Abstract
Purpose
Growth hormone deficiency (GHD) after radiation therapy negatively affects growth and development and quality of life in children with brain tumors.
Patients and Materials
Between 1997 and 2008, 192 pediatric patients with localized primary brain tumors (ependymoma, n = 88; low-grade glioma, n = 51; craniopharyngioma, n = 28; high-grade glioma, n = 23; and other tumor types, n = 2) underwent provocative testing of GH secretion by using the secretogogues arginine and l-dopa before and after (6, 12, 36, and 60 months) conformal radiation therapy (CRT). A total of 664 arginine/l-dopa test procedures were performed.
Results
Baseline testing revealed preirradiation GHD in 22.9% of tested patients. On the basis of data from 118 patients, peak GH was modeled as an exponential function of time after CRT and mean radiation dose to the hypothalamus. The average patient was predicted to develop GHD with the following combinations of the time after CRT and mean dose to the hypothalamus: 12 months and more than 60 Gy; 36 months and 25 to 30 Gy; and 60 months and 15 to 20 Gy. A cumulative dose of 16.1 Gy to the hypothalamus would be considered the mean radiation dose required to achieve a 50% risk of GHD at 5 years (TD50/5).
Conclusion
GH secretion after CRT can be predicted on the basis of dose and time after irradiation in pediatric patients with localized brain tumors. These findings provide an objective radiation dose constraint for the hypothalamus.
INTRODUCTION
Growth hormone deficiency (GHD) is the first and most common adverse effect of hypothalamic irradiation in brain tumor survivors.1 A pooled prevalence of 35.6% has been estimated from studies evaluating GHD in survivors of childhood cancer. GHD is an important and well-documented etiology of poor growth, abnormal body composition, altered energy metabolism,2 poor overall health, and diminished quality of life. Recent evidence suggests that GHD increases cardiovascular risk factors3,4 and contributes to cognitive impairment,5–7 adding to the importance of the problem and our need to understand the risk factors for GHD, including the specific contribution of cranial irradiation.
Our understanding of the contribution of radiation dose and time after treatment to the development of GHD has relied on retrospective information obtained from patients treated to regional or whole-brain volumes from which reasonable estimates of doses to the hypothalamus-pituitary axis were obtained. Stem-cell transplantation regimens using total-body irradiation yield a 25% incidence at 5 to 10 years for 8 to 12 Gy and a 50% incidence at 10 years for 14.4 Gy.8 Cranial irradiation regimens using doses of more than 24 Gy yield a 66% incidence,9,10 and regimens using doses of more than 30 Gy lead to incidences as high as 80% by 10 years.11 In one series of optic pathway tumors, doses in excess of 45 Gy resulted in a 100% incidence of GHD within 2 years.12 These same studies have confirmed that increasing cranial radiation dose and time after treatment are the main risk factors.13
There is a need for well-designed studies to accurately determine the prevalence of endocrinopathies in cancer survivors treated with radiation therapy. We performed prospective serial tests of endocrine function in children with localized brain tumors treated with conformal radiation therapy (CRT) including intensity-modulated radiation therapy. This report describes the results from serial growth hormone (GH) testing in a cohort of patients up to 5 years after the initiation of radiation therapy. The results have applicability in the treatment of children with CNS tumors and patients in whom the hypothalamus-pituitary unit is included in the irradiated volume.
PATIENTS AND METHODS
Pediatric patients (n = 192) with localized primary brain tumors including ependymoma (n = 88), low-grade glioma (n = 51), craniopharyngioma (n = 28), high-grade glioma (n = 23), and other tumor types (n = 2) underwent provocative testing of GH secretion before and after CRT or intensity-modulated radiation therapy. All patients signed consent forms that were approved by the institutional review board.
Endocrine Testing
The arginine tolerance/l-dopa (AT/l-dopa) test was performed before (baseline) and at 6, 12, 36, and 60 months after the initiation of CRT. No patients were receiving dexamethasone or enzyme-inducing antiepileptic drugs at the time of testing. The clinical measure of GH secretory capacity was the peak GH value as determined by chemiluminescence. Patients were determined to have GHD if the peak GH response to the AT/l-dopa test was less than 7 ng/ml. Details regarding this procedure have been described previously.14
CRT and Hypothalamic Dose-Volume Data
The method of CRT has been previously described.15,16 Except for patients with high-grade glioma who were treated by using a 2-cm clinical target volume margin, all patients were treated by using a 1-cm clinical target volume margin surrounding the gross residual tumor or the tumor bed. Patients younger than age 7 years undergoing treatment had general anesthesia. Patients were immobilized with a relocatable stereotactic head frame, a thermoplastic face mask, or a molded vacuum bag.
To assist in the planning process and identification of normal tissue structures, all patients underwent magnetic resonance imaging (MRI) scans to obtain a 3-dimensionally acquired contrast-enhanced T1-weighted data set. The resultant images were registered to the treatment planning computed tomography data set obtained with the patient in the treatment position. The hypothalamus was contoured from the MRI data, and the distribution of the dose through the hypothalamus was calculated for each patient. The mean dose to the hypothalamus was used for the analysis.
Statistical Analysis
Two analyses were carried out for this study. The first was to characterize the baseline peak GH levels, estimate the proportion of patients with GHD before irradiation, and identify clinical factors associated with pre-existing GHD. The second was to characterize the longitudinal trends of peak GH after CRT, estimate the rate of change in peak GH values during the first 5 years after CRT, and quantify the influence of radiation dose and other clinical factors on the rate of change.
A mixed effects (random and fixed effects) model was used for the analysis.17,18 The peak GH, measured by the AT/l-dopa test, was the response variable for the model. The peak GH values were log transformed to achieve the best fit. In the model for the longitudinal analysis, the log peak GH value was modeled as a function of time for the evaluation of each patient and was used to create a regression line. The intercept of the line was the baseline (pre-CRT) log peak GH value, and the slope of the line was the rate of change for the log peak GH value. The intercept and slope of individual patient regression lines were considered random effects and were used to estimate the regression curve for the patient population. The effect of irradiation on the longitudinal trend of peak GH value was estimated from the contributive factor of the mean dose to the hypothalamus in the model. The total effect of CRT on the hypothalamus was modeled as a linear combination of the effects of different levels of radiation dose. The resulting model with estimating parameters was used to predict the longitudinal change in peak GH level.
To estimate the risk of GHD (the probability that the peak GH was lower than 7 ng/mL) for a given mean radiation dose at a specific time after CRT, we assumed that log peak GH was normally distributed with a mean predicted by equation 2 (see Results) and that the standard deviation was 0.64 on the basis of the estimated standard deviation for the log peak GH at baseline in all patients. We assumed that for a subgroup receiving the same mean radiation dose, the mean log peak GH level would change with time but that the standard deviation of the log peak GH level would remain the same.
RESULTS
Pre-Irradiation GHD
Baseline testing was performed on 180 patients. To conservatively estimate the incidence of pre-existing GHD, we excluded eight patients with baseline values ≥ 3 ng/mL and less than 7 ng/mL when subsequent testing showed that peak GH levels at later times recovered to levels ≥ 7 ng/mL. On the basis of this possibility, we excluded one patient who underwent only baseline testing with peak GH ≥ 3 ng/mL and less than 7 ng/mL. None of the patients with baseline values less than 3 ng/mL showed evidence of recovery to the normal range of ≥ 7 ng/mL on subsequent testing; thus, those with only one baseline evaluation value less than 3 ng/mL were included in the analysis of preirradiation GHD. Finally, we excluded the test results for one patient who had a longstanding history of selective serotonin reuptake inhibitor use. Among the 170 patients who were included in the analysis of baseline test results, 39 (22.9%) had preirradiation GHD. Peak GH was less than 3 ng/mL in 25, less than 5 ng/mL in 33, and less than 7 ng/mL in 35 patients.
Pre-CRT GHD could not be predicted significantly by sex, history of pre-CRT chemotherapy, age at the time of CRT, or time interval from diagnosis to CRT. Pre-CRT GHD was less likely in white patients (relative risk [RR], 0.325; P = .0213) than in black patients; in diagnoses other than craniopharyngioma, including ependymoma and both high- and low-grade glioma, the RRs were 0.017, 0.011, and 0.043, respectively (P < .001); and in patients with infratentorial tumors compared with those with supratentorial tumors, the RR was 0.142 (P < .001). Patients who qualitatively appeared to have hydrocephalus were not more likely to have pre-CRT GHD; however, those who required cerebrospinal fluid (CSF) shunting did have a higher risk (RR, 2.085; P = .0480).
Longitudinal Effect of CRT on GH Secretion
The longitudinal change in peak GH values was modeled by using data from 118 patients, including those who did not have preirradiation GHD and who were able to undergo baseline and at least one subsequent evaluation. The number of baseline and subsequent evaluations totaled 469: baseline (n = 118), 6 months (n = 110), 12 months (n = 113), 36 months (n = 72), and 60 months (n = 56).
The longitudinal trend of peak GH level was modeled with the time variable (time after the start of irradiation) and clinical variables, including the mean radiation dose to the hypothalamus (mean dose), the presence or absence of CSF shunting before irradiation (CSF shunt), the baseline value of peak GH (bGH), and tumor location. There was an association between mean dose and CSF shunt (P = .0253), mean dose and tumor location (P < .001), and mean dose and the time interval from diagnosis to CRT (P = .0025). Patients with a CSF shunt had lower baseline levels of peak GH than patients without shunts (P = .5830), and patients with supratentorial tumors had lower baseline levels than those with infratentorial tumors (P = .1667). The mean dose to the hypothalamus was higher in patients with a longer interval from diagnosis to the start of irradiation (P = .0025).
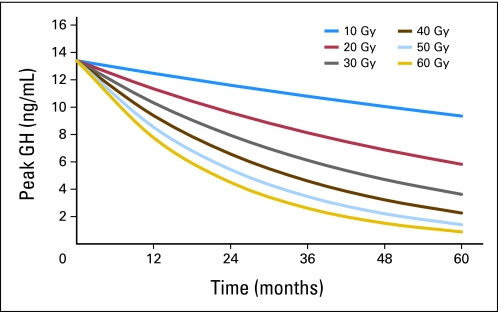
There was a statistically significant (P < .001) exponential decline in peak GH values after the start of irradiation (equation 1), shown by a model with only time as the predictor. The paired interactions of time and mean dose (P < .001), time and CSF shunt (P < .0022), and time and bGH (P = .0484) were significant by a model that included time and mean radiation dose as predictors (equation 2). The exponential decline in peak GH with time is shown by using curves that represent dose at intervals of 10 Gy (Fig 1).
Fig 1.
Peak growth hormone (GH) according to hypothalamic mean dose and time after start of irradiation. According to equation 2, peak GH = exp{2.5947 + time × [0.0019 − (0.00079 × mean dose)]}.
All possible interactions of the four clinical variables were considered in model fitting; the best model is delineated in equation 3. In that model, the interaction between time and mean radiation dose was the most significant (P < .001), followed by time and bGH (P = .0029), and time and CSF shunt (P = .0350). In the composite model, patients without CSF shunts had higher longitudinal values of peak GH. Patients with higher baseline values of peak GH had a greater rate of decline in longitudinal values. Increasing mean dose was inversely correlated with longitudinal peak GH.
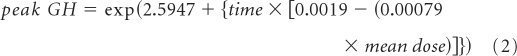 |
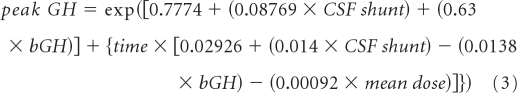 |
Considering attrition from disease progression and the initiation of replacement therapy in those who developed clinically significant GHD during the first years after irradiation (Appendix, online only), we performed a similar analysis by using a data set that was limited to peak GH values obtained through 36 months. In this subset analysis, the interaction between time and mean dose remained highly significant, and the model showed a steeper decline in peak GH as a function of time and dose.
Probability of GHD by Time and Dose
By using the estimating equation that included time and mean dose to the hypothalamus (equation 2), and assuming a standard deviation similar to that of our cohort at baseline, we calculated the probability of GHD (ie, probability of a peak GH lower than 7 ng/mL) at 12, 36, and 60 months after irradiation (Table 1) for each given level of mean radiation dose (5 Gy, 10 Gy, …, 60 Gy). A similar analysis was performed by using the data set for 0 to 36 months. The average patient was predicted to develop GHD with the following combinations of time after CRT and mean dose to the hypothalamus: 12 months and more than 60 Gy, 36 months and 25 to 30 Gy, and 60 months and 15 to 20 Gy.
Table 1.
Probability of GHD by Mean Dose to the Hypothalamus and Time After Irradiation Using Peak GH Data Through 36 and 60 Months After Conformal RT
| Time After RT Start (months) |
Mean Dose to Hypothalamus (Gy) |
36-Month Data (GH, ng/mL)* |
60-Month Data (GH, ng/mL)† |
||||
|---|---|---|---|---|---|---|---|
| Probability of < 7 | Probability of < 3 | Predicted Mean Peak | Probability of < 7 | Probability of < 3 | Predicted Mean Peak | ||
| 12 | 5 | 0.15 | 0.0093 | 13.55 | 0.16 | 0.0107 | 13.07 |
| 10 | 0.18 | 0.0120 | 12.73 | 0.18 | 0.0130 | 12.46 | |
| 15 | 0.20 | 0.0154 | 11.96 | 0.20 | 0.0157 | 11.89 | |
| 20 | 0.23 | 0.0196 | 11.23 | 0.23 | 0.0189 | 11.34 | |
| 25 | 0.26 | 0.0247 | 10.55 | 0.25 | 0.0226 | 10.81 | |
| 30 | 0.29 | 0.0309 | 9.92 | 0.27 | 0.0269 | 10.31 | |
| 35 | 0.33 | 0.0383 | 9.32 | 0.30 | 0.0318 | 9.83 | |
| 40 | 0.36 | 0.0472 | 8.75 | 0.32 | 0.0375 | 9.38 | |
| 45 | 0.40 | 0.0576 | 8.22 | 0.35 | 0.0439 | 8.94 | |
| 50 | 0.44 | 0.0697 | 7.73 | 0.38 | 0.0513 | 8.53 | |
| 55 | 0.48 | 0.0837 | 7.26 | 0.41 | 0.0595 | 8.13 | |
| 60 | 0.52 | 0.0998 | 6.82 | 0.44 | 0.0688 | 7.76 | |
| 36 | 5 | 0.19 | 0.0135 | 12.36 | 0.18 | 0.0131 | 12.44 |
| 10 | 0.28 | 0.0274 | 10.25 | 0.25 | 0.0227 | 10.79 | |
| 15 | 0.38 | 0.0518 | 8.50 | 0.32 | 0.0377 | 9.36 | |
| 20 | 0.50 | 0.0910 | 7.05 | 0.41 | 0.0599 | 8.12 | |
| 25 | 0.61 | 0.1486 | 5.85 | 0.50 | 0.0912 | 7.04 | |
| 30 | 0.72 | 0.2267 | 4.85 | 0.58 | 0.1332 | 6.11 | |
| 35 | 0.81 | 0.3237 | 4.02 | 0.67 | 0.1869 | 5.30 | |
| 40 | 0.88 | 0.4346 | 3.33 | 0.74 | 0.2524 | 4.60 | |
| 45 | 0.93 | 0.5508 | 2.77 | 0.81 | 0.3282 | 3.99 | |
| 50 | 0.96 | 0.6628 | 2.29 | 0.86 | 0.4119 | 3.46 | |
| 55 | 0.98 | 0.7620 | 1.90 | 0.91 | 0.4998 | 3.00 | |
| 60 | 0.99 | 0.8426 | 1.58 | 0.94 | 0.5877 | 2.60 | |
| 60 | 5 | 0.23 | 0.0193 | 11.28 | 0.21 | 0.0160 | 11.84 |
| 10 | 0.40 | 0.0569 | 8.26 | 0.33 | 0.0379 | 9.34 | |
| 15 | 0.59 | 0.1369 | 6.04 | 0.47 | 0.0800 | 7.37 | |
| 20 | 0.76 | 0.2720 | 4.42 | 0.61 | 0.1504 | 5.82 | |
| 25 | 0.89 | 0.4526 | 3.24 | 0.75 | 0.2533 | 4.59 | |
| 30 | 0.95 | 0.6437 | 2.37 | 0.85 | 0.3844 | 3.62 | |
| 35 | 0.99 | 0.8039 | 1.74 | 0.92 | 0.5305 | 2.86 | |
| 40 | 1.00 | 0.9104 | 1.27 | 0.96 | 0.6725 | 2.25 | |
| 45 | 1.00 | 0.9664 | 0.93 | 0.98 | 0.7931 | 1.78 | |
| 50 | 1.00 | 0.9898 | 0.68 | 0.99 | 0.8825 | 1.40 | |
| 55 | 1.00 | 0.9975 | 0.50 | 1.00 | 0.9403 | 1.11 | |
| 60 | 1.00 | 0.9995 | 0.37 | 1.00 | 0.9731 | 0.87 | |
Abbreviations: GH, growth hormone; GHD, growth hormone deficiency; RT, radiation therapy.
36-month model: peak GH = exp(2.6518 + {time × [0.001385 − (0.00104 × mean dose)]}).
60-month model: peak GH = exp(2.5947 + {time × [0.0019 − (0.00079 × mean dose)]}).
Complication Probabilities: TD5/5 and TD50/5
The TD5/5 and TD50/5 represent the minimum (5% risk) and maximum (50% risk) radiation dose tolerance estimated at 5 years. These estimates consider conventional fractionated radiation therapy to the organ at risk by using clinical regimens of 1.8 to 2.0 Gy per day administered 5 days per calendar week. Assuming the standard deviation of the baseline value of log peak GH in our cohort as that for the log peak GH for any given pair of time and mean dose, and assuming a normal distribution for this value, we determined that all patients would have at least a 5% risk of having a peak GH level less than 7 ng/mL, regardless of their mean doses.
By using the same method, we determined that for patients to have less than a 50% risk of peak GH below 7 ng/mL at 5 years, the mean dose to the hypothalamus should not exceed 16.1 Gy over the course of 6 to 6.5 weeks based on the 60-month data set and 12.6 Gy over the course of 6 to 6.5 weeks based on the 36-month data set.
DISCUSSION
GHD after therapeutic cranial irradiation is a treatable late effect of successful cancer therapy that might be reduced or eliminated through careful treatment planning or new methods. Our results suggest that when the mean dose to the hypothalamus can be reduced to less than 16.1 Gy, half the surviving children may be spared from GHD during the first 5 years after treatment. Considering that GHD results from damage to the neurons in the hypothalamus that are considered most sensitive to the effects of irradiation,19 it follows that the incidence of other endocrine deficiencies might also be reduced if and when this threshold dose is observed. Reducing hypothalamic irradiation should be feasible when treating children with brain tumors if the targeted volume is not immediately adjacent to the hypothalamus and when advanced methods of photon or proton therapy are used. That our patients received 30 to 33 fractions of 1.8 Gy over the course of 6 to 6.5 weeks should be considered in the interpretation of these results, since the fractional dose threshold is 0.49 to 0.54 Gy per fraction or 27% to 30% of the prescribed daily dose.
The criteria for diagnosis of GHD vary by institution. Children without any tumor history are often considered to have GHD and qualify for GH therapy when their peak stimulated GH is less than 10 ng/mL. This study provides firm estimates of the radiation dose required to induce GHD by using a more conservative diagnostic level of 7 ng/mL. However, it is clear that other factors in addition to radiation dose contribute to this endocrine deficit. In our study, the incidence of GHD before irradiation was related to CSF shunting, which is standard in the sequelae and treatment of severe hydrocephalus. Pre-existing GHD was also related to tumor diagnosis and tumor location. These variables are often correlated, considering the singular suprasellar location of craniopharyngioma and the fact that the diencephalon or optic pathway is the most commonly irradiated site in childhood low-grade glioma. Because these tumors are intimately associated with the hypothalamus, these patients have a high likelihood of postradiation GHD if it is not already present before irradiation. All factors considered, our data suggest a need for early evaluation and intervention in these patients.
Children with ependymoma often present with obstructive hydrocephalus originating in the posterior fossa. The direct effect of hydrocephalus on the hypothalamus from increased intracranial pressure and expansion of the ventricular system should not be underestimated. Although tumor resection may relieve the obstruction, permanent CSF shunting is required for the most severe cases. In addition to radiation dose to the hypothalamus, CSF shunting is an important risk factor for GHD both before and after irradiation.
Endocrine deficiencies cannot always be predicted by tumor location. This observation highlights the contribution of scattered radiation20 and the need for more accurate estimates of hypothalamic radiation dose. Clinical data describing neuroendocrine effects of irradiation have been derived by using generalized estimates of radiation dose under conditions in which the dose to the hypothalamic-pituitary axis was generally homogeneous and discrete. Examples include patients treated with single-dose or fractionated total-body irradiation (8 to 14 Gy), those given cranial irradiation for acute lymphoblastic leukemia (18 Gy and 24 Gy), and those with tumors of the sella or parasellar region in which the hypothalamic-pituitary axis was uniformly included in the volume of prescribed dose (> 50 Gy). Radiation is a significant contributor to neuroendocrine complications commonly observed after treatment for brain tumors and tumors of the head and neck when the hypothalamus is subtended by the irradiated volume.21 Similar complications are observed when the hypothalamus is incidentally irradiated in the treatment of nasopharyngeal cancer, retinoblastoma, Hodgkin's lymphoma, and pediatric sarcomas of the head and neck.22
For other diseases, the hypothalamus may have been located within the irradiated volume for part or all of the treatment or in the gradient of dose (dose falloff), receiving only a fraction of the daily dose administered. These circumstances make it difficult to assign a dose to the hypothalamus and to determine the risk for late effects. These difficulties are present when the patient is seen by an endocrinologist years after treatment and retrospective dose calculations may be difficult to perform. Newer radiation techniques use 3-dimensional imaging (computed tomography and MRI) and allow for more accurate dose calculation and reporting. Correlated with objective measures of endocrine effects, dosimetry of hypothalamic irradiation will become increasingly valuable in predicting the incidence of specific endocrinopathies.
In pediatric radiation oncology, reducing adverse effects of treatment is an important goal. Reducing adverse effects can be achieved primarily by limiting CNS irradiation to patients for whom the indications are clear and the benefits outweigh the risks. The risk for endocrine-related complications should be carefully considered in planning radiation therapy but should not be used as a reason to avoid curative therapy. Careful follow-up and surveillance will lead to earlier intervention and mitigation of the consequences of cranial radiation.
Appendix
Protocol Performance
This study was designed to perform testing before irradiation and 6, 12, 36, and 60 months after treatment in 202 patients enrolled onto the St Jude RT1 protocol. The number of possible evaluations was 1,010. A total of 664 tests were performed. Baseline testing was limited to 170 patients, and 32 did not have baseline testing. Lack of baseline testing was attributed to the inability to achieve timely intravenous access or the ongoing use of dexamethasone. The former was rare, although the latter was most common in patients with high-grade glioma who were symptomatic from their tumors.
Most important, in conducting this type of study, one must consider attrition from tumor progression and the incidence of hormone replacement therapy after the completion of treatment. Among the 118 patients included in the longitudinal analysis, there were four with craniopharyngioma, 70 with ependymoma, 29 with low-grade glioma, 14 with high-grade glioma, and one with choroid plexus carcinoma. The 3- and 5-year progression-free survival (PFS) rates were 80% ± 4% and 77% ± 4% for the longitudinal analysis cohort, and the cumulative incidences of growth hormone (GH) replacement therapy at 3 and 5 years were 20% ± 4% and 26% ± 4%, respectively.
The-3 year PFS rates were 75% ± 19% for craniopharyngioma, 81% ± 5% for ependymoma, 36% ± 12% for high-grade glioma, and 93% ± 5% for low-grade glioma. The PFS rate at 5 years differed from the 3-year rate only for patients with low-grade glioma (87% ± 6%). The cumulative incidence of GH replacement therapy, after considering tumor progression as a competing risk but not accounting for older patients who may have had growth hormone deficiency (GHD) but for whom GH replacement therapy might not be clearly indicated at 3 years, was 100% for craniopharyngioma, 13% ± 4% (19% ± 5% at 5 years) for ependymoma, 71% ± 8% (71% ± 8% at 5 years) for high-grade glioma, and 30% ± 9% (43% ± 9% at 5 years) for low-grade glioma.
Since those who survived and were tested at 60 months did not have a preponderance of GH replacement therapy, we evaluated the interaction between time and mean dose to the hypothalamus on the basis of data acquired through 36 months. The interaction was statistically significant (P < .001) and was represented by the expression in equation 3. The group tested at 60 months (equation 2) represented those who had good survivorship and achieved 60 months of follow-up without the initiation of GH replacement therapy. (See Longitudinal Effect of CRT on GH Secretion under Results for equations 2 and 3).
Added Information About Low-Grade Glioma
Among the 51 patients with low-grade glioma, 38 of 51 tumors were considered to be diencephalic or optic pathway tumors, 28 of 38 involved the hypothalamus or had the potential to involve the hypothalamus, and 26 of 28 patients underwent baseline testing. Among the 26 patients with involvement or potential involvement of the hypothalamus, eight showed evidence of preirradiation GHD. Although none of the eight patients contributed to the longitudinal analysis, all but one had additional evaluations (total number of evaluations, three to four) through 36 months. All showed further decline in their peak GH value.
Footnotes
See accompanying editorial on page 4743
Supported in part by Grants No. 5 P30 CA21765-28 from the National Cancer Institute, Cancer Center Support, and No. RPG-99-252-01-CCE from the American Cancer Society Research Project and by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Thomas E. Merchant, Susan R. Rose, Xiaoping Xiong, Robert H. Lustig
Administrative support: Thomas E. Merchant
Collection and assembly of data: Thomas E. Merchant, Susan R. Rose, Christina Bosley, Robert H. Lustig
Data analysis and interpretation: Thomas E. Merchant, Shengjie Wu, Xiaoping Xiong, Robert H. Lustig
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Darzy KH, Shalet SM. Hypopituitarism following radiotherapy revisited. Endocr Dev. 2009;15:1–24. doi: 10.1159/000207607. [DOI] [PubMed] [Google Scholar]
- 2.Vance ML, Mauras N. Growth hormone therapy in adults and children. N Engl J Med. 1999;341:1206–1216. doi: 10.1056/NEJM199910143411607. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE, Merriam GR. Growth hormone therapy in adults. Annu Rev Med. 2003;54:513–533. doi: 10.1146/annurev.med.54.101601.152147. [DOI] [PubMed] [Google Scholar]
- 4.Gilchrist FJ, Murray RD, Shalet SM. The effect of long-term untreated growth hormone deficiency (GHD) and 9 years of GH replacement on the quality of life (QoL) of GH-deficient adults. Clin Endocrinol (Oxf) 2002;57:363–370. doi: 10.1046/j.1365-2265.2002.01608.x. [DOI] [PubMed] [Google Scholar]
- 5.Wass JA, Reddy R. Growth hormone and memory. J Endocrinol. 2010;207:125–126. doi: 10.1677/JOE-10-0126. [DOI] [PubMed] [Google Scholar]
- 6.Nieves-Martinez E, Sonntag WE, Wilson A, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puga González B, Ferrández Longás A, Oyarzábal M, et al. The effects of growth hormone deficiency and growth hormone replacement therapy on intellectual ability, personality and adjustment in children. Pediatr Endocrinol Rev. 2010;7:328–338. [PubMed] [Google Scholar]
- 8.Leung W, Ahn H, Rose SR, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 2007;86:215–224. doi: 10.1097/MD.0b013e31812f864d. [DOI] [PubMed] [Google Scholar]
- 9.Shalet SM. Radiation and pituitary dysfunction. N Engl J Med. 1993;328:131–133. doi: 10.1056/NEJM199301143280211. [DOI] [PubMed] [Google Scholar]
- 10.Sklar CA. Growth and neuroendocrine dysfunction following therapy for childhood cancer. Pediatr Clin North Am. 1997;44:489–503. doi: 10.1016/s0031-3955(05)70487-9. [DOI] [PubMed] [Google Scholar]
- 11.Shalet SM, Beardwell CG, Pearson D, et al. The effect of varying doses of cerebral irradiation on growth hormone production in childhood. Clin Endocrinol (Oxf) 1976;5:287–290. doi: 10.1111/j.1365-2265.1976.tb01955.x. [DOI] [PubMed] [Google Scholar]
- 12.Brauner R, Malandry F, Rappaport R, et al. Growth and endocrine disorders in optic glioma. Eur J Pediatr. 1990;149:825–828. doi: 10.1007/BF02072067. [DOI] [PubMed] [Google Scholar]
- 13.Mulder RL, Kremer LC, van Santen HM, et al. Prevalence and risk factors of radiation-induced growth hormone deficiency in childhood cancer survivors: A systematic review. Cancer Treat Rev. 2009;35:616–632. doi: 10.1016/j.ctrv.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Weldon VV, Gupta SK, Klingensmith G, et al. Evaluation of growth hormone release in children using arginine and L-dopa in combination. J Pediatr. 1975;87:540–544. doi: 10.1016/s0022-3476(75)80816-x. [DOI] [PubMed] [Google Scholar]
- 15.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156–3162. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 16.Merchant TE, Kun LE, Wu S, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–3604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York, NY: Springer; 2000. [Google Scholar]
- 18.Venables WN, Ripley BD. Modern Applied Statistics With S-Plus. ed 2. New York, NY: Springer; 1997. [Google Scholar]
- 19.Robinson IC, Fairhall KM, Hendry JH, et al. Differential radiosensitivity of hypothalamo-pituitary function in the young adult rat. J Endocrinol. 2001;169:519–526. doi: 10.1677/joe.0.1690519. [DOI] [PubMed] [Google Scholar]
- 20.Rohrer TR, Beck JD, Grabenbauer GG, et al. Late endocrine sequelae after radiotherapy of pediatric brain tumors are independent of tumor location. J Endocrinol Invest. 2009;32:294–297. doi: 10.1007/BF03345714. [DOI] [PubMed] [Google Scholar]
- 21.Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forstner D, Borg M, Saxon B. Orbital rhabdomyosarcoma: Multidisciplinary treatment experience. Australas Radiol. 2006;50:41–45. doi: 10.1111/j.1440-1673.2005.01526.x. [DOI] [PubMed] [Google Scholar]