Abstract
Many natural broad-spectrum cationic antimicrobial peptides (AMPs) possess a general mode of action that is dependent on lipophilicity and charge. Modulating the lipophilicity of AMPs by the addition of a fatty acid has been an effective strategy to increase the lytic activity and can further broaden the spectrum of AMPs. However, lipophilic modifications that narrow the spectrum of activity and exclusively direct peptides to fungi are less common. Here, we show that short peptide sequences can be targeted to fungi with structured lipophilic biomolecules, such as vitamin E and cholesterol. The conjugates were active against Aspergillus fumigatus, Cryptococcus neoformans, and Candida albicans but not against bacteria and were observed to cause membrane perturbation by transmission electron microscopy and in membrane permeability studies. However, for C. albicans, selected compounds were effective without the perturbation of the cell membrane, and synergism was seen with a vitamin E conjugate and amphotericin B. Moreover, in combination with β-cyclodextrin, antibacterial activity emerged in selected compounds. Biocompatibility for selected active compounds was tested in vitro and in vivo using toxicity assays on erythrocytes, macrophages, and mice. In vitro cytotoxicity experiments led to selective toxicity ratios (50% lethal concentration/MIC) of up to 64 for highly active antifungal compounds, and no in vivo murine toxicity was seen. Taken together, these results highlight the importance of the conjugated lipophilic structure and suggest that the modulation of other biologically relevant peptides with hydrophobic moieties, such as cholesterol and vitamin E, generate compounds with unique bioactivity.
INTRODUCTION
Acquired immunodeficiency results from many processes and diseases, and it necessitates the use of antibiotics in affected individuals. Among other examples, cancer-related chemotherapy, HIV, and immunosuppressive drugs increase the patient's susceptibility to opportunistic microbial infections. In fact, invasive mycoses have emerged as major causes of morbidity and mortality (17, 24, 35), and the vast majority of the invasive fungal infections are caused by Aspergillus and Candida species (26). Other diseases, such as cystic fibrosis, require the chronic use of antimicrobial agents, and in many cases the bacteria common in these patients are resistant to many common antibiotics and require many weeks of therapy, which leads to a higher susceptibility to fungal infections (30). Moreover, the widely used antifungal agent amphotericin B (AmB) is relatively toxic, and great care is needed for the administration or delivery of this compound (40). Therefore, new antimicrobial therapies, especially therapies specific for fungi, are needed.
Antimicrobial peptides (AMPs) are used by the innate immune system of all organisms (33, 44). Many studies on different AMPs have generated a large body of knowledge that will be foundational for future antimicrobial treatments. The modes of action have been reviewed in detail (3, 25, 27, 39, 41), and new modes of action are still emerging (14). Peptides are being found that modulate the immune response or have targets within the cell (19), many peptides bind and permeate cell membranes (1, 29), and others have multiple targets (16). Taking these findings together, AMPs display robust mechanisms that could decrease the possibility for resistance to develop (18, 28).
Native lipopeptides form a class of antimicrobial peptides. Lipopeptides in general possess a broad spectrum of activities that include antibacterial, antifungal, antiviral, and cytolytic action. These compounds have been gaining increased acceptance and attention because of the pressure for new antimicrobial agents against resistant pathogens. Different lipopeptide design strategies include peptide alkylation, acylation, and peptidomimetics, which are being studied preclinically (2, 6, 7, 31, 32). Clinically used lipopeptides include the antifungal agent caspofungin and the antimicrobial agent daptomycin. Previously, we reported that linear ultrashort cationic lipopeptides have potent antimicrobial and antifungal properties, and we described the minimum peptide length necessary for activity. We observed that even small changes in amino acid composition, for example, glycine for alanine residues within a lysine containing tetrapeptide, can alter the antimicrobial potency and have large effects on hemolytic activities. The mode of action of these peptides is the perturbation of the pathogen's membrane (21, 22).
Lipopeptides usually possess a strong affinity for membranes and thus have a broad spectrum of activity (13). A problem with many such peptides is that the toxic dose is close to the therapeutic dose, and the challenge remains to broaden this window in novel ways (20). Strategies to address this challenge include modifications to the peptide to optimize specificity, to decrease the toxicity, and to increase the potency. Selected examples of attempts to resolve these problems have been to optimize the sequence or lipophilic parts of the peptide, for example, the magainin analog MSI-78, or to use drug delivery technologies (15). Here, we aimed to design selective, nontoxic peptides by conjugating a targeted biomolecule to ultrashort peptide sequences and to find the minimal and optimal sequences for high activity. Also, we tested select compounds for synergy with amphotericin B (AmB) and β-cyclodextrin (CD). AmB was used because its mechanism differs from that of the ultrashort peptides, and CD was used as a tool to alter the hydrophobic character of the peptide through noncovalent interactions. CD is a cyclic oligosaccharide that is well known to bind hydrophobic compounds, which in turn modify the physical properties of the inclusion compound. The family of cyclodextrins currently is used in drug delivery applications to, among other things, increase the water solubility of very hydrophobic drugs and for the delivery and penetration of drugs into tissues (4, 11, 36). We observed that the vitamin E (VitE) and cholesterol peptides showed a higher selectivity than the fatty acid-conjugated sequences and exhibited a larger therapeutic window, giving higher 50% lethal concentration (LC50)/MIC ratios than the fatty acid-conjugated sequences.
MATERIALS AND METHODS
Materials.
Rink amide paramethylbenzhydrylamine (MBHA) resin and 9 fluorenylmethoxycarbonyl (Fmoc) amino acids were obtained from Calbiochem Novabiochem AG (Switzerland). (+)-α-Tocopherol succinate (vitamin E succinate), amphotericin B, and gentamicin were purchased from Sigma Chemical Co. (Israel). Other reagents used for peptide synthesis included trifluoroacetic acid (TFA; Sigma), piperidine (Merck), N,N-diisopropylethylamine (DIEA; Sigma), N-methylmorpholine (NMM; Fluka), N-hydroxybenzotriazole hydrate (HOBT; Aldrich), 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU), and dimethylformamide (DMF peptide synthesis grade; Biolab). All other reagents were of analytical grade. Buffers were prepared in double-distilled water. RPMI 1640 was purchased from Biological Industries (Beit Haemek, Israel).
Peptide synthesis and purification.
Peptides were synthesized by an Fmoc solid-phase method on Rink amide MBHA resin by using an ABI 433A automatic peptide synthesizer followed by peptide cleavage from the resin and purification by reverse-phase high-performance liquid chromatography (RP-HPLC) (>98%). Reported retention times were obtained using a run time of 25 min and a C4 column using a gradient of 50 to 95% buffer B (buffer A, H2O + 0.1% TFA; buffer B, CH3CN + 0.1% TFA). The composition of the lipopeptides was confirmed by electrospray mass spectroscopy. Peptides were tested as acetic acid salts for in vivo testing.
Antifungal activity.
The antifungal activity of the lipopeptides was measured using the guidelines of Clinical Laboratory Standards document M27-A3 or M38-A2 for Aspergillus species (9, 10). The peptides were examined in sterile 96-well plates (Nunc F96 microtiter plates) in a final volume of 200 μl containing 100 μl of a suspension containing fungi at a concentration of 2 × 103 CFU/ml in culture medium (RPMI 1640, 0.165 M morpholinepropanesulfonic acid [MOPS], pH 7.4, with l-glutamine and without NaHCO3 medium) was added to 100 μl of water containing the peptide in serial 2-fold dilutions in medium. The fungi were incubated at 35°C for 48 h for Aspergillus fumigatus (ATCC 26430) and Candida albicans (ATCC 10231) and 72 h for Cryptococcus neoformans (ATCC MYA-422), respectively, using a Binder KB115 incubator. Growth inhibition was determined by eye, and antifungal activities were expressed as the MIC (averages of triplicate assays), the concentration at which no growth was observed. Synergy was tested using the checkerboard method as described previously (43). Briefly, using an 8 by 8 grid in sterile 96-well plates, VitE-KkKK was added to the first row and diluted in medium across the plate. AmB was similarly diluted in a second plate along the other axis and added to the first plate. The Candida was prepared as described above and added to the wells, and after appropriate incubation, growth inhibition was determined by eye. Compounds were deemed to be synergistic if ΣFIC < 0.5, where ΣFIC = FICa + FICb and FICx = MICx(in combination)/MICx(alone), where FIC is the fractional inhibitory concentration, a and b are test compounds, and x is a or b.
Antibacterial activity.
The antibacterial activity of the lipopeptides was examined according to CLSI guidelines (8) in sterile 96-well plates (Nunc F96 microtiter plates) in a final volume of 100 μl containing aliquots (50 μl) of a suspension containing bacteria at a concentration of 106 CFU/ml in culture medium added to 50 μl of water containing the peptide (prepared from a stock solution of 1 mg/ml peptide in water) in serial 2-fold dilutions in LB. The inhibition of growth was determined by measuring the absorbance at 492 nm with a Microplate autoreader El309 (Biotek Instruments) after an incubation of 18 to 20 h at 37°C. Antibacterial activities were expressed as the MIC (averages from triplicate assays), the concentration at which no growth was observed after 18 to 20 h of incubation. The bacteria used were Escherichia coli ATCC D21, Enterobacter cloacae, Enterobacter aerogenes, Staphylococcus aureus ATCC 6538P, gentamicin-resistant Acinetobacter baumannii, Enterococcus faecalis ATCC 29212, and Streptococcus pyogenes ATCC 49399. A similar procedure was performed for the other bacteria (see entries 18 to 42 in Table 2), except that a final inoculum of 104 CFU/ml in a volume of 0.2 ml was used. For Arthrobacter crystallopoietes (48 h of incubation with agitation), the inoculum was 107 CFU/ml in a volume of 0.2 ml. The peptides were screened with an agar diffusion assay (Mueller-Hinton agar with 5% sheep blood [BD]) as follows. The indicator strains were grown in tryptic soy broth (TSB; Oxoid). Culture plates were overlaid with 3 ml tryptic soy soft agar inoculated with those growth suspensions. Compounds were diluted in dimethylsulfoxide (DMSO) to a concentration of 1 mg/ml, and 3-μl aliquots of these dilutions were dropped on the agar surface. The lantibiotic nisin was used as a positive control and DMSO as a negative control. The diameter of the inhibition zones was measured after 24 h of incubation at 37°C.
Table 2.
List of bacterial and fungal strains that were screened in this study
| Strain no. | Strain name | Extra informationa |
|---|---|---|
| 1 | Aspergillus fumigatus AF293 | Clinical isolate |
| 2 | Aspergillus fumigatus Af13073 | |
| 3 | Aspergillus fumigatus Af31 | Clinical isolate |
| 4 | Aspergillus niger An20 | Clinical isolate |
| 5 | Aspergillus niger An35 | Clinical isolate |
| 6 | Aspergillus niger An42 | Clinical isolate |
| 7 | Aspergillus flavus Afl3 | Clinical isolate |
| 8 | Aspergillus terreus At6 | Clinical isolate |
| 9 | Aspergillus terreus At9 | Clinical isolate |
| 10 | Candida albicans Ca2901 | |
| 11 | Candida albicans Ca58455 | Clinical isolate |
| 12 | Candida albicans Ca90028 | |
| 13 | Candida krusei Ck6258 | |
| 14 | Candida parapsilosis Cp22019 | |
| 15 | Candida tropicalis Ct20336 | |
| 16 | Mucor racemosus | Clinical isolate |
| 17 | Rhizopus arrhizus | Clinical isolate |
| 18 | Staphylococcus aureus 5185 | MSSA clinical isolate |
| 19 | Staphylococcus aureus I-11574 | MSSA clinical isolate |
| 20 | Staphylococcus aureus LT-1334 | MRSA clinical isolate |
| 21 | Staphylococcus aureus LT-1338 | MRSA clinical isolate |
| 22 | Staphylococcus epidermidis LT-1324 | MRSE clinical isolate |
| 23 | Candida albicans I-11301 | Clinical isolate |
| 24 | Candida albicans I-11134 | Clinical isolate |
| 25 | Citrobacter freundii I-11090 | Clinical isolate |
| 26 | Klebsiella pneumoniae I-10910 | K. pneumoniae subsp. ozeanae clinical isolate |
| 27 | Enterococcus faecium I-11305b | Clinical isolate |
| 28 | Enterococcus faecium I-11054 | Clinical isolate |
| 29 | Echerichia coli I-11276b | Clinical isolate |
| 30 | Echerichia coli O-19592 | Clinical isolate |
| 31 | Stenotrophomonas maltophilia O-16451 | Clinical isolate |
| 32 | Stenotrophomonas maltophilia I-10717 | Clinical isolate |
| 33 | Pseudomonas aeruginosa 4991 | Clinical isolate |
| 34 | Pseudomonas aeruginosa I-10968 | Clinical isolate |
| 35 | Staphylococcus haemolyticus coagulase-negative I-10925 | Clinical isolate |
| 36 | Staphylococcus simulans 22 | Laboratory strain |
| 37 | Micrococcus luteus ATCC 4698 | |
| 38 | Mycobacterium smegmatis ATCC 700084 | |
| 39 | Bacillus subtilis 168 | Laboratory strain |
| 40 | Bacillus megaterium KM ATCC13632 | |
| 41 | Arthrobacter crystallopoietes DSM20117 | |
| 42 | Listeria welchimeri DSM20650 |
MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; MRSE, methicillin-resistant S. epidermidis.
Visualization of damage by the lipopeptides using transmission electron microscopy (TEM).
Candida albicans ATCC 10231 (1 ml; 4 × 107 CFU/ml) was incubated with peptide in medium for 30 min. The sample was centrifuged at 620 × g for 2 min, and the supernatant was replaced with 1% glutaraldehyde in phosphate-buffered saline (PBS) for 15 min. The sample was centrifuged and the pellet washed twice with PBS. The pellet was resuspended in 100 μl PBS, and a drop was deposited onto a carbon-coated grid and negatively stained with 2% uranyl acetate. Grids were examined using a JEOL JEM 100B electron microscope (Japan Electron Optics Laboratory, Tokyo, Japan). For Aspergillus fumigatus ATCC 26430, the spores first were washed five times with PBS and then treated for 8 min with the peptides and prepared similarly to C. albicans. For S. aureus ATCC 6538P, bacteria were grown to mid-log phase, and the medium was replaced with PBS and diluted to an optical density (OD) of 0.1. After treatment with the peptide for 8 min, bacteria were prepared similarly to C. albicans as described above.
Hemolysis of hRBCs.
The hemolysis assay was done by using a final volume of 100 μl of PBS solution containing the peptides and human red blood cells (hRBCs) (final concentration, 4%). The release of hemoglobin was monitored after an incubation time of 60 min at 37°C by measuring the absorbance of the supernatant at 540 nm. Controls for 0% hemolysis (blank) and 100% hemolysis consisted of hRBCs suspended in PBS and 1% Triton X-100, respectively.
Membrane permeation studies.
For Sytox green, the bacteria or fungi were grown in appropriate medium until an OD of 1 was obtained. The sample was centrifuged at 1,000 × g, and the medium was replaced with sodium phosphate buffer (SPB). The samples were diluted to an OD of 0.1, and the dye was added to a final concentration of 1 μM. This solution was incubated, protected from light, for 20 min, and 50 μl was added to a solution containing peptides (50 μl) in an opaque black 96-well plate. Fluorescence was measured with excitation at 485 nm and emission at 530 nm. For A. fumigatus, 100 μl medium containing spores (2,000 spores/ml) was added to each well of an opaque black 96-well plate and incubated overnight at 35°C. Dye was added for a final concentration of 0.2 μM in SPB and left to incubate for 15 min. The peptide then was added, and the fluorescence was measured as described above. For Bacillus megaterium, the strain was grown in half-concentrated MH broth to an OD at 600 nm (OD600) of 0.8 and incubated for 10 to 20 min with the membrane potential-sensitive fluorescent probe bis-(1,3-dibutylbarbituric acid) thrimethine oxonol (DiBAC4; 1 μM; Invitrogen) (3). Compounds were added at a concentration corresponding to 10× the MIC. Fluorescence was measured at excitation and emission wavelengths of 480 and 520 nm, respectively. Nisin was used as a positive control, and DMSO was the negative control.
Cell culture.
In vitro assays were performed on RAW264.7 murine macrophages (ATCC TIB71). Cells were grown in Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), l-glutamine, sodium pyruvate, nonessential amino acids, and antibiotics (Biological Industries, Beit Haemek, Israel). The incubator was set to 37°C with a humidified atmosphere containing 5% CO2.
XTT cytotoxicity assays.
A total of 2 × 105 cells per well were grown overnight on a 96-well plate. The following day, the media were replaced with 100 μl culture medium containing different concentrations of the different peptides. Peptide concentrations ranged from 3.12 to 200 μM. The cells then were incubated for 16 h, and the medium was replaced with 100 μl fresh medium and 50 μl of 2,3-bis-2H-tetrazolium-5-carboxanilide inner salt (XTT) reaction solution (Biological Industries). Viability was determined as described previously (23, 34). The LC50 for each peptide was obtained from the dose-dependent cell viability curves.
Evaluation of toxicity.
Noninfected, immunosuppressed mice (n = 5 per group) received intratracheal (i.t.) PBS or VitE-KAaK at 4.5 mg/kg or VitE-KkKK at 4.5 and 9 mg/kg of body weight on days 1, 4, 7, and 11. Six-week-old female ICR mice were immunocompromised by cortisone acetate injection (37). Cortisone acetate (150 mg/kg PBS with 0.1% Tween 80) was injected subcutaneously at 4 days prior to treatment with the compound, on the day of first treatment, and 2 and 4 days after the first treatment. VitE-KAaK was administered i.t. at 4.5 mg/kg (50 μl) on days 1, 4, 7, and 11, and VitE-KkKK was given similarly at 4.5 and 9 mg/kg. Mice were monitored for 28 days for survival, weight, overall appearance, and behavior. Experiments were done according to the regulations of the animal care facility at the Weizmann Institute of Science.
RESULTS
Design and synthesis.
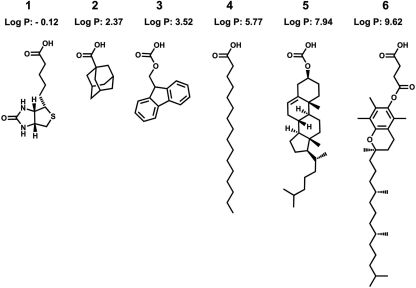
We synthesized a series of positively charged biomolecule-tetrapeptide conjugates based on lysine. Both d- and l-amino acids were used, and the sequences were based on previous studies of fatty acid-conjugated peptides (21, 22). The lipophilic part of the previously studied lipopeptides consisted of a linear fatty acid, for example, palmitic acid, coupled to the N terminus of the peptide sequence. Here, we replaced this component with different biologically relevant or lipophilic compounds, which gave rise to novel active agents with differing specificities and activities. Figure 1 shows the diversity of structures used, including a calculated log P value for the lipophilic part of the peptide conjugates. The structures tested include components native to membrane environments, for example, cholesterol and vitamin E (VitE), but other compounds also were included, such as biotin, adamantanecarboxyllic acid, and the Fmoc-protecting group. Previously reported palmitic acid peptides, derivatives of compound 4 in Fig. 1, were included for comparison.
Fig 1.
Structures and calculated log P values of peptide anchors. Compound 1, biotin; 2, adamantanecarboxylic acid; 3, 9H-fluoren-9-ylmethoxycarbonic acid; 4, palmitic acid; 5, cholesterylcarbonic acid; and 6, vitamin E succinate.
The coupling of the carboxylic acid part of compounds 1, 2, 4, and 6 in Fig. 1 was performed similarly to that for amino acids. A final standard piperidine treatment was omitted to obtain Fmoc-protected peptides, and for the cholesterol conjugates the resin-bound peptide was treated with an excess of cholesteryl chloroformate. Peptides were amidated at the C terminus following acidic cleavage from the resin and purified without complication using HPLC. Table 1 lists the compounds tested together with the HPLC retention times as an indication of the relative hydrophobicity of the active conjugates. After preliminary biological screening, a small series of vitamin E-peptide conjugates also was prepared that contained two to four amino acids.
Table 1.
Hydrophobicity of compounds indicated by CLogPa
| Designation | HPLC retention time (min) | CLogP |
|---|---|---|
| VitE-Kk | 19.91 | 10.09 |
| VitE-KGk | 19.38 | 9.52 |
| VitE-KKK | 17.57 | 9.57 |
| VitE-KkKK | 15.77 | 9.05 |
| VitE-KGGk | 19.39 | 8.95 |
| VitE-KAaK | 20.30 | 9.58 |
| VitE-KLlK | 24.01 | 12.49 |
| Cholesterol-KGGk | 15.55 | 3.48 |
| Cholesterol-KAaK | 16.54 | 4.09 |
| Fmoc-KGGk | NT | 0.63 |
| Fmoc-KAaK | NT | 1.25 |
| Fmoc-KLlK | NT | 4.16 |
| Adamantyl-KGGk | NT | −0.85 |
| Adamantyl-KAaK | NT | −0.24 |
| Adamantyl-KLlK | NT | 2.67 |
| Biotin-KGGk | NT | −3.39 |
| Biotin-KAaK | NT | −2.78 |
| Biotin-KLlK | NT | 0.14 |
CLogP, calculated log P value. VitE and cholesterol compounds also are compared using HPLC (C4 column). NT, not tested on this HPLC column.
VitE-peptide conjugates have unique biological activities.
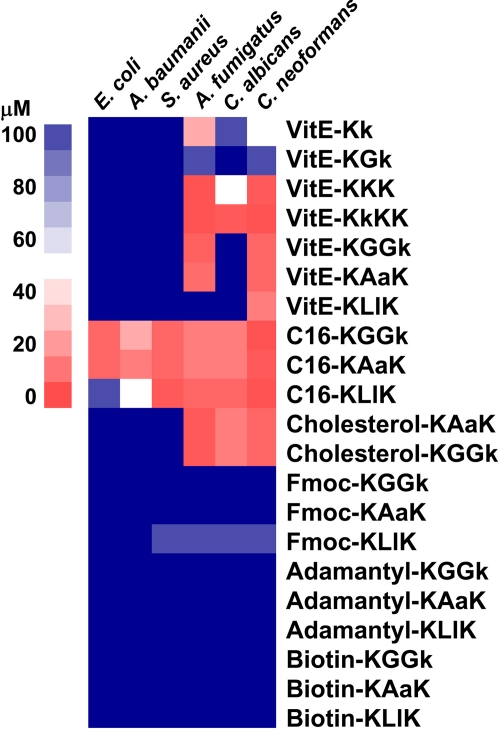
The MICs against Gram-positive and Gram-negative bacteria, as well as fungi, were obtained for the peptide conjugates (Fig. 2). As a control, we used the previously reported palmitoylated peptide sequences (21). The MIC results from multiple assays were averaged and were visualized using the TreeView program, version 1.60 (12), where a numerical MIC value was converted into a color scale. The degree of specificity and activity can be easily compared in this way. For example, the VitE-peptide conjugates were active on certain fungi but inactive against all bacteria tested, whereas the palmitoylated peptide sequences KGGk and KAaK exhibited good activity against E. coli and S. aureus, as well as against the fungi. Furthermore, the specificities of the VitE sequences can be easily observed. For example, KLlK was only active on C. neoformans, KGGk and KAaK were active only on C. neoformans and A. fumigatus (but not C. albicans), and KkKK was active on all three types of fungi tested. Our data clearly exhibited the selectivity of certain conjugates toward fungi, and to further validate this we screened the VitE and cholesterol-containing compounds against 23 strains of clinically relevant bacteria and an additional two strains of C. albicans (Table 2, entries 18 to 42). This confirmed the exclusive antifungal selectivity of the VitE compounds, which gave no detectable activity, but for the cholesterol peptides exceptions were observed in 3/25 strains. For these exceptional strains, B. megaterium, M. luteus, and Arthrobacter crystallopoietes, for example, activity could be detected with cholesterol-KGGk with MICs of 2, 8, and 8 μM, respectively. Taken together, and with few exceptions, a general inactivity on bacteria but very high activity on the fungus, especially A. fumigatus, was seen.
Fig 2.
In vitro inhibition assays with bacteria and fungus. MICs are represented on a color scale.
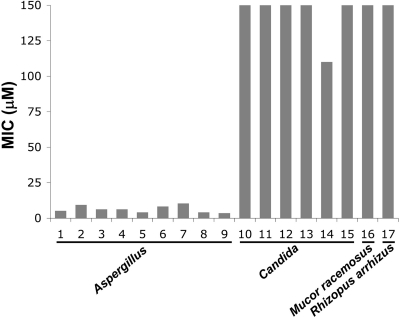
To study the activity differences of VitE-KAaK between different fungi, a panel of 21 strains of Aspergillus and Candida species, among others, was used (Table 2, entries 1 to 17). This confirmed that this peptide conjugate was specific and highly active against all Aspergillus species and was completely inactive against all Candida species (Fig. 3).
Fig 3.
Inhibition assays with a panel of fungi and vitamin E-KAaK. High activity was observed for Aspergillus species (lower MICs). Low activity was observed for Candida species, Mucor racemosus, and Rhizopus arrhizus.
Additionally, hemolytic activity also was obtained for selected active compounds by the incubation of the peptides in a highly diluted solution of human erythrocytes, and cytotoxicity was evaluated using murine macrophages and cell viability assessed using the XTT reagent (Table 3). Using the toxicity against macrophages, good biocompatibility was seen for the highly active compound VitE-KkKK, with LC50/MIC ratios of 64 and 32 for Aspergillus (MIC, 1.56 μM) and Candida (MIC, 3.12 μM), respectively. Other compounds gave lower ratios, and the less selective and less active C16-KGGk gave an LC50/MIC ratio of 8 and 4 for Aspergillus (MIC, 12.5 μM) and Candida (MIC, 25 μM), respectively.
Table 3.
In vitro and in vivo toxicity studies with active compounds
| Compound | Toxicity parameter |
|||
|---|---|---|---|---|
|
In vitro |
In vivoa |
|||
| 50% Hemolysis (μM) | Macrophage LC50 (μM) | Dosage (mg/kg i.t.) | Effect | |
| VitE-KGGk | 60 | >100 | NT | NT |
| VitE-KAaK | 120 | >100 | 4.5 | Not toxic |
| VitE-KKK | 60 | 70 | NT | NT |
| VitE-KkKK | 60 | 100 | 4.5 | Not toxic |
| 9 | Not toxic | |||
| Chol-KAaK | 60 | 70 | NT | NT |
| C16-KGGk | 30 | >100 | NT | NT |
i.t., intratracheal; NT, not tested. Doses were given four times each to groups of five mice.
Vitamin E conjugates nontoxic in vivo.
In addition to the hemolytic assays, in vivo toxicity assays were performed on selected VitE conjugates in mice (Table 3). Immunosuppressed mice (n = 5) were given 4.5 or up to 9 mg/kg of VitE-KAaK or VitE-KkKK i.t., respectively, four times during 11 days, and they were monitored for 28 days. Mice behaved, appeared, and increased in weight similarly to the control group (PBS), indicating nontoxicity.
CD-bioconjugate interaction modifies mechanism of specificity and antifungal activity.
CD is able to form inclusion complexes with fatty acids and cholesterol, thus we hypothesized that it would alter the hydrophobicity, organization, and delivery of our bioconjugates. It was used here as a tool to perturb the properties of our compounds, and it gave rise to interesting changes in their activity and selectivity. Interestingly, at an optimized CD concentration of 2.5 mM, compounds that were highly selective for fungi (VitE-KkKK and VitE-KKK) became active against S. aureus. The doubly charged conjugates VitE-KAaK and VitE-KGGk, being selective for Aspergillus and Cryptococcus, became active against Candida in complex with CD (Table 4).
Table 4.
MICs of peptide conjugates alone and with 2.5 mM CD
| Compound | MIC (μM) against: |
|||||||
|---|---|---|---|---|---|---|---|---|
|
S. aureus |
E. coli |
A. fumigatus |
C. albicans |
|||||
| Alone | With CD | Alone | With CD | Alone | With CD | Alone | With CD | |
| VitE-KkKK | >100 | 6.25 | >100 | >100 | 1.56 | 12.5 | 3.12 | 12.5 |
| VitE-KKK | >100 | 50 | >100 | >100 | 1.56 | 6.25 | 12.5 | 12.5 |
| VitE-KAaK | >100 | >100 | >100 | >100 | 6.25 | 6.25 | >100 | 25 |
| VitE-KGGk | >100 | >100 | >100 | >100 | 3.12 | 6.25 | >100 | 12.5 |
| C16-KGGk | 12.5 | 100 | 12.5 | 100 | 12.5 | >100 | 25 | >100 |
CD alters bioconjugate interaction with bacterial and fungal membranes.
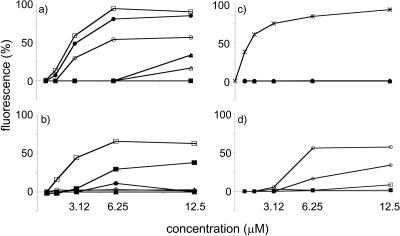
The mode of action was probed using Sytox green, which is a dye that cannot enter an intact cell unless there is severe membrane permeation or damage. Binding to nucleic acids drastically increases the fluorescence. The fluorescence change due to the action of the peptide conjugates was normalized to the maximum fluorescence change of the positive-control melittin as 100%. For S. aureus, VitE-KkKK was active only in complex with CD (MIC, 6.25 μM), and in this case, Sytox green fluorescence was significantly enhanced to intensities similar to those of the active peptides melittin and C16-KGGk (Fig. 4a). Here, maximum fluorescence corresponded to the MIC of the peptides. No fluorescence increase was observed with C16-KGGk in the presence of CD until the high concentration of 100 μM, and this agrees with the loss of biological activity observed in the MIC assay. For E. coli, no Sytox green penetration was seen with VitE-KkKK with or without CD, which agreed with the absence of biological activity (Fig. 4b). For C16-KGGk, a maximum fluorescence was reached at the MIC value, whereas a gradual increase in fluorescence in the presence of CD was seen. Interestingly, no penetration of Sytox green, suggesting no cell membrane permeation, was observed for active conjugates VitE-KkKK (with or without CD) and VitE-KGGk (with CD) with C. albicans (Fig. 4c). For A. fumigatus, the Sytox green fluorescence corresponded to the activity of the compounds. VitE-KkKK, VitE-KAaK, C16-KGGk, and C16-KGGk with CD, with MICs of 1.56, 6.25, 12.5, and >100 μM, respectively, gave decreasing fluorescence responses (Fig. 4d). Cholesterol-KGGk, a compound that showed activity on B. megaterium, was tested in a similar depolarization assay with DiBAC4 (3), and at a concentration of 10× the MIC it gave a fluorescence increase of 50% compared to that of nisin, which was used as a positive control (data not shown).
Fig 4.
Sytox green uptake assay. (a) S. aureus. (b) E. coli. (c) C. albicans. (d) A. fumigatus. ○, VitE-KkKK; ▵, VitE-KGGk; ♦, VitE-KAaK; □, C16-KGGk; ●, VitE-KkKK with CD; ▴, VitE-KGGk with CD; ■, C16-KGGk with CD; ×, melittin. Values are expressed as percent fluorescence relative to that of melittin.
Visualization of cell damage by TEM.
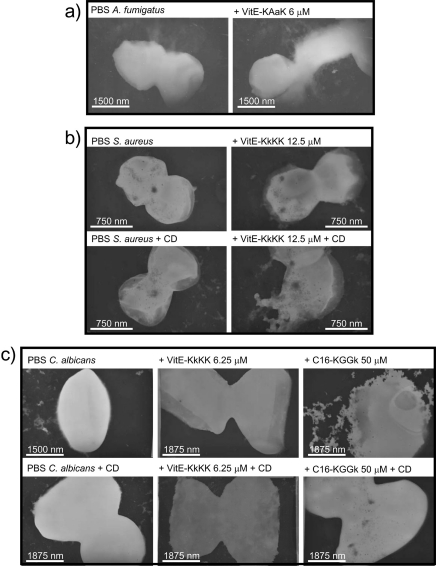
The spores of A. fumigatus were treated with VitE-peptide conjugates at their MICs for 8 min and were visualized by TEM. Pictures were obtained for the selectively active VitE-peptide sequence KAaK at a concentration of 6 μM. The conidia in the control sample had relatively smooth edges, whereas the treated sample showed rough and uneven edges that are indicative of structural damage (Fig. 5a). S. aureus was treated with VitE-KkKK at a concentration of 12.5 μM with and without CD (2.5 mM). This was below the MIC for the peptide conjugate alone but above the MIC for the peptide in combination with CD. Accordingly, intact bacteria were observed in the sample treated with peptide alone, and damage was observed in the sample treated with the peptide in combination with CD (Fig. 5b). Lastly, peptide conjugates were incubated at a concentration above the MIC for 30 min with C. albicans, with and without the addition of 2.5 mM CD, and visualized (Fig. 5c). The addition of CD did not cause any visual change in microbe general shape or structure and was almost identical to the microbe sample in PBS. C. albicans treated with VitE-KkKK showed no damage with or without the addition of CD, indicating an internal target for this compound. The treatment of C. albicans with C16-KGGk at a concentration of 50 μM, which is above the MIC without CD but below the MIC with CD, accordingly showed damage without CD and no damage with CD.
Fig 5.
Transmission electron microscopy revealed different interactions of peptide conjugates with different microorganisms. (a) A. fumigatus treated with VitE-KAaK (6 μM) compared to A. fumigatus in PBS. (b) S. aureus treated with VitE-KkKK (12.5 μM), with and without CD (2.5 mM), compared to S. aureus in PBS. (c) C. albicans incubated with different concentrations of peptide and CD at 2.5 mM.
A vitamin E-peptide conjugate synergizes with AmB.
AmB is a polyene antifungal agent that associates with ergosterol in the fungal membrane, which leads to pores. It is clinically used despite a relatively high toxicity. Finding agents that improve or synergize with AmB could lead to less toxic and more effective antifungal therapy (38). Since no massive membrane damage was observed by TEM and no Sytox green fluorescence increase occurred with C. albicans and VitE-KkKK treatment, we predicted that the VitE conjugate has an alternate or internal target, and it may synergize with a pore-forming antifungal such as AmB. We tested for synergy between the two compounds in a checkerboard MIC assay against C. albicans. The combination of AmB and VitE-KkKK gave an FIC of 0.37, which indicated synergism.
DISCUSSION
Previous studies reported on a family of potent antimicrobial and antifungal ultrashort lipopeptides containing four or fewer amino acids (21, 22). The conjugation of fatty acids to otherwise inactive peptides greatly increased the activity and spectrum. Here, we aimed to incorporate new features into a simple, basic design that would increase specificity toward fungi. We chose to vary the lipophilic fatty acid component of the lipopeptide with biologically relevant compounds while keeping the peptide segment as short as possible. Among the molecules tested, cholesterol and vitamin E succinate conjugated to short peptide sequences were found to be highly biologically active. Cyclodextrin and AmB were tested for possible synergistic effects. Interestingly, VitE-KkKK became active against S. aureus in combination with CD, and it synergized with AmB against C. albicans. Overall, we observed that different selectivity and activity can be obtained with different biomolecules, that fungi can be targeted, that there is a minimum hydrophobicity necessary for short peptides to be active, and that activity can be modulated using noncovalent interactions.
The activity of the bioconjugates was comparable to that of the ultrashort peptides previously reported and, in general, to that of many native AMPs. Biotin, adamantyl, and Fmoc conjugates were inactive; however, cholesterol and VitE conjugates were very active. For the active bioconjugates, low micromolar values were obtained, and they were especially active against the fungus tested. The calculated log P of the hydrophobic moieties correlated to the general selectivity of the peptide. Palmitic acid-conjugated peptides were broadly active against both bacteria and fungi, whereas the more hydrophobic conjugates were active only on fungi, and less hydrophobic conjugates lost all biological activity. The less hydrophobic compounds Fmoc, adamantane, and biotin probably need to be balanced with longer peptide sequences that include hydrophobic amino acids. Others also have reported on the importance of hydrophobicity and have defined an optimal hydrophobicity window for α-helical AMPs (5). However, in our case, selectivity is not controlled only by hydrophobicity, since the number of charges and the small variations to the peptide part of the VitE conjugates gave differences in membrane targeting and activity.
Remarkably, testing VitE-KkKK in combination with CD induced activity with Gram-positive S. aureus but not with E. coli. In contrast, this phenomenon was not seen in other active compounds, for example, the cholesterol-KAaK conjugate remained inactive under the same conditions. The antifungal specificity for the VitE conjugates depended on the number of charges in the peptide and also on the separation of charge. For example, doubly charged Kk and KGk conjugates were not very active, whereas the KGGk and KAaK conjugates were very active, but not toward C. albicans. The activity increased with the triply charged KKK, and it became very active with KkKK toward C. albicans. This extra charge may affect the ability to traverse the membrane for different internal targets or alter the mode of action of these very similar compounds. Interestingly, activity was induced upon the addition of CD with VitE-KAaK and especially VitE-KGGk. Exact reasons for this still are unclear. Possible explanations include cyclodextrin aiding doubly charged compound transport across outer membranes or CD interacting with components in the membrane or the membrane itself for a synergistic effect. CD also may affect critical micelle concentrations and aggregation states.
To shed some light on the mechanism of action of the VitE conjugates, the fluorescent probe Sytox green was used. A comparison was made to the previously studied membrane-active palmitic acid-conjugated peptide C16-KGGk. For C. albicans, no leakage of Sytox green was observed for the VitE conjugates into the cell. The good activity combined with the absence of membrane damage indicate that internal structures, such as mitochondria, were targets for the peptide. Electron microscopy gave further evidence for internal targeting, since at concentrations above the MIC no apparent damage was observed for active VitE-KkKK. Therefore, we hypothesized that an antibiotic with multiple internal targets would benefit from easier access to the cell or would synergize with pore-forming or membrane-disrupting agents. We tested VitE-KkKK in combination with AmB, since AmB targets the membrane by binding to ergosterol and altering the membrane integrity by forming pores. Indeed, a strong synergism was observed (ΣFIC = 0.37) due to possible individual actions on different targets, or because amphotericin causes increased access of VitE-KkKK to the cytosol. Cyclodextrin also may act in this way by aiding or preventing the transport of these compounds across the membrane.
CD either increases or decreases the activity of the compounds through a noncovalent interaction, which modulates the hydrophobicity. These activity changes can be explained in the following ways. CD may cause a perturbation in the lipophilicity of the peptide conjugate for greater partitioning into the membrane, which may cause activity increases. However, an inclusion complex that is too strong may abolish lipophilicity. Palmitic acid and cholesterol are known to bind CD, and these conjugates were most susceptible to being rendered inactive by CD. For the vitamin E compounds that may interact with CD differently, differing effects were observed with C. albicans: a decreased activity for VitE-KkKK but an increased activity for VitE-KXXK.
In general, the biocompatibility of the most active compounds was good. Hemolysis and cytotoxicity studies showed an LC50/MIC ratio of up to 40 and 64, respectively. Preliminary murine in vivo studies were performed to assess toxicity in animals. The intratracheal administration of a compound (up to 9 mg/kg) to immunocompromised mice was used according to a murine lung infection model previously reported for the treatment of A. fumigatus (42). Here, no toxicity was observed, and mouse weight and behavior were identical to those of the control group. This toxicity profile warrants further experimentation in animal infection models.
Cholesterol- and VitE-conjugated peptides containing short, positively charged peptide sequences were highly active and gave a unique selectivity toward fungi. This selectivity could be modulated with CD for increased activity on S. aureus and C. albicans with selected compounds, and CD could drastically reduce hemolytic activity in some cases. Synergism was observed with AmB. Taken together, these results demonstrate the versatility of adding biologically relevant compounds to peptides to modulate activity and selectivity toward eukaryotic cells. CD imparts unique properties to the peptide conjugates and modulates the selectivity and activity. In the past decades, CD drug delivery technology has been extensively developed, and this study supports the notion that this technology is broadly applicable to the field of antimicrobial peptides and lipopeptides. Choosing certain lipid-targeting molecules thus is crucial for the design of biologically active lipopeptides, where high membrane specificity is desired. Fungus specificity was observed using more structurally complex lipophilic compounds, and this strategy could be extended to modify the activity of other known antimicrobial peptides and also to other peptide sequences that exert their function in close proximity to the eukaryotic cell membrane.
ACKNOWLEDGMENTS
We thank Yehuda Marikovsky for help with TEM studies and Alina Iulia Chiriac for her contribution to the antimicrobial study.
This study was supported by a grant from the German Israel Foundation (GIF) to Y.S. and H.G.S.
Footnotes
Published ahead of print 17 October 2011
REFERENCES
- 1. Arnusch CJ, et al. 2007. Enhanced membrane pore formation by multimeric/oligomeric antimicrobial peptides. Biochemistry 46:13437–13442 [DOI] [PubMed] [Google Scholar]
- 2. Avrahami D, Shai Y. 2003. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to d,l-amino acid-containing antimicrobial peptides: a plausible mode of action. Biochemistry 42:14946–14956 [DOI] [PubMed] [Google Scholar]
- 3. Bechinger B. 1999. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim. Biophys. Acta 1462:157–183 [DOI] [PubMed] [Google Scholar]
- 4. Challa R, Ahuja A, Ali J, Khar RK. 2005. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 6:E329–E357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Y, et al. 2007. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob. Agents Chemother. 51:1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chongsiriwatana NP, et al. 2011. Short alkylated peptoid mimics of antimicrobial lipopeptides. Antimicrob. Agents Chemother. 55:417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chu-Kung AF, Nguyen R, Bozzelli KN, Tirrell M. 2010. Chain length dependence of antimicrobial peptide-fatty acid conjugate activity. J. Colloid. Interface Sci. 345:160–167 [DOI] [PubMed] [Google Scholar]
- 8. Clinical Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement (M100–S15), vol. 30 Clinical Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Clinical Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38–A2, 2nd ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Clinical Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27–A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 11. Davis ME, Brewster ME. 2004. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3:1023–1035 [DOI] [PubMed] [Google Scholar]
- 12. Eisen MB, Spellman PT, Brown PO, Botstein D. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 95:14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Epand RM. 1997. Biophysical studies of lipopeptide-membrane interactions. Biopolymers 43:15–24 [DOI] [PubMed] [Google Scholar]
- 14. Epand RM, et al. 2010. Lipid clustering by three homologous arginine-rich antimicrobial peptides is insensitive to amino acid arrangement and induced secondary structure. Biochim. Biophys. Acta 1798:1272–1280 [DOI] [PubMed] [Google Scholar]
- 15. Faber C, et al. 2003. Release of antimicrobial peptide Dhvar-5 from polymethylmethacrylate beads. J. Antimicrob. Chemother. 51:1359–1364 [DOI] [PubMed] [Google Scholar]
- 16. Friedrich CL, Moyles D, Beveridge TJ, Hancock RE. 2000. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groll AH, McNeil Grist L. 2009. Current challenges in the diagnosis and management of invasive fungal infections: report from the 15th International Symposium on Infections in the Immunocompromised Host: Thessaloniki, Greece, 22–25 June 2008. Int. J. Antimicrob. Agents 33:101–104 [DOI] [PubMed] [Google Scholar]
- 18. Hancock RE, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 19. Hilpert K, et al. 2010. Short cationic antimicrobial peptides interact with ATP. Antimicrob. Agents Chemother. 54:4480–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lohner K, Blondelle SE. 2005. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 8:241–256 [DOI] [PubMed] [Google Scholar]
- 21. Makovitzki A, Avrahami D, Shai Y. 2006. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. U. S. A. 103:15997–16002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makovitzki A, Baram J, Shai Y. 2008. Antimicrobial lipopolypeptides composed of palmitoyl Di- and tricationic peptides: in vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry 47:10630–10636 [DOI] [PubMed] [Google Scholar]
- 23. Makovitzki A, Fink A, Shai Y. 2009. Suppression of human solid tumor growth in mice by intratumor and systemic inoculation of histidine-rich and pH-dependent host defense-like lytic peptides. Cancer Res. 69:3458–3463 [DOI] [PubMed] [Google Scholar]
- 24. Minamoto GY, Rosenberg AS. 1997. Fungal infections in patients with acquired immunodeficiency syndrome. Med. Clin. North Am. 81:381–409 [DOI] [PubMed] [Google Scholar]
- 25. Oren Z, Shai Y. 1998. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers 47:451–463 [DOI] [PubMed] [Google Scholar]
- 26. Pappas PG, et al. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 50:1101–1111 [DOI] [PubMed] [Google Scholar]
- 27. Park Y, Hahm KS. 2005. Antimicrobial peptides (AMPs): peptide structure and mode of action. J. Biochem. Mol. Biol. 38:507–516 [DOI] [PubMed] [Google Scholar]
- 28. Peschel A, Sahl HG. 2006. The co-evolution of antimicrobial host defense peptides and microbial peptide resistance. Nat. Rev. Microbiol. 4:529–536 [DOI] [PubMed] [Google Scholar]
- 29. Pieters RJ, Arnusch CJ, Breukink E. 2009. Membrane permeabilization by multivalent anti-microbial peptides. Protein Pept. Lett. 16:736–742 [DOI] [PubMed] [Google Scholar]
- 30. Pihet M, et al. 2009. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis-a review. Med. Mycol. 47:387–397 [DOI] [PubMed] [Google Scholar]
- 31. Porter EA, Wang X, Lee HS, Weisblum B, Gellman SH. 2000. Non-haemolytic beta-amino-acid oligomers. Nature 404:565. [DOI] [PubMed] [Google Scholar]
- 32. Radzishevsky IS, et al. 2007. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat. Biotechnol. 25:657–659 [DOI] [PubMed] [Google Scholar]
- 33. Reddy KV, Yedery RD, Aranha C. 2004. Antimicrobial peptides: premises and promises. Int. J. Antimicrob. Agents 24:536–547 [DOI] [PubMed] [Google Scholar]
- 34. Rosenfeld Y, Papo N, Shai Y. 2006. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides: peptide properties and plausible modes of action. J. Biol. Chem. 281:1636–1643 [DOI] [PubMed] [Google Scholar]
- 35. Segal BH, et al. 2007. Prevention and early treatment of invasive fungal infection in patients with cancer and neutropenia and in stem cell transplant recipients in the era of newer broad-spectrum antifungal agents and diagnostic adjuncts. Clin. Infect. Dis. 44:402–409 [DOI] [PubMed] [Google Scholar]
- 36. Soares AF, Carvalho Rde A, Veiga F. 2007. Oral administration of peptides and proteins: nanoparticles and cyclodextrins as biocompatible delivery systems. Nanomedicine (London) 2:183–202 [DOI] [PubMed] [Google Scholar]
- 37. Spikes S, et al. 2008. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J. Infect. Dis. 197:479–486 [DOI] [PubMed] [Google Scholar]
- 38. Stergiopoulou T, et al. 2009. Comparative pharmacodynamic interaction analysis between ciprofloxacin, moxifloxacin and levofloxacin and antifungal agents against Candida albicans and Aspergillus fumigatus. J. Antimicrob. Chemother. 63:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toke O. 2005. Antimicrobial peptides: new candidates in the fight against bacterial infections. Biopolymers 80:717–735 [DOI] [PubMed] [Google Scholar]
- 40. Torrado JJ, Espada R, Ballesteros MP, Torrado-Santiago S. 2008. Amphotericin B formulations and drug targeting. J. Pharm. Sci. 97:2405–2425 [DOI] [PubMed] [Google Scholar]
- 41. Tossi A, Sandri L, Giangaspero A. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4–30 [DOI] [PubMed] [Google Scholar]
- 42. Vallon-Eberhard A, et al. 2008. Efficient clearance of Aspergillus fumigatus in murine lungs by an ultrashort antimicrobial lipopeptide, palmitoyl-lys-ala-DAla-lys. Antimicrob. Agents Chemother. 52:3118–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. White RL, Burgess DS, Manduru M, Bosso JA. 1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 40:1914–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395 [DOI] [PubMed] [Google Scholar]