Abstract
Emergence of resistance to pentavalent antimonials has become a severe obstacle in the treatment of visceral leishmaniasis (VL) on the Indian subcontinent. The mechanisms operating in laboratory-generated strains are somewhat known, but the determinants of clinical antimony resistance are not well understood. By utilizing a DNA microarray expression profiling approach, we identified a gene encoding mitogen-activated protein kinase 1 (MAPK1) for the kinetoplast protozoan Leishmania donovani (LdMAPK1) that was consistently downregulated in antimony-resistant field isolates. The expression level of the gene was validated by real-time PCR. Furthermore, decreased expression of LdMAPK1 was also confirmed at the protein level in resistant isolates. Primary structure analysis of LdMAPK1 revealed the presence of all of the characteristic features of MAPK1. When expressed in Escherichia coli, the recombinant enzyme showed kinase activity with myelin basic protein as the substrate and was inhibited by staurosporine. Interestingly, overexpression of this gene in a drug-sensitive laboratory strain and a resistant field isolate resulted in increased the sensitivity of the transfectants to potassium antimony tartrate, suggesting that it has a role in antimony resistance. Our results demonstrate that downregulation of LdMAPK1 may be in part correlated with antimony drug resistance in Indian VL isolates.
INTRODUCTION
Leishmaniasis imposes a substantial burden of mortality and morbidity, affecting 12 million people in more than 88 countries in tropical and subtropical zones of the world (1). The traditionally exotic disease leishmaniasis is becoming of greater interest for two main reasons, international travel and the importance of Leishmania as an opportunist pathogen in AIDS patients (http://www.who.int/leishmaniasis/burden/en). It is an emerging tropical disease in the United States, with more than 500 parasitologically confirmed cases among military personnel deployed to areas where visceral leishmaniasis (VL) is endemic (7). This is a disease complex caused by an obligatory intracellular protozoan parasite of the genus Leishmania and is manifested by self-healing skin ulcers to fatal visceral infection if left untreated. Since there are no vaccines against leishmaniasis available at present (24), chemotherapy is the main weapon against this disease and pentavalent antimonials [Sb(V)] are the first-line drugs for all clinical forms. Unfortunately, during the last decade, treatment has been eroded by the development of resistance to this drug, which has become a major obstacle to treatment, especially in India, where more than 60% of VL patients are unresponsive to Sb(V) treatment (50). Various proportions of antimony-resistant Leishmania parasites have also been observed in other regions where VL is endemic, such as Iran, Peru, and Colombia (18, 43, 62). Amphotericin B is in use to treat resistant Leishmania. However, its wider use in regions where VL is endemic is limited by its high cost and toxicity. Miltefosine (hexadecylphosphocholine), originally developed as an antineoplastic agent, has now been approved as the first oral drug for leishmaniasis. It can be used for both antimony-responding and nonresponding patients (51). Besides having good efficacy, it is very expensive and has been reported to double the relapse rate (52). This is a warning that resistance to this drug could develop quickly in the future. Therefore, resistance to a first-line drug(s) has a very big impact on the treatment of leishmaniasis. Prevention and circumvention of resistance to antimonials have become WHO priorities (www.who.int/infectious-diseasereport/2000).
Most of the knowledge of antimony resistance mechanisms in Leishmania spp. has emerged primarily from the study of laboratory-generated drug-resistant cell lines, created through stepwise exposure to antimony (41). Different mechanisms suggested for drug resistance in Leishmania include gene amplification, reduced accumulation of active drug in parasites due to either increased efflux or decreased influx, and unique parasite thiol metabolism (10). In more recent times, several features of drug resistance have been corroborated in antimonial-resistant field isolates and have suggested that natural antimony resistance is multifactorial and may be different from laboratory resistance (3). Microarray technology and proteomic screening have been employed to elucidate a global picture of the mechanisms leading to resistance in the field (30, 46, 56). Various molecules such as a multidrug resistance-associated protein, HSP83, a nucleoside transporter, a long-chain fatty acid–coenzyme A ligase, and a small kinetoplastid calpain-related protein have been identified using these methods. Recently, overexpression of histone 2A has been shown to modulate drug susceptibility in Leishmania parasites (47). In the present study, for the first time, we have established that downregulation of mitogen-activated protein kinase 1 (MAPK1) is associated with resistance to sodium stibogluconate in Leishmania donovani field isolates.
MATERIALS AND METHODS
Parasites. (i) Clinical isolates.
The clinical strains of L. donovani used in this study were isolated from patients at the Kala-Azar Medical Research Center, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, and at its affiliated hospital at Muzaffarpur, Bihar, India. The ethics committee of the Kala-Azar Medical Research Center (Muzaffarpur, India) reviewed and approved the study protocol. The criterion for the diagnosis of VL was the presence of L. donovani bodies in splenic aspirates, which were graded according to standard criteria (9).
(ii) Reference strain.
L. donovani Dd8 promastigotes (World Health Organization designation MHOM/IN/80/Dd8) which were originally obtained from the late P. C. C. Garnham (Imperial College London, London, United Kingdom) were used as the sensitive reference strain. It was maintained at the Central Drug Research Institute in golden hamsters.
Culture conditions.
The splenic aspirates of patients were inoculated into Novy MacNeal Nicolle (NNN) medium, grown at 25°C, and subcultured every 6 days. The positive cultures were then adapted to medium 199 (Sigma, St. Louis, MO) supplemented with 10% fetal calf serum (Gibco/Invitrogen, Carlsbad, CA), 1% penicillin (50 U/ml), and streptomycin (50 g/ml) solution (Sigma) (13). Cryopreserved parasites were used for experimental work within six passages after their isolation from patients.
In vitro drug sensitivity of field isolates.
Parasite isolates (promastigotes) were analyzed for antimony [Sb(III)] susceptibility as described previously (2, 36). The effect of Sb(V) on the amastigote stage within macrophage cells (J774) was evaluated according to the method described previously (16).
Identification of MAPK1 using L. donovani genomic DNA microarray.
Transcriptome analysis was carried out as described earlier (19). An array containing 4,224 genomic fragments was hybridized with fluorescently labeled cDNA synthesized from total RNA isolated from isolates S1 (drug sensitive) and R3 (drug resistant). The hybridized microarray was scanned in a laser scanner (Axon 4000A), visualized, and analyzed using GenePixPro3.0 software. Replicate experiments with three biological preparations were performed comparing antimony-resistant and -sensitive parasites. The local background was subtracted from the intensity value of each spot on the array. Total intensity normalization was performed. Clones with intensity values lower than those of negative controls were not included. The median signal intensity obtained for each spot was calculated, and the normalized median of the ratios (resistant/sensitive) was used to identify genes up- or downregulated in the resistant isolate.
Cloning of MAPK1 ORF and sequence analysis.
BLAST analysis identified three clones, G13H6, G36H1, and G51G7, as homologues of MAPK1 of L. mexicana (60). The complete open reading frame (ORF) of LdMAPK1 was PCR amplified from both sensitive and resistant strains using forward primer 5′-ATGACCTCATACGGCATCGAC-3′ and reverse primer 5′-CTAGTACACGTCTGTTATGTGATAGCGGTA-3′ (designed on the basis of L. major genomic database [www.genedb.org]) with 80 ng genomic DNA of R3 or S1 as the template. The PCR fragments were cloned into pCRII-TOPO (Invitrogen), and the resulting constructs were designated pCRT-Ldpk-S1/3, pCRT-Ldpk-S1/7, and pCRT-Ldpk-R3/5 and sequenced. The construct pCRT-Ldpk-S1/3 was used to amplify LdMAPK-ORF for subcloning and expression.
RNA isolation and real-time PCR (RT-PCR) analysis.
Total RNA was isolated using TRIzol reagent (Invitrogen) from mid-log-phase promastigotes of reference strain Dd8 and the isolates. The total RNA (100 μg) was treated with RNase-free DNase I to avoid any genomic contamination and was further purified using the RNeasy purification kit (Qiagen Inc., Valencia, CA) in accordance with the manufacturer's instructions. cDNA was synthesized from 5 μg of total RNA using SuperScript II RNase H reverse transcriptase and oligo(dT)12-18 primers (Invitrogen) following the manufacturer's instructions. RT-PCR was performed in triplicate with 30-μl volumes using IQ SYBR green Super mix (Bio-Rad) in an RT-PCR machine (Stratagene). The sequences of the primers were as follows: LdMAPK1, 5′-ATGACCTCCTATGGCATCGA-3′ (forward) and 5′-TGATGTTCTGTGCGTGGAGC-3′ (reverse); α-tubulin, 5′-GTCGTGCTGCCTCATGTACC-3′ (forward) and 5′-GTACTCCTCGACGTCCTCCT-3′ (reverse). The relative amount of PCR product generated from each primer set was determined based on the threshold cycle (CT) value of the MAPK1 gene normalized to that of the reference α-tubulin gene using the Livak method (32). The expression ratio was calculated using the formulas ΔCT(field isolate) = CT(MAPK1) − CT(Tub) and ΔCT(Ldd) = CT(MAPK1) − CT(Tub), and the ΔCT of the test sample was normalized to the ΔCT of the calibrator with the formulas ΔΔCT = ΔCT(field isolate) − ΔCT(Ldd) and 2−ΔΔCT = normalized expression ratio. Control experiments were performed by amplification using total RNA but no reverse transcriptase to rule out DNA contamination.
Isolation of genomic DNA and gene copy analysis.
Total genomic DNA was isolated from L. donovani strain Dd8 promastigotes as described earlier (35). Gene copy numbers of L. donovani were determined by Southern analysis. To see any haploid deletion or aneuploidy among the isolates, RT-PCR was conducted with the same amount of genomic DNA (80 ng) of isolates as the template using the same primer set as described above. The relative amount of PCR product generated from each isolate was determined based on the CT value of the MAPK1 gene normalized to that of the α-tubulin reference gene.
Expression and purification of LdMAPK1.
The LdMAPK1 ORF was cloned into the pGEX KG expression vector (American Type Culture Collection) by introducing NcoI restriction sites (underlined) into PCR primers 5′-GGTTCCATGGCCTCCTATGGCATCGAC-3′ (forward) and 5′-GTACCATGGACTAAACGTCTGTAATGT-3′ (reverse). The recombinant constructs pGEXKG PK4 (with the ORF in the right orientation) and pGEXKG PK3 (with the ORF in the wrong orientation) were used to express recombinant LdMAPK1 as a glutathione S-transferase (GST) fusion protein (rgLdMAPK1N) or a truncated fusion protein (rgLdKPAM1), respectively, in BL21(DE3)pLysS cells. rgLdMAPK1N was purified under native conditions through a preequilibrated glutathione-Sepharose 4B column in accordance with the manufacturer's instructions (GE Healthcare). Sample purity was evaluated on a Coomassie-stained 10% SDS-PAGE gel (27). Protein concentrations were determined by the Bradford method using bovine serum albumin as the standard (6).
The kinase activity of purified native recombinant protein rgLdMAPK1N was assayed by the ATP utilization method. Briefly, a reaction mixture with a total volume of 50 μl containing 50 mM morpholinepropanesulfonic acid (MOPS; pH 7.2), 100 mM NaCl, 10 mM MgCl2, 2 mM MnCl2, 100 μM ATP, 10 μg myelin basic protein (MBP; Sigma), and 30 to 100 ng of rgLdMAPK1N or rgLdKPAM1 was incubated at 30°C for 30 min, and residual ATP was measured using the Kinase-Glo Plus luminescent assay kit (Promega) using a luminometer (PolarStar Galaxy instrument). Equal amounts of protein purified from uninduced cultures (UNLdMAPK1) and truncated protein (rgLdKPAM1) were added as enzyme sources to negative-control tubes. The specific activity of rgLdMAPK1N was calculated as nanomoles of ATP consumed/min/mg protein.
Western blot analysis.
The LdMAPK1 ORF was cloned into the pCR-T7/CT-TOPO vector (Invitrogen) in accordance with the manufacturer's protocol. The recombinant LdMAPK1 protein with a His tag at the C-terminal end (rLdMAPK1) was expressed in Escherichia coli strain BL21(DE3) (Invitrogen) and purified using Ni-nitrilotriacetic acid-agarose ion-exchange column chromatography according to the manufacturer's protocol (Qiagen). The recombinant protein was purified to homogeneity by SDS-PAGE and used to immunize rabbits for anti-rLdMAPK1 antibody generation. To demonstrate endogenous MAPK1 in sensitive and resistant strains, mid-log-phase cultures of promastigotes of various strains were harvested by centrifugation (1,000 × g for 10 min), washed in phosphate-buffered saline, and lysed in SDS-PAGE sample buffer. Proteins from equivalent numbers of cells (2 × 106 to 4 × 106) were analyzed by SDS-PAGE, transferred onto nitrocellulose membrane, and processed for Western blot analysis with anti-rLdMAPK1 antibody as described previously (54). Blots were developed using ECL reagent (GE Healthcare) and visualized on X-ray film. The images were scanned, and quantitative assessment was carried out with software provided with the Gel-Doc System (Alpha Innotech).
Overexpression of LdMAPK1 in sensitive and resistant parasites.
The LdMAPK1 ORF was PCR amplified from construct pCRT-ldpk-S1/3 using primers with SpeI restriction sites (underlined) (forward, 5′-CGGCACTAGTATGACCTCCTATGGCATC-3′; reverse, 5′-CCGGACTAGTCTAATAAACGTCTGTTAT-3′) and cloned into Leishmania shuttle vector pKS-Neo. Clone pKS-MAPK1 (ORF in the right orientation) was transfected into one sensitive laboratory strain, Dd8 (Ldd), and one resistant field isolate, R1, by electroporation using a Gene Pulser (Bio-Rad) under conditions described earlier (2). Parasites transfected with the empty vector pKS-NEO and pKS-KPAM1 (ORF in the wrong orientation) were taken as controls. Transfectants were selected and maintained in the presence of G418 (20 or 40 μg/ml). The copy number of the transfected plasmid was reduced in one set of each transfectants by removing drug pressure from the medium.
Statistical analysis.
The 50% inhibitory concentrations (IC50s) of drugs were calculated by regression obtained through probit analysis of log dose/response data on the drug (15). The data were statistically analyzed by the Student t test and are presented as means and standard deviations (SDs) of three determinations from at least two independent experiments. A P value of <0.05 was considered significant.
Nucleotide sequence accession number.
The complete sequence of LdMAPK1 is available in GenBank under accession no. GQ370815.
RESULTS
The clinical and laboratory profiles of VL patient isolates are summarized in Table 1. The trivalent antimony compound is the active form of the drug (10), and it is highly active against both stages of the parasite. The IC50 of Sb(III) (potassium antimony tartrate) was determined as an index of the antimony resistance phenotype of each isolate under laboratory conditions (3). Two of the isolates in Table 1, S1 and R3, had previously been tested for their resistance phenotypes under in vivo condition in a hamster model as well (36). Interestingly, a mixed response was observed. All of the isolates collected from a drug-resistant area, i.e., Muzaffarpur, did not exhibit resistance to Sb(III); e.g., S2 was sensitive to trivalent antimony. Similarly, some of the isolates collected from the drug-sensitive area, i.e., Ballia, did show resistance to Sb(III) compared to strain Dd8; e.g., R5 exhibited 2-fold resistance index (Table 1). The resistance profiles of isolates determined by the Sb(V) intracellular amastigote assay correlated well with those determined by the Sb(III) promastigote assay and confirmed the resistance indices of the isolates. The isolates exhibited almost equal sensitivities to the second-line antileishmanial drug amphotericin B (data not shown).
Table 1.
Clinical resistance profiles and in vitro susceptibilities of field isolates of L. donovani to Sb(III) and Sb(V)
| Straina | Collection area | Clinical drug response | Ldf score | Mean index of resistance ±SD |
|
|---|---|---|---|---|---|
| Sb(III) | Sb(V) | ||||
| Lddb | Laboratory | Sensitive | 1d | 1e | |
| S1c | Ballia (BHU/NMW-5) | Sensitive area | 2+ | 0.667 ± 0.078 | 0.98 ± 0.06 |
| S2 | Muzaffarpur | Resistant area | 1+ | 0.712 ± 0.032 | 0.93 ± 0.07 |
| S3 | Pard 111/3 | Sensitive area | 2+ | 0.868 ± 0.015 | 0.91 ± 0.06 |
| R1c | Muzaffarpur | SAG resistant | NAg | 3.18 ± 0.028 | 2.37 ± 0.13 |
| R2 | Muzaffarpur | Resistant area | 2+ | 1.912 ± 0.039 | 1.77 ± 0.09 |
| R3 | Muzaffarpur | SAG resistant | 1+ | 2.34 ± 0.046 | 1.70 ± 0.06 |
| R4 | Muzaffarpur | Amphotericin B resistant | NA | 1.87 ± 0.043 | 2.05 ± 0.07 |
| R5 | Ballia (BHU/NMW-17) | Sensitive area | 4+ | 2.20 ± 0.042 | 1.83 ± 0.10 |
S, sensitive; R, resistant.
Ldd, L. donovani sensitive laboratory reference strain Dd8.
Field isolates S1 and R3 are the same as those described by Mittal et al. (36). Their responses to SAG (sensitive/resistant) had been confirmed under in vivo conditions in a hamster model. Sb(III) and Sb(V) resistance indices were calculated using the formula IC50isolates/IC50Dd8.
IC50, 31.1 ± 0.99 μg/ml.
IC50, 91.7 ± 4.1 μg/ml.
Ld, Leishman-Donovan bodies.
NA, not applicable.
Identification of L. donovani MAPK1. (i) Cloning and sequence analysis.
Isolates S1 and R3, with confirmed sodium antimony gluconate (SAG) responses under in vivo conditions, were used for DNA microarray expression profiling to identify genes that are differentially expressed in resistant parasites. A microarray containing 4,224 PCR products amplified from randomly sheared L. donovani genomic clones was probed with fluorescent cDNA generated from promastigotes of sensitive and resistant isolates. Analysis of data from two replicates and two dye flip hybridizations showed the differential expression of 14 clones. Interestingly, three out of seven downregulated clones, namely, G13H6, G36D10, and G51G7, independently aligned with the MAPK gene, which suggested that the gene may have some role in resistance; hence, it was taken up for detailed characterization. A 723-bp-long DNA sequence of G13H6 was matched to the MAPK1 homologue of L. mexicana present on chromosome 36. A significant match was also observed for this clone with the 3′ end of the MAPK1 homologue of L. infantum (see Table S1 in the supplemental material). Similarly, DNA sequences of G36D10 (885 bp) and G51G7 (1,105 bp) were matched to the MAPK1 homologue of L. infantum and L. mexicana present on chromosome 36 (see Fig. S1 and Table S1 in the supplemental material).
Subsequently, the full-length coding sequence of the LdMAPK1 gene was amplified by PCR from genomic DNA of both resistant and sensitive L. donovani isolates using primers designed from the L. major MAPK1 sequence annotated in GenBank. Interestingly, the sequence of LdMAPK1 cloned from SAG-resistant isolate R3 was found to be 100% identical to the sequence cloned from SAG-sensitive strain S1, suggesting that a change (point mutation) in the sequence of the gene did not relate to SAG resistance in the isolates (data not shown). A multiple-sequence alignment, a protein domain search, and a phylogenetic relationship analysis indicate that LdMAPK1 is a highly conserved protein among Leishmania spp. (97%) and contains all of the features of MAPKs (see Fig. S2 and S3 in the supplemental material), as described earlier (42, 44, 59).
(ii) Genomic organization.
In a Southern blot analysis of L. donovani genomic DNA, we observed a single band (lanes 2 to 5) with restriction enzymes that do not cut within the LdMAPK1 ORF (BglII, HindIII, and NcoI) and two bands (lane 6) with restriction enzyme SacI, which cuts once in the gene, at bp 887. Therefore, the LdMAPK1 gene is present as a single copy in the L. donovani haploid genome (see Fig. S4 in the supplemental material). Further, we did not observe any difference in the copy number of the gene in field isolates and laboratory strain (see Table S2 in the supplemental material).
(iii) Kinase activity of recombinant LdMAPK1.
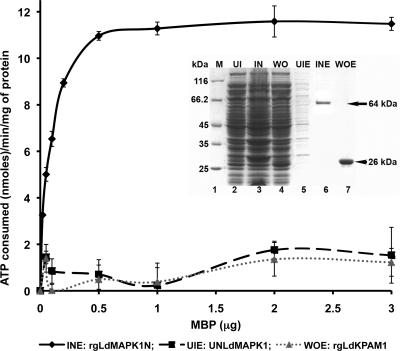
Sequence homology to MAPKs and the presence of typical kinase domains suggest that LdMAPK1 is a functional kinase. To demonstrate its activity, we expressed the protein under native conditions as a GST fusion protein (rgLdMAPK1N), purified it on glutathione-Sepharose columns (Fig. 1, inset), and subjected it to an enzyme assay (Fig. 1). Due to the lack of information about the LdMAPK1 substrate, we used MBP as a substrate, which has been used extensively as a broad-spectrum substrate for other MAPKs of L. mexicana (5). The enzyme activity of rgLdMAPK1N was demonstrated by the consumption of ATP in the phosphorylation of MBP. The residual ATP was measured using the Kinase-Glo Plus luminescent assay kit (Promega). For negative controls, equal amounts of proteins purified from uninduced and induced cultures of the clone with the LdMAPK1 gene in the wrong orientation were used as an enzyme source. With the recombinant native protein (rgLdMAPK1N), an increase in ATP consumption was observed with an increase in the substrate (MBP) concentration, while the protein purified from negative controls did not exhibit any significant ATP consumption. The specific activity of rgLdMAPK1 was found to be 11.66 nmol ATP consumed/min/mg protein. Staurosporine, a broad-spectrum kinase inhibitor (44), inhibited recombinant rgLdMAPK1N-catalyzed ATP consumption in a dose-dependent manner. At a 4 μM concentration, complete inhibition of enzyme activity was observed. The data confirmed that rgLdMAPK1N is a functional kinase.
Fig 1.
Michaelis-Menten plot for kinase activity of recombinant LdMAPK1 protein (rgLDMAPK1N). Engyme activity (nmol ATP consumed per min per mg protein) was determined as a function of the increasing concentration of MBP. The inset shows the purity of rgLdMAPK1N in SD5-PAGE. UI, uninduced culture; IN, induced culture; WO, induced culture having gene in wrong orientation; UIE, elution from uninduced culture (UNLdMAPK1); INE, elution from induced culture (rgLDMAPK1N); WOE, elution from induced culture having gene in wrong orientation (rgLdKPAM1).
(iv) Validation of downregulation of LdMAPK1 in L. donovani SAG-resistant isolates by RT-PCR and Western blotting.
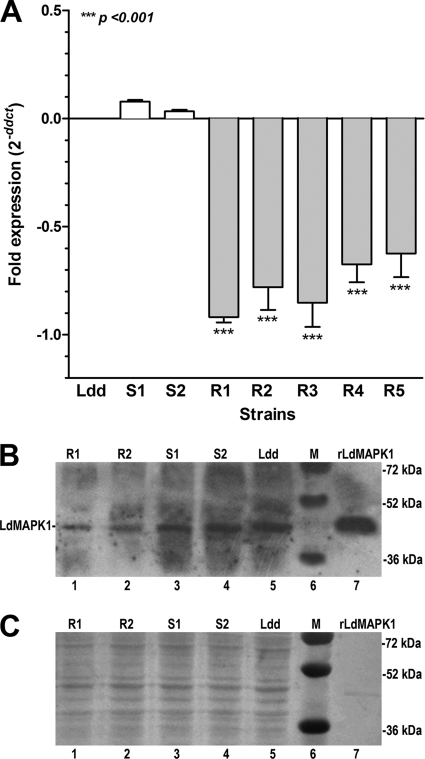
Figure 2A exhibits differences in the expression of LdMAPK1 in L. donovani clinical isolates from that in strain Ldd, as determined by RT-PCR. A significant decrease (2- to 3-fold) in the expression of LdMAPK1 was observed in all of the resistant isolates compared to that in sensitive reference strain Ldd. On the other hand, sensitive isolates S1 and S2 exhibited a slight upregulation (0.5-fold). Therefore, RT-PCR analysis confirmed the downregulation of LdMAPK1 expression in resistant isolates as detected by microarray.
Fig 2.
Validation of microarray results of differential expression of LdMAPK1. (A) Expression of LdMAPK1 in L. donovani clinical isolates relative to that in laboratory reference strain Dd8 (Ldd) as detected by RT-PCR. The data are the mean ± SD of three independent experiments performed with three different RNA preparations. Asterisks denote highly significant differences from Ldd. (B) Verification of LdMAPK1 expression patterns of sensitive and resistant isolates by Western blot analysis using anti-LdMAPK1 antibodies. Results shown are representative of three experiments. (C) Cell lysate loading controls. Ponceau S staining of the membrane for total protein was done prior to antibody incubation. Ldd, sensitive laboratory strain Dd8; S, sensitive; R, resistant; M, molecular size markers.
The downregulation of LdMAPK1 in resistant isolates was also verified at the protein level by Western blotting with anti-LdMAPK1 antibodies. His-tagged recombinant LdMAPK1 protein (rLdMAPK1) was used as a positive control. LdMAPK1 exhibited downregulation (1.5- to 2-fold) in resistant parasites compared to sensitive ones at the protein level (Fig. 2B). To ensure that equal amounts of proteins were loaded in the gel, the membranes were stained with Ponceau S for total protein prior to antibody incubations (Fig. 2C). Taken together, the data suggest that the decrease in the LdMAPK1 levels in resistant isolates may be one of the mechanisms of resistance.
(v) Overexpression of LdMAPK1 in sensitive and resistant Leishmania parasites.
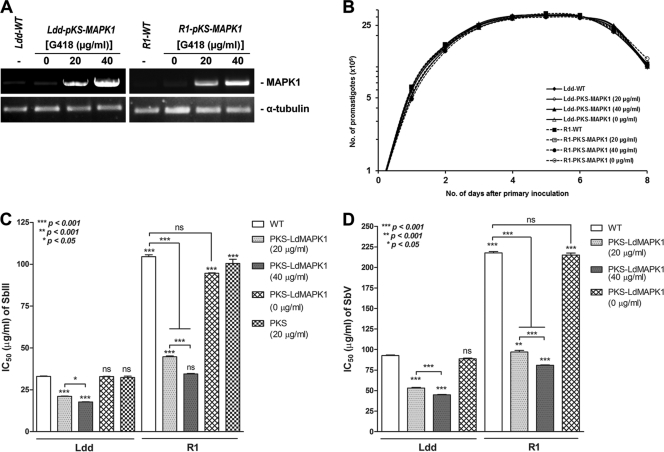
To see whether overexpression of LdMAPK1 would modulate the Sb(III) sensitivity of parasites, the gene was overexpressed in L. donovani sensitive strain Dd8 and resistant isolate R1, which has the highest Sb(III) and Sb(V) resistance indices. The presence of the LdMAPK1 gene in transfectants was confirmed by RT-PCR. Figure 3A depicts very intense bands in the parasites transfected with the pKS-LdMAPK1 construct compared to those in cells transfected with the vector only (Ldd-pKS and R1-pKS), suggesting that the LdMAPK1 transcript was present in the transfectants at high copy numbers. The band intensity of the LdMAPK1 amplicon was 1.8- and 2.96-fold higher (calculated by densitometric analysis using Gel-Doc software) in Ldd-pKS-MAPK1 transfectants maintained at 20 and 40 μg/ml G418, respectively, than in control Ldd-pKS transfectants. Similarly, R1-pKS-MAPK1 transfectants maintained at 20 and 40 μg/ml G418 exhibited LdMAPK1 amplicon band intensities 1.90- and 2.68-fold higher than those of control R1-pKS transfectants. MAPK1 transfectants of Ldd and R1 maintained in the absence of G418 exhibited the same amplicon intensity as controls. Taken together, the data suggest that the copy number of LdMAPK1 in transfectants varied with the G418 concentration; i.e., the higher the concentration, the higher the expression of LdMAPK1.
Fig 3.
Characterization of LdMAPK1-overexpressing L. donovani sensitive laboratory reference strain Dd8 (Ldd-pKS-MAPK1) and resistant isolate R1 (R1-pKS-MAPK1). (A) RT-PCR analysis of overexpression of LdMAPK1 in antimony-sensitive and antimony-resistant transfectants compared to that in controls transfected with the empty vector (Ldd-pKS and R1-pKS). (B) Growth curves of Ldd-pKS-MAPK1 and R-pKS-MAPK1 in comparison with that of the controls, Ldd-pKS and R1-pKS. Each data point on the curve represents the mean value of three separate assays. (C) Sb(III) IC50s for LdMAPK1 transfectants. (D) Sb(V) IC50s for LdMAPK1 transfectants. The values are calculated from mean ± SD of percent inhibition values from three experiments. Asterisks denote significant differences from wild-type (WT) Ldd, as shown (ns, no significant difference).
Figure 3B depicts growth curves of transfectants. The rate of multiplication of LdMAPK1-transfected promastigotes (Ldd-pKS-MAPK1, R1-pKS-MAPK1) was comparable to that of controls transfected with the empty vector (Ldd-pKS and R1-pKS, respectively), indicating that overexpression of LdMAPK1 had no effect on the in vitro growth of the parasites.
Figure 3C and D exhibit the Sb(III) and Sb(V) susceptibilities of the promastigotes and intracellular amastigotes of transfectants (overexpressing LdMAPK1). Resistant strain R1 exhibited 3.18-fold ± 0.028-fold higher Sb(III) IC50s and 2.37-fold ± 0.13-fold higher Sb(V) IC50s than wild-type sensitive strain Ldd. Interestingly, LdMAPK1 transfectants of strain R1 (R1-pKS-MAPK1) maintained in 20 μg/ml G418 exhibited significantly lower (2.33-fold ± 0.06-fold) IC50s of Sb(III) than the wild type. The Sb(III) sensitivity of R1 transfectants was further increased by 3.02-fold ± 0.09-fold (almost as sensitive as wild-type Ldd) after the copy number of LdMAPK1 was increased by increasing the concentration of G418 to 40 μg/ml. Similar results were observed in Ldd transfectants (Ldd-pKS-MAPK1). Ldd transfectants exhibited significantly higher sensitivity to Sb(III) than the wild type, 1.56-fold ± 0.04-fold (G418, 20 μg/ml) and 1.85-fold ± 0.03-fold (G418, 40 μg/ml). The IC50 of Sb(V) was also significantly decreased, by 2.24-fold ± 0.08-fold and 2.69-fold ± 0.03-fold, in R1-LdMAPK1 maintained in 20 and 40 μg/ml G418, respectively (almost as sensitive as wild-type Ldd). The Sb(V) sensitivity was also increased significantly, by 1.74-fold ± 0.07-fold and 2.05-fold ± 0.04-fold, in Ldd-LdMAPK1 maintained in 20 and 40 μg/ml G418, respectively. The increase in the sensitivity of transfectants to Sb(III) or Sb(V), depending on the copy number of LdMAPK1, suggests a direct relationship between LdMAPK1 expression and antimony sensitivity. The overexpression of LdMAPK1 in the wrong orientation and transfection with the empty vector did not have any effect on parasite sensitivity to Sb(III) or Sb(V) (data not shown). Taken together, the data clearly suggest that the overexpression of LdMAPK1 significantly increased the susceptibility of promastigotes to Sb(III) and that of intracellular amastigotes to Sb(V).
DISCUSSION
Sb(V) are first-line drugs for the treatment of leishmanial diseases, and resistance to these drugs is a very serious clinical problem not only on the Indian subcontinent but also throughout the world (18, 31, 43, 62). So far, studies on field isolates revealed that the mechanism of natural antimony resistance is multifactorial and may differ from laboratory resistance (3). Therefore, exploration of the mechanisms contributing to the clinical ineffectiveness of pentavalent antimony is a challenging area of research. Unraveling of the mechanisms responsible for resistance could help/lead to effective drug treatment strategies.
DNA microarray, a powerful tool, has been employed to study the drug resistance mechanism in cancer cells and microorganisms (8, 60), including Leishmania (17, 46, 55). These studies have validated not only observations of previous studies on resistant laboratory mutants but have also pinpointed new genes that exhibit differential expression in resistant and sensitive field isolates (47). For similar purpose, we compared the transcriptome profile of SAG-sensitive and -resistant field isolates of L. donovani using DNA microarray containing 4,224 genomic fragments of L. donovani and identified three clones that were consistently downregulated in the resistant field isolate. The clinical phenotype (resistant/sensitive) of the isolates used in the transcriptome profiling had been confirmed under both in vitro and in vivo conditions (36) and had not been modulated/maintained by in vitro drug pressure. Sequencing and annotation of these clones revealed significant homology to different parts (3′ untranslated region and ORF) of the same protein, i.e., MAPK homologue 1 of L. mexicana (LmxMAPK1) and L. major (see Table S1 and Fig. S1 in the supplemental material); hence, it was named LdMAPK1.
MAPKs are well-known mediators of signal transduction of higher eukaryotes regulating important processes such as proliferation, differentiation, cell shape, stress response, and apoptosis. Out of the 17 MAPKs and MAPK-like kinases identified in Leishmania (25, 42, 58), the functional role in the parasite has been shown for only a few; e.g., LmxMPK1 was shown to be essential for the differentiation of promastigotes to amastigotes and for the survival of amastigotes in infected mammalian hosts (58), LmxMPK2 was shown to be essential for establishment of infection in BALB/c mice (59), LmxMPK3 and LmxMPK9 were found to be involved in the regulation of flagellar length of promastigotes (5, 14), and LmxMPK4 was found to be essential for promastigotes, as well as the amastigote stage, as a homozygous gene deletion mutant could not be obtained (57).
Interestingly, the protein encoded by the LdMAPK1 gene exhibited maximum homology, i.e., 98% with L. infantum MAPK1, followed by 96% with LmxMAPK1 (see Fig.S2A and B in the supplemental material). The observed homology was very significant in the kinase domains and invariant amino acids present in the phosphate anchor ribbon, the P+1 specificity pocket, the catalytic loop, the dual phosphorylation motif, the DFG motif, and the invariant Lys residue in subdomain II. The sequence also exhibited the presence of a MAPK signature sequence and the presence of Ser150, confirming that LdMAPK1 is a putative MAPK. Interestingly, nine residues in subdomain XI of LdMAPK1 were found highly conserved in tyrosine-autophosphorylating protein kinases, indicating that it may autophosphorylate at tyrosine residues to become active (45). In LdMAPK1, the region between subdomains VII and VIII is 14 amino acids long, which is similar to that of ERK proteins (39). Further, the X residue in the activation site (TXY) of LdMAPK1 is TDY, similar to that of ERK proteins, suggesting again that LdMAPK1 may be a member of the ERK subfamily. The LdMAPK1 protein exhibited significant homology to protein kinases and MAPKs of other organisms, particularly in conserved domains (see Fig. 2A in the supplemental material). However, leishmanial MAPK1s represent a distinct group of MAPKs, clustering with their own branch of the phylogenic tree (data not shown).
Southern blot analysis revealed that the LdMAPK1 gene is present as a single copy per haploid genome of L. donovani, as was also observed in the case of LmxMPK1 of L. mexicana (58). Further, RT-PCR using equal amount of genomic DNA as the template resulted in almost equal amounts of MAPK1 fragment amplification from both resistant and sensitive isolates, suggesting that the decrease in LdMAPK1 expression in resistant isolates may not be due to haploid deletion (see Table S2 in the supplemental material).
The recombinant LdMAPK1 protein expressed in E. coli exhibited typical protein kinase activity, i.e., phosphorylated MBP at the expense of ATP (Fig. 1). MBP is a general protein kinase substrate already used to demonstrate the kinase activity of other leishmanial MAPKs (37). Interestingly, inhibition of LdMAPK1-mediated MBP phosphorylation in the presence of staurosporine, a potent but nonspecific protein kinase inhibitor, further confirmed the kinase activity of recombinant LdMAPK1. This is the first demonstration of kinase activity of leishmanial MAPK1. The microarray data, i.e., downregulated expression of LdMAPK1 in resistant isolates, were validated by RT-PCR and Western blot analysis. Five resistant strains, two sensitive strains, and one sensitive laboratory strain were taken for RT-PCR analysis. The clinical resistance phenotypes of all of the isolates used in this study were also confirmed under laboratory conditions (Table 1) using promastigote Sb(III) and intracellular amastigote Sb(V) sensitivity as resistance indices. A correlation had already been established between the clinical resistance phenotypes of the isolates and the in vitro sensitivity of promastigotes to Sb(III) or the sensitivity of amastigotes to SAG (36, 61). All 5 of the resistant clinical isolates exhibited significant (∼2- to 3-fold) downregulation of MAPK1 compared to sensitive isolates, including reference strain Ldd (Fig. 2A), showing that the microarray data had revealed a trend that was more generally observable in resistant strains. Similar downregulation was also observed at the protein level in some representative strains (Fig. 2B), suggesting that it has a functional role. Recently, an upregulation of MAPK1 was demonstrated in antimony-resistant Leishmania parasites (47). Interestingly, in that study, the resistance phenotype of isolate K80 was maintained under in vitro drug pressure. This suggests that the expression of MAPK1 may differ under different conditions of resistance, i.e., clinical isolates, clinical isolates maintained under laboratory conditions, and laboratory-generated resistant mutants. However, this speculation has to be confirmed with more studies.
To establish the role of LdMAPK1 in antimony resistance, we overexpressed the gene in L. donovani sensitive laboratory strain Dd8 (Ldd) and resistant isolate R1 (Fig. 3). Further, we modulated the extent of LdMAPK1 expression by varying the G418 drug pressure. At a higher concentration of G418 (40 μg/ml), transfectants of both Ldd and R1 exhibited around 2.7- to 3-fold higher expression of LdMAPK1 than transfectants maintained at a lower concentration (20 μg/ml). It was observed that overexpression of LdMAPK1 conferred 2- to 3-fold increased sensitivity to Sb(III) or Sb(V) on the parasite compared to that of the corresponding strains transfected with the vector alone. Interestingly, increased expression of LdMAPK1 caused by increased G418 pressure resulted in further increased sensitivity of parasites to antimony. The transfectants (parasites overexpressing this protein) of both strains (sensitive and resistant) were killed by half or less than half of the Sb(III) or Sb(V) concentrations required to kill the corresponding controls transfected with the empty plasmid or the wild types. The data clearly suggest that the observed downregulation of this gene in resistant field isolates accounts in part for their resistance phenotype. Moreover, the overexpression of LdMAPK1 has no effect on morphology or the growth pattern of transfectants (Fig. 3B). The present study is the first ever report demonstrating the association of MAPK1 with the susceptibility of Leishmania parasites to antimony.
The roles of MAPKs in chemoresistance in cancer cells have been studied extensively. Generally, MAPKs are the last kinases in signal transduction cascades relaying signals augmented by environmental stress or stimuli which can ultimately lead to changes in gene expression profiles. It is well established that Sb(III) and the related metal As(III) induce apoptosis in a caspase- and reactive oxygen species-dependent manner in lymphoid tumor cells, human myeloid leukemia HL60 cells, other mammalian cells, and Leishmania amastigotes too (11, 21, 28, 29, 38, 49, 53). Traditionally, JNK and p38 kinases are associated with stress responses, including antimony- and arsenite-stimulated apoptosis (12, 20, 23, 34), while ERK1/2 are thought to be activated in response to growth stimuli for proliferation and survival. However, recent studies have challenged this view and suggested their activation in stress responses like in neurodegeneration (22, 33) in Parkinson's disease after application of the toxin 6-hydroxydopamine (26), in glutamate-induced oxidative toxicity, and in peroxynitrite-induced apoptosis (40, 48). Hence, under some conditions, ERK can induce apoptosis. The role of ERK in drug resistance was also demonstrated recently. The expression level of ERK2 was found to be decreased in human cervical carcinoma HeLa cells resistant to cisplatin (4), suggesting that it has a role in the toxicity pathway and resistance.
The present study suggests a possible role for LdMAPK1 in antimony resistance in field-isolated parasite strains. The downregulation of LdMAPK1 in antimony-resistant clinical isolates and the increase in the sensitivity of overexpressing parasites to Sb(III) suggest that this protein may also be investigated for a role in the observed cell death pathway induced by antimony (49). The results of the present study provide the first ever indication of a role for MAPK in resistance, and studies on the mechanism are under way. By exploring the regulation of LdMAPK1 expression and by targeting it, new drugs could be developed that will be effective against antimony-resistant parasites.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Department of Science and Technology, India (SR/SO/BB-037/2009), and Department of Biotechnology, India (BT/PR2792/Med/14/383/2001), grants. CSIR is gratefully acknowledged for financial support to Ashutosh and Mansi Garg.
Footnotes
Published ahead of print 7 November 2011
This article is CDRI communication 8160.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Alvar J, Yactayo S, Bern C. 2006. Leishmaniasis and poverty. Trends Parasitol. 22:552–557 [DOI] [PubMed] [Google Scholar]
- 2. Ashutosh, Gupta S, Ramesh, Sundar S, Goyal N. 2005. Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrob. Agents Chemother. 49:3776–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashutosh, Sundar S, Goyal N. 2007. Molecular mechanisms of antimony resistance in Leishmania. J. Med. Microbiol. 56:143–153 [DOI] [PubMed] [Google Scholar]
- 4. Basu A, Tu H. 2005. Activation of ERK during DNA damage-induced apoptosis involves protein kinase C delta. Biochem. Biophys. Res. Commun. 334:1068–1073 [DOI] [PubMed] [Google Scholar]
- 5. Bengs F, Scholz A, Kuhn D, Wiese M. 2005. LmxMPK9, a mitogen-activated protein kinase homologue affects flagellar length in Leishmania mexicana. Mol. Microbiol. 55:1606–1615 [DOI] [PubMed] [Google Scholar]
- 6. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 7. CDC 2004. Update: cutaneous leishmaniasis in U.S. military personnel—Southwest/Central Asia, 2002-2004. MMWR Morb. Mortal. Wkly. Rep. 53:264–265 [PubMed] [Google Scholar]
- 8. Cheok MH, et al. 2003. Treatment-specific changes in gene expression discriminate in vivo drug response in human leukemia cells. Nat. Genet. 34:85–90 [DOI] [PubMed] [Google Scholar]
- 9. Chulay JD, Bryceson ADM. 1983. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 32:475–479 [DOI] [PubMed] [Google Scholar]
- 10. Croft SL, Sundar S, Fairlamb AH. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davison K, Côté S, Mader S, Miller WH. 2003. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia 17:931–940 [DOI] [PubMed] [Google Scholar]
- 12. Davison K, Mann KK, Waxman S, Miller WH., Jr 2004. JNK activation is a mediator of arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells. Blood 103:3496–3502 [DOI] [PubMed] [Google Scholar]
- 13. Debrabant A, Gottilieb M, Dwyer DM. 1995. Isolation and characterization of gene encoding the surface membrane 3-nucleotidase/nuclease of Leishmania donovani. Mol. Biochem. Parasitol. 71:51–63 [DOI] [PubMed] [Google Scholar]
- 14. Erdmann M, Scholz A, Melzer IM, Schmetz C, Wiese M. 2006. Interacting protein kinases involved in the regulation of flagellar length. Mol. Biol. Cell 17:2035–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finney DJ. 1971. Probit analysis, 3rd ed Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 16. Goyal N, et al. 2006. Cloning and characterization of angiotensin converting enzyme related dipeptidylcarboxypeptidase from Leishmania donovani. Mol. Biochem. Parasitol. 145:147–157 [DOI] [PubMed] [Google Scholar]
- 17. Guimond C, et al. 2003. Modulation of gene expression in Leishmania drug resistant mutants as determined by targeted DNA microarrays. Nucleic Acids Res. 31:5886–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hadighi R, et al. 2006. Unresponsiveness to glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 3(5):e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanks SK, Quinn AM. 1991. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 200:38–62 [DOI] [PubMed] [Google Scholar]
- 20. Huang C, Ma WY, Li J, Dong Z. 1999. Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res. 59:3053–3058 [PubMed] [Google Scholar]
- 21. Huang H, Shu SC, Shih JH, Kuo CJ, Chiu ID. 1998. Antimony trichloride induces DNA damage and apoptosis in mammalian cells. Toxicology 129:113–123 [DOI] [PubMed] [Google Scholar]
- 22. Irving EA, Bamford M. 2002. Role of mitogen- and stress-activated kinases in ischemic injury. J. Cereb. Blood Flow Metab. 22:631–647 [DOI] [PubMed] [Google Scholar]
- 23. Kajiguchi T, et al. 2003. Sustained activation of c-jun-terminal kinase (JNK) is closely related to arsenic trioxide-induced apoptosis in an acute myeloid leukemia (M2)-derived cell line, NKM-1. Leukemia 17:2189–2195 [DOI] [PubMed] [Google Scholar]
- 24. Kedzierski L, et al. 2009. Leishmaniasis: current treatment and prospects for new drugs and vaccines. Curr. Med. Chem. 16:599–614 [DOI] [PubMed] [Google Scholar]
- 25. Kuhn D, Wiese M. 2005. LmxPK4, a mitogen-activated protein kinase homologue of Leishmania mexicana with a potential role in parasite differentiation. Mol. Microbiol. 56:1169–1182 [DOI] [PubMed] [Google Scholar]
- 26. Kulich SM, Chu CT. 2001. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J. Neurochem. 77:1058–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 28. Lecureur V, et al. 2002. Potassium antimonyl tartrate induces caspase- and reactive oxygen species-dependent apoptosis in lymphoid tumoral cells. Br. J. Haematol. 119:608–615 [DOI] [PubMed] [Google Scholar]
- 29. Leonard SS, Harris GK, Shi X. 2004. Metal-induced oxidative stress and signal transduction. Free Radic. Biol. Med. 37:1921–1942 [DOI] [PubMed] [Google Scholar]
- 30. Leprohon P, et al. 2009. Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic Acids Res. 37:1387–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lira R, et al. 1999. Evidence that incidence of treatment failure in Indian kala-azar is due to the emergence of antimony resistant strains of Leishmania donovani. J. Infect. Dis. 180:564–567 [DOI] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 33. MacKeigan JP, et al. 2002. Inactivation of the antiapoptotic phosphatidylinositol 3-kinase-Akt pathway by the combined treatment of taxol and mitogen-activated protein kinase kinase inhibition. Clin. Cancer Res. 8:2091–2099 [PubMed] [Google Scholar]
- 34. Mann K. K., et al. 2006. Antimony trioxide-induced apoptosis is dependent on SEK1/JNK signaling. Toxicol. Lett. 160:158–170 [DOI] [PubMed] [Google Scholar]
- 35. Medina-Acosta E, Cross GA. 1993. Rapid isolation of DNA from trypanosomatid protozoa using a simple ‘mini-prep’ procedure. Mol. Biochem. Parasitol. 59:327–329 [DOI] [PubMed] [Google Scholar]
- 36. Mittal MK, et al. 2007. Characterization of natural antimony resistance in Leishmania donovani isolates. Am. J. Trop. Med. Hyg. 76:681–688 [PubMed] [Google Scholar]
- 37. Morales MA, Renaud O, Faigle W, Shorte SL, Späth GF. 2007. Over-expression of Leishmania major MAP kinases reveals stage-specific induction of phosphotransferase activity. Int. J. Parasitol. 37:1187–1199 [DOI] [PubMed] [Google Scholar]
- 38. Müller S, Miller WH, Jr, Dejean A. 1998. Trivalent antimonials induce degradation of the PML-RAR oncoprotein and reorganization of the promyelocytic leukemia nuclear bodies in acute promyelocytic leukemia NB4 cells. Blood 92:4308–4316 [PubMed] [Google Scholar]
- 39. Nakashima S, et al. 1999. Molecular cloning and expression of a stress-responsive mitogen-activated protein kinase-related kinase from Tetrahymena cells. J. Biol. Chem. 274:9976–9983 [DOI] [PubMed] [Google Scholar]
- 40. Oh-Hashi K, Maruyama W, Isobe K. 2001. Peroxynitrite induces GADD34, 45, and 153 VIA p38 MAPK in human neuroblastoma SH-SY5Y cells. Free Radic. Biol. Med. 30:213–221 [DOI] [PubMed] [Google Scholar]
- 41. Ouellette M, Drummelsmith J, Papadopoulou B. 2004. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Updat. 7:257–266 [DOI] [PubMed] [Google Scholar]
- 42. Parsons M, Worthey EA, Ward PN, Mottram JC. 2005. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics 6:127–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rojas R, et al. 2006. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J. Infect. Dis. 193:1375–1383 [DOI] [PubMed] [Google Scholar]
- 44. Rüegg UT, Burgess GM. 1989. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol. Sci. 10(6):218–220 [DOI] [PubMed] [Google Scholar]
- 45. Seger R, et al. 1991. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc. Natl. Acad. Sci. U. S. A. 88:6142–6146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh N, et al. 2007. Differential gene expression analysis in antimony-unresponsive Indian kala azar (visceral leishmaniasis) clinical isolates by DNA microarray. Parasitology 134(Pt. 6):777–787 [DOI] [PubMed] [Google Scholar]
- 47. Singh R, Kumar D, Duncan RC, Nakhasi HL, Salotra P. 2010. Overexpression of histone H2A modulates drug susceptibility in Leishmania parasites. Int. J. Antimicrob. Agents 36:50–57 [DOI] [PubMed] [Google Scholar]
- 48. Stanciu M, DeFranco DB. 2002. Prolonged nuclear retention of activated extracellular signal-regulated protein kinase promotes cell death generated by oxidative toxicity or proteasome inhibition in a neuronal cell line. J. Biol. Chem. 277:4010–4017 [DOI] [PubMed] [Google Scholar]
- 49. Sudhandiran G, Shaha C. 2003. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes J. Biol. Chem. 278:25120–25132 [DOI] [PubMed] [Google Scholar]
- 50. Sundar S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6:849–854 [DOI] [PubMed] [Google Scholar]
- 51. Sundar S, et al. 1999. Oral treatment of visceral leishmaniasis with miltefosine. Ann. Trop. Med. Parasitol. 93:589–597 [DOI] [PubMed] [Google Scholar]
- 52. Sundar S, Murray HW. 2005. Availability of miltefosine for the treatment of kala-azar in India. Bull. World Health Organ. 83:394–395 [PMC free article] [PubMed] [Google Scholar]
- 53. Tirmenstein MA, et al. 1995. Antimony-induced oxidative stress and toxicity in cultured cardiac myocytes. Toxicol. Appl. Pharmacol. 130:41–47 [DOI] [PubMed] [Google Scholar]
- 54. Towbin H, Staehelin T, Falgout B. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some application. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ubeda JM, et al. 2008. Modulation of gene expression in drug resistant Leishmania is associated with gene amplification, gene deletion and chromosome aneuploidy. Genome Biol. 9:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vergnes B, et al. 2007. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol. Cell Proteomics 6:88–101 [DOI] [PubMed] [Google Scholar]
- 57. Wang Q, Melzer IM, Kruse M, Sander-Juelch C, Wiese M. 2005. LmxMPK4, a mitogen-activated protein (MAP) kinase homologue essential for promastigotes and amastigotes of Leishmania mexicana. Kinetoplastid Biol. Dis. 4:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wiese M. 1998. A mitogen-activated protein (MAP) kinase homologue of Leishmania mexicana is essential for parasite survival in the infected host. EMBO J. 17:2619–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wiese M. 2007. Leishmania MAP kinases—familiar proteins in an unusual context. Int. J. Parasitol. 37:1053–1062 [DOI] [PubMed] [Google Scholar]
- 60. Wilson M, et al. 1999. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc. Natl. Acad. Sci. U. S. A. 96:12833–12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wyllie S, et al. 2010. Elevated levels of tryparedoxin peroxidase in antimony unresponsive Leishmania donovani field isolates. Mol. Biochem. Parasitol. 173:162–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yardley V, et al. 2006. American tegumentary leishmaniasis: is antimonial treatment outcome related to parasite drug susceptibility? J. Infect. Dis. 194:1168–1175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.