Abstract
Axon degeneration is a characteristic event in many neurodegenerative conditions including stroke, glaucoma, and motor neuropathies. However, the molecular pathways that regulate this process remain unclear. Axon loss in chronic neurodegenerative diseases share many morphological features with those in acute injuries, and expression of the Wallerian degeneration slow (WldS) transgene delays nerve degeneration in both events, indicating a common mechanism of axonal self-destruction in traumatic injuries and degenerative diseases. A proposed model of axon degeneration is that nerve insults lead to impaired delivery or expression of a local axonal survival factor, which results in increased intra-axonal calcium levels and calcium-dependent cytoskeletal breakdown.
Introduction
As the primary signal conduit in neurons, axon fibers are on average 20,000 times larger than the cell body in length and total surface area (Friede, 1963). When the normal functions of this neuronal compartment are compromised by various insults such as trauma, blockade of axonal transport, or chemical toxicity, distinct morphological and molecular changes known as Wallerian degeneration result in cytoskeletal disassembly and granular degeneration of the axon distal to the injury site. This is followed by breakdown of the blood–brain barrier and infiltration of reactive glial cells to aid the removal of axonal and myelin debris (Fig. 1; Waller, 1850; Griffin et al., 1995; Vargas and Barres, 2007). Delaying axon degeneration prevents the progression of these subsequent glial events, suggesting that physical breakdown of the axonal cytoskeleton is required to trigger surrounding glial reactivation during Wallerian degeneration (Lunn et al., 1989; George and Griffin, 1994).
Figure 1.
Course of Wallerian axon degeneration. As early as 5–30 min after nerve injury, the axonal segments proximal (left) and distal (right) to the injury site exhibit short-distance acute axon degeneration (AAD), an event that is principally mediated by extracellular Ca2+ influx and activation of the intracellular Ca2+-dependent protease calpain. This event is followed by a slower axonal retraction and formation of axonal bulbs at the injury sites (arrowheads). For the next 24 to 48 h after injury there is a period of relative latency in which the distal axon remains morphologically stable and electrically excitable. Although beading occurs along the distal axon at irregular intervals, there are few signs of physical fragmentation. At more than 72 h after injury, rapid fragmentation and cytoskeletal breakdown occur along the full length of the distal axon, followed by increased glial (consisting primarily of astrocytes, macrophages and, in the PNS, Schwann cells) influx to clear axonal remnants (blue circles) and to possibly promote regenerative attempts by the proximal axon.
In many neurodegenerative diseases, prominent axonal pathology often precedes cell body loss in the form of “dying back,” in which axons from the synaptic regions gradually degenerate toward the cell body. Despite differences in the rate of degeneration, this process mirrors many morphological features of transected nerves, including axonal swelling, microtubule disassembly, and eventual fragmentation of the axonal cytoskeleton (Cavanagh, 1964; Griffin et al., 1995). Furthermore, molecular manipulations to delay the degeneration of severed axons also attenuate the progression of clinical pathologies in animal models of peripheral motor neuropathies, multiple sclerosis, ischemic stroke, and glaucoma (Sagot et al., 1995; Ferri et al., 2003; Samsam et al., 2003; Kaneko et al., 2006; Howell et al., 2007; Meyer zu Horste et al., 2011; Verghese et al., 2011), indicating that similar mechanisms regulate axon loss in both acute injuries and chronic diseases. Therefore, understanding the mechanisms of nerve degeneration in simple traumatic injuries allows us to model how axons are lost in more complex neurodegenerative conditions.
Much of our understanding into the nature of axon degeneration has come from imaging studies and pharmacological disruption of the process, but also from studying the Wallerian degeneration slow (WldS) mutation, which robustly delays degeneration of the distal axon from a variety of injuries including nerve transection (Lunn et al., 1989) and chemotoxic insults (Wang et al., 2001). Expression of the WldS gene product alone is sufficient to confer axon protection to wild-type neurons (Mack et al., 2001), and the ability of severed WldS axons to survive for weeks even after the cell bodies have degenerated (Deckwerth and Johnson, 1994) is evidence that axon degeneration involves mechanisms distinct from those that govern neuronal cell body death (Raff et al., 2002). This significant delay also provides an opportunity to study the mechanisms that regulate axon survival and degradation in wild-type neurons. However, distinction should be made between Wallerian axon degeneration after nerve injuries and axon pruning that occurs normally during development. Although several downstream molecules are shared, separate mechanisms control each event as WldS expression delays injury-induced axon degeneration but not axonal pruning (Hoopfer et al., 2006). The molecular pathways involved in developmental axon pruning are covered extensively in other excellent reviews (Low and Cheng, 2005; Luo and O’Leary, 2005; Saxena and Caroni, 2007). Interestingly, WldS expression does prevent the pruning of dendrites in Drosophila (Schoenmann et al., 2010; Tao and Rolls, 2011), suggesting that developmental remodeling of dendritic, but not axonal arbors involves processes akin to Wallerian degeneration. Here we focus on recent understanding of the molecular events that occur in the axon after injury, and suggest a model to explain how these events orchestrate the axonal self-destruction program.
A molecular time course of axon degeneration
Previous studies examining the sequence of events in transected axons, the simplest model of axon degeneration, reveal at least three morphologically discernible phases (Fig. 1). These consist of an acute degeneration stage that affects both the proximal and distal stumps of the axon immediately upon injury (Kerschensteiner et al., 2005), a latency period in which the distal axon remains morphologically intact and electrically excitable for brief periods (Tsao et al., 1994), and an abrupt granular degeneration phase involving the rapid fragmentation of the entire axonal cytoskeleton distal to the injury site (Lubińska, 1982; Griffin et al., 1995). These sequences of events are quickly completed by 24–48 h in vivo (Lubińska, 1977; George and Griffin, 1994) and 12–24 h in vitro in rodent axons (Glass et al., 1993; Araki et al., 2004; Wang et al., 2005). However, altering physiological conditions up to a few hours after injury, such as by lowering ambient temperature and reducing extracellular calcium levels or by genetically expressing the WldS transgene, significantly prolongs the latency period, during which the injured axons remain in a vulnerable but functional state (George et al., 1995; Tsao et al., 1999; Conforti et al., 2000). Therefore, identifying the underlying molecular events during the different morphological phases may reveal specific targets to delay or even rescue axon degeneration.
Phase I: Acute axon degeneration
It was previously thought that transected axons are structurally dormant from the time of injury until the onset of axonal fragmentation. However, recent in vivo real-time imaging studies reveal that the injured axons are far more dynamic shortly after injury. As soon as a few minutes after axotomy, the axonal segments immediately proximal and distal to the injury site rapidly degenerate by several hundred micrometers in either direction in a process that lasts between 5 and 60 min (Kerschensteiner et al., 2005). This early response to injury is followed by a slower formation of dystrophic bulb structures at the terminals of both transected ends due to accumulation of axoplasmic organelles from ongoing anterograde and retrograde transport (Fig. 1; Griffin et al., 1995).
The short, early-onset degenerative event, termed acute axonal degeneration (AAD), has been observed in both dorsal spinal sensory and optic nerves in vivo (Kerschensteiner et al., 2005; Knöferle et al., 2010). Channel-mediated influx of extracellular Ca2+ is critical for initiating AAD, as Ca2+ channel blockers prevent the early intra-axonal rise in Ca2+ and attenuate the progression of AAD. Moreover, addition of Ca2+ ionophores significantly increases the number of injured axons undergoing AAD (Knöferle et al., 2010).
The primary mechanism by which Ca2+ leads to cytoskeletal breakdown in AAD is via Ca2+-dependent activation of the serine-threonine protease calpain, which is capable of cleaving axonal neurofilament and microtubule-associated components such as spectrin and tubulin (Billger et al., 1988; Johnson et al., 1991). Increased calpain cleavage of spectrin occurs as early as 30 min after injury in vivo (Kampfl et al., 1996), corresponding to the onset of AAD. Other degradative processes such as autophagy are also triggered by the initial Ca2+ influx after axotomy (Knöferle et al., 2010) and may contribute to the severity and duration of axon loss in AAD. However, chemical inhibition of calpain fully abrogates this short distance degeneration at the severed ends of spinal cord axons (Kerschensteiner et al., 2005), indicating that calpain activity is the primary effector of AAD.
What is the purpose of AAD, and does it contribute to subsequent Wallerian degeneration of the entire distal axon? Earlier studies suggest that calpain-mediated proteolysis of neurofilaments aid in cytoskeletal restructuring and formation of growth cones in regenerating axons (Spira et al., 2003). Furthermore, the space created by this short-distance axon degeneration may enable glial proliferation at the lesion site and provide a more permissive environment for regeneration of the proximal axon. Thus, AAD may be the principal mechanism by which injured proximal axons are lost to allow neurite regrowth.
However, whether AAD affects degeneration of the distal axonal segment is less clear. Expression of the WldS transgene, which delays Wallerian degeneration, also prevents the onset of AAD (Kerschensteiner et al., 2005), suggesting that the two events may share a common mechanism. Where the events converge is not completely understood, although increased Ca2+ influx and Ca2+-dependent activation of proteases are critical in both processes (George et al., 1995; Knöferle et al., 2010). Moreover, measuring Ca2+ levels in the distal ends of transected nerves reveals distinct phases of Ca2+ elevation involving an initial Ca2+ wave front that propagates anterogradely from the injury site within seconds after axotomy (Ziv and Spira, 1993), followed by a slower rise in Ca2+ throughout the axon that occurs hours later (LoPachin et al., 1990). Although the evidence is only correlative, we surmise that such bimodal rise of Ca2+ in the axon may underlie the separate stages of axon degeneration in AAD and Wallerian degeneration. Experiments addressing whether specifically blocking AAD affects the progression of subsequent axon degeneration, such as by chelating Ca2+ or reversibly blocking Ca2+ channels only within the immediate minutes after axotomy, will help elucidate the mechanistic relationship between AAD and Wallerian degeneration.
Phase II: Latency in the distal axon
In contrast to the proximal stump, which begins to produce axoplasmic sprouts toward the lesion site only a few hours after axotomy (Kerschensteiner et al., 2005), the distal axons exhibit a period of structural quiescence after AAD, though these axons are still capable of physiological functions. For instance, severed axons of motor neurons retain their ability to conduct action potentials up to 24 h after injury in vivo, though the evoked potentials and conduction velocity progressively decay (Moldovan et al., 2009). Additionally, both anterograde and retrograde transport activities continue in the distal axon (Smith and Bisby, 1993). What is the molecular basis of this physiological latency in the injured axon, and more importantly what triggers the abrupt transition from this phase to rapid, irreversible physical degeneration? We examine the molecular events that have been shown to modulate the duration of axon survival, and discuss their roles as potential triggers for the switch to axonal degradation.
Increased intra-axonal calcium.
As an early and critical event in AAD, intra-axonal rise in Ca2+ levels is also necessary and sufficient for the subsequent Wallerian degeneration of the distal axon. Culturing neurons in a reduced calcium environment by switching to low Ca2+ media (below 200uM) or chelating external Ca2+ with EGTA robustly delays axon degeneration for 4 d after axotomy, whereas adding Ca2+ ionophores is sufficient to revert the protective phenotype and induce degeneration in uninjured neurites (Schlaepfer and Bunge, 1973; George et al., 1995). Similar to AAD, the Ca2+-dependent protease calpain is also activated in the distal axons from rising Ca2+ levels after axotomy (Glass et al., 2002); however, chemically inhibiting calpain activity only delays axon degeneration for 12–24 h in vitro (Glass et al., 1994; Wang et al., 2000). The significantly weaker axonal protection from calpain inhibition alone compared with Ca2+ chelation (Finn et al., 2000; Zhai et al., 2003) suggests that additional Ca2+-dependent proteases or pathways mediate the cytoskeletal breakdown in Wallerian degeneration.
What are the major causes of elevated intra-axonal calcium after traumatic nerve injury? As the exposed axonal membrane at the transected ends is quickly sealed by Ca2+-dependent fusion of vesicles (Eddleman et al., 1998), and as the highest axonal Ca2+ levels actually occur at significant distances away from the injury site (Ziv and Spira, 1993), the transient Ca2+ leak from openings at the cut ends is unlikely to account for the bulk of Ca2+ rise in the distal axon. Instead, the sources of axonal Ca2+ that contribute to Wallerian degeneration likely come from channel-mediated Ca2+ influx and intracellular Ca2+ release from storage sites in the injured axon itself.
Indeed, it was previously shown that L-type, but not N-type calcium channel blockers significantly delay axon degeneration for 4 d after axotomy (George et al., 1995), indicating that Ca2+ influx is required for the progression of Wallerian degeneration and is mediated by ion-specific channels. However, the signal or driving force that promotes Ca2+ entry into the injured axon is less well defined. Several reports suggest that decreased activity of Na+/K+ ATPase due to energy failure or mechanical disruption of surface membrane from nerve injuries leads to initial Na+ influx through tetrodotoxin (TTX)-sensitive Na+ channels. This then depolarizes the membrane to open voltage-gated Ca2+ channels as well as reverses the normal direction of Na+/Ca2+ exchanger activity to collectively drive Ca2+ influx (Stirling and Stys, 2010). However, addition of TTX, which blocks the inward Na+ current and attenuates intra-axonal Ca2+ levels (Wolf et al., 2001), fails to delay axon degeneration after axotomy (George et al., 1995; Press and Milbrandt, 2008), suggesting that Na+ entry is not the primary signal to trigger Ca2+ influx, or does not lead to sufficient Ca2+ entry to initiate Wallerian axon degeneration. Curiously, a decrease in intra-axonal K+ is reported to precede the rise of Ca2+ and Na+ levels in transected sciatic nerves, (LoPachin et al., 1990), raising the possibility that loss of intra-axonal K+ potential may provide a more critical electrochemical driving force for Ca2+ influx after nerve injuries.
In addition to extracellular influx, significant sources of intracellular Ca2+ are also sequestered in membranous organelles (Verkhratsky, 2005). These Ca2+ stores are tightly regulated by organelle efflux and uptake, but may be released in excess subsequent to extracellular Ca2+ entry or as a direct response to nerve injury. For instance, the mitochondria can buffer cytosolic Ca2+ through selective uniporters (Kirichok et al., 2004; Perocchi et al., 2010), and are capable of Ca2+ release by opening the permeability transition pore (PTP) complex when mitochondrial Ca2+ levels rise precipitously (Rasola and Bernardi, 2007). In fact, increasing the threshold for mitochondrial Ca2+ release by genetically ablating cyclophilin D, a critical component of the PTP in promoting mitochondrial Ca2+ efflux (Baines et al., 2005), limits white matter loss in traumatic brain injury (Büki et al., 1999) and temporarily delays axon degeneration from axotomy for up to 4 h (Barrientos et al., 2011). Moreover, at least in ischemic nerve injuries, Ca2+ is released from the ER through ryanodine and IP(3) receptors (Ouardouz et al., 2003; Nikolaeva et al., 2005), while axotomy rapidly depletes ER Ca2+ stores in the proximal axon (Rigaud et al., 2009). In more distal axons a similar system of ER-derived endomembranous tubules, collectively termed “axoplasmic reticulum” (Ellisman and Porter, 1980; Lindsey and Ellisman, 1985), expresses structural elements that functionally resemble the smooth ER in the soma (Merianda et al., 2009) and contains significant Ca2+ deposits (Henkart et al., 1978), suggesting that additional Ca2+ may be specifically stored and released from these endomembranous organelles in the axon. Further evidence of physical exchange of Ca2+ between the ER and mitochondria at regions of high Ca2+ concentration (Rizzuto et al., 1998) delineate a dynamic relationship between sites of Ca2+ storage to maintain homeostatic levels of intracellular Ca2+. Injury to the axon is likely to perturb this balance by increasing extracellular Ca2+ influx and/or triggering the release of intracellular Ca2+ stores, which overwhelms the endogenous buffering capacity and results in catastrophic rise of Ca2+ levels in the axon (Fig. 2).
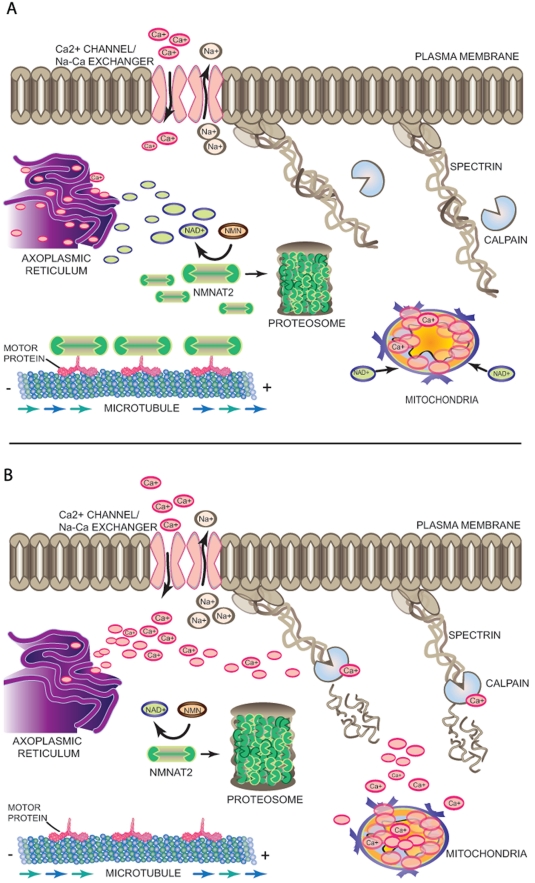
Figure 2.
A molecular model of axon degeneration. (A) In the absence of injury, there is a balance between continuous somal supply via anterograde transport of an axon survival signal such as Nmnat2 and its degradation by the proteasome, resulting in sustained level of the molecule in the axon. Sufficiently high local NAD+ levels, resulting from enzymatic activity of Nmnat2, may keep axonal Ca2+ levels low by regulating the movement of Ca2+ in and out of axonal Ca2+ storage sites such as axoplasmic reticulum and mitochondria through a so-far unidentified mechanism. This regulation of Ca2+ levels in the axoplasm prevents the activation of Ca2+-dependent proteases from cleaving cytoskeletal proteins such as spectrin and preserves the structural and functional integrity of the axon. (B) Nerve injury results in impaired somal supply of Nmnat2 to the axon, resulting in diminished levels of the protein as well as axonal NAD+. Lack of NAD+-dependent regulation of Ca2+ levels lead to increased Ca2+ channel-mediated influx, reverse activity of the Na+/Ca2+ exchanger, or release of Ca2+ from internal storage sites, which together contribute to a catastrophic rise in intra-axonal Ca2+. High Ca2+ levels activate Ca2+-dependent proteases and initiate proteolytic degradation of spectrin and other axonal cytoskeletal components.
Is rising axonal Ca2+ concentration an early trigger to activate the degeneration program after injury, or an effector of more upstream signaling events? Recent evidence suggests that the latter is more likely to be the case. Events similar to Wallerian axon degeneration also occur in genetic mutants such as pmn that are impaired in axonal transport (Martin et al., 2002), where there is an absence of direct trauma and where the source of Ca2+ influx into the axon is not apparent (Coleman, 2005). Moreover, exogenous addition of Ca2+ is sufficient to abolish WldS-mediated axonal protection (Glass et al., 1994), indicating that the calcium-dependent activities in the axon are downstream of molecular components that suppress the activation of Wallerian degeneration. Thus, although Ca2+ signaling is clearly critical for axonal degradation, more upstream cellular processes likely trigger the increased intra-axonal Ca2+ levels by promoting channel-mediated influx or modulating intracellular Ca2+ buffering and release, or both, while subsequent Ca2+-dependent proteolytic events mediate the physical destruction of the axon.
Intra-axonal signaling of axon death/survival signals.
The above conclusion invites the question of what, if any, signaling event is sufficient to trigger the Ca2+-dependent degeneration program in the axon? Two potential mechanisms may be used by the cell to signal nerve injury and initiate axon degeneration. First, axonal injury may directly activate or uncage a death signal to trigger a Ca2+-dependent degeneration program. Alternatively, nerve injury may impair the transport or expression of an axonal trophic factor, causing the molecule to fall below a critical threshold to support axonal integrity and function (Lubińska, 1982; Coleman, 2005). There is recent evidence supporting both hypotheses.
Consistent with the “death signal” hypothesis, recent reports implicate a network of kinases that promotes axonal degeneration after nerve injuries. For instance, loss-of-function mutations in mammalian DLK or its Drosophila homologue wallenda, a member of the mitogen-activated kinase kinase kinase family, as well as use of a chemical inhibitor of JNK kinase, a target of DLK, all result in axon protection up to 48 h after axotomy (Miller et al., 2009). Moreover, both genetic knockdown by shRNA and chemical inhibition of GSK3 or IKKB were also sufficient to delay axon degeneration by 24 h in vitro after the same injury to neurites (Gerdts et al., 2011). In all these events, the axonal protection occurs only when the kinase activity is inhibited between time of axotomy and up to 3 h after injury (Miller et al., 2009), suggesting that these molecules may function as early sensors of axonal injury. Yet it is unclear how injury leads to activation of these kinases, and whether increased kinase activity is sufficient to induce spontaneous axon degeneration or abolish WldS-mediated axon protection. Moreover, the extent of axon protection from inhibiting these molecules alone remains considerably weaker than that from WldS expression or Ca2+ chelation, indicating that activation of these molecules may be one of several parallel injury response events rather than the principal trigger of axon degeneration. In another study supporting the presence of an axonal self-destruction signal, Nikolaev et al. (2009) observed that trophic starvation of neurons leads to cleavage and secretion of APP, a precursor of β-amyloid protein and a marker of axonal damage (Coleman, 2005). The secreted APP binds to the TNF family receptor DR6 as an autocrine signal to trigger a BAX-dependent, caspase-6–mediated breakdown of axonal cytoskeleton (Nikolaev et al., 2009). However, blocking the release of APP or chemically inhibiting caspase-6 fails to delay axon degeneration after axotomy (Vohra et al., 2010), suggesting that trophic starvation triggers a distinct axonal self-destruction program from that caused by traumatic injuries.
At the same time, there is also evidence suggesting that a constitutively transported or expressed factor normally supports axonal survival, and its absence or degradation after injury triggers axon degeneration. Recently, Gilley and Coleman (2010) observed that focal inhibition of protein translation in the cell body, but not in the axon, results in spontaneous axon degeneration of uninjured neurites. This suggests that synthesis of a protein factor in the soma and its delivery to the axon, rather than local axonal translation, maintains axon viability. Remarkably, the group identifies Nmnat2, a primarily ER and Golgi-localized protein that catalyzes the rate-limiting step of NAD+ synthesis (Berger et al., 2005), as one of the factors required for the survival of wild-type axons. In the CNS, Nmnat2 is a highly neuronal-specific protein (Cahoy et al., 2008). It is expressed in the axons and its expression quickly decreases within 4 h after axotomy or after blockade of axonal transport (Gilley and Coleman, 2010). This turnover time for the protein correlates with the latent period between axon injury and the initial appearance of axonal blebbing (Beirowski et al., 2004). Moreover, depletion of Nmnat2, but not other isoforms of Nmnat enzyme using siRNA specifically induces degeneration in uninjured axons, while overexpression of Nmnat2 delays the degeneration of wild-type axons from axotomy for up to 48 h (Gilley and Coleman, 2010; Yan et al., 2010). These exciting findings indicate that Nmnat2 is continuously required to promote endogenous axon survival, and that decreased level of the protein—caused by impaired axonal transport from the soma, local degradation in the axon, or both—leads to failure to suppress a default axon degeneration program (Fig. 2).
Ubiquitin–proteasome system.
Previous studies demonstrate that inhibiting the ubiquitin–proteasome system (UPS) by blocking proteasome activity prevents axon pruning during development (Watts et al., 2003) and delays axon degeneration from injury up to 3 d in vivo in mammalian nerves and 5–10 d in Drosophila axons (Zhai et al., 2003; MacInnis and Campenot, 2005; Hoopfer et al., 2006). How does proteasome inhibition protect axons, and through what mechanism does axonal injury affect ubiquitination or proteasome activity?
As the primary function of the proteasome is to regulate protein turnover, a likely explanation is that proteasome inhibition helps sustain intracellular levels of molecules that promote axonal survival by maintaining transport of the factor to the axon or by preventing the degradation of the molecule itself. Consistent with the first mechanism, proteasome inhibition in axotomized nerves attenuates microtubule disassembly and preserves axonal transport by preventing the turnover of microtubule-associated factors such as MAP1 and tau that help stabilize the microtubule network (Zhai et al., 2003). Moreover, inhibiting proteasome activity may also directly interfere with the degradation of a putative axonal survival factor such as Nmnat2. Indeed, the turnover of Nmnat2 is shown to be dependent on proteasome activity as its levels in the transected axon remain high when proteasome activity is blocked (Gilley and Coleman, 2010). Thus, in axonal injuries where the somal supply of the factor is lost, the level of the survival factor in the axon is then solely determined by its rate of proteasome-dependent degradation, whereas proteasome inhibition helps sustain sufficient levels of the factor in the axon to delay the onset of axon degeneration (Fig. 2).
Phase III: Granular fragmentation
The latent phase of Wallerian degeneration is followed by an abrupt switch to explosive, asynchronous fragmentation that is completed along the length of the axolemma within 1–2 h from the onset, with a rate of up to 24 mm/h in vivo and 0.4 mm/h in culture (Sievers et al., 2003; Beirowski et al., 2005). Interestingly, focal, severe injuries such as axotomy result in a proximal-to-distal direction of axon degeneration, whereas in more chronic injuries the axons degenerate from synaptic ends to the cell body in a retrograde pattern (Beirowski et al., 2005). The basis for the injury-dependent differences in the direction of degeneration is not fully understood. A recent report suggests that increased macroautophagy activity that is dependent on AKT/mTOR signaling mediates retrograde axon degeneration in dopaminergic neurons after acute chemotoxic injury (Cheng et al., 2011). However, whether this mechanism participates in Wallerian degeneration and whether the signaling events occur broadly in other CNS neurons is unclear. On the other hand, the proximal-to-distal direction of degeneration from acute nerve transection may be accounted for by the survival factor hypothesis, as depletion of the survival factor would occur at the proximal tip of the distal stump first if anterograde transport continues in the axon (Lubińska, 1982). The segment closest to the cut site would likely experience the earliest loss of the survival factor, and therefore be the first to undergo degeneration after axotomy.
Delayed axonal degeneration: Molecular mechanisms of WldS axon protection
The discovery of the WldS mouse mutant, which robustly protects both CNS and PNS nerves from physical injury, chemotoxic insult, and neurodegenerative conditions (Lunn et al., 1989; Perry et al., 1991; Wang et al., 2001; Ferri et al., 2003; Howell et al., 2007) changed our understanding of the nature of axon degeneration in injury and disease. In contrast to complete fragmentation of wild-type axons within 48 h after axotomy, the transected WldS axons remain structurally intact and electrically excitable for weeks in vivo and up to a week in culture (Lunn et al., 1989; Ludwin and Bisby, 1992; Wang et al., 2005). Transgenic expression of the WldS protein also leads to axon protection in many species, including rats (Adalbert et al., 2005) and Drosophila (Hoopfer et al., 2006; MacDonald et al., 2006). This protection, which is dose dependent (Mack et al., 2001), also extends to the synapses as WldS expression preserves synaptic integrity and function in the peripheral neuromuscular junction after axotomy (Wang et al., 2001; Adalbert et al., 2005) as well as in the CNS striatum after cortical lesions (Gillingwater et al., 2006a; Wright et al., 2010). Continued axon protection after grafting WldS nerves to wild-type hosts shows that WldS-mediated protection is intrinsic to the axon (Glass et al., 1993), and the mechanism is distinct from simply inhibiting the classical apoptotic pathways that govern cell body degeneration (Deckwerth and Johnson, 1994; Sagot et al., 1995; Finn et al., 2000). Thus, determining the molecular components of the degeneration pathway that WldS is interfering with is critical to understanding how axons are normally lost after injury.
The WldS mutation results in the formation of a chimeric gene product consisting of the first 70 amino acids of a ubiquitination factor (Ube4b) and the full sequence of a NAD+ synthetic enzyme (Nmnat1; Mack et al., 2001). The N-terminal 70 amino acids of Ube4b within WldS contain no enzymatic activity, though it contains a binding site for VCP (Laser et al., 2006), a cytoplasmic protein with diverse cellular functions (Wang et al., 2004). This VCP binding domain, along with the enzymatic activity of Nmnat1, are both required for WldS-mediated axon protection (Avery et al., 2009; Conforti et al., 2009). However, overexpression of mutant Nmnat that lacks the enzymatic domain in Drosophila still protects photoreceptor cells from SCA-1–induced degeneration (Zhai et al., 2006). This nonenzymatic protection by Nmnat has been shown to be a consequence of chaperone functions of the protein (Zhai et al., 2008) and suggests that, at least in nonmammalian species, Nmnat is able to confer neuroprotection independent of its enzymatic properties. However, whether this phenotype is axonal specific or an indirect result of broader protection of the cell bodies remains unclear.
Although the WldS protein is predominantly localized in the nucleus due to endogenous nuclear localization of Nmnat1, emerging evidence instead points to the trace amount of extranuclear WldS protein as the critical component for axon protection (Coleman and Freeman, 2010). Indeed, WldS protein has recently been detected in the axoplasm and in axonal organelles including the mitochondria and phagosomes (Beirowski et al., 2009; Yahata et al., 2009). Moreover, misexpression of Nmnat1 alone outside of the nucleus by deleting its nuclear localizing sequence (Beirowski et al., 2009; Sasaki et al., 2009), virally transducing Nmnat1 in injured axons (Sasaki and Milbrandt, 2010), and fusing it to the N-terminal sequence of APP protein to increase expression in axonal compartments (Babetto et al., 2010) all lead to robust axon protection comparable to that of WldS neurons. Thus, extranuclear WldS expression, most likely due to protein interaction between WldS and the cytoplasmic VCP protein (Laser et al., 2006), results in sufficient ectopic Nmnat activity to confer axon protection. Although one cannot rule out the additional contribution of gene changes caused by WldS expression (Gillingwater et al., 2006b) toward the protective phenotype, current data suggest that the likely basis of WldS-mediated axon protection is through mistargeting of the normally nuclear Nmnat1 protein and its enzymatic activity to extranuclear, possibly axonal compartments.
How does the WldS protein mediate axon protection, and where does this activity intersect with the normal degenerative process? Interestingly, both WldS and Nmnat2 share the same enzymatic domain for NAD+ synthesis (Sorci et al., 2007) that is required for axon protection (Jia et al., 2007; Yan et al., 2010). And as endogenous Nmnat2 activity is essential for axonal survival, it is suggested that the WldS protein protects axons by augmenting or substituting for Nmnat2 to maintain sufficient levels of Nmnat enzyme activity in the distal axons after injury (Gilley and Coleman, 2010). Consistent with this hypothesis, in vivo tracing of GFP-tagged WldS protein in uninjured nerves shows that WldS is normally present in axonal regions where Nmnat2 is also expressed. Moreover, Nmnat2 is found to rapidly degrade, even in WldS nerves, within 4 h after nerve injury, whereas the WldS protein remains stable in the distal axon (Gilley and Coleman, 2010). These reports strongly argue that a molecular mechanism by which the WldS protein confers axon protection is by sustaining key levels of Nmnat activity in the axon that would normally diminish from decreasing Nmnat2 expression after injury (Fig. 3).
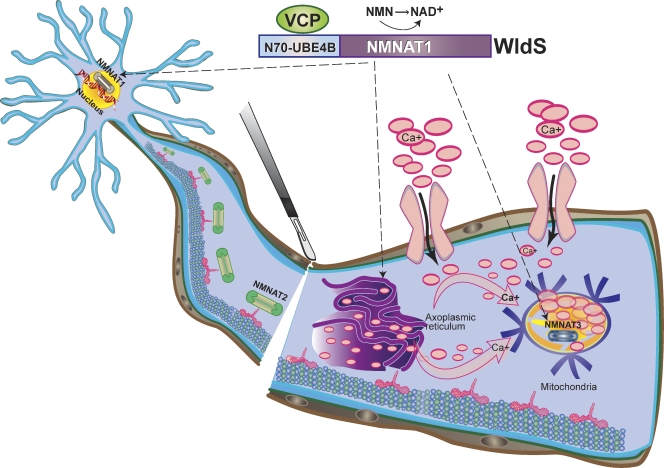
Figure 3.
A molecular model of WldS-mediated axon protection. The WldS fusion protein, consisting of the full-length Nmnat1 and the first 70 amino acids of Ube4b, is predominantly localized in the nucleus; however, it is also expressed in axonal cytoplasm and organelles such as mitochondria (broken arrows denote known neuronal sites of WldS expression) likely due to interaction with the cytoplasmic VCP protein. Expression of either WldS or extranuclear forms of Nmnat1 is sufficient to protect axons from degeneration upon injury, and this may result from substituting for the activity of Nmnat2 protein, which is degraded quickly after nerve injury. The WldS protein may also augment the enzymatic activity of Nmnat3, a mitochondrial Nmnat isoform, to confer axon protection. The combined result of ectopic Nmnat activities in WldS neurons may be less intracellular Ca2+ release from axoplasmic reticulum or greater Ca2+ buffering by the mitochondria via increased NAD+ production in these organelles, leading to overall decrease in intra-axonal Ca2+ levels (pink arrows denote net direction of Ca2+ flux).
Moreover, as additional Nmnat isoforms exist in different subcellular regions, WldS protein may also promote axonal survival by augmenting the activity of other Nmnat enzymes. Consistent with this, overexpression of Nmnat3, a mitochondrial Nmnat isoform (Berger et al., 2005), results in robust axon protection from traumatic injuries comparable to that of WldS expression in both mammalian and Drosophila models (Avery et al., 2009; Sasaki et al., 2009; Yahata et al., 2009). Although Nmnat3 is not required for normal maintenance of axonal survival (Gilley and Coleman, 2010), its overexpression results in significantly stronger axonal protection than from overexpression of Nmnat2, though this may be due to the labile nature of Nmnat2 protein (Gilley and Coleman, 2010). As the WldS protein is also identified in the mitochondria (Yahata et al., 2009), it raises the notion that WldS expression increases Nmnat activity at multiple intracellular sites to delay axonal degeneration. Assessing whether expression of WldS continues to protect axons in Nmnat2 or Nmnat3 knockouts will help reveal the critical site of Nmnat activity for conferring axonal protection.
Yet, precisely how increased Nmnat enzymatic activity in WldS, Nmnat2, or Nmnat3 protects the axons remains a mystery. As all three proteins contain the highly conserved catalytic domain for the synthesis of NAD+ (Berger et al., 2005), the common metabolite of Nmnat enzyme activities, NAD+ has emerged as an attractive molecular mediator of WldS axon protection. Indeed, exogenous NAD+ is sufficient to protect axons in vitro (Wang et al., 2005; Sasaki et al., 2009). However, this axon protection is only observed at extracellular concentrations above 1 mM, which far exceeds physiological levels, although this may be due to saturation or limited uptake of extracellular NAD+ by putative NAD+ channels on the surface membrane (Alano et al., 2010). Surprisingly, no appreciable difference in NAD+ levels is detected between WldS and wild-type neurons (Mack et al., 2001), and globally reducing neuronal NAD+ levels by chemically inhibiting NAMPT, an enzyme that synthesizes the precursor to NAD+, does not abate WldS axon protection in vitro. Although inhibiting NAMPT activity partially abolishes WldS protection in vivo (Conforti et al., 2009), whether this is due to toxicity from prolonged drug exposure cannot be ruled out. Conversely, increasing overall NAD+ levels in the neuron by blocking PARP and CD38, both NAD+ consuming enzymes, also fails to protect wild-type axons after injury (Sasaki et al., 2009).
A possible explanation for this paradoxical lack of axon protection when cytoplasmic NAD+ levels are raised is that axonal survival requires high NAD+ levels within specific regions or organelles in the axon. Indeed, both Nmnat2 and Nmnat3 are primarily restricted to neuronal ER/Golgi and mitochondrial compartments, respectively (Berger et al., 2005), suggesting that these intra-axonal compartments and membranous organelles may regulate NAD+ concentrations independently from the cytoplasmic NAD+ pool. The presumed effect of a local increase in NAD+ at these intracellular sites in the axon is unclear, though ATP levels drop precipitously after nerve injury, and exogenous NAD addition sustains neuronal ATP levels (Wang et al., 2005). This suggests that rising NAD+ levels may exert axonal protection through increased local energy production. However, Press and Milbrandt (2008) recently reported that increasing mitochondrial Nmnat3 activity is sufficient to delay axon degeneration even from rotenone, a blocker of mitochondrial oxidative phosphorylation. This axonal protection is independent of ATP levels as the rate of ATP loss is similar between Nmnat3-expressing and wild-type axons treated with rotenone (Press and Milbrandt, 2008). Moreover, neither lowering neuronal ATP levels by adding deoxyglucose, an inhibitor of glycolytic enzymes, nor raising ATP levels through TTX, which attenuates ATP consumption by Na/K ATPase, affects axon survival, indicating that maintenance of ATP is unlikely to be the principal mechanism by which increased NAD+ levels promote axon protection. Alternatively, as both the ER/Golgi and mitochondria are capable of sequestering Ca2+, an attractive hypothesis is that sufficient NAD+ levels in these organelles may directly regulate Ca2+ balance in the axon. Indeed, aside from serving as cofactor in oxidative phosphorylation, NAD+ is also capable of modulating ion channel opening (Tamsett et al., 2009). This indicates the possibility that local increase in Nmnat activity, and therefore increase in NAD+ levels, may lower intra-axonal Ca2+ concentrations and protect axons by increasing Ca2+ buffering capacity of mitochondria or decreasing intracellular release by axoplasmic Ca2+ storage sites (Fig. 3). Further work in analyzing differences in Ca2+ flow in subcellular axonal compartments between WldS and wild-type neurons, as well as identifying NAD+-interacting proteins that may gate Ca2+ movement in the axon will be critical in identifying the relationship between Ca2+ regulation and Nmnat activity in promoting axonal survival.
Concluding remarks
Previous studies have independently established the requirements for axonal Ca2+ rise, activities by the ubiquitin–proteasome system and intracellular proteases in axonal degradation. However, how each molecular component interacts to orchestrate the initiation and execution of axonal self-destruction after injury is unclear. A critical insight is gleaned from an early study by Lubińska et al. (1982), who observed that the latency period before the distal axons degenerate is lengthened when the axonal transection is made closer to the cell body. Lubińska and colleagues reasoned that a survival factor is maintained at a critical level in the axon, and when an injury occurs close to the cell body there is a greater level of the trophic factor remaining in the larger distal stump than when the injury occurs more distally. Building upon Lubińska’s initial hypothesis, an appealing unifying model of axon degeneration is that after axonal injury, there is impaired axoplasmic delivery of an axonal survival factor from the soma, which along with continual turnover of the survival factor by the proteasome causes its expression to fall below a critical threshold in the axon. This decreased survival factor activity is sensed by the axon, perhaps via decreased local levels of NAD+, to trigger an execution signal or program that increases intra-axonal Ca2+ levels due to extracellular influx or intracellular Ca2+ release, which then initiates Ca2+-dependent cytoskeletal breakdown (Fig. 2). Such a trophic delivery model helps explain many features of Wallerian axon degeneration, including the physiological latency due to the turnover rate of the survival factor, and the injury-specific directionality of axon degeneration due to the predicted time course of survival factor loss after disrupted transport. It also helps explain the mechanism of WldS protection as one of sustaining Nmnat activity, and thus NAD+ levels above critical threshold in axonal compartments (Fig. 3). The recent identification of the labile molecule Nmnat2, whose endogenous levels in the axon are influenced by proteasomal turnover and whose enzymatic activity is necessary for axon survival, provides exciting experimental support for the survival factor hypothesis.
This survival factor model is also useful for guiding future experiments to answer several key unanswered questions. First, is NAD+, the common enzymatic product of Nmnat2, Nmnat3, and WldS proteins, the critical molecule that mediates axon survival? Identifying the putative molecular targets of NAD+ in the axon, as well as determining whether other metabolites are produced by Nmnat through a metabolomics screen, provide complimentary approaches to identify the key molecular events downstream of Nmnat activity responsible for maintaining axon survival. Second, what are the intermediate events between Nmnat activity and physical proteolysis of the axon? Although direct gating of intra-axonal Ca2+ levels by Nmnat or even NAD+ is appealing in its simplicity, it has yet to be demonstrated experimentally. Finally, do other pathways known to modulate axon degeneration, including several of the “death signals,” interact or converge with Nmnat activity? Interestingly, increased Nmnat activity protects against both Wallerian degeneration and axon degradation from trophic withdrawal (Vohra et al., 2010), suggesting that the final commitment to irreversible axon degeneration may intersect at the levels of Nmnat activity in the axon. Thus, addressing the downstream effectors of WldS/Nmnat-mediated axon protection will be instrumental in identifying therapeutic targets to protect the white matter from many insults besides traumatic injuries. With a working molecular model, one may begin to answer these questions and accelerate the process of identifying the full molecular pathways that underlie axon degeneration in injury and disease.
Acknowledgments
We thank Mariko Howe, Mauricio Vargas, and Tom Clandinin for critical reading of the manuscript. The authors apologize to colleagues whose work was not included due to space restrictions.
This work was supported by National Institutes of Health grant EY11310 and the Adelson Medical Research Foundation. J.T. Wang was supported by a Howard Hughes Medical Scientist Research Training Grant and an American Heart Association Pre-doctoral Fellowship.
Footnotes
Abbreviations used in this paper:
- AAD
- acute axonal degeneration
- TTX
- tetrodotoxin
- WldS
- Wallerian degeneration slow
References
- Adalbert R., Gillingwater T.H., Haley J.E., Bridge K., Beirowski B., Berek L., Wagner D., Grumme D., Thomson D., Celik A., et al. 2005. A rat model of slow Wallerian degeneration (WldS) with improved preservation of neuromuscular synapses. Eur. J. Neurosci. 21:271–277 10.1111/j.1460-9568.2004.03833.x [DOI] [PubMed] [Google Scholar]
- Alano C.C., Garnier P., Ying W., Higashi Y., Kauppinen T.M., Swanson R.A. 2010. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J. Neurosci. 30:2967–2978 10.1523/JNEUROSCI.5552-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T., Sasaki Y., Milbrandt J. 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 305:1010–1013 10.1126/science.1098014 [DOI] [PubMed] [Google Scholar]
- Avery M.A., Sheehan A.E., Kerr K.S., Wang J., Freeman M.R. 2009. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J. Cell Biol. 184:501–513 10.1083/jcb.200808042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babetto E., Beirowski B., Janeckova L., Brown R., Gilley J., Thomson D., Ribchester R.R., Coleman M.P. 2010. Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo. J. Neurosci. 30:13291–13304 10.1523/JNEUROSCI.1189-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., et al. 2005. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 434:658–662 10.1038/nature03434 [DOI] [PubMed] [Google Scholar]
- Barrientos S.A., Martinez N.W., Yoo S., Jara J.S., Zamorano S., Hetz C., Twiss J.L., Alvarez J., Court F.A. 2011. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J. Neurosci. 31:966–978 10.1523/JNEUROSCI.4065-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B., Berek L., Adalbert R., Wagner D., Grumme D.S., Addicks K., Ribchester R.R., Coleman M.P. 2004. Quantitative and qualitative analysis of Wallerian degeneration using restricted axonal labelling in YFP-H mice. J. Neurosci. Methods. 134:23–35 10.1016/j.jneumeth.2003.10.016 [DOI] [PubMed] [Google Scholar]
- Beirowski B., Adalbert R., Wagner D., Grumme D.S., Addicks K., Ribchester R.R., Coleman M.P. 2005. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 6:6 10.1186/1471-2202-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B., Babetto E., Gilley J., Mazzola F., Conforti L., Janeckova L., Magni G., Ribchester R.R., Coleman M.P. 2009. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J. Neurosci. 29:653–668 10.1523/JNEUROSCI.3814-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F., Lau C., Dahlmann M., Ziegler M. 2005. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 280:36334–36341 10.1074/jbc.M508660200 [DOI] [PubMed] [Google Scholar]
- Billger M., Wallin M., Karlsson J.O. 1988. Proteolysis of tubulin and microtubule-associated proteins 1 and 2 by calpain I and II. Difference in sensitivity of assembled and disassembled microtubules. Cell Calcium. 9:33–44 10.1016/0143-4160(88)90036-X [DOI] [PubMed] [Google Scholar]
- Büki A., Okonkwo D.O., Povlishock J.T. 1999. Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury. J. Neurotrauma. 16:511–521 10.1089/neu.1999.16.511 [DOI] [PubMed] [Google Scholar]
- Cahoy J.D., Emery B., Kaushal A., Foo L.C., Zamanian J.L., Christopherson K.S., Xing Y., Lubischer J.L., Krieg P.A., Krupenko S.A., et al. 2008. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28:264–278 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.B. 1964. The significance of the “dying back” process in experimental and human neurological disease. Int. Rev. Exp. Pathol. 3:219–267 [PubMed] [Google Scholar]
- Cheng H.C., Kim S.R., Oo T.F., Kareva T., Yarygina O., Rzhetskaya M., Wang C., During M., Talloczy Z., Tanaka K., et al. 2011. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J. Neurosci. 31:2125–2135 10.1523/JNEUROSCI.5519-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. 2005. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 6:889–898 10.1038/nrn1788 [DOI] [PubMed] [Google Scholar]
- Coleman M.P., Freeman M.R. 2010. Wallerian degeneration, wld(s), and nmnat. Annu. Rev. Neurosci. 33:245–267 10.1146/annurev-neuro-060909-153248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L., Tarlton A., Mack T.G., Mi W., Buckmaster E.A., Wagner D., Perry V.H., Coleman M.P. 2000. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc. Natl. Acad. Sci. USA. 97:11377–11382 10.1073/pnas.97.21.11377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L., Wilbrey A., Morreale G., Janeckova L., Beirowski B., Adalbert R., Mazzola F., Di Stefano M., Hartley R., Babetto E., et al. 2009. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J. Cell Biol. 184:491–500 10.1083/jcb.200807175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckwerth T.L., Johnson E.M., Jr 1994. Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis). Dev. Biol. 165:63–72 10.1006/dbio.1994.1234 [DOI] [PubMed] [Google Scholar]
- Eddleman C.S., Ballinger M.L., Smyers M.E., Fishman H.M., Bittner G.D. 1998. Endocytotic formation of vesicles and other membranous structures induced by Ca2+ and axolemmal injury. J. Neurosci. 18:4029–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M.H., Porter K.R. 1980. Microtrabecular structure of the axoplasmic matrix: visualization of cross-linking structures and their distribution. J. Cell Biol. 87:464–479 10.1083/jcb.87.2.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri A., Sanes J.R., Coleman M.P., Cunningham J.M., Kato A.C. 2003. Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr. Biol. 13:669–673 10.1016/S0960-9822(03)00206-9 [DOI] [PubMed] [Google Scholar]
- Finn J.T., Weil M., Archer F., Siman R., Srinivasan A., Raff M.C. 2000. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J. Neurosci. 20:1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede R.L. 1963. The relationship of body size, nerve cell size, axon length, and glial density in the cerebellum. Proc. Natl. Acad. Sci. USA. 49:187–193 10.1073/pnas.49.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George E.B., Glass J.D., Griffin J.W. 1995. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci. 15:6445–6452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R., Griffin J.W. 1994. Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp. Neurol. 129:225–236 10.1006/exnr.1994.1164 [DOI] [PubMed] [Google Scholar]
- Gerdts J., Sasaki Y., Vohra B., Marasa J., Milbrandt J. 2011. Image-based screening identifies novel roles for IKK and GSK3 in axonal degeneration. J. Biol. Chem. 286:28011–28018 10.1074/jbc.M111.250472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J., Coleman M.P. 2010. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 8:e1000300 10.1371/journal.pbio.1000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater T.H., Ingham C.A., Parry K.E., Wright A.K., Haley J.E., Wishart T.M., Arbuthnott G.W., Ribchester R.R. 2006a. Delayed synaptic degeneration in the CNS of Wlds mice after cortical lesion. Brain. 129:1546–1556 10.1093/brain/awl101 [DOI] [PubMed] [Google Scholar]
- Gillingwater T.H., Wishart T.M., Chen P.E., Haley J.E., Robertson K., MacDonald S.H., Middleton S., Wawrowski K., Shipston M.J., Melmed S., et al. 2006b. The neuroprotective WldS gene regulates expression of PTTG1 and erythroid differentiation regulator 1-like gene in mice and human cells. Hum. Mol. Genet. 15:625–635 10.1093/hmg/ddi478 [DOI] [PubMed] [Google Scholar]
- Glass J.D., Brushart T.M., George E.B., Griffin J.W. 1993. Prolonged survival of transected nerve fibres in C57BL/Ola mice is an intrinsic characteristic of the axon. J. Neurocytol. 22:311–321 10.1007/BF01195555 [DOI] [PubMed] [Google Scholar]
- Glass J.D., Schryer B.L., Griffin J.W. 1994. Calcium-mediated degeneration of the axonal cytoskeleton in the Ola mouse. J. Neurochem. 62:2472–2475 10.1046/j.1471-4159.1994.62062472.x [DOI] [PubMed] [Google Scholar]
- Glass J.D., Culver D.G., Levey A.I., Nash N.R. 2002. Very early activation of m-calpain in peripheral nerve during Wallerian degeneration. J. Neurol. Sci. 196:9–20 10.1016/S0022-510X(02)00013-8 [DOI] [PubMed] [Google Scholar]
- Griffin J.W., George E.B., Hsiesh S.T., Glass J.D. 1995. Axonal degeneration and disorders of the axonal cytoskeleton. The Axon: Structure, Function and Pathophysiology. Waxman S.G., Kocsis J.D., Sytys P.K., Oxford University Press, NY: 3750–3790 [Google Scholar]
- Henkart M.P., Reese T.S., Brinley F.J., Jr 1978. Endoplasmic reticulum sequesters calcium in the squid giant axon. Science. 202:1300–1303 10.1126/science.725607 [DOI] [PubMed] [Google Scholar]
- Hoopfer E.D., McLaughlin T., Watts R.J., Schuldiner O., O’Leary D.D., Luo L. 2006. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 50:883–895 10.1016/j.neuron.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Howell G.R., Libby R.T., Jakobs T.C., Smith R.S., Phalan F.C., Barter J.W., Barbay J.M., Marchant J.K., Mahesh N., Porciatti V., et al. 2007. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 179:1523–1537 10.1083/jcb.200706181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Yan T., Feng Y., Zeng C., Shi X., Zhai Q. 2007. Identification of a critical site in Wld(s): essential for Nmnat enzyme activity and axon-protective function. Neurosci. Lett. 413:46–51 10.1016/j.neulet.2006.11.067 [DOI] [PubMed] [Google Scholar]
- Johnson G.V., Litersky J.M., Jope R.S. 1991. Degradation of microtubule-associated protein 2 and brain spectrin by calpain: a comparative study. J. Neurochem. 56:1630–1638 10.1111/j.1471-4159.1991.tb02061.x [DOI] [PubMed] [Google Scholar]
- Kampfl A., Posmantur R., Nixon R., Grynspan F., Zhao X., Liu S.J., Newcomb J.K., Clifton G.L., Hayes R.L. 1996. mu-calpain activation and calpain-mediated cytoskeletal proteolysis following traumatic brain injury. J. Neurochem. 67:1575–1583 10.1046/j.1471-4159.1996.67041575.x [DOI] [PubMed] [Google Scholar]
- Kaneko S., Wang J., Kaneko M., Yiu G., Hurrell J.M., Chitnis T., Khoury S.J., He Z. 2006. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 26:9794–9804 10.1523/JNEUROSCI.2116-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner M., Schwab M.E., Lichtman J.W., Misgeld T. 2005. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 11:572–577 10.1038/nm1229 [DOI] [PubMed] [Google Scholar]
- Kirichok Y., Krapivinsky G., Clapham D.E. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 427:360–364 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Knöferle J., Koch J.C., Ostendorf T., Michel U., Planchamp V., Vutova P., Tönges L., Stadelmann C., Brück W., Bähr M., Lingor P. 2010. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl. Acad. Sci. USA. 107:6064–6069 10.1073/pnas.0909794107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laser H., Conforti L., Morreale G., Mack T.G., Heyer M., Haley J.E., Wishart T.M., Beirowski B., Walker S.A., Haase G., et al. 2006. The slow Wallerian degeneration protein, WldS, binds directly to VCP/p97 and partially redistributes it within the nucleus. Mol. Biol. Cell. 17:1075–1084 10.1091/mbc.E05-04-0375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J.D., Ellisman M.H. 1985. The neuronal endomembrane system. III. The origins of the axoplasmic reticulum and discrete axonal cisternae at the axon hillock. J. Neurosci. 5:3135–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin R.M., Jr, LoPachin V.R., Saubermann A.J. 1990. Effects of axotomy on distribution and concentration of elements in rat sciatic nerve. J. Neurochem. 54:320–332 10.1111/j.1471-4159.1990.tb13317.x [DOI] [PubMed] [Google Scholar]
- Low L.K., Cheng H.J. 2005. A little nip and tuck: axon refinement during development and axonal injury. Curr. Opin. Neurobiol. 15:549–556 10.1016/j.conb.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Lubińska L. 1977. Early course of Wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 130:47–63 10.1016/0006-8993(77)90841-1 [DOI] [PubMed] [Google Scholar]
- Lubińska L. 1982. Patterns of Wallerian degeneration of myelinated fibres in short and long peripheral stumps and in isolated segments of rat phrenic nerve. Interpretation of the role of axoplasmic flow of the trophic factor. Brain Res. 233:227–240 10.1016/0006-8993(82)91199-4 [DOI] [PubMed] [Google Scholar]
- Ludwin S.K., Bisby M.A. 1992. Delayed wallerian degeneration in the central nervous system of Ola mice: an ultrastructural study. J. Neurol. Sci. 109:140–147 10.1016/0022-510X(92)90160-M [DOI] [PubMed] [Google Scholar]
- Lunn E.R., Perry V.H., Brown M.C., Rosen H., Gordon S. 1989. Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. Eur. J. Neurosci. 1:27–33 10.1111/j.1460-9568.1989.tb00771.x [DOI] [PubMed] [Google Scholar]
- Luo L., O’Leary D.D. 2005. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28:127–156 10.1146/annurev.neuro.28.061604.135632 [DOI] [PubMed] [Google Scholar]
- MacDonald J.M., Beach M.G., Porpiglia E., Sheehan A.E., Watts R.J., Freeman M.R. 2006. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 50:869–881 10.1016/j.neuron.2006.04.028 [DOI] [PubMed] [Google Scholar]
- MacInnis B.L., Campenot R.B. 2005. Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol. Cell. Neurosci. 28:430–439 10.1016/j.mcn.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Mack T.G., Reiner M., Beirowski B., Mi W., Emanuelli M., Wagner D., Thomson D., Gillingwater T., Court F., Conforti L., et al. 2001. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 4:1199–1206 10.1038/nn770 [DOI] [PubMed] [Google Scholar]
- Martin N., Jaubert J., Gounon P., Salido E., Haase G., Szatanik M., Guénet J.L. 2002. A missense mutation in Tbce causes progressive motor neuronopathy in mice. Nat. Genet. 32:443–447 10.1038/ng1016 [DOI] [PubMed] [Google Scholar]
- Merianda T.T., Lin A.C., Lam J.S., Vuppalanchi D., Willis D.E., Karin N., Holt C.E., Twiss J.L. 2009. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol. Cell. Neurosci. 40:128–142 10.1016/j.mcn.2008.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Horste G., Miesbach T.A., Muller J.I., Fledrich R., Stassart R.M., Kieseier B.C., Coleman M.P., Sereda M.W. 2011. The Wlds transgene reduces axon loss in a Charcot-Marie-Tooth disease 1A rat model and nicotinamide delays post-traumatic axonal degeneration. Neurobiol. Dis. 42:1–8 10.1016/j.nbd.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Miller B.R., Press C., Daniels R.W., Sasaki Y., Milbrandt J., DiAntonio A. 2009. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat. Neurosci. 12:387–389 10.1038/nn.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan M., Alvarez S., Krarup C. 2009. Motor axon excitability during Wallerian degeneration. Brain. 132:511–523 10.1093/brain/awn332 [DOI] [PubMed] [Google Scholar]
- Nikolaev A., McLaughlin T., O’Leary D.D., Tessier-Lavigne M. 2009. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 457:981–989 10.1038/nature07767 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nikolaeva M.A., Mukherjee B., Stys P.K. 2005. Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J. Neurosci. 25:9960–9967 10.1523/JNEUROSCI.2003-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M., Nikolaeva M.A., Coderre E., Zamponi G.W., McRory J.E., Trapp B.D., Yin X., Wang W., Woulfe J., Stys P.K. 2003. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron. 40:53–63 10.1016/j.neuron.2003.08.016 [DOI] [PubMed] [Google Scholar]
- Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E., Mootha V.K. 2010. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 467:291–296 10.1038/nature09358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V.H., Brown M.C., Lunn E.R. 1991. Very slow retrograde and Wallerian degeneration in the CNS of C57BL/Ola mice. Eur. J. Neurosci. 3:102–105 10.1111/j.1460-9568.1991.tb00815.x [DOI] [PubMed] [Google Scholar]
- Press C., Milbrandt J. 2008. Nmnat delays axonal degeneration caused by mitochondrial and oxidative stress. J. Neurosci. 28:4861–4871 10.1523/JNEUROSCI.0525-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M.C., Whitmore A.V., Finn J.T. 2002. Axonal self-destruction and neurodegeneration. Science. 296:868–871 10.1126/science.1068613 [DOI] [PubMed] [Google Scholar]
- Rasola A., Bernardi P. 2007. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 12:815–833 10.1007/s10495-007-0723-y [DOI] [PubMed] [Google Scholar]
- Rigaud M., Gemes G., Weyker P.D., Cruikshank J.M., Kawano T., Wu H.E., Hogan Q.H. 2009. Axotomy depletes intracellular calcium stores in primary sensory neurons. Anesthesiology. 111:381–392 10.1097/ALN.0b013e3181ae6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 280:1763–1766 10.1126/science.280.5370.1763 [DOI] [PubMed] [Google Scholar]
- Sagot Y., Dubois-Dauphin M., Tan S.A., de Bilbao F., Aebischer P., Martinou J.C., Kato A.C. 1995. Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J. Neurosci. 15:7727–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsam M., Mi W., Wessig C., Zielasek J., Toyka K.V., Coleman M.P., Martini R. 2003. The Wlds mutation delays robust loss of motor and sensory axons in a genetic model for myelin-related axonopathy. J. Neurosci. 23:2833–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Milbrandt J. 2010. Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J. Biol. Chem. 285:41211–41215 10.1074/jbc.C110.193904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Vohra B.P., Lund F.E., Milbrandt J. 2009. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J. Neurosci. 29:5525–5535 10.1523/JNEUROSCI.5469-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Caroni P. 2007. Mechanisms of axon degeneration: from development to disease. Prog. Neurobiol. 83:174–191 10.1016/j.pneurobio.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Schlaepfer W.W., Bunge R.P. 1973. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J. Cell Biol. 59:456–470 10.1083/jcb.59.2.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmann Z., Assa-Kunik E., Tiomny S., Minis A., Haklai-Topper L., Arama E., Yaron A. 2010. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J. Neurosci. 30:6375–6386 10.1523/JNEUROSCI.0922-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers C., Platt N., Perry V.H., Coleman M.P., Conforti L. 2003. Neurites undergoing Wallerian degeneration show an apoptotic-like process with Annexin V positive staining and loss of mitochondrial membrane potential. Neurosci. Res. 46:161–169 10.1016/S0168-0102(03)00039-7 [DOI] [PubMed] [Google Scholar]
- Smith R.S., Bisby M.A. 1993. Persistence of axonal transport in isolated axons of the mouse. Eur. J. Neurosci. 5:1127–1135 10.1111/j.1460-9568.1993.tb00967.x [DOI] [PubMed] [Google Scholar]
- Sorci L., Cimadamore F., Scotti S., Petrelli R., Cappellacci L., Franchetti P., Orsomando G., Magni G. 2007. Initial-rate kinetics of human NMN-adenylyltransferases: substrate and metal ion specificity, inhibition by products and multisubstrate analogues, and isozyme contributions to NAD+ biosynthesis. Biochemistry. 46:4912–4922 10.1021/bi6023379 [DOI] [PubMed] [Google Scholar]
- Spira M.E., Oren R., Dormann A., Gitler D. 2003. Critical calpain-dependent ultrastructural alterations underlie the transformation of an axonal segment into a growth cone after axotomy of cultured Aplysia neurons. J. Comp. Neurol. 457:293–312 10.1002/cne.10569 [DOI] [PubMed] [Google Scholar]
- Stirling D.P., Stys P.K. 2010. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol. Med. 16:160–170 10.1016/j.molmed.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamsett T.J., Picchione K.E., Bhattacharjee A. 2009. NAD+ activates KNa channels in dorsal root ganglion neurons. J. Neurosci. 29:5127–5134 10.1523/JNEUROSCI.0859-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Rolls M.M. 2011. Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J. Neurosci. 31:5398–5405 10.1523/JNEUROSCI.3826-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao J.W., Brown M.C., Carden M.J., McLean W.G., Perry V.H. 1994. Loss of the compound action potential: an electrophysiological, biochemical and morphological study of early events in axonal degeneration in the C57BL/Ola mouse. Eur. J. Neurosci. 6:516–524 10.1111/j.1460-9568.1994.tb00295.x [DOI] [PubMed] [Google Scholar]
- Tsao J.W., George E.B., Griffin J.W. 1999. Temperature modulation reveals three distinct stages of Wallerian degeneration. J. Neurosci. 19:4718–4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M.E., Barres B.A. 2007. Why is Wallerian degeneration in the CNS so slow? Annu. Rev. Neurosci. 30:153–179 10.1146/annurev.neuro.30.051606.094354 [DOI] [PubMed] [Google Scholar]
- Verghese P.B., Sasaki Y., Yang D., Stewart F., Sabar F., Finn M.B., Wroge C.M., Mennerick S., Neil J.J., Milbrandt J., Holtzman D.M. 2011. Nicotinamide mononucleotide adenylyl transferase 1 protects against acute neurodegeneration in developing CNS by inhibiting excitotoxic-necrotic cell death. Proc. Natl. Acad. Sci. USA. 108:19054–19059 10.1073/pnas.1107325108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. 2005. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 85:201–279 10.1152/physrev.00004.2004 [DOI] [PubMed] [Google Scholar]
- Vohra B.P., Sasaki Y., Miller B.R., Chang J., DiAntonio A., Milbrandt J. 2010. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J. Neurosci. 30:13729–13738 10.1523/JNEUROSCI.2939-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller A. 1850. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. Lond. 140:423–429 10.1098/rstl.1850.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.S., Wu Y., Culver D.G., Glass J.D. 2000. Pathogenesis of axonal degeneration: parallels between Wallerian degeneration and vincristine neuropathy. J. Neuropathol. Exp. Neurol. 59:599–606 [DOI] [PubMed] [Google Scholar]
- Wang M.S., Fang G., Culver D.G., Davis A.A., Rich M.M., Glass J.D. 2001. The WldS protein protects against axonal degeneration: a model of gene therapy for peripheral neuropathy. Ann. Neurol. 50:773–779 10.1002/ana.10039 [DOI] [PubMed] [Google Scholar]
- Wang Q., Song C., Li C.C. 2004. Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J. Struct. Biol. 146:44–57 10.1016/j.jsb.2003.11.014 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhai Q., Chen Y., Lin E., Gu W., McBurney M.W., He Z. 2005. A local mechanism mediates NAD-dependent protection of axon degeneration. J. Cell Biol. 170:349–355 10.1083/jcb.200504028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts R.J., Hoopfer E.D., Luo L. 2003. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 38:871–885 10.1016/S0896-6273(03)00295-2 [DOI] [PubMed] [Google Scholar]
- Wolf J.A., Stys P.K., Lusardi T., Meaney D., Smith D.H. 2001. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 21:1923–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A.K., Wishart T.M., Ingham C.A., Gillingwater T.H. 2010. Synaptic protection in the brain of WldS mice occurs independently of age but is sensitive to gene-dose. PLoS ONE. 5:e15108 10.1371/journal.pone.0015108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N., Yuasa S., Araki T. 2009. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J. Neurosci. 29:6276–6284 10.1523/JNEUROSCI.4304-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T., Feng Y., Zheng J., Ge X., Zhang Y., Wu D., Zhao J., Zhai Q. 2010. Nmnat2 delays axon degeneration in superior cervical ganglia dependent on its NAD synthesis activity. Neurochem. Int. 56:101–106 10.1016/j.neuint.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Zhai R.G., Cao Y., Hiesinger P.R., Zhou Y., Mehta S.Q., Schulze K.L., Verstreken P., Bellen H.J. 2006. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 4:e416 10.1371/journal.pbio.0040416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R.G., Zhang F., Hiesinger P.R., Cao Y., Haueter C.M., Bellen H.J. 2008. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature. 452:887–891 10.1038/nature06721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q., Wang J., Kim A., Liu Q., Watts R., Hoopfer E., Mitchison T., Luo L., He Z. 2003. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 39:217–225 10.1016/S0896-6273(03)00429-X [DOI] [PubMed] [Google Scholar]
- Ziv N.E., Spira M.E. 1993. Spatiotemporal distribution of Ca2+ following axotomy and throughout the recovery process of cultured Aplysia neurons. Eur. J. Neurosci. 5:657–668 10.1111/j.1460-9568.1993.tb00531.x [DOI] [PubMed] [Google Scholar]