Abstract
The impact of the adequacy of empirical therapy on outcome for patients with bloodstream infections (BSI) is key for determining whether adequate empirical coverage should be prioritized over other, more conservative approaches. Recent systematic reviews outlined the need for new studies in the field, using improved methodologies. We assessed the impact of inadequate empirical treatment on the mortality of patients with BSI in the present-day context, incorporating recent methodological recommendations. A prospective multicenter cohort including all BSI episodes in adult patients was performed in 15 hospitals in Andalucía, Spain, over a 2-month period in 2006 to 2007. The main outcome variables were 14- and 30-day mortality. Adjusted analyses were performed by multivariate analysis and propensity score-based matching. Eight hundred one episodes were included. Inadequate empirical therapy was administered in 199 (24.8%) episodes; mortality at days 14 and 30 was 18.55% and 22.6%, respectively. After controlling for age, Charlson index, Pitt score, neutropenia, source, etiology, and presentation with severe sepsis or shock, inadequate empirical treatment was associated with increased mortality at days 14 and 30 (odds ratios [ORs], 2.12 and 1.56; 95% confidence intervals [95% CI], 1.34 to 3.34 and 1.01 to 2.40, respectively). The adjusted ORs after a propensity score-based matched analysis were 3.03 and 1.70 (95% CI, 1.60 to 5.74 and 0.98 to 2.98, respectively). In conclusion, inadequate empirical therapy is independently associated with increased mortality in patients with BSI. Programs to improve the quality of empirical therapy in patients with suspicion of BSI and optimization of definitive therapy should be implemented.
INTRODUCTION
The empirical antibiotic treatment of patients with potentially serious infections is a challenging task. Providing appropriate empirical coverage is proving more and more difficult as antibiotic resistance increases in both the hospital and the community (1). In such situations, physicians face a dilemma: to provide a very-broad-spectrum empirical coverage, accepting that on many occasions it will be excessive and might contribute to further resistance selection, or to use a narrower-spectrum empirical regimen, accepting that it may not cover the causative pathogen and might require correction once the susceptibility results are known (19).
A key aspect of this decision-making process is the prognostic impact of empirical therapy. A meta-analysis recently showed reduced mortality rates for sepsis patients who received appropriate empirical therapy (22), although the studies analyzed were heterogeneous in terms of populations and types of infection covered. In both this study and another systematic review focusing on the methodological aspects of the topic (18), the need for new studies with improved methodologies was outlined. Bloodstream infection (BSI) has some advantages as a model for this kind of research. Although patients with bacteremia are only a subset of the pool of patients suffering sepsis, infections are readily detectable in routine practice, and the causative microorganisms and susceptibility are known by definition. The objective of this study was to assess the impact of adequate empirical therapy on the mortality of patients with BSI in the present context of antibiotic resistance, including methodological recommendations (18, 22).
MATERIALS AND METHODS
We followed the recommendations of the STROBE statement for reporting observational studies (30).
Design overview, sites, and procedures.
This analysis is part of the SAEI/SAMPAC Bacteremia Project, a project aimed at investigating the epidemiology, clinical features, and prognosis of BSI. A prospective cohort study was conducted in 15 public hospitals (10 tertiary and 5 community) in Andalucía, Spain. All consecutive episodes of clinically significant BSI in adult patients (>14 years old) from participating hospitals between 15 October 2006 and 15 December 2006 (tertiary centers) or 15 March 2007 (community centers) were included in the cohort. A detailed epidemiologic analysis of the cohort has been reported elsewhere (25).
Episodes were detected through daily review of blood culture results. The recommendations of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) were followed for performing, processing, and interpreting the blood cultures (17). Susceptibility results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) recommendations (6). All patients were monitored by an infectious disease or internal medicine specialist at each participant hospital, who collected the data by direct patient evaluation and by reviewing the chart records from the date of diagnosis of BSI until discharge; for patients discharged before day 30, mortality was assessed by consulting the electronic hospital charts and the official mortality registry. The information was collected in a predefined structured questionnaire. The study was approved by the ethics committee of the Hospital Universitario Virgen Macarena, which waived the need to obtain informed consent.
Variables and definitions.
Clinically significant BSI was defined as the isolation of a pathogen in a blood culture drawn from a patient with sepsis, according to standard criteria (15). The day the blood cultures were drawn was considered to be day 0. The outcome variables were all-cause mortality at days 14 and 30. The main exposure variable was inadequate empirical therapy. Antimicrobials were considered empirical if they were administered before susceptibility test results were available (typically, 48 to 72 h after blood cultures were performed) and were considered definitive thereafter. Empirical therapy was considered adequate when all of the following criteria were fulfilled: (i) at least one antimicrobial was administered as recommended following Spanish guidelines (5), including drug, route, and dosage; (ii) all organisms isolated from blood were susceptible in vitro; and (iii) the first dose was administered within the first 24 h after the blood culture had been drawn. Otherwise, empirical therapy was considered inadequate. The decision was made by the local investigator and reviewed by two study coordinators (Pilar Retamar and Jesús Rodríguez-Baño), who were blinded to outcome when reviewing the data. Discrepancies were solved by discussion.
Other exposure variables collected included acquisition type, classified into hospital acquired, health care associated, or community acquired (8); demographics; intensive care unit (ICU) admission; presence of underlying chronic diseases; severity of underlying chronic conditions according to the Charlson index (3); neutropenia (<500 neutrophils per μl) at day 0 (or expected to occur in <3 days); source of BSI, determined from clinical and microbiological data and using CDC criteria (12); invasive procedures performed during the previous week (for major surgery, the previous month was considered); antimicrobial use in the preceding 2 months; severity of illness the day before the onset of bacteremia (day −1), determined using the Pitt score (10); severity of systemic inflammatory response syndrome (SIRS) on day 0, according to predefined criteria (15); and etiology.
Statistical analysis.
The associations between the different variables and mortality were estimated by calculating crude relative risks (RR) with 95% confidence intervals (95% CI). To control for confounding, we first performed an exploratory multivariate analysis, using logistic regression, which included all variables related to mortality at a conservative significance level of <0.2, plus all variables of potential clinical importance. Variables were selected using a stepwise backward process; a P value of <0.2 was used as cutoff for keeping the variables in the model. The effect modification between the exposure of interest and other variables was investigated. The validity of the model was evaluated by the Hosmer-Lemeshow test for estimating goodness of fit to the data, and its discrimination ability was evaluated by using the area under the receiver operating characteristics (ROC) curve.
To further investigate the impact of inadequate empirical therapy on mortality, we then performed a propensity score-based matched analysis (7). The propensity score—the estimated probability (value of 0 to 1) that a patient had received adequate empirical therapy—was calculated using a nonparsimonious multivariable logistic regression model in which adequate empirical therapy was the dependent variable and all variables with P values of <0.2 for the association with empirical therapy in the univariate analysis were included as independent factors. Model validity was assessed as explained above. Patients receiving inadequate empirical therapy were matched on a 1:1 basis with patients receiving adequate empirical therapy, using individual propensity scores. The matching scheme used the minimum absolute distance between scores. The matching tolerance was a propensity score difference of 0.05, meaning that a patient receiving inadequate empirical therapy was matched with one receiving adequate empirical therapy only when the estimated probability of the latter receiving inadequate empirical therapy lay within 5% of the estimated probability of his or her counterpart receiving adequate therapy. Whenever more than one match was possible, the selection was made by simple randomization. Crude comparisons of the matched cohorts were performed by the McNemar test; odds ratios (ORs) with 95% CI were calculated by conditional logistic regression. The association between inadequate empirical therapy and mortality in the matched cohorts was adjusted for other variables by using a multivariate conditional regression logistic analysis in which potential confounders were selected using a stepwise forward process. All analyses were carried out using the SPSS 15.0 software package (SSPP Inc., Chicago, IL).
RESULTS
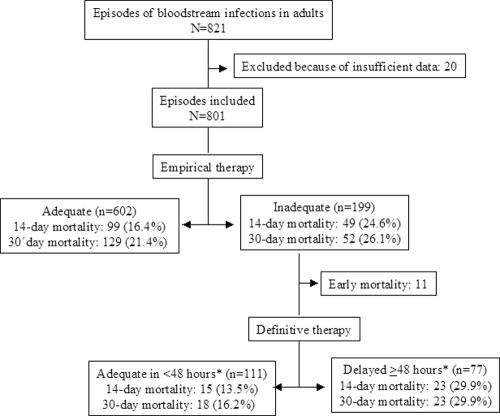
During the study period, 821 episodes of BSI were diagnosed in the participating centers; 20 episodes (2.4%) were excluded because the available data were incomplete (exposure to important variables could not be assessed in 15 cases, and postdischarge mortality could not be assessed in 5 cases). Thus, 801 episodes in 756 patients were included (Fig. 1). Inadequate empirical therapy was administered in 199 episodes (24.8%). The characteristics of the patients are shown in Table 1, listed according to adequate or inadequate empirical therapy. All-cause mortality was 18.5% (148 patients) at day 14 and 22.6% (181 patients) at day 30.
Fig 1.
Patients with BSI included in the cohort, with mortality shown according to antimicrobial therapy.
Table 1.
Features of 801 episodes of bloodstream infections, according to adequate or inadequate empirical therapy
| Characteristic | Valuea |
P valueb | |
|---|---|---|---|
| Inadequate empirical therapy (n = 199) | Adequate empirical therapy (n = 602) | ||
| Hospital-acquired infection | 142 (71.4) | 318 (52.8) | <0.001 |
| Male gender | 116 (58.3) | 357 (59.3) | 0.8 |
| Median age (yr) (interquartile range) | 67 (56–76) | 66 (51–75) | 0.3 |
| Tertiary hospital | 154 (77.4) | 483 (80.2) | 0.3 |
| ICU admission | 43 (21.6) | 1112 (18.6) | 0.3 |
| Median Charlson index (interquartile range) | 2 (1–4) | 2 (1–3) | 0.2 |
| Median Pitt score (interquartile range) | 1 (0–3) | 1 (0–3) | 0.9 |
| Cancer | 67 (33.7) | 160 (26.6) | 0.05 |
| Diabetes mellitus | 48 (24.1) | 159 (26.4) | 0.5 |
| Chronic pulmonary disease | 25 (12.6) | 80 (13.3) | 0.7 |
| Chronic renal insufficiency | 20 (10.1) | 70 (11.6) | 0.5 |
| Chronic liver disease | 18 (9) | 55 (9.1) | 0.9 |
| Neutropenia | 9 (4.5) | 37 (6.1) | 0.3 |
| Central venous catheter | 77 (38.7) | 168 (27.9) | 0.004 |
| Urinary catheter | 81 (40.7) | 195 (32.4) | 0.03 |
| Mechanical ventilation | 28 (14.1) | 66 (11) | 0.2 |
| Parenteral hyperalimentation | 18 (9) | 26 (4.3) | 0.01 |
| Previous antimicrobial use | 98 (49.2) | 231 (38.5) | 0.008 |
| Surgery | 39 (19.6) | 75 (12.5) | 0.01 |
| Source of bacteremia | 0.002 | ||
| Unknown | 55 (27.6) | 135 (22.4) | |
| Urinary tract | 23 (11.6) | 137 (22.8) | |
| Intra-abdominal infection | 29 (14.5) | 110 (18.2) | |
| Vascular catheter | 51 (25.6) | 82 (13.6) | |
| Respiratory tract | 25 (12.6) | 77 (12.8) | |
| Other source | 16 (8) | 61 (10.1) | |
| Etiology | <0.001 | ||
| Escherichia coli | 34 (17) | 188 (31.2) | |
| Coagulase-negative staphylococci | 60 (30.1) | 78 (12.9) | |
| Staphylococcus aureus | 24 (12) | 74 (12.2) | |
| Klebsiella pneumoniae | 13 (6.5) | 58 (9.6) | |
| Enterococcus spp. | 21 (10.5) | 38 (6.3) | |
| Streptococcus pneumoniae | 3 (1.5) | 36 (5.9) | |
| Pseudomonas aeruginosa | 11 (5.5) | 28 (4.6) | |
| Enterobacter spp. | 9 (4.5) | 18 (2.9) | |
| Acinetobacter baumannii | 13 (6.5) | 10 (1.6) | |
| Other | 34 (17) | 109 (18.1) | |
| Polymicrobial etiology | 23 (11.6) | 35 (5.8) | 0.007 |
| Severe sepsis or septic shock | 42 (21.1) | 165 (25.9) | 0.07 |
Data are expressed as numbers (percentages) of cases, except where specified.
P values were calculated using the chi-square test, except for age, Charlson index, and Pitt score (Mann-Whitney U test).
Table 2 shows the crude analysis of the associations between exposure to different variables and 14-day mortality. The associations between the various sources and etiologies of BSI and mortality are shown in more detail in Table 3. Since mortality rates associated with the biliary tract, the urinary tract, and use of a vascular catheter were similar and also significantly lower than that associated with other sources, the variable “source” was dichotomized into low-risk (including those mentioned above) and high-risk sources. Furthermore, since mortality rates associated with monomicrobial episodes caused by Klebsiella spp., Escherichia coli, and coagulase-negative staphylococci were similar and also lower than that caused by other microorganisms or polymicrobial episodes, etiology likewise was dichotomized into low- and high-risk etiologies. The mortality rate of patients receiving inadequate empirical therapy was higher than that of patients receiving adequate empirical therapy (Table 2).
Table 2.
Univariate analysis of associations between exposure to different variables and 14-day mortality in 801 episodes of bloodstream infection
| Variable | Mortality at 14 days (no. of deaths/no. of infections [%]) | RR (95% CI) | P valuec |
|---|---|---|---|
| Gender | |||
| Male | 96/473 (20.3) | Reference | |
| Female | 52/318 (15.9) | 0.71 (0.57–1.06) | 0.1 |
| Age (yr) | |||
| ≤55 | 25/204 (12.3) | Reference | |
| >55 | 123/597 (20.6) | 1.68 (1.13–2.51) | 0.007 |
| Type of acquisition | |||
| Community | 22/149 (14.8) | Reference | |
| Health care associated | 34/192 (17.7) | 1.20 (0.73–1.96) | 0.4 |
| Hospital | 92/560 (20) | 1.35 (0.88–2.08) | 0.1 |
| Type of hospital | |||
| Tertiary | 112/637 (17.6) | Reference | |
| Community | 36 (164 (22.0) | 1.25 (0.89–1.74) | 0.1 |
| Charlson index | |||
| 0–1 | 42/334 (12.6) | Reference | |
| 2 | 41/212 (19.3) | 1.54 (1.04–2.28) | 0.03 |
| ≥3 | 65/255 (25.5) | 2.03 (1.52–3.74) | <0.001 |
| Neutropenia | |||
| No | 133/755 (17.6) | Reference | |
| Yes | 15/46 (32.6) | 1.85 (1.19–2.88) | 0.01 |
| Pitt score | |||
| 0-1 | 47/491 (9.6) | Reference | |
| 2 | 15/92 (16.3) | 1.70 (1.00–2.91) | 0.05 |
| ≥3 | 86/218 (39.4) | 4.12 (3.00–5.66) | <0.001 |
| ICU admission | |||
| No | 90/646 (13.9) | Reference | <0.001 |
| Yes | 58/155 (37.4) | 2.69 (2.03–3.55) | |
| Severity of SIRS at presentation | |||
| Sepsis | 62/594 (10.4) | Reference | |
| Severe sepsis | 37/100 (37.0) | 3.16 (2.19–4.56) | <0.001 |
| Septic shock | 53/107 (49.5) | 4.75 (3.50–6.43) | <0.001 |
| Sourcea | |||
| Low risk | 36/352 (10.2) | Reference | |
| High risk | 112/449 (24.9) | 2.44 (1.72–3.46) | <0.001 |
| Etiologyb | |||
| Low risk | 48/386 (24.1) | Reference | |
| High risk | 100/415 (24.1) | 1.94 (1.41–2.66) | <0.001 |
| Empirical therapy | |||
| Adequate | 99/602 (16.4) | Reference | |
| Inadequate | 49/199 (24.6) | 1.47 (1.10–2.02) | 0.01 |
Low-risk sources include the biliary tract, urinary tract, and vascular catheter. For further explanation, see the text and Table 3.
Low-risk etiology includes monomicrobial episodes caused by Klebsiella spp., Escherichia coli, and coagulase-negative staphylococci. For further explanation, see the text and Table 3.
P values were calculated by the chi-square test.
Table 3.
Mortality at day 14 in patients with bacteremia, according to source of infection and etiology
| Variable | Mortality at 14 days (no. of deaths/no. of infections [%]) | RR (95% CI) | P valuea |
|---|---|---|---|
| Source | |||
| Biliary tract | 5/59 (8.5) | Reference | |
| Urinary tract | 16/160 (10.0) | 1.18 (0.45–3.08) | 0.7 |
| Vascular catheter | 15/133 (11.3) | 1.33 (0.51–3.49) | 0.5 |
| Unknown source | 40/190 (21.1) | 2.48 (1.03–6.00) | 0.02 |
| Intra-abdominal infectionb | 24/80 (30.0) | 3.54 (1.44–8.73) | 0.002 |
| Respiratory tract | 33/102 (32.4) | 3.82 (1.58–9.24) | <0.001 |
| Other | 15/77 (24.1) | 2.30 (0.89–5.97) | 0.07 |
| Etiologyc | |||
| Klebsiella spp. | 5/60 (8.3) | Reference | |
| Escherichia coli | 24/203 (11.8) | 1.42 (0.57–3.56) | 0.4 |
| Coagulase-negative staphylococci | 19/123 (15.4) | 1.85 (0.73–4.72) | 0.1 |
| Streptococcus pneumoniae | 9/37 (18.9) | 2.92 (1.06–8.04) | 0.02 |
| Staphylococcus aureus | 19/95 (20.0) | 2.40 (0.95–6.09) | 0.05 |
| Enterobacter spp. | 6/23 (26.1) | 3.13 (1.06–9.27) | 0.03 |
| Enterococcus spp. | 11/40 (27.5) | 3.30 (1.24–8.78) | 0.01 |
| Pseudomonas aeruginosa | 10/32 (31.3) | 3.75 (1.40–10.03) | 0.004 |
| Other microorganisms | 27/130 (20.7) | 2.49 (1.01–6.15) | 0.03 |
| Polymicrobial bacteremia | 18/58 (31.0) | 3.72 (1.48–9.37) | 0.001 |
| Antimicrobial resistanced | |||
| Multidrug resistant | 25/112 (22.3) | 1.25 (0.85–1.83) | 0.2 |
| Not multidrug resistant | 123/689 (17.9) | Reference |
P values were calculated by the chi-square test.
Other than the biliary tract.
Only monomicrobial episodes were considered for each specific microorganism.
Includes methicillin-resistant S. aureus, extended-spectrum-beta-lactamase-producing enterobacteria, and all meropenem- or imipenem-resistant Gram-negative bacilli.
Overall, infections caused by methicillin-resistant Staphylococcus aureus, extended-spectrum-beta-lactamase (ESBL)-producing E. coli or Klebsiella, or carbapenem-resistant Pseudomonas aeruginosa or Acinetobacter baumannii were associated with an increased crude risk of 14-day mortality compared with infections caused by their susceptible counterparts (17/78 episodes [21.7%] versus 31/324 episodes [9.5%]; RR = 2.28 [95% CI = 1.33 to 3.90]; P = 0.002).
The univariate analysis of variables potentially associated with mortality at day 30 showed similar results for most variables (data not shown); however, there was no significant difference in mortality between patients receiving adequate and inadequate empirical therapies (26.4% versus 21.4%; RR = 1.21 [95% CI = 0.92 to 1.61]; P = 0.1).
Among the 199 patients who received inadequate empirical therapy, 11 died before the susceptibility test results were available (Fig. 1). Of the 188 patients who survived until then, definitive therapy was delayed ≥48 h after the susceptibility test results were available for 77 patients, and 111 patients were changed to an active antimicrobial in less than 48 h. The 14-day mortality among patients with delayed definitive therapy was 29.9%, compared to 13.5% among those whose therapy was not delayed (RR = 2.21 [95% CI = 1.24 to 3.96]; P = 0.006), and 30-day mortality rates were 29.9% and 16.2%, respectively (RR = 1.84 [95% CI = 1.07 to 3.17]; P = 0.02).
An exploratory multivariate analysis of variables associated with 14- and 30-day mortality was then performed. The variables introduced were age, type of acquisition, type of hospital, Charlson index, neutropenia, Pitt score, ICU admission, severity of SIRS at presentation, source, etiology, and empirical therapy. The interactions between empirical therapy and source, etiology, and severity of SIRS were also studied. All variables were dichotomized with the aim of simplifying interpretation. Table 4 presents the data for the final models. Inadequate empirical therapy was associated with an increased risk of death at days 14 and 30 after controlling for age, Charlson index, neutropenia, source, etiology, Pitt score, and severity of SIRS at presentation. Pitt score and age were kept in both final models (14- and 30-day mortality) even if they were not significantly associated in one of them because they were important in the other; removing them from models in which no significant association was found did not change the results. The P value for the Hosmer-Lemeshow goodness-of-fit test was 0.69 for the 14-day mortality model and 0.21 for the 30-day mortality model. The areas under the ROC curves were 0.79 and 0.76, respectively. Inclusion of specific etiologies or sources in the models did not significantly change the impact of inappropriate empirical therapy. A conditional analysis which included the participating hospitals disclosed similar results (data not shown).
Table 4.
Final logistic regression model for exploratory multivariate analysis of variables associated with mortality in patients with bloodstream infections
| Variable | β coefficient | OR (95% CI) | P value |
|---|---|---|---|
| Mortality at day 14 | |||
| Age of >55 yr | 0.62 | 1.86 (1.10–3.15) | 0.02 |
| Charlson index of ≥2 | 0.63 | 1.88 (1.21–2.92) | 0.005 |
| Neutropenia | 0.76 | 2.14 (0.99–4.65) | 0.05 |
| High-risk source | 0.84 | 2.32 (1.46–3.67) | <0.001 |
| High-risk etiology | 0.53 | 1.71 (1.11–2.63) | 0.01 |
| Pitt score of ≥2 | 0.35 | 1.42 (0.81–2.51) | 0.21 |
| Severe sepsis or septic shock at presentation | 1.49 | 4.45 (2.51–3.34) | <0.001 |
| Inadequate empirical therapy | 0.75 | 2.12 (1.34–3.34) | 0.001 |
| Mortality at day 30 | |||
| Age of >55 yr | 0.36 | 1.45 (0.92–2.28) | 0.11 |
| Charlson index of ≥2 | 0.49 | 1.64 (1.10–2.44) | 0.01 |
| Neutropenia | 0.62 | 1.86 (0.88–3.91) | 0.09 |
| High-risk source | 0.79 | 2.21 (1.45–3.35) | <0.001 |
| High-risk etiology | 0.65 | 1.91 (1.29–2.84) | 0.001 |
| Pitt score of ≥2 | 0.67 | 1.96 (1.18–3.23) | 0.009 |
| Severe sepsis or septic shock at presentation | 1.09 | 2.93 (1.76–4.99) | <0.001 |
| Inadequate empirical therapy | 0.44 | 1.56 (1.01–2.40) | 0.04 |
We next used propensity score analysis to further adjust for confounding. Type of service, type of acquisition, a diagnosis of cancer, use of a central venous catheter, use of a urinary catheter, parenteral hyperalimentation, surgery, previous antimicrobial use, BSI source, and severity of SIRS at presentation showed crude P values of <0.2 in association with inadequate empirical therapy (Table 1) and were introduced into a nonparsimonious multivariate logistic regression model (etiology was not considered, since this factor is unknown when empirical therapy is indicated). The P value of the Hosmer-Lemeshow test for the model was 0.67, and the area under the ROC curve was 0.78. One hundred ninety-one pairs of patients were matched according to their probability of receiving inadequate empirical therapy according to this model. Pairs were matched suitably by age, gender, type of acquisition, ICU admission, Charlson index, underlying diseases, invasive procedures, previous antimicrobial use, BSI source, Pitt score, and severity of SIRS at presentation (Table 5). Eight patients with inadequate treatment (4%) could not be matched because of the preestablished cutoff for matching tolerance. Mortality at day 14 was 24.6% (47 patients) for those with inadequate empirical therapy and 11.0% (21 patients) for those who received adequate therapy; at day 30, mortality was 26.2% (20 patients) and 17.3% (31 patients) for those with inadequate and adequate empirical therapy, respectively. With respect to the 191 matched pairs, both members of the matched pair survived for 14 days in 135 cases, and both died in 12 cases; the patient who received inadequate empirical therapy died and the matched counterpart survived in 35 cases; and the opposite occurred in 9 cases. The corresponding data for 30-day mortality were 128, 20, 30, and 13 pairs, respectively. ORs (95% CI) calculated by conditional logistic regression were 3.58 (1.86 to 8.0) and 2.0 (1.07 to 3.71) for 14- and 30-day mortality, respectively, and P values obtained using McNemar's test were <0.001 and 0.02, respectively. Conditional multivariate analysis showed that inadequate empirical therapy was associated with 14-day mortality when controlling for source, etiology, Pitt score, and severity of SIRS at presentation; the adjusted OR (95% CI) was 3.03 (1.60 to 5.74) (P = 0.001). The adjusted OR (95% CI) for 30-day mortality obtained using the same model was 1.70 (0.97 to 2.98) (P = 0.06).
Table 5.
Comparison of matched patients receiving inadequate and adequate empirical therapya
| Variable | No. (%) of patients |
P value | |
|---|---|---|---|
| Inadequate empirical therapy (n = 191) | Adequate empirical therapy (n = 191) | ||
| Male gender | 110 (57.6) | 123 (64.4) | 0.1 |
| Age of >55 yr | 148 (77.5) | 140 (73.3) | 0.3 |
| Tertiary center | 149 (78.0) | 156 (81.7) | 0.3 |
| ICU admission | 41 (21.5) | 46 (24.1) | 0.4 |
| Nosocomial BSI | 134 (70.2) | 133 (69.6) | 0.9 |
| Charlson index of ≥2 | 116 (60.7) | 111 (58.1) | 0.4 |
| Cancer | 65 (34.0) | 66 (34.6) | 1.0 |
| Central venous catheter | 73 (38.2) | 66 (34.6) | 0.5 |
| Parenteral hyperalimentation | 16 (8.4) | 17 (8.9) | 0.6 |
| Surgery | 36 (18.8) | 40 (20.9) | 0.4 |
| Previous antimicrobial treatment | 92 (48.2) | 94 (49.2) | 0.8 |
| High-risk etiology | 102 (53.4) | 97 (50.8) | 0.4 |
| High-risk source | 110 (57.6) | 110 (57.6) | 1.0 |
| Pitt score of ≥2 | 74 (38.7) | 66 (34.6) | 0.6 |
| Presentation with severe sepsis or septic shock | 38 (21.6) | 31 (17.3) | 0.3 |
| Mortality at day 14 | 47 (24.6) | 21 (11.0) | <0.001 |
| Mortality at day 30 | 50 (26.2) | 33 (17.3) | 0.02 |
Patients were matched using a propensity score. P values were calculated by the McNemar test.
Table 6 shows the associations between inadequate empirical therapy and mortality for all analyses. In the case of crude analysis, we included ORs instead of RRs to facilitate comparison.
Table 6.
Odds ratios and 95% confidence intervals for inadequate empirical therapy and mortality
| Cohort | OR (95% CI) |
|
|---|---|---|
| 14-day mortality | 30-day mortality | |
| Complete, crude | 1.66 (1.13–2.50) | 1.29 (0.84–1.88) |
| Complete, adjusted | 2.12 (1.34–3.34) | 1.56 (1.01–2.40) |
| Matched | 3.58 (1.86–8.0) | 2.0 (1.07–3.71) |
| Matched, adjusted | 3.03 (1.60–5.74) | 1.70 (0.97–2.98) |
The most frequent empirical regimens used were monotherapy with a β-lactam (343 patients [42.8%]) or fluoroquinolone (74 patients [9.2%]) or combination therapy with a β-lactam plus a fluoroquinolone (99 patients [12.3%]), a β-lactam plus a glycopeptide (78 patients [9.7%]), or a β-lactam plus an aminoglycoside (47 patients [5.8%]). Overall, combination therapy was associated with a higher probability of appropriate empirical therapy than monotherapy (80.1% versus 72.3%; crude RR = 1.11 [95% CI = 1.02 to 1.20]; P = 0.001), although mortality rates at day 14 or 30 did not differ significantly (22% versus 16.5% for day 14 [RR = 1.29 {95% CI = 0.96 to 1.73}; P = 0.1] and 25.8% versus 20.9% for day 30 [RR = 1.24 {95% CI = 0.95 to 1.60}; P = 0.1]).
DISCUSSION
Our data showed that receipt of inadequate empirical therapy was independently associated with increased mortality in patients with BSI. It was estimated that patients who received inadequate empirical therapy had an adjusted 2-fold greater probability of dying by day 14; the results of the propensity score-based matched cohorts showed even higher ORs. As expected, the magnitude of the association was lower for 30-day than for 14-day mortality, meaning that the impact of adequate therapy is higher on early mortality. Since BSI affects between 100 and 200 people per 100,000 population-year and the incidence seems to be increasing (24, 25, 28), these results are relevant for the management of patients.
Our results are consistent with those of a recent meta-analysis which analyzed the impact of appropriate empirical therapy on patients with sepsis (22). The nature of the studies included in the meta-analysis was heterogeneous, and many considered only certain types of patients, infections, or microorganisms. Definitions of inadequate therapy also varied, with mostly only in vitro activity being considered. The need for new studies using improved methodology to analyze the association between antibiotic therapy and mortality has been outlined (18, 22), with specific recommendations that (i) empirical and definitive therapies should be assessed separately; (ii) the definition of adequate therapy should include in vitro susceptibility data, dosage, and route and pattern of administration, rather than considering only in vitro activity (for which the term “appropriate” is frequently used); (iii) the results should be adjusted according to the severity of the patient's illness (see below); (iv) the results should be reported according to the STROBE recommendations (30); and (v) mortality should be assessed at day 30 (22) or in a manner that best represents the underlying construct within the biologically plausible window of effect (18). Taking this into account, we analyzed both early (14-day) and late (30-day) mortality; our results suggest that 14-day mortality might be an adequate time frame for evaluating the impact of antimicrobial therapy in patients with BSI, since later mortality may be influenced more by other conditions. Our study provides data for patients with BSI irrespective of the etiology, acquisition type, source, patient characteristics, or service received. Other strengths of our study are its prospective and multicenter nature.
With regard to controls for confounding potentially caused by the severity of the patient's illness, we assessed both the severity of chronic underlying conditions, using the Charlson comorbidity index, and the acute severity of the illness the day before the onset of bacteremia, using the Pitt score, both of which have been used extensively as mortality predictors for patients with BSI and sepsis (2, 4, 10, 11, 14, 20, 23, 26, 27). We did not use more sophisticated scores, such as the APACHE II score, because much of the data required to calculate it are not available for non-ICU patients. There is controversy about the convenience of controlling for the severity of SIRS at presentation. Some authors consider this an intermediate variable (18), but we agree with others that it is an intermediate variable only for analyzing a prognosis associated with the causative microorganism, not for evaluating the impact of empirical therapy, since empirical therapy is administered after severe sepsis or shock has developed whenever the severity of SIRS is measured at presentation (22, 29). Other variables found to be predictors of mortality in our study included age, neutropenia, source, and the etiology of BSI. We think that it is crucial to control for these variables in future studies concerning the prognosis of BSI.
Beyond controlling for confounding by using multivariate analysis, we also used a propensity score-based analysis (7). As far as we know, this technique has been used in 4 previous studies to analyze the impact of empirical therapy. In 2 of these, the propensity score for each patient's likelihood of receiving inadequate empirical therapy was introduced into the multivariate models. Harbarth et al. found inadequate empirical therapy to be associated with increased 28-day mortality in 904 patients with microbiologically documented severe sepsis or shock (468 had bacteremia) (9). Lin et al. found that delaying active antimicrobial treatment was associated with increased 30-day mortality in bacteremic patients with severe neutropenia (16). Two other studies used the propensity score to conduct a matched cohort analysis. Kim et al. did not find that appropriate empirical therapy was independently associated with increased mortality after 12 weeks of follow-up in 238 patients with BSI due to S. aureus (13), although the statistical power of the study was limited. Paul et al. studied 920 patients with microbiologically documented infections, and by multivariate analysis, appropriate empirical therapy was associated with decreased 30-day mortality (OR, 0.63; 95% CI, 0.39 to 1.01); however, in the propensity-matched cohort (574 patients), the OR was 0.75 (95% CI, 0.44 to 1.27) (21). The mortality rate in that series was 14.3%, much lower than that in our study, which is probably explained by the fact that only 39.5% of patients were bacteremic.
Our study has some limitations. We cannot rule out the influence of unmeasured variables. The efficacies of the different empirical antibiotic regimens may not have been homogeneous. We did not measure other outcome variables, such as duration of hospital stay or the ecological impact of superfluous empirical regimens. The data were obtained in a relatively short time and reflect sepsis and BSI treatment practices in one part of Spain in 2006-2007 that may not be the same in other parts of the world. Finally, even though antibiotic therapy is considered a key aspect in the management of patients with sepsis, inadequate empirical therapy might be just a surrogate marker for poor quality of care of patients with sepsis.
These results emphasize the importance of providing early adequate empirical therapy to all patients suspected of BSI. To achieve this, several conditions must be met (24). First, patients at risk of BSI must be recognized promptly. Second, features that may influence the etiology of infection should be assessed, including the patient's predisposing conditions, the severity of SIRS, and the potential source of infection. Third, appropriate antibiotic guidelines, adapted to the local epidemiology, must be followed. Finally, whenever empirical therapy is found to be inadequate or superfluous, treatment should be adjusted as soon as possible. We recently proposed some quality indicators that would be useful in analyzing the impact of interventions aimed at improving the management of patients with bacteremia (24).
ACKNOWLEDGMENTS
This study was funded by the Consejería de Salud, Junta de Andalucía (0063/2006 and PI0048/2008), the Fondo de Investigación Sanitaria (PI10/02021), and the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, cofinanced by the “A Way to Achieve Europe” European Development Regional Fund, Spanish Network for Research in Infectious Diseases (REIPI RD06/0008).
The funding organizations had no role in design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
We thank the physicians, nurses, and staff of the participating hospitals for facilitating the collection of data.
J.R.-B. has been a member of advisory boards for Merck, Pfizer, Novartis, and Janssen, has served as a speaker for Merck, Pfizer, Novartis, Astra-Zeneca, and Janssen, and has received research support from Novartis. All other authors declare no conflicts of interest.
Members of the SAEI/SAMPAC Bacteremia Group in Spain include Clara Natera, Servicio de Microbiología, Hospital Universitario Reina Sofía, Córdoba; Enrique Nuño, Unidad Clínica de Enfermedades Infecciosas y Microbiología, Hospital Universitario Virgen de la Victoria, Málaga; Marta Herrero, Unidad Clínica de Enfermedades Infecciosas, Microbiología y Medicina Preventiva, Hospital Universitario Virgen del Rocío, Seville; Fernando Fernández-Sánchez, Servicio de Microbiología, Hospital Costa del Sol, Marbella, Málaga; María J. Pérez-Santos, Servicio de Microbiología, Hospital de la Serranía, Ronda, Málaga; Francisco Téllez, Unidad de Enfermedades Infecciosas, Hospital de La Línea, Cádiz; Berta Becerril, Unidad Clínica de Enfermedades Infecciosas y Microbiología, Hospital Punta de Europa, Algeciras, Cádiz; Ana García-Tapia, Servicio de Microbiología, Hospital Puerta del Mar, Cádiz; Inmaculada Carazo, Servicio de Microbiología, Complejo Hospitalario de Jaén, Jaén; Juan C. Alados, Servicio de Microbiología, Hospital de Antequera, Málaga; Raquel Moya, Unidad Clínica de Microbiología y Enfermedades Infecciosas, Hospital del SAS, Jerez de la Frontera, Cádiz; Carmen Florez, Unidad Clínica de Enfermedades Infecciosas y Microbiología, Hospital Universitario de Valme, Seville; Petra Navas, Servicio de Microbiología, Hospital Torrecárdenas, Almería; and Leopoldo Muñoz, Unidad de Enfermedades Infecciosas, Hospital Universitario San Cecilio, Granada.
Footnotes
Published ahead of print 17 October 2011
REFERENCES
- 1. Arias CA, Murray BE. 2009. Antibiotic-resistant bugs in the 21st century: a clinical super-challenge. N. Engl. J. Med. 360:439–443 [DOI] [PubMed] [Google Scholar]
- 2. Chang FY, et al. 2003. Staphylococcus aureus bacteremia. Recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore, MD) 82:323–329 [DOI] [PubMed] [Google Scholar]
- 3. Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383 [DOI] [PubMed] [Google Scholar]
- 4. Chow JW, et al. 1991. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann. Intern. Med. 115:585–590 [DOI] [PubMed] [Google Scholar]
- 5. Cisneros-Herreros JM, Cobo-Reinoso J, Pujol-Rojo M, Rodríguez-Baño J, Salavert-Lletí M. 2007. Guía para el diagnóstico y tratamiento del paciente con bacteriemia. Guías de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC). Enferm. Infecc. Microbiol. Clin. 25:111–130 [DOI] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing. 20th informational supplement. Approved standard M100–S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. D'Agostino RB., Jr 1998. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 17:2265–2281 [DOI] [PubMed] [Google Scholar]
- 8. Friedman ND, et al. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 130:791–797 [DOI] [PubMed] [Google Scholar]
- 9. Harbarth S, et al. 2003. Inappropriate initial antimicrobial therapy and its effect on survival on a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 115:529–535 [DOI] [PubMed] [Google Scholar]
- 10. Hilf M, et al. 1989. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am. J. Med. 87:540–546 [DOI] [PubMed] [Google Scholar]
- 11. Hill PC, et al. 1991. Prospective study of 424 cases of Staphylococcus aureus bacteremia: determination of factors affecting incidence and mortality. Intern. Med. J. 31:97–103 [PubMed] [Google Scholar]
- 12. Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36:309–332 [DOI] [PubMed] [Google Scholar]
- 13. Kim SH, et al. 2006. Outcome of inappropriate empirical antibiotic therapy in patients with Staphylococcus aureus bacteraemia: analytical strategy using propensity scores. Clin. Microbiol. Infect. 12:13–21 [DOI] [PubMed] [Google Scholar]
- 14. Lesens O, et al. 2003. Role of comorbidity in mortality related to Staphylococcus aureus bacteremia: a prospective study using the Charlson weighted index of comorbidity. Infect. Control Hosp. Epidemiol. 24:890–896 [DOI] [PubMed] [Google Scholar]
- 15. Levy MM, et al. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference. Crit. Care Med. 31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 16. Lin MY, Weinstein RA, Hota B. 2008. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob. Agents Chemother. 52:3188–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loza Fernández de Bobadilla E, Planes Reig A, Rodríguez-Creixems M. 2003. Hemocultivos. In Procedimientos en Microbiología Clínica. Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica, Madrid, Spain: http://www.seimc.org/documentos/protocolos/microbiologia Accessed 30 March 2010 [Google Scholar]
- 18. McGregor JC, et al. 2007. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin. Infect. Dis. 45:329–337 [DOI] [PubMed] [Google Scholar]
- 19. Paterson DL, Rice LB. 2003. Empirical antibiotic choice for the seriously ill patient: are minimization of selection of resistant organisms and maximization of individual outcome mutually exclusive? Clin. Infect. Dis. 36:1006–1012 [DOI] [PubMed] [Google Scholar]
- 20. Paterson DL, et al. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial infections. Ann. Intern. Med. 140:26–32 [DOI] [PubMed] [Google Scholar]
- 21. Paul M, Fraser AA, Leibovici L. 2007. Propensity-matched analysis of appropriate empirical antibiotic treatment. Clin. Infect. Dis. 44:1251–1252 [DOI] [PubMed] [Google Scholar]
- 22. Paul M, et al. 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob. Agents Chemother. 54:4851–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhee JY, et al. 2009. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock 31:146–150 [DOI] [PubMed] [Google Scholar]
- 24. Rodríguez-Baño J, de Cueto M, Retamar P, Gálvez-Acebal J. 2010. Current management of bloodstream infections. Expert Rev. Anti Infect. Ther. 8:815–819 [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez-Baño J, et al. 2010. Epidemiology and clinical features of community-acquired, healthcare associated and nosocomial bloodstream infections in tertiary and community hospitals. Clin. Microbiol. Infect. 16:1408–1413 [DOI] [PubMed] [Google Scholar]
- 26. Rodríguez-Baño J, et al. 2003. Bacteriemias por Acinetobacter baumannii: características clínicas y pronósticas. Enferm. Infecc. Microbiol. Clin. 21:242–247 [DOI] [PubMed] [Google Scholar]
- 27. Rodríguez-Baño J, et al. 2010. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin. Infect. Dis. 50:40–48 [DOI] [PubMed] [Google Scholar]
- 28. Rodríguez-Creixems M, et al. 2008. Bloodstream infections. Evolution and trends in the microbiology workload, incidence, and etiology, 1985–2006. Medicine 87:234–249 [DOI] [PubMed] [Google Scholar]
- 29. Soriano A, Martínez JA, Mensa J. 2008. Septic shock should be included in multivariable models analysing the effect of empirical antibiotic therapy on mortality. Clin. Infect. Dis. 46:1484–1485 [DOI] [PubMed] [Google Scholar]
- 30. von Elm E, et al. 2008. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61:344–349 [DOI] [PubMed] [Google Scholar]