Abstract
Tenofovir gel (1%) is being developed as a microbicide for the prevention of human immunodeficiency virus (HIV) infection and has been shown to reduce transmission to women by 39%. The gel also prevents infection in macaques when applied intravaginally or intrarectally prior to challenge with simian-human immunodeficiency virus (SHIV), but very little pharmacokinetic information for macaques is available to help extrapolate the data to humans and thus inform future development activities. We have determined the pharmacokinetics of tenofovir in macaques following intravaginal and intrarectal administration of 0.2, 1, and 5% gels. Plasma and vaginal and rectal fluid samples were collected up to 24 h after dosing, and at 24 h postdosing biopsy specimens were taken from the vaginal wall, cervix, and rectum. Following vaginal and rectal administration, tenofovir rapidly distributed to the matrices distal to the site of administration. In all matrices, exposure increased with increasing dose, and with the 1% and 5% formulations, concentrations remained detectable in most animals 24 h after dosing. At all doses, concentrations at the dosing site were typically 1 to 2 log units higher than those in the opposite compartment and 4 to 5 log units higher than those in plasma. Exposure in vaginal fluid after vaginal dosing was 58 to 82% lower than that in rectal fluid after rectal dosing, but plasma exposure was 1- to 2-fold greater after vaginal dosing than after rectal dosing. These data suggest that a tenofovir-based microbicide may have the potential to protect when exposure is via vaginal or anal intercourse, regardless of whether the microbicide is applied vaginally or rectally.
INTRODUCTION
In 2009, an estimated 2.6 million people became newly infected with human immunodeficiency virus type 1 (HIV-1), bringing the total number of people living with HIV to an estimated 33.3 million (17). In the same year, 1.8 million died from AIDS-related illnesses. Although some prevention strategies such as male and female condoms and behavioral programs promoting abstinence and monogamy have had some level of success (5, 15, 26), they are inadequate to significantly impact the epidemic.
Most new infections occur in developing countries, and in 2009, 68% of all people living with HIV, 69% of new infections, and 72% of AIDS-related deaths were in sub-Saharan Africa (17). In particular, women and girls are increasingly bearing the brunt of the epidemic; in sub-Saharan Africa, more women than men are living with HIV, and young women in this region aged 15 to 24 years are up to eight times more likely to be HIV positive than men. About 40% of all adult women with HIV live in southern Africa (17). Therefore, there is a clear need for safe and efficacious prevention technologies that are also sufficiently inexpensive to be widely used in developing countries. Topical microbicides are products that can be applied to the vagina or rectum to prevent HIV transmission, and their use represents one of the most promising prophylactic strategies currently under development. They are self-administered and can be formulated in a variety of dosage forms to suit regional and individual preferences.
Tenofovir {9-[(R)-2-(phosphonomethoxy)propyl]adenine monohydrate} is an inhibitor of viral reverse transcriptase (RT), an essential enzyme in the transcription of viral RNA into DNA prior to incorporation into the host cell DNA. The active form of tenofovir is tenofovir diphosphate (tenofovir-DP), which is formed in vivo through phosphorylation by intracellular kinases (22, 24). The oral prodrug tenofovir disoproxil fumarate (TDF) has been marketed as Viread by Gilead Sciences, Inc. (Foster City, CA), for the treatment of HIV/AIDS since 2001. In a double-blind, randomized, placebo-controlled clinical trial in women (CAPRISA 004), vaginal application of a gel formulation containing 1% tenofovir was shown to reduce HIV acquisition by an estimated 39% (P = 0.017) overall and by 54% (P = 0.025) in women with high gel application adherence (1). This trial represents the first proof of concept for the vaginal use of topical antiretroviral drugs for the prevention of male-to-female transmission of HIV and clearly supports tenofovir gel as a viable prevention product for use in developing countries. More recently, a trial in men who have sex with men (iPrEx) showed that once-daily oral administration of Truvada (Gilead Sciences, Inc., Foster City, CA), containing 300 mg of TDF and 200 mg of emtricitabine, reduced infections by 44% overall (P = 0.005) (12), although a trial in women using the same medication (FEM-PrEP) was recently halted because it was considered highly unlikely that the trial would be able to demonstrate the effectiveness of Truvada (9).
The nonhuman primate model has been widely used as a preclinical model of microbicide efficacy (25), and in a study in female pig-tailed macaques, intravaginal administration of 1% tenofovir gel 30 min prior to challenge with simian-human immunodeficiency virus strain SF162p3 (SHIVSF162p3) on a twice-weekly dose-and-challenge schedule prevented infection even after 20 exposures to virus (21). In a separate study, four out of six female pigtail macaques receiving weekly intravaginal doses of 1% tenofovir gel remained uninfected when challenged with SHIVSF162p3 at 30 min and 3 days after each dose for 10 weeks (a total of 20 challenges) (7). In addition, it was shown that six out of nine rhesus macaques given 1% tenofovir gel intrarectally up to 2 h prior to rectal challenge with SIVmac251/32H were protected from infection, and an additional two animals that were infected showed either a delay in the appearance of viremia or a low, transient viremia (6).
Although the pharmacodynamic data in macaques show some consistency with the efficacy results of the CAPRISA 004 trial, very little information on the pharmacokinetics of tenofovir following administration via either the vaginal or rectal route is currently available to help understand the data from this model or extrapolate the data to trials in humans. Therefore, we have conducted a study to determine the relative and absolute drug levels in vaginal and rectal fluids, cervical, vaginal, and rectal tissue biopsy specimens, and plasma following delivery of a single dose of one of three concentrations of tenofovir gel both intravaginally and intrarectally.
MATERIALS AND METHODS
Animals.
Female rhesus macaques (Macaca mulatta; Valley Biosystems, CA) were used for this study under the oversight of the Valley Biosystems Institutional Animal Care and Use Committee and according to the Guide for the Care and Use of Laboratory Animals (16). Animals weighed 5.43 to 7.88 kg at the start of the study and were between 5 years 3 months and 9 years 9 months old at study initiation. Animals were housed, as per USDA regulations for nonhuman primates, on a 12-h light/12-h dark cycle with lights on at 0600 h, with a 100% fresh air supply at 10 to 15 changes per hour, room temperature of between 66 and 76°F, and relative humidity of 30 to 70%. Water was provided ad libitum, and animals were fed certified primate diet in daily quantities appropriate to the age, sex, and weight of each individual.
Tenofovir gel.
Tenofovir gels were formulated by Particle Sciences Inc. (Bethlehem, PA) with either 0.2, 1, or 5% tenofovir (Gilead Sciences, Inc., Foster City, CA) in a gel containing purified water, edentate disodium, citric acid, glycerin, propylparaben, methylparaben, and hydroxycellulose that was adjusted to pH 4 to 5. The composition of the gels was the same as that used in the CAPRISA 004 clinical trial.
Study design.
At approximately 30 days prior to tenofovir administration, each animal received a single 30-mg intramuscular injection of depomedroxyprogesterone acetate (Depo Provera; Pharmacia & Upjohn Company, Division of Pfizer Inc., New York, NY) to synchronize their menstrual cycles. The study was then divided into two phases.
(i) Phase A.
Groups of 6 animals received a single intravaginal dose of 0.2, 1, or 5% tenofovir gel at a volume of 0.6 ml/kg of body weight using a rigid stainless steel feeding tube with an atraumatic tip.
(ii) Phase B.
The same groups of 6 animals from phase A received a single intrarectal dose of 0.2, 1, or 5% tenofovir gel at a volume of 0.6 ml/kg using a rigid stainless steel feeding tube with an atraumatic tip.
The dose concentration used in CAPRISA 004 was selected to be the intermediate concentration. The low and high concentrations were selected to be 5-fold lower and 5-fold higher, respectively, than the clinical concentration. The dose volume was selected to provide a total dose similar to that used in previous macaque challenge studies (7, 21) (3 ml of gel was administered to macaques weighing 5 to 11 kg).
Animals were held for a 3-week washout period between the intravaginal and intrarectal doses.
Prior to administration of Depo Provera and tenofovir and before collection of blood and tissue samples as described below, each animal was immobilized with ketamine hydrochloride (10 mg/kg intramuscularly [i.m.]).
Sample collection.
Blood samples were collected from a saphenous, cephalic, or femoral vein into lithium heparin anticoagulant before dosing and at 15 min and 1, 4, 8, and 24 h after intravaginal and intrarectal dosing. The plasma was separated and stored deep-frozen. At the same time points, Weck-Cel surgical spears (Medtronic Xomed, Inc., Mystic, CT) were used to collect samples of cervicovaginal and rectal fluid as follows. A speculum was inserted into the vagina or anus, and a preweighed Weck sponge was carefully placed into the vagina or rectum. For rectal samples, the sponge was moistened with 100 μl of phosphate-buffered saline prior to weighing. The speculum was removed and the sponge was allowed to absorb vaginal or rectal fluid for 10 min. Upon removal, the sponge was weighed, placed in a labeled 15-ml conical tube, and immediately frozen on dry ice.
Immediately following the 24-h blood and fluid sample collections, the animals were given medetomidine (0.03 ml/kg i.m.) in addition to the ketamine to provide additional anesthesia and analgesia. Tissue biopsy specimens were taken from the cervix and from the vaginal wall using Baby Ticshler biopsy forceps (4.2 mm by 2.3 mm). Similarly sized samples were obtained from the rectum using a cupped biopsy forceps and surgical scissors. The tissue samples were immediately rinsed with phosphate-buffered saline and snap-frozen in a slurry of isopentane and dry ice. Where necessary, sterile swabs saturated in ferric subsulfate (Monsel's) solution were used to reduce excessive vaginal bleeding, and tampons were also used to reduce both vaginal and rectal bleeding. The animals were returned to their home cages and given atipamezole (0.03 ml/kg) i.m. to reverse the effects of the medetomidine. The animals also received nonsteroidal anti-inflammatory drugs for 3 days following the biopsies for analgesia.
All plasma, cervicovaginal fluid, and rectal fluid samples and all biopsy specimens were frozen following collection and stored at −80°C.
Samples were subsequently shipped deep-frozen to the UNC Center for AIDS Research, Clinical Pharmacology/Analytical Chemistry Core, University of North Carolina, Chapel Hill, NC, and analyzed for tenofovir concentrations using liquid chromatography (LC)-UV and LC-tandem mass spectrometry (MS/MS). Tissue samples were also analyzed for tenofovir-DP.
Linear ranges for the assays were 0.5 to 200 ng/ml for tenofovir in plasma, 25 to 1,000,000 ng/ml for tenofovir in the sponges, 2 to 2,000 ng/ml for tenofovir in tissues, and 10 to 10,000 ng/ml for tenofovir-DP in tissues. The internal standard varied with the matrix and assay detection method as follows: adefovir with plasma, 2′,3-dideoxyuridine (ddU) with sponges, and stable labeled [13C]tenofovir (for tenofovir) and lamivudine triphosphate (for tenofovir diphosphate) with tissues.
For plasma, protein precipitation was performed prior to solid-phase extraction (Varian Bond-elut column, C18, 200 mg, 1 ml). Extracted samples were evaporated to dryness and reconstituted in 50 μl of a 5% water-acetonitrile solution. A gradient elution system through a Waters ultraperformance liquid chromatography (UPLC) BEH C18 analytical column (3.0 by 50 mm; particle size, 1.7 μm) was used to separate tenofovir from the other components in the plasma matrix, and concentrations were measured using an Agilent 6410 tandem MS detector with electrospray ionization in the positive mode. Data were collected using Agilent MassHunter chromatography software.
Sponges were exposed to multiple washes using an acetate buffer which contained 10 ng/ml of ddU. These were combined and subjected to solid-phase extraction (Varian Bond-elut column, C18, 200 mg, 3 ml). Extracted samples were evaporated to dryness and reconstituted in 50 μl of a 5% methanolic solution. A gradient elution system through a Waters Atlantis dC18 analytical column (3.9 by 150 mm; particle size, 5 μm) was used to separate tenofovir from the other components of the matrix, and concentrations were measured using an Agilent 1100 high-performance liquid chromatography (HPLC)-diode array detector (DAD) system. Data were collected using Agilent Chemstation chromatography software.
Tissue homogenization was performed using a Precellys homogenization system. After homogenization and centrifugation, 700 μl of the supernatant was evaporated to dryness and reconstituted in 300 μl of a 1 mM ammonium phosphate buffer. Analytes were separated on a Waters UPLC BEH C18 analytical column (3.0 by 50 mm; particle size, 1.7 μm) by gradient elution and measured by Agilent 6410 tandem MS detection using electrospray ionization in the positive mode. Data were collected using Agilent MassHunter chromatography software.
Data analysis.
Data from all samples were collated, and pharmacokinetic parameters were generated using a noncompartmental analysis with WinNonLin software (version 5.1; Pharsight Corporation, Mountain View, CA).
RESULTS
Following vaginal and rectal administration, tenofovir was detectable by 15 min after dosing in all matrices (plasma and vaginal and rectal fluids) distal to where the dose was administered (vagina or rectum). After vaginal and rectal dosing, at least five of the six macaques tested in the 1% and 5% groups had detectable tenofovir concentrations in all matrices 24 h after dosing. Tenofovir was also often detectable at 24 h in samples collected from animals treated with the 0.2% gel. At all doses, concentrations at the dosing site were typically 1 to 2 orders of magnitude higher than those in the opposite compartment and 4 to 5 orders of magnitude higher than those in the plasma. Vaginal dosing resulted in local median values of the vaginal fluid maximum concentration (Cmax) and the area under the concentration-time curve from time zero to 24 h (AUC0–24) that were 58 to 82% lower than those that were achieved in rectal fluid with rectal dosing. Conversely, vaginal dosing resulted in median plasma Cmax and AUC0–24 values that were 1- to 2-fold greater than those that were achieved in plasma after rectal dosing. AUC0–24 values in plasma ranged from 0.02 to 0.04% of those in vaginal fluid after vaginal dosing and from 0.002 to 0.008% of those in rectal fluid after rectal dosing.
Intravaginal dosing.
The median tenofovir concentration data for vaginal fluids, rectal fluids, and plasma after intravaginal administration of 0.2%, 1%, and 5% tenofovir gels are presented in Table 1, and the corresponding median tenofovir concentration-versus-time curves are presented in Fig. 1. The dose proportionality of the AUC0–24s and Cmaxs in plasma, rectal fluid, and vaginal fluid following intravaginal dosing are shown in Fig. 2.
Table 1.
Median pharmacokinetics of tenofovir in macaques following intravaginal administration of 0.2%, 1%, and 5% tenofovir gela
| Compartment and dose (%) | Cmax (μg/ml) | Tmax (h) | Clast (μg/ml) | AUC0–24 (μg · h/ml) |
|---|---|---|---|---|
| Vaginal fluid | ||||
| 0.2 | 515.81 ± 99.378 | 0.3 ± 1.531 | 11.97 ± 57.484 | 853.35 ± 679.061 |
| 1 | 2,103.53 ± 749.275 | 0.3 ± 0.387 | 57.27 ± 158.197 | 7,056.89 ± 8,731.087 |
| 5 | 4,051.54 ± 739.806 | 0.6 ± 0.411 | 75.94 ± 54.127 | 13,419.19 ± 4,848.739 |
| Rectal fluid | ||||
| 0.2 | 28.97 ± 41.907 | 1.0 ± 1.775 | 4.879 ± 27.268 | 92.98 ± 311.615 |
| 1 | 142.77 ± 340.000 | 2.5 ± 1.801 | 4.053 ± 12.876 | 561.38 ± 1,083.378 |
| 5 | 361.31 ± 447.089 | 2.5 ± 3.690 | 13.17 ± 23.796 | 1,373.11 ± 2,978.195 |
| Blood plasma | ||||
| 0.2 | 0.056 ± 0.0269 | 1.00 ± 0.0 | 0.000 ± 0.0007 | 0.172 ± 0.0724 |
| 1 | 0.418 ± 0.2879 | 1.00 ± 0.0 | 0.001 ± 0.0006 | 1.13 ± 0.614 |
| 5 | 2.30 ± 0.936 | 1.00 ± 0.0 | 0.006 ± 0.0014 | 5.59 ± 2.192 |
Values are medians ± standard deviations. Clast, last concentration measured.
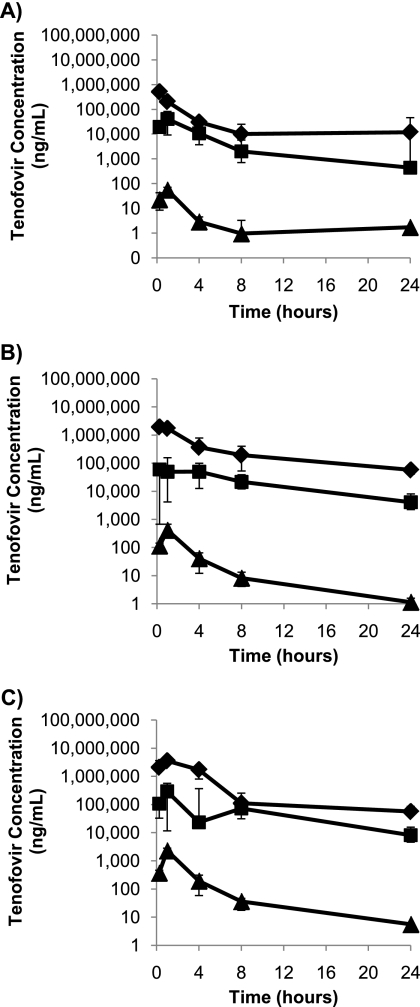
Fig 1.
Median concentrations (n = 6) of tenofovir in vaginal fluid (♦), rectal fluid (■), and blood plasma (▲) following intravaginal administration of 0.2% tenofovir gel (A), 1% tenofovir gel (B), and 5% tenofovir gel (C). Bars represent the 25th and 75th percentiles.
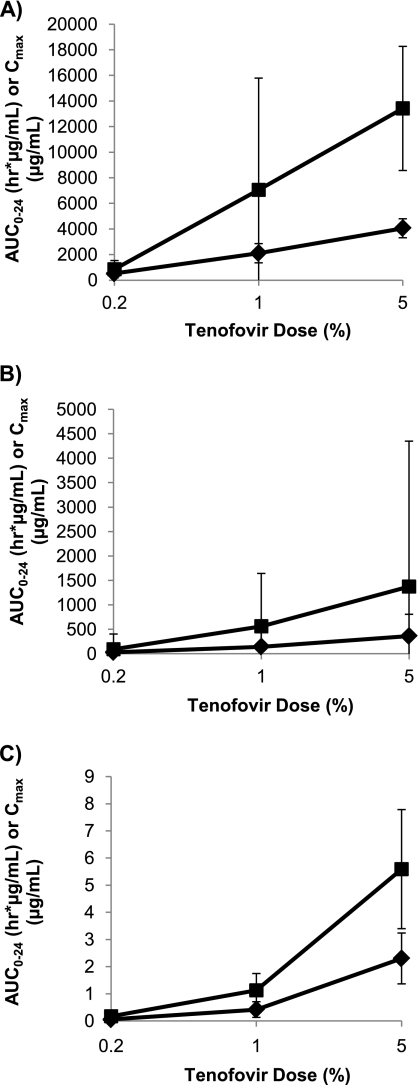
Fig 2.
Dose relationship of AUC0–24 (■) and Cmax (♦) in vaginal fluid (A), rectal fluid (B), and blood plasma (C) following intravaginal administration of 0.2%, 1%, and 5% tenofovir gel. Values represent the medians for 6 macaques, with the 25th and 75th percentiles indicated by the bars.
The median plasma AUC0–24 value following intravaginal dosing was approximately dose proportional, with an ∼5-fold increase in AUC0–24 with each 5-fold increase in dose. However, the median plasma Cmaxs were not dose proportional, with a 10-fold increase between the 0.2% and 1% doses and a 5-fold increase between the 1% and 5% doses.
Median AUC0–24s and Cmaxs in vaginal fluids showed poor dose proportionality following vaginal dosing, with AUC0–24 increasing ∼8-fold and ∼2-fold with dose increases from 0.2% to 1% and 1% to 5%, respectively, while the Cmax doubled with each 5-fold increase in dose level. However, the drug concentrations in vaginal fluid were high, and by 24 h after dosing, median concentrations were still 12, 57, and 76 μg/ml for the 0.2%, 1%, and 5% doses, respectively. The time to Cmax (Tmax) was less than 1 h at all doses.
Tenofovir exposure in rectal fluids following intravaginal dosing was also dose related, but with poor dose proportionality for both AUC0–24 and Cmax. The median AUC0–24 increased 6-fold and 2-fold as the dose increased from 0.2% and 1% and 1% to 5%, respectively, while the median Cmax increased 5-fold between the 0.2% and 1% doses but only 2.5-fold between the 1% and 5% doses. As would be expected, the median Tmax for rectal fluid was later than that for vaginal fluid and ranged from 1 to 2.5 h after dosing, and concentrations at 24 h after dosing remained greater than 4 μg/ml across all dose groups.
Intrarectal dosing.
The median tenofovir concentration data for vaginal fluids, rectal fluids, and plasma after intrarectal administration of 0.2%, 1%, and 5% tenofovir gels are presented in Table 2, and the corresponding median tenofovir concentration-versus-time curves are presented in Fig. 3. The dose proportionality of the AUC0–24s and Cmaxs in plasma, rectal fluid, and vaginal fluid following intrarectal dosing are shown in Fig. 4.
Table 2.
Pharmacokinetics of tenofovir in macaques following intrarectal administration of 0.2%, 1%, and 5% tenofovir gela
| Compartment and dose (%) | Cmax (μg/ml) | Tmax (h) | Clast (μg/ml) | AUC0–24 (μg · h/ml) |
|---|---|---|---|---|
| Vaginal fluid | ||||
| 0.2 | 18.22 ± 159.066 | 4.0 ± 2.934 | 2.61 ± 14.039 | 85.16 ± 203.622 |
| 1 | 37.93 ± 151.221 | 2.5 ± 9.020 | 9.11 ± 4.457 | 377.58 ± 615.747 |
| 5 | 196.650 ± 624.735 | 1.0 ± 9.338 | 108.45 ± 52.008 | 2,207.20 ± 1,008.223 |
| Rectal fluid | ||||
| 0.2 | 2,644.62 ± 1,616.916 | 0.30 ± 0.306 | 2.17 ± 18.009 | 4,870.16 ± 3,976.541 |
| 1 | 6,614.20 ± 2,472.813 | 0.30 ± 0.306 | 11.28 ± 44.224 | 30,581.94 ± 27,528.478 |
| 5 | 12,094.96 ± 2,317.247 | 0.30 ± 0.387 | 45.09 ± 115.301 | 31,742.73 ± 57,633.168 |
| Blood plasma | ||||
| 0.2 | 0.052 ± 0.0238 | 0.25 ± 0.387 | 0.001 ± 0.0006 | 0.121 ± 0.0667 |
| 1 | 0.278 ± 0.0870 | 0.25 ± 0.387 | 0.001 ± 0.0010 | 0.739 ± 0.2220 |
| 5 | 1.06 ± 0.513 | 1.00 ± 0.306 | 0.002 ± 0.0095 | 2.532 ± 1.151 |
Values are medians ± standard deviations. Clast, last concentration measured.
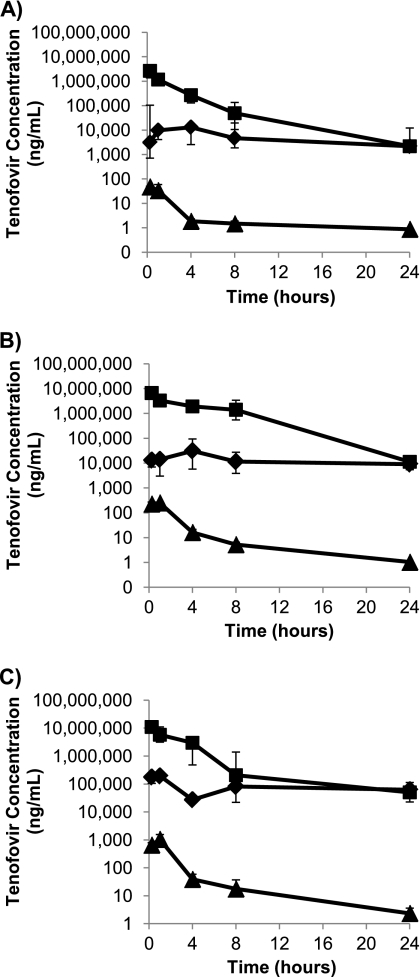
Fig 3.
Median concentrations (n = 6) of tenofovir in vaginal fluid (♦), rectal fluid (■), and blood plasma (▲) following intrarectal administration of 0.2% tenofovir gel (A), 1% tenofovir gel (B), and 5% tenofovir gel (C). Bars represent the 25th and 75th percentiles.
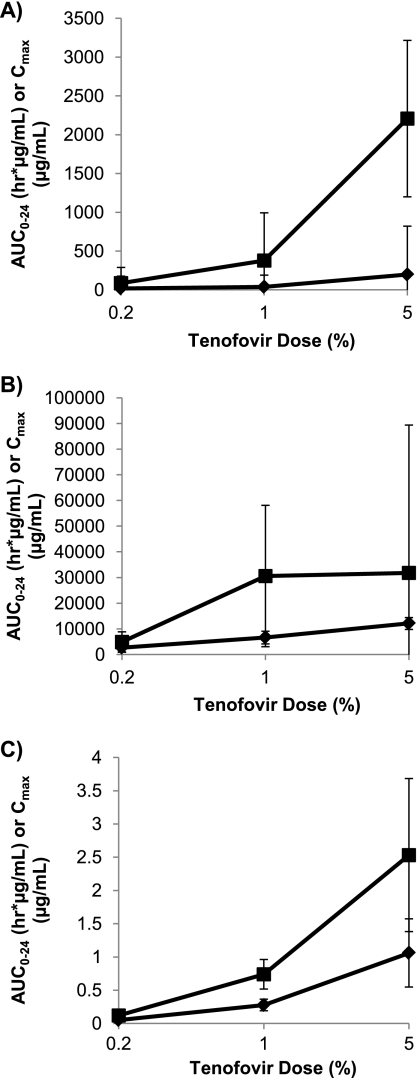
Fig 4.
Dose relationship of AUC0–24 (■) and Cmax (♦) in vaginal fluid (A), rectal fluid (B), and blood plasma (C) following intrarectal administration of 0.2%, 1%, and 5% tenofovir gel. Values represent the medians for 6 macaques, with the 25th and 75th percentiles indicated by the bars.
Following rectal dosing, plasma concentrations showed good dose proportionality, with 6-fold and 3-fold increases in AUC0–24 and 5-fold and 4-fold increases in Cmax between the 0.2% and 1% doses and between the 1% and 5% doses, respectively.
The median Cmax data in rectal fluids showed a doubling of the tenofovir concentration between the 0.2% and 1% doses and again between the 1% and 5% doses. However, the AUC0–24 data for the rectal fluids appeared to show a saturation in exposure, with a 6-fold increase in values between the 0.2% and 1% doses but with no appreciable difference in the median AUC0–24 between the 1.0% and 5.0% doses. As with intravaginal dosing, the median Tmax for the rectal fluids was within 1 h of dosing, and the highest tenofovir concentrations were in the rectal fluids.
Tenofovir exposure (AUC0–24) in vaginal fluids following rectal dosing was roughly dose proportional (∼4-fold increase in the medians between the 0.2% and 1% doses and ∼6-fold increase between the 1% and 5% doses), but the median Cmax level doubled between the 0.2% and 1% doses and then increased 5-fold between the 1% and 5% doses. Tmax values for vaginal fluids were highly variable following intrarectal dosing, ranging from 4 h at 0.2% tenofovir to 1 h at 5% tenofovir and with considerable interanimal variability. AUC0–24s and Cmaxs in vaginal fluids after rectal dosing were lower than the corresponding values in rectal fluids after vaginal dosing. Levels in the vaginal fluid appeared to decline more slowly than those in the rectal fluid, such that by 24 h after dosing, tenofovir levels for the 0.2% and 1% doses were similar in both compartments, and for the 5% dose, tenofovir levels were higher in the vaginal fluid than in the rectal fluid.
Tissue levels of tenofovir and tenofovir diphosphate.
The tissue concentrations of tenofovir following vaginal and rectal dosing are shown in Table 3. Interanimal variation was considerable for tenofovir concentrations in all three tissues. Following intravaginal dosing of the 0.2 and 1% gels, median tenofovir concentrations in cervical and vaginal tissue were similar, whereas concentrations in the rectal tissue were much lower, with only two animals dosed with 0.2% having detectable concentrations. After intravaginal administration of the 5% gel, the median concentrations in the cervical, vaginal, and rectal tissues were 1.5, 3.3, and 7.8 times higher than those in the same tissues after the 1% vaginal dose, and the median concentration in vaginal tissue after the 5% dose was 2-fold higher than that in cervical tissue, which in turn was approximately 3-fold higher than that in rectal tissue. After intrarectal administration, tenofovir concentrations were the highest in rectal tissue, with a 7-fold increase in concentration between the 0.2% and 1% gels but only a 2-fold increase between the 1% and 5% gels. In vaginal tissue, the concentration after administration of the 5% gel was 3-fold lower than that in rectal tissue at the same dose, and the relative concentrations at the 0.2% and 1% doses were lower still in vaginal tissue than rectal tissue, with similar tissue concentrations at both doses. Concentrations in cervical tissue were lower than those in rectal and vaginal tissues.
Table 3.
Tenofovir concentrations in cervical, vaginal, and rectal tissue 24 h after intravaginal and intrarectal administration of 0.2%, 1%, and 5% tenofovir gel to macaques
| Dose (%) | Concn (μg/g) in tissue aftera: |
|||||
|---|---|---|---|---|---|---|
| Vaginal dose |
Rectal dose (μg/g) |
|||||
| Cervical | Vaginal | Rectal | Cervical | Vaginal | Rectal | |
| 0.2 | 1.89 ± 0.740 | 2.11 ± 0.920 | 0.00 ± 0.767 | 0.10 ± 0.119 | 0.53 ± 2.783 | 3.81 ± 15.253 |
| 1 | 19.33 ± 16.740 | 18.32 ± 5.655 | 1.17 ± 3.500 | 0.20 ± 0.105 | 0.50 ± 4.814 | 27.20 ± 54.225 |
| 5 | 29.87 ± 12.732 | 60.11 ± 54.140 | 9.184 ± 1.404 | 2.34 ± 3.184 | 18.78 ± 15.775 | 60.32 ± 78.650 |
Values are medians ± standard deviations.
Attempts to measure tenofovir diphosphate concentrations in biopsy specimens of the cervical, vaginal, and rectal tissue samples collected 24 h after dosing proved unsuccessful. In all samples, values were below the lower limit of quantification for the assay (200 fmol/mg tissue).
DISCUSSION
Following vaginal or rectal administration of tenofovir gel, the drug was rapidly distributed to the opposite compartment, with substantial concentrations being detectable within 15 min after dosing (the first time point at which samples were collected). The mechanism for such rapid transfer is unclear. The relatively low tenofovir concentrations measured in peripheral blood were very unlikely to be sufficient for distribution through the systemic circulation to account for the observed concentrations in the undosed compartment, especially given that it has been shown in women that following a single oral dose of tenofovir, the AUC in the genital tract is only slightly higher (35%) than the AUC in the plasma (8). Other possibilities, based on the close proximity of the vagina and rectum, include passive diffusion and/or transport via the lymphatic system. Other investigators have reported countercurrent exchange processes in the vaginal region that may also contribute to this effect (4). Regardless of the mechanism behind this transfer, there are important implications for the use of tenofovir as a microbicide. Workers in the field have questioned the potential confounding effects of anal intercourse in vaginal microbicide efficacy trials. There are increasing data that suggest that receptive anal intercourse (RAI) is practiced by women in developed and developing countries (2, 11, 13, 18, 20). Although the frequency of RAI in women may be low, the increased risk of infection associated with anal intercourse (19) suggests that such practices have the potential to significantly impact the outcome in these trials. However, on the basis of the data for macaques presented here, it is possible that a tenofovir-based microbicide applied vaginally may have the potential to protect against rectal exposure to HIV-1, and vice versa, although the actual concentrations required for protection are not known. Further clinical trials are warranted to determine whether the same rapid transfer of tenofovir from one compartment to the other occurs in women in a manner similar to that seen in macaques.
As a nucleotide reverse transcriptase inhibitor, the site of action for tenofovir is within the target CD4+ cells for HIV. Mean concentrations of tenofovir in the cervical, vaginal, and rectal tissues at 24 h after dosing were 19.33, 18.32, and 1.17 μg/g, respectively, after vaginal administration of the 1% gel and 0.20, 0.50, and 27.20 μg/g, respectively, after rectal administration of the same dose. In comparison, a single intravaginal dose of 1% tenofovir gel in women was shown to result in 7.4 μg/ml in vaginal tissue 24 h after dosing (23), which is about equal to 7.4 ng/g on the basis of the assumption that 1 ml weighs approximately 1 g.
In order to demonstrate antiviral activity, tenofovir needs to undergo two phosphorylation steps to become the active form, tenofovir diphosphate (22, 24). Consequently, tenofovir diphosphate is the important moiety for efficacy. Analysis of biopsy specimens of the cervical, vaginal, and rectal tissue samples collected 24 h after dosing was unable to detect the diphosphate even at a lower limit of quantification of 200 fmol/mg tissue. The measurement of tenofovir diphosphate has been technically challenging. The analytical method used at the time of this analysis was the industry standard, but more recently, data have been obtained in humans using a more sensitive method that show that following intravaginal and oral administration, tenofovir diphosphate concentrations are approximately 5 to 15% of the tenofovir concentrations in the same compartment (14). Using this adjustment for the data obtained in our study, it can be estimated that the diphosphate concentrations 24 h after intravaginal administration of the 1% gel would be at least 967, 916, and 59 ng/g in the cervical, vaginal, and rectal tissues, respectively, and after intrarectal dosing the corresponding values would be in the regions of at least 10, 25, and 1,360 ng/g. However, since it is not possible to distinguish tenofovir levels that are actually in the tissue from those that may be present on the surface or otherwise associated with the tissue, it is possible that these values represent overestimates of the true tissue concentrations. Again, more recent data obtained in humans using more sensitive analytical methods have shown that tenofovir diphosphate concentrations are in the region of 20 to 100 fmol/mg (equivalent to 6.1 to 30.5 pg/g) in rectal tissue following a single rectal dose of 1% tenofovir gel and that accumulation of tenofovir diphosphate in cells may be a relatively slow process, with multiple dosing required to achieve concentrations greater than 200 fmol/mg tissue (3). Following vaginal dosing of 1% tenofovir gel in women, vaginal tissue concentrations of tenofovir diphosphate were in the region of 1 to 10 pmol/mg (equivalent to 300 to 3,052 pg/g) (14).
Relating the data obtained in this study to the effectiveness of oral Truvada in protecting men who have sex with men in the iPrEx trial or to the apparent lack of protection in women in the FEM-PrEP trial is difficult because of the limited pharmacokinetic data available from these trials. However, it may be possible to address these issues in additional pharmacokinetic and virus challenge studies in macaques. In macaques, a single oral dose of Truvada containing 22 mg/kg of TDF resulted in low or undetectable concentrations of tenofovir in rectal secretions at 2 and 5 h after dosing, but by 24 h, the median concentration had increased to 1,186 ng/ml (10). This is much lower than the maximum concentrations measured in rectal fluid following intrarectal or intravaginal administration of 0.2%, 1%, or 5% tenofovir gel to macaques, despite the total dose of tenofovir administered being substantially higher (in the oral study, a 5-kg macaque would have received 110 mg TDF, whereas in our study, a macaque of the same weight treated with 0.2% gel received only approximately 6 mg tenofovir).
Conclusion.
The intravaginal administration of tenofovir gel to rhesus macaques resulted in rapid distribution of the drug to the rectum. Similarly, intrarectal administration of tenofovir resulted in rapid distribution to the vagina. Although these data have been obtained only in a preclinical study species, they suggest that a tenofovir-based microbicide may have the potential to protect women against infection with HIV when they are exposed via vaginal or anal intercourse, regardless of whether the microbicide is administered vaginally or rectally. Therefore, it is recommended that future evaluation of tenofovir gel as an HIV prevention technology include pharmacokinetic studies in women using a variety of dosing strategies.
ACKNOWLEDGMENTS
We thank Garry Gwozdz of Particle Sciences, Inc., for formulating the tenofovir gels, Robin Shattock of St. George's, University of London, for his advice on the study design, and Jonathon Holt of the International Partnership for Microbicides for his assistance in the preparation of the manuscript.
Footnotes
Published ahead of print 10 October 2011
REFERENCES
- 1. Abdool Karim Q, et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdool Karim SS, Ramjee G. 1998. Anal sex and HIV transmission in women. Am. J. Public Health 88:1265–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anton P, et al. 2011. RMP-02/MTN-006: a phase 1, placebo-controlled trial of rectally applied 1%vaginal tenofovir gel with comparison to oral tenofovir disoproxil fumarate, abstr 34LB, p 82 Abstr. 18th Conf. Retroviruses Opportunistic Infect., Boston, MA [Google Scholar]
- 4. Cicinelli E, de Ziegler D. 1999. Transvaginal progesterone: evidence for a new functional ‘portal system’ flowing from the vagina to the uterus. Hum. Reprod. Update 5:365–372 [DOI] [PubMed] [Google Scholar]
- 5. Cohen SA. 2003. Beyond slogans: lessons from Uganda's experience with ABC and HIV/AIDS. Guttmacher Rep. Public Policy 6(5):1–3 [Google Scholar]
- 6. Cranage M, et al. 2008. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local preexposure application of tenofovir gel. PLoS Med. 5:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dobard CW, et al. 2010. Protection by TFV gel against vaginal SHIV infection in macaques three days after gel application and its relationship to tissue drug levels, abstr 39, p 71 Abstr. Microbicides 2010, Pittsburgh, PA [Google Scholar]
- 8. Dumond JB, et al. 2007. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 21:1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FHI FHI statement on the FEM-PrEP HIV prevention study. http://www.fhi.org/en/AboutFHI/Media/Releases/FEM-PrEP_statement041811.htmAccessed 22 April 2011
- 10. García-Lerma JG, et al. 2010. Intermittent prophylaxis with oral Truvada protects macaques from rectal SHIV infection. Sci. Transl. Med. 2:14–4 [DOI] [PubMed] [Google Scholar]
- 11. Gorbach PM, et al. 2009. Anal intercourse among young heterosexuals in three sexually transmitted disease clinics in the United States. Sex. Transm. Dis. 36:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grant RM, et al. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gross M, et al. 2000. Anal sex among HIV-seronegative women at high risk of HIV exposure. The HIVNET Vaccine Preparedness Study 2 Protocol Team. J. Acquir. Immune Defic. Syndr. 24:393–398 [DOI] [PubMed] [Google Scholar]
- 14. Hendrix C, et al. 2011. MTN-001: a phase 2 crossover study of daily oral and vaginal TFV in healthy, sexually active women results in significantly different product acceptability and vaginal tissue drug concentrations, abstr 35LB, p 82 Abstr. 18th Conf. Retroviruses and Opportunistic Infections, Boston, MA [Google Scholar]
- 15. Holmes K, Levine R, Weaver M. 2004. Effectiveness of condoms in preventing STIs. Bull. World Health Organ. 82:399–478 [PMC free article] [PubMed] [Google Scholar]
- 16. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 17. Joint United Nations Programme on HIV/AIDS (UNAIDS) 2010. UNAIDS report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland [Google Scholar]
- 18. Kalichman S, Simbayi L, Cain D, Jooste S. 2009. heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sex. Transm. Infect. 85:411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minces LR, McGowan I. 2010. Advances in the development of microbicides for the prevention of HIV infection. Curr. Infect. Dis. Rep. 12:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misegades L, Page-Shafer K, Halperin D, McFarland W. 2001. Anal intercourse among young low-income women in California: an overlooked risk factor for HIV? AIDS 15:534–535 [DOI] [PubMed] [Google Scholar]
- 21. Parikh UM, et al. 2009. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 83:10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pruvost A, et al. 2005. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 49:1907–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz J, et al. 2009. A multi-compartment, single and multiple dose pharmacokinetic study of the candidate vaginal microbicide 1% tenofovir gel, abstr. LBPEC03. Abstr. 5th IAS Conf. HIV Pathog., Treatment Prevention Cape Town, South Africa [Google Scholar]
- 24. Stein DS, Moore KHP. 2001. Phosphorylation of nucleoside analog antiretrovirals: a review for clinicians. Pharmacotherapy 21:11–34 [DOI] [PubMed] [Google Scholar]
- 25. Veazey RS. 2008. Microbicide safety/efficacy studies in animals: macaques and small animal models. Curr. Opin. HIV AIDS 3:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weller S, Davis K. 2002. Condom effectiveness in reducing heterosexual HIV transmission (Cochrane Review). Cochrane Database Syst. Rev. 1:CD003255. [DOI] [PubMed] [Google Scholar]