Abstract
Background/purpose
NGX-4010 (QUTENZA™; NeurogesX Inc, San Mateo, CA), a capsaicin 8% dermal patch, is licensed in the European Union for the treatment of peripheral neuropathic pain (PNP) in nondiabetic adults and in the United States for the treatment of neuropathic pain associated with postherpetic neuralgia (PHN). While NGX-4010 treatment is associated with a low risk of systemic adverse events, patch application-related pain is common and may be managed with local cooling and/or oral analgesics. This article characterizes the tolerability of NGX-4010 and will help to guide any pain management.
Methods
This integrated analysis of tolerability data collected from the NGX-4010 clinical study program included 1696 patients with PNP. Patch application-related pain on the treatment day was captured as Numeric Pain Rating Scale (NPRS) “pain now” scores while “average pain for the past 24 hours” NPRS scores were analyzed for 7 days following treatment. Other tolerability assessments included the percentage of patients completing ≥90% of the intended treatment duration and patients using medication for patch application-related pain.
Results
The mean maximum change in “pain now” NPRS scores from pretreatment levels during and after patch application was 2.6 for all patients. This pain was transient and resolved following patch removal. Mean “average pain for the past 24 hours” NPRS scores returned to baseline by the evening of the treatment day for patients with PHN, and the evening of day 2 for patients with human immunodeficiency virus-associated distal sensory polyneuropathy or painful diabetic neuropathy. Repeated NGX-4010 applications did not affect the intensity of patch application-related pain. Almost all patients (≥98%) completed ≥90% of the full treatment duration, regardless of the number of treatments received.
Conclusion
Transient patch application-related pain with NGX-4010 can be managed with local cooling and/or oral analgesics in nearly all cases. Patient adherence to the full intended treatment duration indicated that patch application-related pain was not a barrier to NGX-4010 use.
Keywords: capsaicin 8% patch, NGX-4010, patch application-related pain, neuropathic pain
Introduction
Peripheral neuropathic pain (PNP) conditions, such as postherpetic neuralgia (PHN) and human immunodeficiency virus-associated distal sensory polyneuropathy (HIV-DSP), exert a heavy burden of illness on affected patients.1,2 Conditions such as HIV-DSP are particularly difficult to treat, and most available systemic therapies for PNP provide fewer than half of patients with meaningful pain relief.3,4
The transient receptor potential vanilloid 1 (TRPV1) receptor is highly expressed on peripheral nociceptors and is a key modulator of pain transmission.5 Altered nociceptor expression of TRPV1 may play a role in PNP,6–8 and makes this receptor a logical target for treating such pain. Capsaicin is a selective agonist of the TRPV1 receptor5; the initial exposure of these receptors to high capsaicin concentrations causes nociceptor excitation and consequently pain, perceived as a hot, burning sensation. However, prolonged stimulation with capsaicin causes a reversible defunctionalization and reduction of the nerve fibers in the epidermis, and results in the inhibition of pain transmission.9–11 NGX-4010 (QUTENZA™; NeurogesX Inc, San Mateo, CA) is a capsaicin 8% patch designed to rapidly deliver capsaicin to peripheral nociceptors. In the European Union (EU), NGX-4010 is approved for the treatment of PNP in nondiabetic adults either alone or in combination with other neuropathic pain (NP) medications. It is also approved by the United States (US) Food and Drug Administration for the treatment of NP associated with PHN. Before application of NGX-4010, the skin is pretreated with topical anesthetic to reduce capsaicin-induced discomfort. Up to four patches, designed for cutting to the treatment area size and shape, can be applied for 60 minutes (US indication) or 30 minutes to the feet and 60 minutes to any other area (EU indication), excluding the face, scalp, and mucous membranes.
Single NGX-4010 applications have resulted in rapid, prolonged (up to 12 weeks) pain reductions in randomized, controlled, clinical studies of patients with PHN and HIVDSP. 12–16 Moreover, both single and repeated NGX-4010 applications were well tolerated.12–17 Apart from transient increases in blood pressure, likely associated with the pain experienced during the application procedure, NGX-4010 was not associated with systemic adverse events. This is as expected, since there is minimal systemic absorption of capsaicin.18 Application-site adverse events, such as patch application-related pain and erythema, were the most commonly reported adverse events. Although generally mild or moderate in severity and transient, patch applicationrelated pain needs adequate management during clinical practice. As NGX-4010 moves from clinical trials into clinical practice, clinicians have raised questions about the degree of patch application-related pain, and it has been suggested that epidural injections or intravenous opioids be used pre- and post-NGX-4010 application.19 However, neither intervention was routinely required in the clinical trials; patch application-related pain was treated with oral analgesics (eg, short-acting opioids) during and following NGX-4010 application and/or local cooling (eg, cold packs) after patch removal.
This manuscript describes integrated data analyzing patch application-related pain experienced by patients with PHN, HIV-DSP, or PDN who were treated with NGX-4010 during the clinical study program, and the measures taken to alleviate this pain.
Methods
Patients
Safety and tolerability data from 12 studies of NGX-4010 that included patients with PNP due to PHN, HIV-DSP, or PDN were integrated and analyzed. The ClinicalTrials. gov identifiers of the registered trials, along with the dates of first registration, are as follows: NCT00034710 (May 1, 2002), NCT00061776 (June 3, 2003), NCT00068081 (September 5, 2003), NCT00115310 (June 21, 2005), NCT00300222 (March 6, 2006), NCT00061152 (May 21, 2003), NCT00064623 (July 10, 2003), NCT00085761 (June 14, 2004), NCT00321672 (May 2, 2006), NCT00082316 (May 5, 2004), and NCT00233155 (October 3, 2005).
Eight of the studies were randomized, controlled studies or contained a randomized, controlled period, three were open-label, uncontrolled studies, and one was an openlabel, uncontrolled extension study. Patients were recruited directly by the study centers from their existing patient database, or through print advertising, or radio, or television advertisements. Enrolled patients had an average Numeric Pain Rating Scale (NPRS) score20 of 3–9 (3–8 in two studies). Patients with PHN were eligible if at least 6 months had elapsed since shingle vesicle crusting (at least 3 months in two studies). Patients with PDN had to have NP for at least 3 months and patients with HIV-DSP had to be at least 2 months from diagnosis (3 months in one study).
Patients taking chronic analgesics had to be on a stable dose of those medications for at least 21 days before the day of study-patch application and throughout the study period. Exclusion and inclusion criteria were similar between studies, as described previously.12–15
Studies were approved by Institutional Review Boards and/or independent Ethics Committees at all participating sites, and conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable regulatory requirements. Written informed consent was obtained from all participating patients before initiating study-related procedures.
Procedures
Studies included a baseline screening period of 7–14 days followed by a treatment day (day 0), and a 12-week post-treatment assessment period (4 weeks in one study) with periodic clinic visits. In the randomized, controlled studies, patients were randomized to NGX-4010 (capsaicin 640 μg/cm2, 8% w/w) or a control patch that was identically formulated, but contained a lower capsaicin concentration (3.2 μg/cm2, 0.04% w/w). The low-concentration capsaicin control patches were used in place of placebo patches to provide effective blinding in the studies, since topical capsaicin can produce local erythema and a burning sensation. The treatment assigned to individual patients was determined by a randomization scheme prepared by Fisher Clinical Services (Allentown, PA) or Cardinal Health (Morrisville, NC). In the uncontrolled extension studies or the extension phase of the double-blind studies, patients were allowed to receive openlabel 60-minute NGX-4010 treatments at least 12 weeks apart (6 weeks in the extension study to NCT00034710).
All patients were pretreated with a topical anesthetic (lidocaine 4%; ELA-Max4® or LMX4® [Ferndale Laboratories, Inc, Ferndale, MI]; Topicaine® Gel [ESBA Laboratories, Inc, Jupiter, FL] or Betacaine® Enhanced Gel 4 [Theraderm, Inc, Tampa, FL]) for 60 minutes. After removal of the topical anesthetic, the treatment area was washed with soap and water and thoroughly dried, and NGX-4010 patches were applied for 30, 60, or 90 minutes directly to the painful area(s) (up to 1120 cm2), depending on the study protocol. After patch removal, the area was cleansed with a proprietary cleansing gel formulated to remove residual capsaicin. Patients were monitored for 2 hours (4 hours in one study) after patch removal. Oxycodone oral solution (1 mg/mL) or a similar oral opioid analgesic could be administered at the onset of patch application-related pain and as needed thereafter in the clinic. After patch removal, local cooling (eg, cold packs) could also be used to relieve patch application-related pain. Patients could also take short-acting opioid medication (hydrocodone bitartrate 5 mg/acetaminophen 500 mg) for up to 3–7 days (depending on the study) after patch application for patch applicationrelated pain, as needed.
Assessments and analyses
Tolerability assessments included pain assessment before, during, and after the treatment procedure using NPRS scores for “pain now.” The mean absolute change in “pain now” NPRS score from the pretreatment time point to several post- treatment time points on the treatment day were calculated. In addition, the mean maximum change in “pain now” NPRS score on the treatment day, the number and percentage of patients completing ≥90% of the patch application duration, and the number and percentage of patients who reported an increase in NPRS score of >2 were calculated and subgroup analyses performed, comparing patients who were or were not taking concomitant NP medication (defined as anticonvulsants, nonselectiveserotonin reuptake inhibitor antidepressants, or opioids at day -1 for at least 7 consecutive days). The percentage of patients using medication for patch application-related pain was also determined.20 Mean absolute changes in “average pain for the past 24 hours” NPRS score from baseline for the 7 days following NGX-4010 application were calculated from the daily “average pain for the past 24 hours” NPRS scores captured at 21:00 hours every evening in a paper diary throughout the study period.
Results
Demographics
Demographic and baseline characteristics of the 1696 patients who received NGX-4010 are shown by indication in Table 1. The majority of patients had PHN (54%) and patients with HIV-DSP and PDN comprised 40% and 5% of the population, respectively. Approximately 75% of patients received a single treatment, 20% received two or three treatments, and approximately 5% received four treatments. No patients with PDN were treated more than once. The mean treatment area size was 620 cm2; patients with HIV-DSP or PDN tended to have larger mean treatment areas (935 cm2 and 989 cm2, respectively) than patients with PHN (349 cm2). The average baseline pain level was 5.8 and was comparable for each indication. About half of PHN or PDN patients and 69% of HIV-DSP patients were taking concomitant NP medication (defined as anticonvulsants, nonselective-serotonin reuptake inhibitor antidepressants, or opioids) at study entry and during the studies.
Table 1.
Demographic and baseline characteristics by indication
| Characteristics | NGX-4010 | |||
|---|---|---|---|---|
| PHN (n = 920) | HIV-DSP (n = 685) | PDN (n = 91) | Total (n = 1696) | |
| Age (years) on day of first treatment | ||||
| Mean (SD) | 71 (11) | 49 (8) | 59 (11) | 61 (15) |
| Minimum, maximum | 21, 94 | 22, 74 | 37, 79 | 21, 94 |
| ≥65, n (%) | 693 (75) | 26 (4) | 28 (31) | 747 (44) |
| ≥75, n (%) | 389 (42) | 0 | 8 (9) | 397 (23) |
| Sex | ||||
| Male, n (%) | 432 (47) | 616 (90) | 52 (57) | 1100 (65) |
| Race | ||||
| Asian, n (%) | 15 (2) | 5 (1) | 1 (1) | 21 (1) |
| Black, n (%) | 27 (3) | 187 (27) | 10 (11) | 224 (13) |
| Caucasian, n (%) | 826 (90) | 424 (62) | 67 (74) | 1317 (78) |
| Other, n (%) | 52 (6) | 69 (10) | 13 (14) | 134 (8) |
| Treatment area (cm2) | ||||
| Mean (SD) | 349 (231) | 935 (249) | 989 (200) | 620 (378) |
| Duration of pain (years) | ||||
| Mean (SD) | 3.5 (4.1) | 5.7 (3.8) | 5.0 (4.7) | 4.5 (4.1) |
| Baseline NPRS score for “average pain for the past 24 hours”a | ||||
| Mean (SD) | 5.6 (1.6) | 6.0 (1.6) | 5.8 (1.4) | 5.8 (1.6) |
| On concomitant neuropathic pain medication,b n (%) | ||||
| Yes | 481 (52) | 472 (69) | 46 (51) | 999 (59) |
| Opioids only | 84 (9) | 64 (9) | 4 (4) | 152 (9) |
| Anticonvulsants only | 168 (18) | 128 (19) | 19 (21) | 315 (19) |
| Non-SSRI antidepressant only | 57 (6) | 66 (10) | 8 (9) | 131 (8) |
| Opioids and anticonvulsants | 85 (9) | 64 (9) | 7 (8) | 156 (9) |
| Opioids and non-SSRI antidepressants | 13 (1) | 23 (3) | 3 (3) | 39 (2) |
| Anticonvulsants and non-SSRI antidepressants | 46 (5) | 81 (12) | 3 (3) | 130 (8) |
| All three | 28 (3) | 46 (7) | 2 (2) | 76 (4) |
| Number of treatments received, n (%) | ||||
| One | 691 (75) | 485 (71) | 91 (100) | 1267 (75) |
| Two | 100 (11) | 76 (11) | 0 | 176 (10) |
| Three | 85 (9) | 75 (11) | 0 | 160 (9) |
| Four | 44 (5) | 49 (7) | 0 | 93 (5) |
Notes: Baseline NPRS scores for “average pain for the past 24 hours” were recorded in the evening (at 21:00 hours), beginning on the day of the screening visit (usually day–14) to day–1, as part of diary completion prior to study drug treatment;
patient is defined as being on concomitant pain medication if he/she is on an anticonvulsant, nonselective-SSRI antidepressant, or opioid that was used on day–1 and was taken for a total duration of at least 7 consecutive days.
Abbreviations: non-SSRI, nonselective-serotonin reuptake inhibitor; HIV-DSP, human immunodeficiency virus-distal sensory polyneuropathy; NPRS, Numeric Pain Rating Scale; PDN, painful diabetic neuropathy; PHN, postherpetic neuralgia; SD, standard deviation.
NPRS scores during and after NGX-4010 application
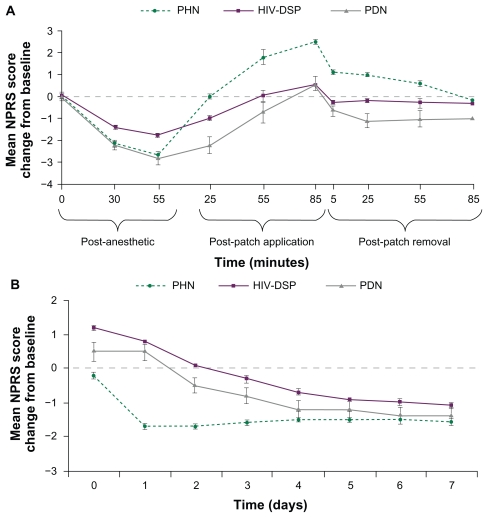
Mean “pain now” NPRS scores on the day of treatment are shown by indication in Figure 1A. In all cohorts, pain decreased during topical anesthetic application and increased during and after NGX-4010 application. Patients with PHN showed a mean maximum change in NPRS score of 2.8 and patients with HIV-DSP or PDN showed a mean maximum change of 2.3 (Table 2). For all patients, a maximum change in NPRS score of 2.6 was observed during patch application and up to 85 minutes post-treatment. In addition, 48% of patients reported an increase in NPRS score of >2 points during and after patch application, including the evening of the treatment day. Subgroup analyses comparing patients who were or were not taking concomitant NP medication revealed no difference in the tolerability of NGX-4010 on the treatment day (data not shown). NPRS score increases were transient and returned, on average, to baseline within 85 minutes following treatment (Figure 1A).
Figure 1.
Mean (± standard error) “pain now” Numeric Pain Rating Scale (NPRS) score change from the pretreatment time point during and immediately following the application procedure on the treatment day (A) and mean (± standard error) “average pain for the past 24 hours” NPRS score change from baseline on days 0 to 7 (B) for each indication.
Abbreviations: HIV-DSP, human immunodeficiency virus-distal sensory polyneuropathy; PDN, painful diabetic neuropathy; PHN, postherpetic neuralgia.
Table 2.
Summary of tolerability on the day of treatment by indication
| NGX-4010 | ||||
|---|---|---|---|---|
| PHN (n = 919) | HIV-DSP (n = 685) | PDN (n = 91) | Total (n = 1696) | |
| Maximum change in NPRS scorea during topical anesthetic application, mean (SD) | −1.6 (2.0) | −1.0 (1.6) | −1.4 (1.8) | −1.3 (1.8) |
| Maximum change in NPRS scorea during and after patch application,b mean (SD) | 2.8 (2.7) | 2.3 (2.7) | 2.3 (3.1) | 2.6 (2.7) |
| Subjects with an increase in NPRS score of >2 points on day 0,b n (%) | 477 (52) | 299 (44) | 42 (46) | 818 (48) |
| Subjects completing at least 90% of the intended patch application duration, n (%) | 901 (98) | 681 (99) | 91 (100) | 1673 (99) |
| Subjects using medication for treatment-related pain on days 0–5, n (%) | 451 (49) | 460 (67) | 13 (14) | 924 (55) |
Notes: Change from preanesthetic time point;
includes the evening of the treatment day.
Abbreviations: HIV-DSP, human immunodeficiency virus-distal sensory polyneuropathy; NPRS, Numeric Pain Rating Scale; PDN, painful diabetic neuropathy; PHN, postherpetic neuralgia; SD, standard deviation.
Mean “average pain for the past 24 hours” NPRS scores for patients with PHN returned to baseline by the evening of the treatment day, decreased below baseline on day 1, and continued to fall below baseline on days 2–7 following treatment (Figure 1B). In patients with HIV-DSP or PDN, mean “average pain for the past 24 hours” NPRS scores returned to baseline by the evening of day 2, indicating a slightly longer duration of patch application-related pain in patients with these conditions. By day 3, patients in all cohorts had less pain, on average, than at baseline. There was no evidence of a return of patch application-related pain after day 0 in any patient population (Figure 1B).
Medications for patch application-related pain and patient compliance
Medications required for patch application-related pain were used by 55% of patients treated with NGX-4010 (Table 2). A higher percentage of patients with HIV-DSP compared with the other two cohorts reported using such medications. Medications were most commonly used on the treatment day and use decreased considerably by day 5.
Nearly all patients (99%) were able to tolerate NGX-4010 treatment to complete ≥90% of the full treatment duration (Table 2).
Tolerability following repeated NGX-4010 applications
With repeated applications of NGX-4010 (Table 3) the maximum change in NPRS scores during and after NGX- 4010 application remained the same or slightly decreased in patients with PHN or HIV-DSP. The number of patients who reported an increase in NPRS score of >2 also remained stable with repeated applications (Table 3).
Table 3.
Summary of tolerability on the day of treatment by indication and treatment cycle
| Change from pre-LMX4® time point | NGX-4010 treatment cycle | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| PHN | n = 370 | n = 229 | n = 129 | n = 44 |
| Maximum change in NPRS score during topical anesthetic application, mean (SD) | −1.7 (2.0) | −1.8 (2.0) | −1.6 (1.9) | −2.1 (1.5) |
| Maximum change in NPRS score during and after patch application,a mean (SD) | 2.6 (2.8) | 2.4 (2.6) | 2.2 (2.8) | 1.6 (2.7) |
| Patients with an increase in NPRS score of >2 points on day 0,a n (%) | 169 (46) | 96 (42) | 54 (42) | 17 (39) |
| Patients with at least 90% of the intended patch application duration, n (%) | 364 (98) | 227 (99) | 127 (98) | 44 (100) |
| Patients using medication for patch application-related pain on days 0–5, n (%) | 198 (54) | 107 (47) | 60 (47) | 20 (45) |
| HIV-DSP | n = 337 | n = 200 | n = 124 | n = 49 |
| Maximum change in NPRS score during topical anesthetic application, mean (SD) | −1.1 (1.6) | −1.2 (1.6) | −1.1 (1.3) | −1.3 (1.7) |
| Maximum change in NPRS score during and after patch application,a mean (SD) | 2.3 (2.8) | 1.9 (2.4) | 1.5 (2.5) | 1.1 (3.0) |
| Patients with an increase in NPRS score of >2 points on day 0,a n (%) | 120 (36) | 66 (33) | 41 (33) | 18 (37) |
| Patients with at least 90% of the intended patch application duration, n (%) | 333 (99) | 199 (100) | 123 (99) | 49 (100) |
| Patients using medication for patch application-related pain on days 0–5, n (%) | 216 (64) | 125 (63) | 75 (60) | 27 (55) |
Note: Includes the evening of the treatment day.
Abbreviations: HIV-DSP, human immunodeficiency virus-distal sensory polyneuropathy; NPRS, Numeric Pain Rating Scale; PHN, postherpetic neuralgia; SD, standard deviation.
Patient compliance was not affected by repeated applications; nearly all patients completed ≥90% of the full retreatment duration, regardless of the number of treatments received (Table 3). The percentage of patients using medication for patch application-related pain on days 0–5 also remained the same or slightly decreased with repeated NGX-4010 applications.
Discussion
Analyses of the tolerability data from the NGX-4010 clinical study program demonstrate that patch application-related pain increases were transient and generally began to resolve following removal of NGX-4010. Interestingly, despite the fact that the patch application-related pain increase during and shortly after patch application was higher in patients with PHN, the average pain score in the PHN cohort returned to baseline on the day of treatment, whereas in the PDN and HIV-DSP cohorts pain scores declined more gradually. These results appear to indicate a slower rate of pain decrease after the treatment procedure in patients with HIV-DSP or PDN than in those with PHN. These differences may be due to the difference in skin thickness between the trunk (PHN) and the feet (HIV-DSP or PDN). This study also found that repeated applications of NGX-4010 did not affect patch applicationrelated pain and tolerability.
During the clinical studies, patch application-related pain was treated with oxycodone oral solution (1 mg/mL) as needed in the clinic on the day of treatment. Patients could also take short-acting opioid medication (hydrocodone bitartrate 5 mg/acetaminophen 500 mg) for up to 3–7 days (depending on the study) after patch application for patch application-related pain, as needed. Just over half of all patients in the NGX-4010 clinical trials used medication for patch application-related pain, with most medication used on the treatment day and decreasing rapidly thereafter. Previous NGX-4010 reports have shown oxycodone use on the treatment day to be 10.6–17.1 mg in patients with PHN and 12.3–31.7 mg in patients with HIV-DSP.15,17,21 While design of the NGX-4010 clinical trials mandated that medication for patch application-related pain could not be administered until after its onset, future studies to investigate the usefulness of preemptive analgesia with oral opioids or other oral analgesics (eg, nonsteroidal anti-inflammatory drugs, aspirin) as an alternative to topical anesthetic pretreatment could be considered. It is important to note that the use of concomitant NP medications did not have an effect on patch application-related pain.
During the clinical trials, cooling measures such as sealed cool gel packs, gauze misted with cool water, or fans were applied to the treatment area after patch removal. These measures were found to relieve patch applicationrelated pain in ≥90% of the patients in whom they were used. In the absence of capsaicin, the TRPV1 receptor is activated at approximately 43°C; the binding of capsaicin to TRPV1 is thought to lower the activation temperature of the receptor, allowing for activation at body temperature and a feeling of application-site burning.22 It is theoretically possible that cooling the treatment area during NGX-4010 application may make it harder for capsaicin to activate the receptor, resulting in reduced efficacy. Although analysis of clinical study data has not shown any apparent effect of the use of cooling on the efficacy of NGX-4010 (data on file), in the clinical studies cooling measures were mostly used following patch removal. As only limited data exist regarding the use of cooling measures during the application of the NGX-4010 patch, it is recommended that cooling should be applied following patch removal. If cooling is required during the application procedure, cool packs should only be applied towards the end of the procedure, in response to capsaicin-induced pain, and never preemptively at the start of the patch application. Patients should be advised that the treated area may be sensitive to heat, including hot showers and baths, direct sunlight, and vigorous exercise, for a few days post-treatment.
During the NGX-4010 clinical program, several measures were taken to alleviate and manage patch application-related pain. Although, other measures that might provide additional benefit in patients’ management were not systematically evaluated, psychological measures such as reassurance, cognitive behavioral therapy, and distraction techniques, which have been shown to be important in reducing pain sensation, might help with post-patch application-related pain.23–25 Therefore, in addition to treatment with a topical anesthetic, informing patients that they may experience patch application-related pain, ie, a burning sensation, that is usually temporary lasting a few hours to a few days, could have an impact on the patients’ ability to tolerate the pain. Further, reaffirming patients that the potential relief from PNP may last for 12 weeks13–15,26 could also help patients to tolerate patch application-related pain. The use of distraction techniques has also been found to be useful in pain management24 and providing patients with reading materials, a television, a snack, lunch, and also by staying with them, particularly during the last 15 minutes of treatment, may enhance the patients’ ability to complete treatment.
In conclusion, single and repeated NGX-4010 treatments are generally well tolerated and, on average, patch application-related pain is transient and can be satisfactorily controlled by oral analgesics and/or local cooling. Using these approaches, nearly all patients completed ≥90% of the full treatment duration, indicating that patch applicationrelated pain is not a barrier to adherence to the full intended treatment duration. The vast majority of patients who required intervention for treatment-related pain were adequately managed with simple, inexpensive approaches, local cooling, and/or oral medications. Aggressive pain control interventions such as intravenous opioids and epidural injections were not routinely required. Since more aggressive interventions do place patients at an added burden of physical risk and expense, the current findings are important as the capsaicin 8% patch begins to be used more commonly by health care providers. In the authors’ experience, patient education prior to patch application, reassurance, and distraction techniques are also important components that can assist patients in tolerating patch application-related pain.
Acknowledgments
The research described in this paper was funded by NeurogesX, Inc. The authors were not compensated in any way for the writing of this manuscript. Editorial assistance was provided by Adelphi Communications, supported by Astellas Europe Ltd.
Footnotes
Disclosure
John F Peppin, Kristine Majors, Lynn R Webster, and David M Simpson are consultants for NeurogesX, Inc and Astellas Pharma Europe, Ltd, and David M Simpson is a consultant for NeurogesX, Inc and receives speaker honoraria from Astellas Pharma Europe, Ltd. Jeffrey K Tobias and Geertrui F Vanhove are NeurogesX, Inc employees and own NeurogesX, Inc stock.
References
- 1.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4(9):543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 2.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18(6):350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 Suppl):S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353(9168):1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 6.Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271(10):1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 7.Facer P, Casula MA, Smith GD, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma W, Zhang Y, Bantel C, Eisenach JC. Medium and large injured dorsal root ganglion cells increase TRPV-1, accompanied by increased alpha2C-adrenoceptor co-expression and functional inhibition by clonidine. Pain. 2005;113(3):386–394. doi: 10.1016/j.pain.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Bley KR. TRPV1 agonist approaches for pain management. In: Gomtsyan A, Faltynek CR, editors. Vanilloid Receptor TRPV1 in Drug Discovery. Hoboken, NJ: John Wiley & Sons, Inc; 2010. pp. 325–347. [Google Scholar]
- 10.Kennedy WR, Vanhove GF, Lu SP, et al. A randomized, controlled, openlabel study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J Pain. 2010;11(6):579–587. doi: 10.1016/j.jpain.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51(2):159–212. [PubMed] [Google Scholar]
- 12.Backonja MM, Wallace MS, Blonsky ER, et al. NGX-4010, a high- concentration capsaicin patch, for the treatment of postherpetic neuralgia: A randomised, double-blind study. Lancet Neurol. 2008;7(12):1106–1112. doi: 10.1016/S1474-4422(08)70228-X. [DOI] [PubMed] [Google Scholar]
- 13.Backonja MM, Malan TP, Vanhove GF, Tobias JK. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an openlabel extension. Pain Med. 2010;11(4):600–608. doi: 10.1111/j.1526-4637.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 14.Irving GA, Backonja MM, Dunteman E, et al. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med. 2011;12(1):99–109. doi: 10.1111/j.1526-4637.2010.01004.x. [DOI] [PubMed] [Google Scholar]
- 15.Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70(24):2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- 16.Webster LR, Malan TP, Tuchman MM, Mollen MD, Tobias JK, Vanhove GF. A multicenter, randomized, double-blind, controlled dose finding study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. J Pain. 2010;11(10):972–982. doi: 10.1016/j.jpain.2010.01.270. [DOI] [PubMed] [Google Scholar]
- 17.Simpson DM, Gazda S, Brown S, et al. Long-term safety of NGX- 4010, a high-concentration capsaicin patch, in patients with peripheral neuropathic pain. J Pain Symptom Manage. 2010;39(6):1053–1064. doi: 10.1016/j.jpainsymman.2009.11.316. [DOI] [PubMed] [Google Scholar]
- 18.Babbar S, Marier JF, Mouksassi MS, et al. Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic pain. Ther Drug Monit. 2009;31(4):502–510. doi: 10.1097/FTD.0b013e3181a8b200. [DOI] [PubMed] [Google Scholar]
- 19.Gustoff B. First experience with QUTENZA: Case reports. International Association for the Study of Pain 13th World Congress on Pain; 2010; Montreal, QC. Discussion. [Google Scholar]
- 20.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 21.Webster LR, Tark M, Rauck R, Tobias JK, Vanhove GF. Effect of duration of postherpetic neuralgia on efficacy analyses in a multicenter, randomized, controlled study of NGX-4010, an 8% capsaicin patch evaluated for the treatment of postherpetic neuralgia. BMC Neurol. 2010;10:92. doi: 10.1186/1471-2377-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace M, Pappagallo M. Qutenza®: A capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11(1):15–27. doi: 10.1586/ern.10.182. [DOI] [PubMed] [Google Scholar]
- 23.Eccleston C. Role of psychology in pain management. Br J Anaesth. 2001;87(1):144–152. doi: 10.1093/bja/87.1.144. [DOI] [PubMed] [Google Scholar]
- 24.Johnson MH. How does distraction work in the management of pain? Curr Pain Headache Rep. 2005;9(2):90–95. doi: 10.1007/s11916-005-0044-1. [DOI] [PubMed] [Google Scholar]
- 25.Linton SJ, McCracken LM, Vlaeyen JW. Reassurance: help or hinder in the treatment of pain. Pain. 2008;134(1–2):5–8. doi: 10.1016/j.pain.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Backonja MM, Walk D, Edwards RR, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25(7):641–647. doi: 10.1097/AJP.0b013e3181a68c7e. [DOI] [PubMed] [Google Scholar]