by popular demand. Dimens. Crit. Care Nurs. 30: 94–96 [DOI] [PubMed] [Google Scholar]
- 153. Hwang SW, Svoboda T, De Jong IJ, Kabasele KJ, Gogosis E. 2005. Bed bug infestations in an urban environment. Emerg. Infect. Dis. 11: 533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hypsa V, Aksoy S. 1997. Phylogenetic characterisation of two transovarially transmitted endosymbionts of the bedbug Cimex lectularius (Heteroptera: Cimicidae). Insect Mol. Biol. 6: 301–304 [DOI] [PubMed] [Google Scholar]
- 155. Idge Acici MM, Igde FA, Umur S. 2009. Carpet beetle Anthrenus verbasci, Linnaeus 1767: a new seasonal indoor allergen. Case report. Turk. Klin. Tip Bilim. 29: 1729–1731 [Google Scholar]
- 156. Jacobs A. 27 November 2005. Just try to sleep tight. The bedbugs are back. New York Times. http://query.nytimes.com/gst/fullpage.html?res=9A04EFD61731F934A15752C1A9639C8B63&pagewanted=1 [Google Scholar]
- 157. Jimenez-Diaz C, Sanchez-Cuenca B. 1935. Asthma produced by susceptibility to unusual allergens: linseed, insects, tobacco, and chicory. JAMA 6: 397–403 [Google Scholar]
- 158. Johnson CG. 1941. The ecology of the bed-bug, Cimex lectularius L., in Britain. J. Hyg. (Lond.) 41: 345–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Jorg ME. 1992. Cimex lectularius L., (the common bed bug) a vector of Trypanosoma cruzi. Rev. Soc. Bras. Med. Trop. 24: 277–278 [DOI] [PubMed] [Google Scholar]
- 160. Jorg ME, Natula ON. 1982. Cimex lectularius, L. (the common bedbug) vector of Trypanosoma cruzi. Prensa Med. Argent. 69: 528–533 [Google Scholar]
- 161. Jupp PG, Lyons SF. 1987. Experimental assessment of bedbugs (Cimex lectularius and Cimex hemipterus) and mosquitoes (Aedes aegypti formosus) as vectors of human immunodeficiency virus. AIDS 1: 171–174 [PubMed] [Google Scholar]
- 162. Jupp PG, McElligott SE. 1979. Transmission experiments with hepatitis B surface antigen and the common bedbug (Cimex lectularius L). S. Afr. Med. J. 56: 54–57 [PubMed] [Google Scholar]
- 163. Jupp PG, McElligott SE, Lecatsas G. 1983. The mechanical transmission of hepatitis B by the common bedbug (Cimex lectularius L.) in South Africa. S. Afr. Med. J. 63: 77–81 [PubMed] [Google Scholar]
- 164. Jupp PG, Prozesky OW, McElligott SE. 1980. Absence of biological multiplication of hepatitis B virus in the common bedbug. S. Afr. Med. J. 57: 36. [PubMed] [Google Scholar]
- 165. Jupp PG, Prozesky OW, McElligott SE, van Wyk LAS. 1978. Infection of the common bedbug (Cimex lectularius L.) with hepatitis B virus in South Africa. S. Afr. Med. J. 53: 598–600 [PubMed] [Google Scholar]
- 166. Jupp PG, Purcell RH, Phillips JM, Shapiro M, Gerin JL. 1991. Attempts to transmit hepatitis B virus to chimpanzees by arthropods. S. Afr. Med. J. 79: 320–322 [PubMed] [Google Scholar]
- 167. Karunaratne SHPP, Damayanthi BT, Fareena MHJ, Imbuldeniya V, Hemingway J. 2007. Insecticide resistance in the tropical bedbug Cimex hemipterus. Pestic. Biochem. Physiol. 88: 102–107 [Google Scholar]
- 168. Kells SA. 2006. Control of bed bugs in residences, information for pest control companies. University of Minnesota, Minneapolis, MN [Google Scholar]
- 169. Kettle DS. 1984. Medical and veterinary entomology. Croom Helm, London, United Kingdom [Google Scholar]
- 170. Kilpinen O, Jensen KMV, Kristensen M. 2008. Bed bug problems in Denmark, with a European perspective, p 395–399 In Robinson WH, Bajomi D. (ed), Proceedings of the 6th International Conference on Urban Pests, Budapest, Hungary, 13 to 16 July 2008 OOK-Press, Pápai, Hungary [Google Scholar]
- 171. Kilpinen O, Kristensen M, Jensen KMV. 2011. Resistance differences between chlorpyrifos and synthetic pyrethroids in Cimex lectularius population from Denmark. Parasitol. Res. 109: 1461–1464 [DOI] [PubMed] [Google Scholar]
- 172. Kinnear J. 1948. Epidemic of bullous erythema on legs due to bed bugs. Lancet ii: 55. [DOI] [PubMed] [Google Scholar]
- 173. Knight M. 1 February 2010. The bedbug decider. New Yorker. www.newyorker.com/talk/2010/02/01/100201ta_talk_knight [Google Scholar]
- 174. Kock R, et al. 2010. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill. 15(41): 19688. [DOI] [PubMed] [Google Scholar]
- 175. Kolb A, Needham GR, Neyman KM, High WA. 2009. Bedbugs. Dermatol. Ther. 22: 347–352 [DOI] [PubMed] [Google Scholar]
- 176. Kulkarni DK, Kumbhojkar MS, Upadhye AS. 1995. Ethnobiological resources used in traditional medicines by Mahadeo koli tribe from western Maharashtra. Flora Fauna 1: 65–66 [Google Scholar]
- 177. Lee I-Y, Ree H-I, An S-J, Linton JA, Yong T-S. 2008. Reemergence of the bedbug Cimex lectularius in Seoul, Korea. Korean J. Parasitol. 46: 269–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Lefferts A, Parkhill A, Cadigan D, Clayton M, Dugan-Merkler M. 2009. Battle and ancient nuisance. Home Healthcare Nurse 27: 598–606 [DOI] [PubMed] [Google Scholar]
- 179. Leininger-Hogan A. 2011. Bedbugs in the intensive care unit. Crit. Care Nurs. Q. 34: 150–153 [DOI] [PubMed] [Google Scholar]
- 180. Lestch C, Fanelli J. 3 July 2010. Bedbugs force yet another store to shutter its doors, this time it's Abercrombie & Fitch at Seaport. New York Daily News. http://articles.nydailynews.com/2010-07-03/local/27068877_1_bedbugs-clothing-store-abercrombie-fitch [Google Scholar]
- 181. Leverkus M, et al. 2006. Bullous allergic hypersensitivity to bed bug bites mediated by IgE against salivary nitrophorin. Soc. Invest. Dermat. 126: 91–96 [DOI] [PubMed] [Google Scholar]
- 182. Liebold K, Schliemann-Willers S, Wollina U. 2003. Disseminated bullous eruptions with systemic reaction caused by Cimex lectularius. J. Eur. Acad. Dermatol. Venereol. 17: 461–463 [DOI] [PubMed] [Google Scholar]
- 183. Lilly DG, Doggett SL, Orton C, Russell RC. 2009. Bed bug product efficacy under the spotlight, part 1. Prof. Pest Manag. 13–14: 19–20 [Google Scholar]
- 184. Lilly DG, Doggett SL, Orton C, Russell RC. 2009. Bed bug product efficacy under the spotlight, part 2. Prof. Pest Manag. 13–14: 15, 18 [Google Scholar]
- 185. Lilly DG, Doggett SL, Zalucki MP, Orton C, Russell RC. 2009. Bed bugs that bite back, confirmation of insecticide resistance in the common bed bug, Cimex lectularius. Prof. Pest Manag. 13: 22–24 [Google Scholar]
- 186. Lilly DG, Jones G, Doggett SL, Orton C, Russell RC. 2 July 2011. Use your Bed Bug Code of Practice to get more money from jobs by educating your customers, session 3B. Abstr. Aust. Environ. Pest Manag. Assoc. NSW Conf. 2011, Sydney, Australia, 2 June 2011 [Google Scholar]
- 187. Lockhart B. 2 March 2011. Bill would require bedbug warnings Stamford Advocate. www.stamfordadvocate.com/local/article/Law-sought-to-warn-of-hotel-apartment-bed-bug-1037245.php
- 188. Lowe CF, Romney MG. 2011. Bedbugs as vectors for drug-resistant bacteria. Emerg. Infect. Dis. 17: 1132–1134 (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lupo L. 2009. Who you gunna call? Pest Control Technol. 37: 97–100 [Google Scholar]
- 190. Lyons SF, Jupp PG, Schoub BD. 1986. Survival of HIV in the common bedbug. Lancet ii: 45. [DOI] [PubMed] [Google Scholar]
- 191. Madge O. 2011. European code of practice, bed bug management. Bed Bug Foundation, London, United Kingdom: http://www.bedbugfoundation.org/downloads/45632_BedBug_ECoP_v9_forWeb.pdf [Google Scholar]
- 192. Madge O. 2011. European code of practice, bed bug management, draft 1. Bed Bug Foundation, London, United Kingdom: http://www.bedbugfoundation.org/downloads/Bedbug140311.pdf [Google Scholar]
- 193. Madge O, Doggett SL. 2010. Bed bugs—the American way. Int. Pest Control 2010(November-December): 308–309 [Google Scholar]
- 194. Madhu R. 2010. Papular urticaria. Indian J. Pract. Pediatr. 12: 100–104 [Google Scholar]
- 195. Mankin RW, et al. 2010. Acoustic indicators for targeted detection of stored product and urban insect pests by inexpensive infrared, acoustic, and vibrational detection of movement. J. Econ. Entomol. 103: 1636–1646 [DOI] [PubMed] [Google Scholar]
- 196. Masetti M, Bruschi F. 2007. Bedbug infestations recorded in central Italy. Parasitol. Int. 56: 81–83 [DOI] [PubMed] [Google Scholar]
- 197. Reference deleted.
- 198. Mayans MV, et al. 1990. Risk factors for transmission of hepatitis B virus to Gambian children. Lancet 366: 1107–1109 [DOI] [PubMed] [Google Scholar]
- 199. Mayans MV, et al. 1994. Do bedbugs transmit hepatitis B? Lancet 343: 761–763 [DOI] [PubMed] [Google Scholar]
- 200. Mellanby K. 1939. The physiology and activity of the bed bug (Cimex lectularius L.) in a natural infestation. Parasitology 31: 200–211 [Google Scholar]
- 201. Messer AK. 2011. Bed bugs, public health, and social justice. J. Environ. Health 73(10): 40, 33. (Letter to the editor.) [PubMed] [Google Scholar]
- 202. Miller D. 2010. Bed bug action plan for apartments. Virginia Tech, Blacksburg, VA: http://www.vdacs.virginia.gov/pesticides/pdffiles/bb-apt1.pdf [Google Scholar]
- 203. Miller D. 2010. Bed bug action plan for hotels. Virginia Tech, Blacksburg, VA: http://www.vdacs.virginia.gov/pesticides/pdffiles/bb-hotels1.pdf [Google Scholar]
- 204. Miller D, Fisher ML. 2008. Bed bug (Hemiptera: Cimicidae) response to fumigation using sulfuryl fluoride, p 123–127 In Robinson WH, Bajomi D. (ed), Proceedings of the 6th International Conference on Urban Pests, Budapest, Hungary, 13 to 16 July 2008 OOK-Press, Pápai, Hungary [Google Scholar]
- 205. Miller R. 30 January 2011. Franklin County homeless shelter to reopen after bedbug infestation. Herald Mail. http://www.herald-mail.com/news/tristate/hm-franklin-county-homeless-shelter-to-reopen-after-bedbug-infestation-20110130,0,2918568.story [Google Scholar]
- 206. MMBWG 2010. Michigan manual for the prevention and control of bed bugs. Michigan Bed Bug Working Group, Michigan Department of Community Health; Lansing, MI: http://www.michigan.gov/documents/emergingdiseases/Bed_Bug_Manual_v1_full_reduce_326605_7.pdf [Google Scholar]
- 207. Moore DJ, Miller DM. 2006. Laboratory evaluations of insecticide product efficacy for control of Cimex lectularius. J. Econ. Entomol. 99: 2080–2086 [DOI] [PubMed] [Google Scholar]
- 208. Moore DJ, Miller DM. 2008. Field evaluations of insecticide treatment regimens for control of the common bed bug, Cimex lectularius (L.). Pest Manag. Sci. 65: 332–338 [DOI] [PubMed] [Google Scholar]
- 209. Myamba J, Maxwell CA, Asidi A, Curtis CF. 2002. Pyrethroid resistance in tropical bedbugs, Cimex hemipterus, associated with use of treated bednets. Med. Vet. Entomol. 16: 448–451 [DOI] [PubMed] [Google Scholar]
- 210. Nagro A. 2011. Opportunity to educate. Bed bug supplement. Pest Control Technol. 39: 111 [Google Scholar]
- 211. Nagro A. 2011. Treat before you heat. Pest Control Technol. 39: 80–81 [Google Scholar]
- 212. Naylor RA, Boase CJ. 2008. Effiacy of (s)-methoprene against Cimex lectularius, p 115–121 In Robinson WH, Bajomi D. (ed), Proceedings of the 6th International Conference on Urban Pests, Budapest, Hungary, 13 to 16 July 2008 OOK-Press, Pápai, Hungary [Google Scholar]
- 213. Naylor RA, Boase CJ. 2010. Practical solutions for treating laundry infested with Cimex lectularius (Hemiptera: Cimicidae). J. Econ. Entomol. 103: 136–139 [DOI] [PubMed] [Google Scholar]
- 214. Newberry K. 1988. Production of a hybrid between the bedbugs Cimex hemipterus and Cimex lectularius. Med. Vet. Entomol. 2: 297–300 [DOI] [PubMed] [Google Scholar]
- 215. Newberry K. 1989. The effects on domestic infestations of Cimex lectularius bedbugs of interspecific mating with C. hemipterus. Med. Vet. Entomol. 3: 407–414 [DOI] [PubMed] [Google Scholar]
- 216. Newberry K. 1990. The tropical bedbug Cimex hemipterus near the southernmost extent of its range. Trans. R. Soc. Trop. Med. Hyg. 84: 745–747 [DOI] [PubMed] [Google Scholar]
- 217. Newberry K, Mchunu ZM. 1989. Changes in the relative frequency of occurrence of infestations of two sympatric species of bedbug in northern Natal and KwaZulu, South Africa. Trans. R. Soc. Trop. Med. Hyg. 83: 262–264 [DOI] [PubMed] [Google Scholar]
- 218. Newkirk MM, Downe AER, Simon JB. 1975. Fate of ingested hepatitis-B antigen (HbAg) in blood-sucking insects. Gastroenterology 69: 982–987 [PubMed] [Google Scholar]
- 219. NMHC 2010. Quick facts: resident demographics. National Multi Housing Council, Washington, DC: http://www.nmhc.org/Content/ServeContent.cfm?ContentItemID=1152 [Google Scholar]
- 220. NPMA 2011. Bed bugs in America: new survey reveals impact on everyday life. National Pest Management Association, Fairfax, VA: http://www.pestworld.com/bedbugs-in-america [Google Scholar]
- 221. NPMA 2011. NPMA BMP bed bugs best management practices. National Pest Management Association, Fairfax, VA: http://www.npmapestworld.org/publicpolicy/documents/NPMABedBugBMPAPPROVED20110124_prettified.pdf [Google Scholar]
- 222. NPMA 2011. Registering new/existing/legacy pesticides for the professional market for the control of bed bugs. National Pest Management Association, Fairfax, VA: https://www.npmapestworld.org/publicpolicy/documents/March132011RegisteringNewProductsPP.pdf [Google Scholar]
- 223. NYCDHMH 2009. The NYC Environmental Public Health and Sustainability Tracking Portal. New York City Department of Mental Health and Hygiene, New York, NY: https://gis.nyc.gov/doh/track/ [Google Scholar]
- 224. Ogston CW, London WT. 1980. Excretion of hepatitis B surface antigen by the bed bug Cimex hemipterus Fabr. Trans. R. Soc. Trop. Med. Hyg. 74: 823–825 [DOI] [PubMed] [Google Scholar]
- 225. Ogston CW, Wittenstein FS, London WT, Millman I. 1979. Persistence of hepatitis B surface antigen in the bed bug Cimex hemipterus. J. Infect. Dis. 140: 411–414 [DOI] [PubMed] [Google Scholar]
- 226. Omori N. 1941. Comparative studies on the ecology and physiology of common and tropical bed bugs, with special references to the reactions to temperature and moisture. J. Med. Assoc. Formos. 60: 555–729 [Google Scholar]
- 227. Omudu EA, Kuse CN. 2010. Bedbug infestation and its control practices in Gbajimba: a rural settlement in Benue state, Nigeria. J. Vector Borne Dis. 47: 222–227 [PubMed] [Google Scholar]
- 228. Orton CJ. 2009. Guidelines for the establishment and management of AEPMA code-of-practice working parties (V2. 1.3). Westmead Hospital, Sydney, Australia: http://medent.usyd.edu.au/bedbug/guidelines_cop_wp.pdf [Google Scholar]
- 229. Oudhia P. 2001. Traditional medicinal knowledge about bed bug Cimex lectularius L. (Hemiptera: Cimicidae) in Chhattisgarh. Insect Environ. 7: 23 [Google Scholar]
- 230. Parsons DJ. 1955. Bed bug bite anaphylaxis misinterpreted as coronary occlusion. Ohio Med. J. 51: 669. [PubMed] [Google Scholar]
- 231. Parvez N, et al. 2010. Universal MRSA nasal surveillance: characterization of outcomes at a tertiary care center and implications for infection control. South. Med. J. 103: 1084–1091 [DOI] [PubMed] [Google Scholar]
- 232. Paul J, Bates J. 2000. Is infestation with the common bedbug increasing? BMJ 320: 1141. [PMC free article] [PubMed] [Google Scholar]
- 233. Pereira RM, Koehler PG. 2011. Use of heat, volatile insecticide, and monitoring tools to control bed bugs (Heteroptera: Cimicidae), p 325–329 In Robinson WH, Bajomi D. (ed), Proceedings of the 7th International Conference on Urban Pests, Ouro Preto, Brazil, Instituto Biologico, Sao Paulo, Brazil [Google Scholar]
- 234. Pereira RM, Koehler PG, Pfiester M, Walker W. 2009. Lethal effects of heat and use of localized heat treatment for control of bed bug infestations. J. Econ. Entomol. 102: 1182–1188 [DOI] [PubMed] [Google Scholar]
- 235. Perron S, King N, Lajoie L, Jacques L. 25 January 2010. Les punaises de lit, retour vers le futur. Bull. Inform. Environ., p 1–9 http://www.inspq.qc.ca/bise/post/2010/01/25/Les-punaises-de-lit-retour-vers-le-futur.aspx [Google Scholar]
- 236. Pfiester M, Koehler PG, Pereira RM. 2008. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J. Econ. Entomol. 101: 1389–1396 [DOI] [PubMed] [Google Scholar]
- 237. Phisalix M. 1922. Animaux venimeux. Et venins. La fonction venimeuse chez tous, les animaux, les appareils venimeux, les venins et leurs propriétés: les fonctions et usages des venins; l'envenimation et son traitement. Masson & Cie, Paris, France [Google Scholar]
- 238. Pinto L. 1999. Bed bugs…they're back. Pest Control 67: 10–12 [Google Scholar]
- 239. Pinto LJ, Cooper R, Kraft SK. 2007. Bed bug handbook. The complete guide to bed bugs and their control. Pinto & Associates, Mechanicsville, MD [Google Scholar]
- 240. Potter MF. 2011. The history of bed bug management. Am. Entomol. 57: 14–25 [Google Scholar]
- 241. Potter MF, et al. 2010. The sensitivity spectrum: human reactions to bed bug bites. Pest Control Technol. 38: 70–74, 100 [Google Scholar]
- 242. Potter MF, Haynes KF, Goodman M, Stamper S, Sams S. 2010. Blast from the past. Pest Manag. Prof. 2010(March): 46, 47, 49–52 [Google Scholar]
- 243. Potter MF, Haynes KF, Romero A, Hardeback E, Wickemeyer W. 2008. Is there a new bed bug answer? Pest Control Technol. 36: 116, 118–124 [Google Scholar]
- 244. Potter MF, Romero A, Haynes KF, Hardebeck E. 2007. Killing them softly: battling bed bugs in sensitive places. Pest Control Technol. 35: 24–32 [Google Scholar]
- 245. Potter MF, Romero A, Haynes KF, Jarzynka T. 2008. Bed bugs, heat and hotel rooms. Pest Control Technol. 36: 106–109, 112, 114, 116, 118, 120–121 [Google Scholar]
- 246. Potter MF, Rosenberg B, Henrikson M. 2010. Bugs without borders, defining the global bed bug resurgence. Pestworld 2010(September-October): 1–12 [Google Scholar]
- 247. Pritchard MJ, Hwang SW. 2009. Severe anemia from bedbugs. CMAJ 181: 287–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Przybilla B, Eben R. 2008. Insect allergy—elicited by millions of species? Allergologie 31: 361–365 [Google Scholar]
- 249. Ravinder K, Janaiah C, Reddy SM. 1992. Fungicidal activity of scent secretions of certain heteropterian bugs. Natl. Acad. Sci. Lett. 15: 103–105 [Google Scholar]
- 250. Reinhardt K, Kempke D, Naylor R, Siva-Jothy MT. 2009. Sensitivity to bites by the bedbug, Cimex lectularius. Med. Vet. Entomol. 23: 163–166 [DOI] [PubMed] [Google Scholar]
- 251. Reinhardt K, Naylor R, Siva-Jothy MT. 2005. Potential sexual transmission of environmental microbes in a traumatically inseminating insect. Ecol. Entomol. 30: 607–611 [Google Scholar]
- 252. Reinhardt K, Siva-Jothy MT. 2006. Biology of the bed bugs (Cimicidae). Annu. Rev. Entomol. 52: 351–374 [DOI] [PubMed] [Google Scholar]
- 253. Reynolds E. 26 September 2008. Patients flee hospital infested by bed bugs. Daily Mirror, p 21 www.thefreelibrary.com/Patients+flee+hospital+infested+by+bed+bugs%3B+PEST+CONTROL+MOVES+IN…-a0185576297 [Google Scholar]
- 254. Richards L, Boase CJ, Gezan S, Cameron MM. 2009. Are bed bug infestations on the increase within greater London? J. Environ. Health Res. 9: 17–24 [Google Scholar]
- 255. Robinson W. 2004. Bed bugs knock roaches off the list. Pest Control 72: 51–53 [Google Scholar]
- 256. Robinson WH, Boase CJ. 2011. Bed bug (Hemiptera: Cimicidae) resurgence: plotting the trajectory, p 315–318 In Robinson WH, Bajomi D. (ed), Proceedings of the 7th International Conference on Urban Pests, Ouro Preto, Brazil. Instituto Biologico, Sao Paulo, Brazil [Google Scholar]
- 257. Romero A, Potter MF, Haynes KF. 2009. Are dusts the bed bug bullet? Pest Manag. Prof. 2009(May): 22–23, 26, 28, 30 [Google Scholar]
- 258. Romero A, Potter MF, Haynes KF. 2009. Evaluation of piperonyl butoxide as a deltamethrin synergist for pyrethroid-resistant bed bugs. J. Econ. Entomol. 102: 2310–2315 [DOI] [PubMed] [Google Scholar]
- 259. Romero A, Potter MF, Haynes KF. 2009. Behavioral responses of the bed bug to insecticide residues. J. Med. Entomol. 46: 51–57 [DOI] [PubMed] [Google Scholar]
- 260. Romero A, Potter MF, Haynes KF. 2010. Evaluation of chlorfenapyr for control of the bed bug, Cimex lectularius L. Pest Manag. Sci. 66: 1243–1248 [DOI] [PubMed] [Google Scholar]
- 261. Romero A, Potter MF, Potter DA, Haynes KF. 2007. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J. Med. Entomol. 44: 175–178 [DOI] [PubMed] [Google Scholar]
- 262. Roos TC, Alam M, Roos S, Merk HF, Bickers DR. 2001. Pharmacotherapy of ectoparasitic infections. Drugs 61: 1067–1088 [DOI] [PubMed] [Google Scholar]
- 263. Rush WA, Francy DB, Smith GC, Cropp CB. 1980. Transmission of an arbovirus by a member of the family Cimicidae. Ann. Entomol. Soc. Am. 73: 315–318 [Google Scholar]
- 264. Russell RC. 2001. The medical significance of Acari in Australia, p 535–546 In Halliday RB, Walter DE, Proctor HC, Norton RA, Colloff MJ. (ed), Proceedings of the 10th International Congress of Acarology. CSIRO Publishing, Melbourne, Australia [Google Scholar]
- 265. Sakamoto JM, Feinstein J, Rasgon JL. 2006. Wolbachia infections in the Cimicidae: museum specimens as an untrapped resource for endosymbiont surveys. Appl. Environ. Microbiol. 72: 3161–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Sansom JE, Reynolds NJ, Peachey RDG. 1992. Delayed reaction to bed bug bites. Arch. Dermatol. 128: 272–273 [DOI] [PubMed] [Google Scholar]
- 267. Scarupa MD, Economides AE. 2006. Bedbug bites masquerading as urticaria. J. Allergy Clin. Immunol. 117: 1508–1509 (Letter.) [DOI] [PubMed] [Google Scholar]
- 268. Schapiro R. 21 August 2010. Bedbugs found in small area in basement of Empire State Building. New York Daily News. http://articles.nydailynews.com/2010-08-21/local/27073221_1_bedbugs-tallest-building-empire-state-building [Google Scholar]
- 269. Schwarz J. 29 October 2010. Bedbugs hitch a ride to downtown office buildings. NBC Chicago, Chicago, IL: http://www.nbcchicago.com/news/local/Bed-Bugs-Make-The-Leap-To-Downtown-Office-Buildings-106297913.html [Google Scholar]
- 270. Scott HG. 1963. Household and stored-food insects of public health importance and their control. U.S. Department of Health, Education, and Welfare Communicable Disease Center, Atlanta, GA [Google Scholar]
- 271. Siff A. 16 July 2010. Something lingering in the lingerie? Bed bugs hit Victoria's Secret. NBC New York, New York, NY: http://www.nbcnewyork.com/news/local/Dont-Mention-It-Bed-Bugs-Hit-Victorias-Secret-98621039.html [Google Scholar]
- 272. Sigurdson K, Ayas NT. 2007. The public health and safety consequences of sleep disorders. Can. J. Physiol. Pharmacol. 85: 179–183 [DOI] [PubMed] [Google Scholar]
- 273. Silverman AL, et al. 2001. Assessment of hepatitis B virus DNA and hepatitis C virus RNA in the common bedbug (Cimex lectularius L.) and kissing bug (Rhodnius prolixus). Am. J. Gastroenterol. 96: 2194–2198 [DOI] [PubMed] [Google Scholar]
- 274. Skerritt J. 21 April 2011. Katz pledges summit on bug registry. Winnipeg Free Press; www.winnipegfreepress.com/local/katz-pledges-summit-on-bug-registry-120342999.html [Google Scholar]
- 275. St. Aubin F. 1991. Everything old is new again. Pest Control Technol. 19: 50, 52, 102 [Google Scholar]
- 276. Steen CJ, Carbonaro PA, Schwartz RA. 2004. Arthropods in dermatology. J. Am. Acad. Dermatol. 50: 819–842 [DOI] [PubMed] [Google Scholar]
- 277. Sternberg L. 1929. A case of asthma caused by the Cimex lectularius. Med. J. Rec. 129: 622 [Google Scholar]
- 278. Stevens K. 2003. Sleeping with the enemy. New York Times 25(December): 5 [Google Scholar]
- 279. Straub RD, Salvaggio HL, Adams DR, Zaenglein AL. 2009. Diffuse clusters of vesicles on the face and extremities of a 10-month-old girl. Pediatr. Dermatol. 26: 747–748 [DOI] [PubMed] [Google Scholar]
- 280. Stucki A, Ludwig R. 2008. Bedbug bites. N. Engl. J. Med. 359: 1047. [DOI] [PubMed] [Google Scholar]
- 281. Surender P, Janaiah C, Reddy VK, Reddy SM. 1988. Bacterial activity of certain volatile scent components of heteropteran bugs. Proc. Indian Natl. Sci. Acad. Part B Biol. Sci. 54: 315–316 [Google Scholar]
- 282. Suwannayod S, Chanbang Y, Buranapanichpan S. 2010. The life cycle and effectiveness of insecticide against the bed bugs of Thailand. Southeast Asian J. Trop. Med. Public Hyg. 41: 548–554 [PubMed] [Google Scholar]
- 283. Taisey AA, Neltner T. 2010. What's working for bed bug control in multifamily housing: reconciling best practices with research and the realities of implementation. National Center for Healthy Housing, Columbia, MD: http://www.nchh.org/Portals/0/Contents/Bed_Bug_Report_2-12-10.pdf [Google Scholar]
- 284. Tawatsin A, et al. 2011. Insecticides resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae). J. Med. Entomol. 48: 1023–1030 [DOI] [PubMed] [Google Scholar]
- 285. Taylor P, Morrison J. 1980. Cimex lectularius as a vector of hepatitis B. Cent. Afr. J. Med. 26: 96–100 [PubMed] [Google Scholar]
- 286.Terminix. Annual list identifies where bedbugs bite most across U. S. 2011. http://www.terminix.com/Media/PressReleases.aspx.
- 287. Ter Poorten MC, Prose NS. 2005. The return of the common bed bug. Pediatr. Dermatol. 22: 183–187 [DOI] [PubMed] [Google Scholar]
- 288. Tharakaram S. 1999. Bullous eruption due to Cimex lectularius. Clin. Exp. Dermatol. 24: 241–242 [DOI] [PubMed] [Google Scholar]
- 289. Thomas I, Kihiczak CG, Schwartz RA. 2004. Bedbug bites: a review. Int. J. Dermatol. 43: 430–433 [DOI] [PubMed] [Google Scholar]
- 290. Thompson J. 1983. Bed bug. Agfacts information leaflet. Department of Agriculture, Sydney, New South Wales, Australia [Google Scholar]
- 291. Thorburn PT, Riha RL. 2010. Skin disorders and sleep in adults: where is the evidence? Sleep Med. Rev. 14: 351–358 [DOI] [PubMed] [Google Scholar]
- 292. Tomar BS. 1998. Hepatitis E in India. Acta Paediatr. Sin. 39: 150–156 [PubMed] [Google Scholar]
- 293. Tonn RJ, Nelson M, Espinola H, Cardozo JV. 1982. Cimex hemipterus and Rhodnius prolixus from an area of Venezuela endemic for Chagas' disease. Bull. Soc. Vector Ecol. 7: 49–50 [Google Scholar]
- 294. Trudnowski RJ, Rico RC. 1974. Specific gravity of blood and plasma at 4 and 37°C. Clin. Chem. 20: 615–616 [PubMed] [Google Scholar]
- 295. Turell MJ. 1988. Horizontal and vertical transmission of viruses by insect and tick vectors, p 127–152 In Monath TP. (ed), The arboviruses: epidemiology and ecology, vol 1 CRC Press, Boca Raton, FL [Google Scholar]
- 295a. U.S. Court of Appeals, Seventh Circuit 2003. Mathias v, Accor Economy Lodging. U.S. Court of Appeals, Seventh Circuit. http://scholar.google.com.au/scholar_case?case=7866068039935648138&hl=en&as_sdt=2&as_vis=1&oi=scholarr
- 296. U. S. House of Representatives 2011. HR 967: bed bug management, prevention, and research act. U.S. House of Representatives, Washington, DC: http://www.govtrack.us/congress/billtext.xpd?bill=h112-967 [Google Scholar]
- 297. Usinger RL. 1966. Monograph of Cimicidae. Thomas Say Foundation, College Park, MD [Google Scholar]
- 298. Vaickus LJ, Bouchard J, Kim J, Natarajan S, Remick DG. 2010. Oral tolerance inhibits pulmonary eosinophilia in a cockroach allergen induced model of asthma: a randomized laboratory study. Respir. Res. 11: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Valenzuela JG, Chuffe OM, Ribeiro JM. 1996. Apyrase and anti-platelet activities from the salivary glands of the bed bug Cimex lectularius. Insect Biochem. Mol. Biol. 21: 557–562 [Google Scholar]
- 300. Valenzuela JG, Guimaraes JA, Ribeiro JM. 1996. A novel inhibitor of factor X activation from the salivary glands of the bed bug Cimex lectularius. Exp. Parasitol. 83: 184–190 [DOI] [PubMed] [Google Scholar]
- 301. Valenzuela JG, Walker FA, Ribeiro JMC. 1995. Salivary nitrophorin (nitric-oxide-carrying hemoprotein) in the bedbug Cimex lectularius. J. Exp. Biol. 198: 1519–1526 [DOI] [PubMed] [Google Scholar]
- 302. Vandam J. 2 November 2003. Sleep tight, and don't let…oh, just forget about it. New York Times. www.nytimes.com/2003/11/02/nyregion/neighborhood-report-greenpoint-sleep-tight-and-don-t-let-oh-just-forget-about-it.html [Google Scholar]
- 303. Vargo EL, et al. 2011. Genetic analysis of bed bug infestations and populations, p 319–323 In Robinson WH, Bajomi D. (ed), Proceedings of the 7th International Conference on Urban Pests, Ouro Preto, Brazil. Instituto Biologico, Sao Paulo, Brazil [Google Scholar]
- 304. Vaughan JA, Azad AF. 1993. Patterns of erythrocyte digestion by bloodsucking insects: constraints on vector competence. J. Med. Entomol. 30: 214–216 [DOI] [PubMed] [Google Scholar]
- 305. Venkatachalam PS, Belavady B. 1962. Loss of haemoglobin iron due to excessive biting by bed bugs. A possible aetiological factor in the iron deficiency anaemia of infants and children. Trans. R. Soc. Trop. Med. Hyg. 56: 218–221 [DOI] [PubMed] [Google Scholar]
- 306. Walker W, Glover K, Koehler P, Thoms E, Hobelmann E. 2008. Fumigation, steam, dusting and labor. Pest Control Technol. 36: 40, 42, 44–46, 48, 50 [Google Scholar]
- 307. Walpole DE, Newberry K. 1988. A field study of mating between species of bedbug in northern KwaZulu, South Africa. Med. Vet. Entomol. 2: 293–296 [DOI] [PubMed] [Google Scholar]
- 308. Wang C, Cooper R. 2011. Detection tools and techniques. Pest Control Technol. 39: 72, 74, 76, 78–79, 112 [Google Scholar]
- 309. Wang C, Gibb T, Bennett GW. 2009. Evaluation of two least toxic integrated pest management programs for managing bed bugs (Heteroptera: Cimicidae) with discussion of a bed bug intercepting device. J. Med. Entomol. 46: 566–571 [DOI] [PubMed] [Google Scholar]
- 310. Wang CL, Gibb T, Bennett GW, McKnight S. 2009. Bed bug (Heteroptera: Cimicidae) attraction to pitfall traps baited with carbon dioxide, heat, and chemical lure. J. Econ. Entomol. 102: 1580–1585 [DOI] [PubMed] [Google Scholar]
- 311. Wang CL, Tsai W-T, Cooper R, White J. 2011. Effectiveness of bed bug monitors for detecting and trapping bed bugs in apartments. J. Econ. Entomol. 104: 274–278 [DOI] [PubMed] [Google Scholar]
- 312. WanZhen F, KaiShong Y. 1995. A clinical study of the relationship between bed bugs and allergic asthma. Chin. J. Vector Biol. Control 6: 54–57 [Google Scholar]
- 313. WanZhen F, KaiShong Y. 1997. Relationship between bedbug antigen and bronchial asthma. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 15: 386–387 [Google Scholar]
- 314. Waters F, Bucks ES. 2011. Neuropsychological effects of sleep loss: implication for neuropsychologists. J. Int. Neuropsychol. Soc. 17: 571–586 [DOI] [PubMed] [Google Scholar]
- 315. Webb PA, et al. 1989. Potential for insect transmission of HIV: experimental exposure of Cimex hemipterus and Toxorhynchites amboinensis to human immunodeficiency virus. J. Infect. Dis. 160: 970–977 [DOI] [PubMed] [Google Scholar]
- 316. Weeks ENI, Birkett MA, Cameron MM, Pickett JA, Logan JG. 2010. Semiochemicals of the common bed bug, Cimex lectularius L. (Hemiptera: Cimicidae), and their potential for use in monitoring and control. Pest Manag. Sci. 67: 10–20 [DOI] [PubMed] [Google Scholar]
- 317. Whitford F, et al. 2006. The pesticide marketplace, discovering and developing new products. Purdue University, West Lafayette, IN: http://www.ppp.purdue.edu/Pubs/ppp-71.pdf [Google Scholar]
- 318. Wikipedia 2011. Demographics of the United States. Wikipedia. http://en.wikipedia.org/wiki/US_population [Google Scholar]
- 319. Wikipedia 2011. Demographics of Australia. Wikipedia. http://en.wikipedia.org/wiki/Australian_population [Google Scholar]
- 320. Williams JE, Imlarp S, Top FH, Jr, Cavanaugh DC, Russell PK. 1976. Kaeng Khoi virus from naturally infected bedbugs (Cimicidae) and immature freetailed bats. Bull. World Health Organ. 53: 365–369 [PMC free article] [PubMed] [Google Scholar]
- 321. Wills W, et al. 1977. Hepatitis-B virus in bedbugs (Cimex hemipterus) from Senegal. Lancet ii: 217–219 [DOI] [PubMed] [Google Scholar]
- 322. Yeap HL, et al. 2011. Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics 187: 583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Yoon KS, et al. 2008. Biochemical and molecular analysis of deltamethrin resistance in the common bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 45: 1092–1101 [DOI] [PubMed] [Google Scholar]
- 324. Zahner GEP, Kasl SV, While M, Will J. 1985. Psychological consequences of infestation of the dwelling unit. Am. J. Public Health 75: 1303–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Zhu F, et al. 2010. Widespread distribution of knockdown resistance mutations in the bed bug, Cimex lectularius (Hemiptera: Cimicidae), populations in the United States. Arch. Insect Biochem. 73: 245–257 [DOI] [PubMed] [Google Scholar]
- 326. Zhu IY, Stiller MJ. 2002. Arthropods and skin diseases. Int. J. Dermatol. 41: 533–549 [DOI] [PubMed] [Google Scholar]