Abstract
New broad-spectrum β-lactamases such as KPC enzymes and CTX-M-15 enzymes threaten to markedly reduce the utility of our armamentarium of β-lactam agents, even our most potent drugs, such as carbapenems. NXL104 is a broad-spectrum non-β-lactam β-lactamase inhibitor. In this evaluation, we examined organisms carrying defined β-lactamases and identified doses and schedules of NXL104 in combination with the new cephalosporin ceftaroline, which would maintain good bacterial cell kill and suppress resistance emergence for a clinically relevant period of 10 days in our hollow-fiber infection model. We examined three strains of Klebsiella pneumoniae and one isolate of Enterobacter cloacae. K. pneumoniae 27-908M carried KPC-2, SHV-27, and TEM-1 β-lactamases. Its isogenic mutant, K. pneumoniae 4207J, was “cured” of the plasmid expressing the KPC-2 enzyme. K. pneumoniae 24-1318A carried a CTX-M-15 enzyme, and E. cloacae 2-77C expressed a stably derepressed AmpC chromosomal β-lactamase. Dose-ranging experiments for NXL104 administered as a continuous infusion with ceftaroline at 600 mg every 8 h allowed identification of a 24-h area under the concentration-time curve (AUC) for NXL104 that mediated bactericidal activity and resistance suppression. Dose fractionation experiments identified that “time > threshold” was the pharmacodynamic index linked to cell kill and resistance suppression. Given these results, we conclude that NXL104 combined with ceftaroline on an 8-hourly administration schedule would be optimal for circumstances in which highly resistant pathogens are likely to be encountered. This combination dosing regimen should allow for optimal bacterial cell kill (highest likelihood of successful clinical outcome) and the suppression of resistance emergence.
INTRODUCTION
Over the last decade, a number of new β-lactamases have emerged, which have put much of our antimicrobial chemotherapy armamentarium in jeopardy. Enzymes such as CTX-M-15 have spread worldwide (8) and are able to hydrolyze many of the later-generation cephalosporins. Over the course of the last decade, we have come to depend on carbapenems as the agents for treating our most resistant hospital-acquired Gram-negative infections. Unfortunately, KPC enzymes have emerged in many cities across the United States (9) and have made the organisms carrying these enzymes very resistant to carbapenems. A longer-term problem is represented by AmpC-type β-lactamases (7), which reside on the chromosome of many Gram-negative organisms (generally, the SPICE organisms: Serratia marcescens, Pseudomonas aeruginosa, indole-positive Proteeae, Citrobacter spp., and Enterobacter spp.), which can hydrolyze many cephalosporins and penicillins.
One way to restore the potency of these β-lactam agents is to coadminister a second agent to serve as a β-lactamase inhibitor. There are multiple examples of such successful combinations, such as ticarcillin-clavulanate, amoxicillin-clavulanate, ampicillin-sulbactam, and piperacillin-tazobactam. Each of these combinations has a history of good clinical utility. Unfortunately, the clavams and the currently extant penicillanic acid sulfones do not inhibit newer enzymes, especially KPC enzymes, and also have little to no inhibitory potential for the AmpC-type β-lactamases.
NXL104 is a new non-β-lactam β-lactamase inhibitor with the ability to inhibit a broad spectrum of enzymes, including these newer and troublesome enzymes. Little has been done in the past to identify the pharmacodynamically linked index for β-lactamase inhibition (10). It was the purpose of this series of studies to identify the pharmacodynamically linked index for enzyme inhibition for NXL104 and to identify a regimen of NXL104 plus ceftaroline that would provide robust activity against several isolates of Klebsiella pneumoniae and Enterobacter cloacae expressing these clinically problematic enzymes.
MATERIALS AND METHODS
Microorganisms.
All organisms studied were kindly supplied by JMI Laboratories (North Liberty, IA): K. pneumoniae 27-908M, a bacterium that expresses KPC-2, SHV-27, and TEM-1 β-lactamases; K. pneumoniae 24-1318A (carrying a CTX-M-15 β-lactamase); and Enterobacter cloacae 2-77C (carrying an AmpC β-lactamase). A third K. pneumoniae strain, 4207J, was used as a control for some of the hollow-fiber experiments. This strain is isogenic to K. pneumoniae 27-908M; it was cured of the KPC-2-containing plasmid but still possesses the genes blaTEM-1 and blaSHV-27.
MIC determination.
MIC values were determined by Clinical and Laboratory Standards Institute microdilution broth and agar dilution methodologies for ceftaroline alone and in combination with NXL104, using a fixed concentration of 4 mg of NXL104/liter, as well as in a doubling-dilution series for both drugs. The mutation frequency to resistance was estimated by plating 5 ml of log-phase growth suspensions of each strain onto agar containing 3× the baseline MIC of ceftaroline alone and with 4 mg of NXL104/liter. The concentration of microbes in the bacterial suspension was determined by quantitative cultures. The ratio of these provided the estimate of the subpopulation frequency to resistance. This was done on at least three occasions. From each resistance plate, at least three colonies were randomly picked and tested for change in the MIC from the baseline.
Hollow-fiber infection model.
The hollow-fiber bioreactor system (HFS) was first described by Blaser et al. (2) for use as a pharmacodynamic system for bacteria and for HIV pharmacodynamic studies by Bilello et al. (1, 4, 5). A schematic diagram of the system and a description of its use were presented previously in our study of Mycobacterium tuberculosis (6).
The specific dosages/exposures and schedules of administration for ceftaroline and NXL104 (both supplied by Cerexa, Inc., Oakland, CA) that were simulated in individual hollow-fiber experiments are described in Results in order to minimize repetition of information. Ceftaroline was administered to simulate the free (non-protein-bound) human serum concentration-time profile for a regimen of 600 mg given every 8 h (q8h; except for one experiment, where a regimen of a 900-mg dose given q12h was simulated). For dose-ranging experiments, NXL104 was administered by continuous infusion. The continuous-infusion design for NXL104 allowed the effective 24-h area under the concentration-time curve (AUC) to be identified. The dynamically linked index could then be most easily identified in dose fractionation experiments. In dose fractionation experiments, NXL104 was administered as a continuous infusion, the whole exposure was administered once daily, half the exposure was administered twice daily, and one-third of the total exposure was administered every 8 h. Both drugs were administered with a 2.5-h half-life.
The inoculum was prepared by growing three medium-sized overnight-grown colonies of the organisms cited above in cation-adjusted Mueller-Hinton II broth (Ca-MHB) at 35°C. Hollow-fiber systems were maintained at 35°C in a humidified incubator. Approximately 15 ml of bacterial culture in late-log-phase growth (1.5 × 107 to 1.5 × 108 CFU/ml) was infused into hollow-fiber cartridges.
Treatment was given for 10 days. Over the first 48 h of each experiment, serial samples of media were collected from each hollow-fiber system, and the concentrations of ceftaroline and/or NXL104 were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) to validate that the targeted concentration-time profiles for each drug were achieved (see below). At 0, 5, and 24 h after treatment initiation and then on days 2, 3, 4, 6, 8, and 10, bacterial samples were collected from each hollow-fiber system. The bacterial samples were washed twice to prevent drug carryover and were then quantitatively cultured onto antibiotic-free agar to enumerate the effect of each treatment regimen on the total bacterial population. A portion of each bacterial sample was also quantitatively cultured onto agar supplemented with 3× the MIC of the combination of ceftaroline plus NXL104 to define the effect of each regimen on the less susceptible bacterial populations (on one occasion, 1.5× the MIC was used in error).
Nitrocefin assay for measurement of β-lactamase.
A nitrocefin assay was obtained from Calbiochem (catalog no. 484400; Darmstadt, Germany). Using the spectrophotometric assay to determine the β-lactamase activity, the working solution of nitrocefin was prepared by dissolving 1 mg of nitrocefin in 100 μl of dimethyl sulfoxide, followed by dilution with 1.9 ml of phosphate buffer (100 mM, pH 7), to produce a 2-ml total volume. The working solution of nitrocefin was then diluted 10-fold in phosphate buffer (0.1 M phosphate, 1 mM EDTA; pH 7.0), and 20 μl was added to 100 μl of each sample. The samples were then incubated at 35°C for 30 min, after which the optical density is read at 490 nm using a Spectromax M5 spectrophotometer (Molecular Devices, Sunnyvale, CA).
A standard curve was prepared by growing the bacteria overnight on tryptic soy agar plus 5% sheep blood overnight at 35°C. Three to four colonies were placed in a flask containing Mueller-Hinton II broth at 35°C with shaking at 100 rpm. Samples of supernatant were collected every 30 min, and then the β-lactamase standard curve was generated by using the nitrocefin assay.
Samples of supernatant were collected at each hollow-fiber quantitative sample time point. The amount of β-lactamase in each sample was quantitated by using the standard curve generated previously.
Analytical method.
The LC-MS/MS method used to analyze NXL104 and ceftaroline is presented below as a summary of the high-pressure liquid chromatography (HPLC) and MS/MS parameters.
Instrumentation and settings. (i) LC system.
A Shimadzu CBM-20A controller with Shimadzu LC-20AD HPLC pumps, a Shimadzu DGU-20A degasser, a Shimadzu SIL-20AC autosampler, and a Shimadzu CTO-20A column oven were used. The column was a Thermo Scientific Hypersil Gold (5 μm, 150 by 4.6 mm). The mobile phase was 1.20-ml/min isocratic (split 1:1 before LC-MS/MS) 10 mM ammonium formate (pH 3.75), water, methanol, and isopropanol (100:780:80:40). The injection volume was 5 μl. The autosampler rinse was composed of 50:50 methanol-water.
(ii) MS system.
An Applied Biosystems/MDS Sciex API 5000 LC/MS/MS system with Analyst 1.4.1 software was used. The ionization mode was turbospray, with negative and positive ions. The nebulizer gases were nitrogen at a setting of 45 (gas 1) and nitrogen at a setting of 45 (gas 2). Curtain gas was nitrogen at a setting of 15, and the collision gas was nitrogen at a setting of 6.00. The nebulizer temperature was 550°C. The ion spray voltage was −4,000 V (negative ion) and 4,000 V (positive ion). The scan mode was multiple reaction monitoring (for NXL104: negative ion, 0 to 2.5 min; MS/MS transition, m/z 264 → m/z 96; for ceftaroline: positive ion, 2.5 to 5.0 min; MS/MS transition, m/z 605 → m/z 208).
The assay was linear over a range of 0.05 to 25.0 mg/liter (r2 > 0.995) for both NXL104 and ceftaroline. The interday coefficients of variation for the quality-control samples analyzed in replicates of three at three different concentrations (0.100, 1.00, and 10.0 mg/liter) on each analysis day were 4.85% or less for NXL104 and 5.89% or less for ceftaroline. Assay accuracies (percent recovery) ranged between 96.0 and 105% for NXL104 and between 95.0 and 108% for ceftaroline.
RESULTS
Experiments with K. pneumoniae 27-908M and 4207J. (i) Susceptibility studies.
Susceptibility studies of K. pneumoniae 27-908M and 4207J to ceftaroline and NXL104 were conducted alone and in combination using broth microdilution and agar dilution methods. Combination MICs were determined by using two dilution schemes. In the first method, MICs were determined using serial 2-fold dilutions of both drugs. In the second method, the concentration of NXL104 was fixed at 4 mg/liter, while the concentration of ceftaroline was serially diluted.
For K. pneumoniae 27-908M and 4207J, the MICs of ceftaroline alone were 1,024 and 4 mg/liter, respectively. The MIC of NXL104 was >64 mg/liter for both bacterial isolates. For susceptibility studies in which ceftaroline and NXL104 were examined in combination using geometric serial dilutions for both compounds, the MICs for K. pneumoniae 27-908M and 4207J were 0.75 and 4 mg/liter and 0.375 and 2 mg/liter, respectively. For susceptibility studies in which geometric serial dilutions of ceftaroline were examined with a fixed concentration of NXL104 of 4 mg/liter, the MICs for these strains were 0.75 and 4 mg/liter and 0.375 and 4 mg/liter, respectively.
(ii) Mutation frequency studies.
The mutation frequencies to 3× the baseline MICs for the combination of ceftaroline and NXL104 were −6.86 ± 0.06 log CFU for K. pneumoniae 27-908M in three trials and <−7.95 log CFU in one trial for K. pneumoniae 4207J.
Dose-range hollow-fiber study 1.
K. pneumoniae 27-908M and 4207J were examined in this dose-ranging study using an inoculum of 107 CFU/ml (15-ml volume) per hollow-fiber system arm. The experimental arms were as follows: (arm A) a no-treatment control with K. pneumoniae 27-908M (a KPC-2 expressing strain); (arm B) a no-treatment control with the strain cured of the KPC-2 plasmid (isolate 4207J); (arm C) ceftaroline alone against the plasmid-containing strain, K. pneumoniae 27-908M; (arm D) ceftaroline alone against the plasmid-cured strain, K. pneumoniae 4207J; (arm E) ceftaroline plus NXL104 at 2 mg/liter given as a continuous infusion versus strain 27-908M; (arm F) ceftaroline plus NXL104 at 4 mg/liter given as a continuous infusion versus strain 27-908M; (arm G) ceftaroline plus NXL104 at 6 mg/liter given as a continuous infusion versus strain 27-908M; (arm H) ceftaroline plus NXL104 at 8 mg/liter given as a continuous infusion versus strain 27-908M; (arm I) NXL104 at 8 mg/liter alone with the plasmid-containing strain, 27-908M; and (arm J) NXL104 8 mg/liter alone with the plasmid-cured strain, 4207J.
For experimental arms in which ceftaroline was used, the non-protein-bound human concentration-time profile reported for ceftaroline at 600 mg given q8h was simulated. The targeted non-protein-bound (free) Cmax was 22.3 mg/liter. The simulated serum half-life was 2.5 h, and the free 24-h AUC was 266.35 mg · h/liter.
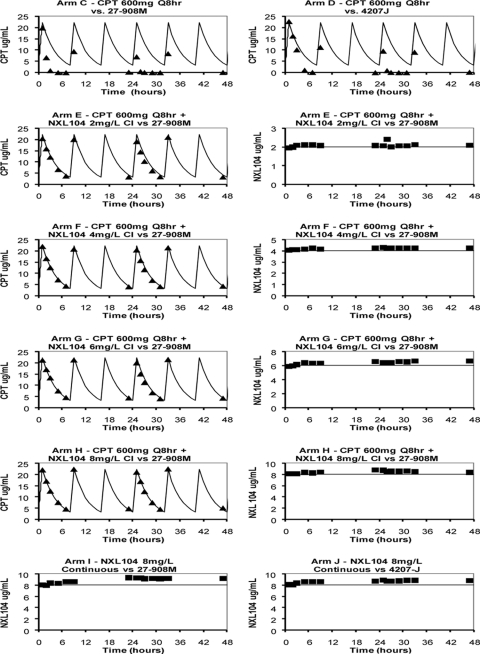
The targeted pharmacokinetic (PK) profiles for the ceftaroline and NXL104 regimens were well simulated for regimens in which ceftaroline and NXL104 were administered together (Fig. 1, arms E to H). In hollow-fiber systems where ceftaroline was administered as a single agent, the concentrations of this drug progressively decreased and were lower than the targeted values (Fig. 1, arms C and D). Measurement of the pharmacokinetic profile for ceftaroline before these hollow-fiber system arms were inoculated with bacteria showed that the targeted concentration-time profiles for ceftaroline were simulated correctly (data not shown). Measurement of β-lactamase concentrations in the media in these arms after the systems were inoculated with K. pneumoniae using a quantitative nitrocefin assay showed a rapid increase in β-lactamase content in these arms (data not shown). It is likely that these β-lactamases inactivated a fraction of the cephalosporin antibiotic, resulting in ceftaroline exposures that were significantly lower than targeted. Of note, β-lactamase activity was not detected in arms in which ceftaroline was administered in combination with NXL104 and in arms in which NXL104 was given as a single agent (data not shown).
Fig 1.
Pharmacokinetic profiles for the first dose-ranging hollow-fiber experiment (K. pneumoniae 27-908M and 4207J). Targeted (solid curves) and measured concentration-time profiles for ceftaroline (triangles) and NXL104 (squares) in hollow-fiber systems are shown. For experimental arms that received both drugs (arms E to H), the PK profiles for each drug are shown in separate graphs for clarity. Arms A and B are not shown because they were the nontreatment controls for K. pneumoniae 27-908M and 4207J. For experimental arms in which the bacterial strain is not stated, the KPC-2 β-lactamase-producing strain, K. pneumoniae 27-908M, was examined (i.e., arms E through I). CPT, ceftaroline.
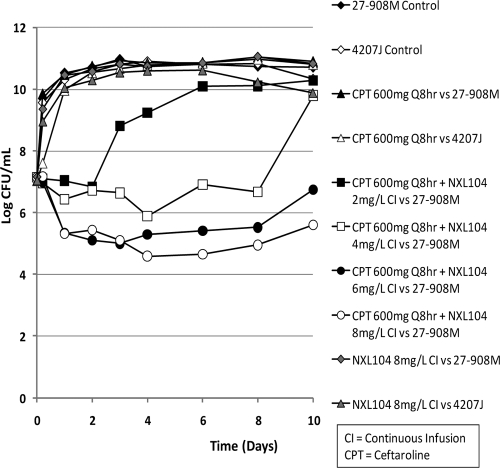
Bacteria in the no-treatment control arms grew well. Simulated exposures for ceftaroline at 600 mg q8h as a monotherapy were ineffective against both the KPC-2-containing (27-908M) and the plasmid-cured (4207J) K. pneumoniae strains (Fig. 2). The K. pneumoniae strains that were treated with 8 mg of NXL104/liter, given as a continuous infusion as a single agent, had growth profiles that were similar to those of the no-treatment controls.
Fig 2.
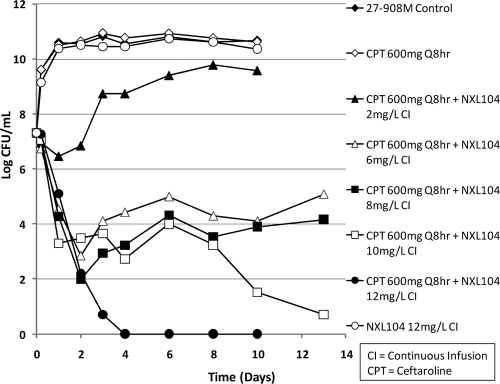
First dose-ranging hollow-fiber study (HFS) for ceftaroline alone and in combination with a continuous infusion of NXL104 against K. pneumoniae 27-908M, a strain that produces TEM-1, SHV-27, and KPC-2 β-lactamases, and an isogenic strain, K. pneumoniae 4207J (cured of the KPC-2 carrying plasmid but still expressing TEM-1 and SHV-27 β-lactamases). The effect of each regimen on the total bacterial population is shown. K. pneumoniae 4207J was used in the second, fourth, and tenth experimental arms. All other arms used K. pneumoniae 27-908M.
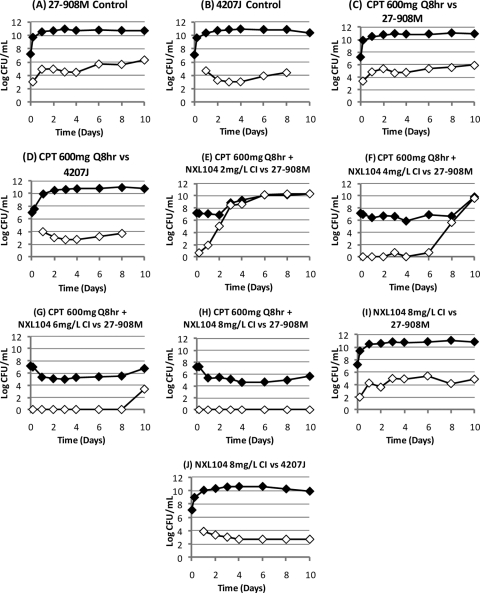
In contrast, the simulated regimen of ceftaroline at 600 mg q8h in combination with incremental levels of NXL104 given as continuous infusions showed an exposure-response relationship (Fig. 2). Ceftaroline in combination with a continuous infusion of 2 mg of NXL104/liter failed by day 3 of therapy. Ceftaroline in combination with 4 mg of NXL104 (continuous infusion)/liter failed by day 10 of treatment. The regimens of ceftaroline in combination with continuous infusions of 6 and 8 mg of NXL104/liter initially killed ∼1.7 log CFU of bacteria/ml relative to the 0-h value. The bacterial densities in these hollow-fiber arms remained at a concentration of ∼5 log CFU/ml until at least day 8 of therapy. The quantitative culture results on day 10 suggested that the bacterial concentrations at this time point were increasing. Plating of a portion of the bacterial suspensions on both antibiotic-free and antibiotic-supplemented agar showed that the slight upswing in the total bacterial population in the arm treated with ceftaroline in combination with a continuous infusion of 6 mg of NXL104/liter was associated with amplification of resistant mutants (Fig. 3). Resistance amplification was not seen in the experimental arm in which ceftaroline was used in combination with a continuous infusion of 8 mg of NXL104/liter.
Fig 3.
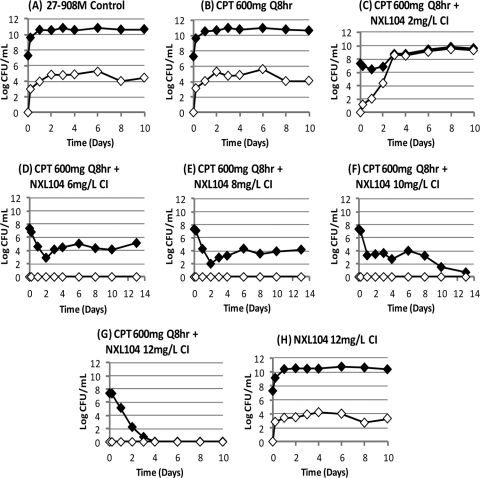
Effect of each treatment regimen on the total bacterial population (black diamond) and the subpopulation with MICs that were ≥3× the baseline MIC of ceftaroline in combination with NXL104 (white diamond) for the first dose-ranging study. The baseline MICs of ceftaroline/NXL104 for K. pneumoniae 27-908M were 0.75/4 mg/liter. For K. pneumoniae 4207J, the MICs were 0.375/4 mg/liter for this antibiotic combination. CPT, ceftaroline.
Susceptibility studies were conducted on a subset of the bacterial colonies that grew on agar that were supplemented with 3× the baseline MIC of ceftaroline in combination with NXL104. Experimental arms that yielded these isolates included the control arms and the treatment regimens that failed. The baseline ceftaroline/NXL104 MICs for the parent K. pneumoniae 27-908M isolate that was inoculated into the hollow-fiber systems were 0.75/4 mg/liter. Strains amplified with suboptimal ceftaroline/NXL104 regimens had MICs of 1.5/8 to >6/32 mg/liter when susceptibility studies were conducted using serial dilutions of both drugs. When susceptibilities were determined using a fixed concentration of NXL104 (4 mg/liter), the ceftaroline/NXL104 MICs increased from 0.75/4 mg/liter at baseline to 1.5/4 to >24/4 mg/liter.
Dose-range hollow-fiber study 2 (for K. pneumoniae 27-908M only).
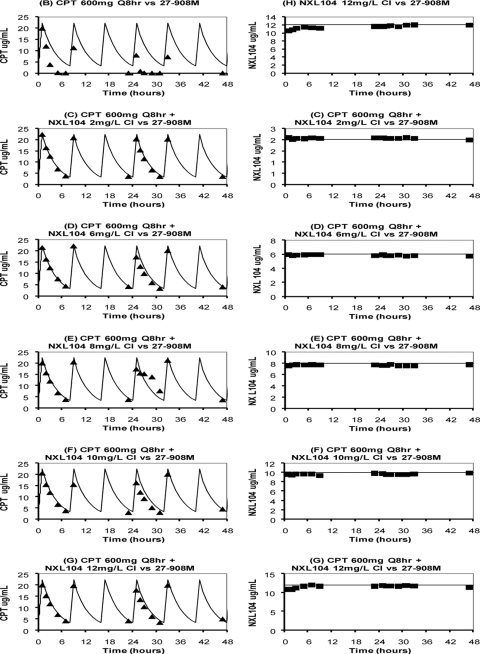
Because it was uncertain whether a continuous infusion of NXL104 at 8 mg/liter in combination with ceftaroline would have failed if treatment was extended beyond 10 days of combination therapy, the duration of therapy examined in the second dose-ranging hollow-fiber experiment was extended from 10 to 13 days for arms that had not failed by day 10 of treatment. Also, the range of concentrations of NXL104 was extended from a maximal concentration of 8 mg/liter in the first dose-ranging experiment to 12 mg/liter in the second dose-ranging study. As with the first dose-ranging study, NXL104 was administered as a continuous infusion alone and together with a simulated regimen for ceftaroline at 600 mg given q8h. The bacterial inoculum for each hollow-fiber arm was similar to the first dose-range experiment (107 CFU/ml, 15-ml volume).
In this repeat dose-range study, only K. pneumoniae 27-908M, the strain that possessed the KPC-2 plasmid, was evaluated. The experimental arms for the second dose-ranging hollow-fiber experiment were as follows: (arm A) no-treatment control; (arm B) ceftaroline at 600 mg q8h as monotherapy; (arm C) ceftaroline at 600 mg q8h plus NXL104 at 2 mg/liter as a continuous infusion; (arm D) ceftaroline at 600 mg q8h plus NXL104 at 6 mg/liter as a continuous infusion; (arm E) ceftaroline at 600 mg q8h plus NXL104 at 8 mg/liter as a continuous infusion; (arm F) ceftaroline at 600 mg q8h plus NXL104 at 10 mg/liter as a continuous infusion; (arm G) ceftaroline at 600 mg q8h plus NXL104 at 12 mg/liter as a continuous infusion; and (arm H) NXL104, 12 mg/liter continuous infusion, as monotherapy.
In the first dose-ranging study, the concentration-time profiles for ceftaroline were lower than expected in arms that received this drug as monotherapy. In an attempt to correct for the possible effect of preexisting β-lactamases inactivating ceftaroline early in the treatment course, the bacterial suspension used in the second dose-ranging study was washed twice with warm medium before it was inoculated into the hollow-fiber systems.
The targeted and measured PK profiles for the regimens simulated within the hollow-fiber systems are shown in Fig. 4. The targeted concentration-time profiles were well achieved in the hollow-fiber systems in which ceftaroline was administered together with NXL104 and in systems that received NXL104 as a single agent. In the experimental arm in which only ceftaroline was given, washing the bacterial suspension prior to inoculating it into hollow-fiber systems resulted in a slightly higher concentration of ceftaroline with the first administered dose, but the concentrations of drug rapidly decreased with subsequent drug infusions (Fig. 4). Increases in β-lactamase levels in the media of these experimental arms correlated with the marked reductions in ceftaroline exposures that were observed (data not shown).
Fig 4.
Pharmacokinetic profiles for the second dose-ranging hollow-fiber experiment (for K. pneumoniae 27-908M only). Targeted (solid curves) and measured concentration-time profiles for ceftaroline (triangles) and NXL104 (squares) in hollow-fiber systems are shown. For experimental arms that received both drugs (arms C to G), the PK profiles for each drug are shown in separate graphs for clarity. The bacterial suspension was washed to remove preformed β-lactamases before it was inoculated into hollow-fiber systems. Arm A is not shown because this arm was the nontreatment control for K. pneumoniae 27-908M. CPT, ceftaroline; CI, continuous infusion.
Bacteria in the nontreated control arm grew well. Regimens of ceftaroline in combination with 0 to 12 mg of NXL104/liter showed a clear exposure-response effect. Bacteria in the ceftaroline monotherapy arm grew in parallel with the control arm (Fig. 5). Ceftaroline in combination with a continuous infusion of 2 mg/liter of NXL104 failed by day 3 of therapy. Failure was due to amplification of less-susceptible bacterial populations (Fig. 6).
Fig 5.
Second 10-day dose-ranging hollow-fiber study (HFS) using only K. pneumoniae strain 27-908M, the KPC-2-producing isolate. This strain also has the genes that encode TEM-1 and SHV-27 β-lactamases. The bacterial suspension was washed to remove preformed β-lactamases before it was inoculated into hollow-fiber systems. The effect of each regimen on the total bacterial population is shown. Experimental arms that had not failed (noted by outgrowth) by day 10 were continued to day 13 to better define the effect of their treatment regimens on microbiological outcomes. In this experiment, NXL104 was given as a continuous infusion.
Fig 6.
Effect of each treatment regimen on the total bacterial population (black diamond) and the subpopulation with MICs that were ≥3× the baseline MIC of K. pneumoniae 27-908M (white diamond). CPT, ceftaroline; CI, continuous infusion.
Ceftaroline plus 6 mg of NXL104/liter resulted in a 2- to 3-log CFU/ml reduction in bacterial counts compared to the 0-h time point. In the first dose-ranging study, this regimen selected for resistance. However, in the second dose-ranging experiment, this arm did not amplify the less-susceptible bacterial population. Ceftaroline in combination with 8 mg of NXL104/liter showed a 3-log CFU/ml reduction in bacterial density after day 10 of therapy and did not select for resistance.
The combination of ceftaroline and 10 mg of NXL104 (continuous infusion)/liter showed a two-stage decrease in bacterial densities. A 4-log CFU/ml reduction in bacterial counts (compared to 0-h values) was seen from days 1 to 8 of therapy. Thereafter, the bacterial count in this experimental arm progressively decreased such that a reduction of >6-log CFU/ml in bacterial density was seen by day 13 of therapy (Fig. 5). The combination of ceftaroline and a continuous infusion of 12 mg of NXL104/liter performed best. It decreased the bacterial counts in its hollow-fiber system to below the limit of reliable quantification of bacterial density (2-log CFU/ml) by day 4 of treatment. After 13 days of therapy, the entire volume of medium within the hollow-fiber system was cultured for bacteria. These cultures were sterile, showing that this regimen eradicated the bacterial population.
Suboptimal regimens failed due to amplification of subpopulations of K. pneumoniae 27-908M with decreased susceptibilities to ceftaroline/NXL104. The ceftaroline/NXL104 MICs for these strains were 1.5/8 mg/liter compared to baseline MICs of 0.75/4 mg/liter when susceptibility testing was conducted using serial dilutions of both drugs. For susceptibility studies in which the concentration of NXL104 was fixed at 4 mg/liter, the mutants amplified with suboptimal treatment regimens had ceftaroline/NXL104 MICs of 1.5/4 to 24/4 mg/liter.
The two dose-ranging studies demonstrated an exposure-response relationship for incremental dosages of NXL104 when examined in combination with a fixed ceftaroline regimen. Ceftaroline monotherapy was not active against the KPC-2-producing K. pneumoniae 27-908M, or its isogenic strain in which the KPC-2 plasmid was cured but SHV-27 and TEM-1 remained. Also, for K. pneumoniae 27-908M, simulated regimens of ceftaroline at 600 mg q8h, when given alone and in combination with continuous infusions of NXL104 at ≤4 mg/liter, failed due to the selection of resistant mutants. Ceftaroline in combination with a continuous infusion of 6 mg of NXL104/liter failed in one trial but succeeded in the second trial. Regimens of ceftaroline at 600 mg q8h in combination with continuous infusions of ≥8 mg of NXL104/liter consistently killed the bacterial population and did not amplify the more resistant bacterial subpopulations.
Dose fractionation hollow-fiber study for NXL104 against K. pneumoniae 27-908M.
In the two dose-range studies, the continuous infusion of 8 mg of NXL104/liter in combination with ceftaroline at 600 mg q8h consistently succeeded in killing the K. pneumoniae strain that expressed KPC-2 β-lactamase (Fig. 2 and 5) and did not amplify for resistance. In the dose fractionation study for NXL104, simulated concentration-time profiles for ceftaroline at 600 mg q8h were examined in combination with different schedules of NXL104 administration. Each schedule of NXL104 administration generated approximately the same 24-h AUC associated with a continuous infusion at a concentration of 8 mg/liter (24-h AUC of 192 mg · h/liter).
For the dose fractionation study, the following regimens for NXL104 were given in combination with a simulated regimen of ceftaroline (600 mg q8h for all arms): (arm A) ceftaroline plus a continuous infusion of 8 mg of NXL104/liter (24-h AUC of 192 mg · h/liter); (arm B) ceftaroline plus NXL104 AUC of 192 mg · h/liter per dose given once q24h; (arm C) ceftaroline plus NXL104 AUC of 96 mg · h/liter per dose given q12h; and (arm D) ceftaroline plus NXL104 AUC of 64 mg · h/liter per dose given q8h.
In addition to the above dose fractionation regimens for NXL104, the following control arms were also tested in this dose fractionation experiment: (arm E) no-treatment control arm; (arm F) ceftaroline at 600 mg q8h, alone; (arm G) NXL104 at 8 mg/liter, administered as a continuous infusion; and (arm H) ceftaroline at 600 mg q8h plus NXL104 at a concentration of 2 mg/liter, given as a continuous infusion (failure control). The pharmacodynamic parameters associated with the ceftaroline and the fractionated regimens of NXL104 are shown in Table 1.
Table 1.
Free (non-serum-protein-bound) pharmacokinetic-pharmacodynamic values generated with the fractionated NXL104 regimensa
| Regimen | Free time-above MIC (mg/liter) | Free Cmax (mg · h/liter) | Free 24-h AUC (h/%) |
|---|---|---|---|
| Ceftaroline, 600 mg, q8h | 22.3 | 266 | 0/0 |
| Ceftaroline in combination with NXL104 (free 24-h AUC of 192 mg · h/liter) administered as: | |||
| Total daily dose q24h | 46.5 | 192 | 9.6/40 |
| One-half the total daily dose q12h | 24.0 | 192 | 14.8/62 |
| One-third the total daily dose q8h | 17.7 | 192 | 19.2/80 |
| Continuous infusion | 8.0 | 192 | 24.0/100 |
For K. pneumoniae 27-908M, the MIC for ceftaroline monotherapy was 1,048 mg/liter. The MICs of ceftaroline in combination with NXL104 were 0.75 mg/liter for CPT and 4 mg/liter for NXL104 (all NXL104 concentrations were fixed at 4 mg/liter). For the combination therapies, time ≥ MICs were calculated for the duration (hours and percentage of 24 h) in which the concentration of ceftaroline was ≥0.75 mg/liter and the concentration of NXL104 was ≥4 mg/liter.
The hollow-fiber experiment was to last for 10 days. However, we extended the experiment from 10 to 13 days for treatment arms that had not clearly failed by day 10 of therapy. The targeted concentration-time profiles for ceftaroline and NXL104 were well achieved in experimental arms in which ceftaroline was given in combination with NXL104 and when NXL104 was given alone (data not shown).
Bacteria in the ceftaroline monotherapy and the NXL104 monotherapy (8 mg/liter, continuous infusion) arms showed growth profiles that were similar to the no-treatment control arm. Ceftaroline in combination with 2 mg of NXL104/liter, given as a continuous infusion, showed a delay in time to failure, similar to what was seen in the dose-ranging experiments (Fig. 7).
Fig 7.
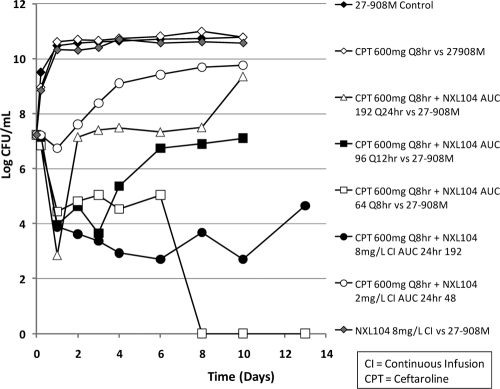
Dose fractionation study for NXL104 24-h AUC of 192 mg · h/liter in combination with a fixed regimen of ceftaroline at 600 mg q8h. The bacterium evaluated was K. pneumoniae 27-908M, the KPC-2 expression strain. The effect of each regimen on the total bacterial population is shown. For these experiments, the bacteria were washed to remove preformed β-lactamases prior to inoculating the bacteria into the hollow-fiber systems. Treatment arms that did not fail within 10 days of therapy were continued to day 13 to better delineate the effect of each regimen on microbiological outcomes. AUC, area under the concentration-time curve.
For the arms in which ceftaroline was administered in combination with different schedules of administration of NXL104 (all achieving a total daily AUC of 192 mg · h/liter for NXL104), giving the total daily dose as a single dose once daily failed by day 2 of treatment (Fig. 8C). The regimen in which one-half of the total daily dose was given every 12 h in combination with ceftaroline failed on day 4 of therapy (Fig. 7). Microbiological failure allowed the total population to return to baseline, but without a less-susceptible population being captured on antibiotic-containing agar plates (Fig. 8D).
Fig 8.
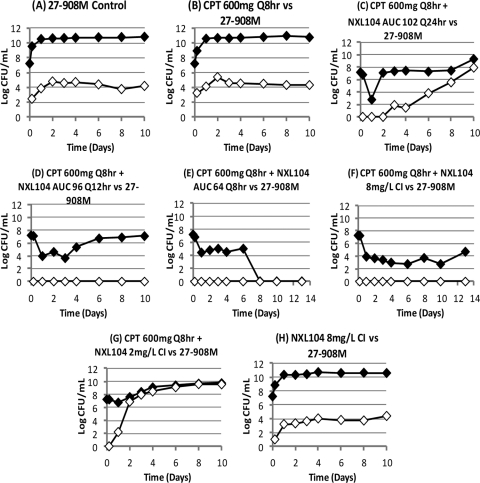
Dose fractionation study results for K. pneumoniae 27-908M: effect of each regimen on the total bacterial population (black diamond) and the bacterial subpopulation with MICs that were >3× the baseline MIC (white diamond) of the combination of ceftaroline (CPT) and NXL104. CI, continuous infusion.
In contrast, the regimens of ceftaroline in combination with the more fractionated schedules of administration (NXL104 given as three divided doses every 8 h and the total daily dose given over 24 h as a continuous infusion) were successful. These regimens reduced the total K. pneumoniae 27-908M population for the duration of the 10-day experiment and did not amplify the less susceptible bacterial subpopulations. Since the more fractionated regimens maximize the “time > threshold” (time that drug concentrations exceed a specific important concentration), the dose fractionation study demonstrated that “time > threshold” is the pharmacodynamic index linked with efficacy and with prevention of emergence of resistance during therapy. Based on the pharmacodynamic values presented in Table 1 (concentrations needed to be above the MIC for ceftaroline and the amount of NXL104 used in the in vitro MIC test [4 mg/liter]), the time-above-the-MIC value for NXL104 of between 14.8 and 19.2 h (or 62 to 80% of a dosing interval) was needed for treatment success when this β-lactamase inhibitor was used in combination with ceftaroline at 600 mg given q8h.
Hollow-fiber study with K. pneumoniae 24-1318A (CTX-M-15 β-lactamase).
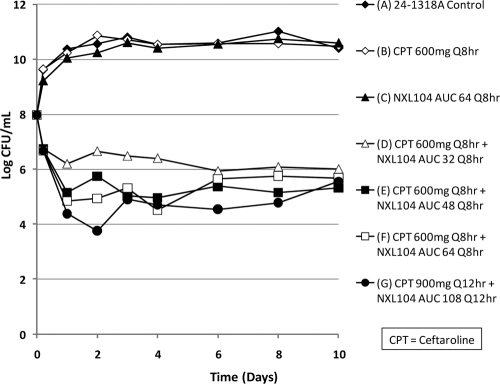
This hollow-fiber study examined the efficacy of ceftaroline in combination with NXL104 for the treatment of K. pneumoniae 24-1318A, a strain that expressed a CTX-M-15 β-lactamase. This bacterial strain had a ceftaroline MIC of 2,048 mg/liter and an NXL104 MIC of 16 mg/liter. For the combination of ceftaroline and NXL104, the MICs were 0.12/4 mg/liter when susceptibility testing was conducted using a serial 2-fold dilution of ceftaroline together with a fixed concentration of 4 mg of NXL104/liter. With a fixed 2:1 dilution scheme, the MICs were 1.0/0.5 mg/liter. The mutation frequency of this strain to 1.5× the MIC of ceftaroline in combination with 4 mg/liter of NXL104 was −6.07 log CFU. The design of the experiment follows: (arm A) control; (arm B) ceftaroline at 600 mg q8h as monotherapy; (arm C) NXL104 AUC 64 q8h as monotherapy; (arm D) ceftaroline at 600 mg q8h + NXL104 AUC 32 q8h; (arm E) ceftaroline at 600 mg q8h plus NXL104 AUC 48 q8h; (arm F) ceftaroline at 600 mg q8h plus NXL104 AUC 64 q8h; and (arm G) ceftaroline at 900 mg q12h plus NXL104 AUC 108 q12h. The starting inoculum in the hollow-fiber systems was 107 CFU/ml (15-ml volumes). Treatment was for 10 days.
The effects of each regimen on the total bacterial population are shown in Fig. 9. The monotherapies for ceftaroline and for NXL104 failed. Outgrowth in the ceftaroline monotherapy arm was due to mutants with ceftaroline/NXL104 MICs that were >3/4 mg/liter. All of the other regimens produced similar degrees of killing, although there was a bias toward slightly less antimicrobial effect for ceftaroline at 600 mg q8h in combination with NXL104 at 400 mg q8h, the lowest dosage of NXL104 examined. Ceftaroline in combination with NXL104 was effective for the treatment of a K. pneumoniae isolate that expressed a CTX-M-15 β-lactamase at the dosages simulated.
Fig 9.
Dose-range study for ceftaroline plus NXL104 on K. pneumoniae strain 24-1318A, a strain that produces the CTX-M-15 β-lactamase.
The targeted concentration-time profiles for ceftaroline in combination with NXL104 and for NXL104 as monotherapy were well simulated within the hollow-fiber systems. As has been reported above, the measured concentration-time profile simulated for ceftaroline as monotherapy was consistently less than the targeted profile due to degradation of the drug by the β-lactamase produced by the K. pneumoniae isolate (data not shown).
Dose fractionation hollow-fiber study examining the efficacy of ceftaroline and NXL104 against an E. cloacae strain that expressed an AmpC β-lactamase.
The MICs for this E. cloacae isolate (strain 2-77C) were as follows: ceftaroline, 1,024 mg/liter; and ceftaroline/NXL104, 0.75/4 mg/liter or 1/4 mg/liter when the NXL104 concentration was fixed at 4 mg/liter (depending on the ceftaroline dilution series) and 2/1 mg/liter using serial dilutions for both drugs at a 2:1 ratio.
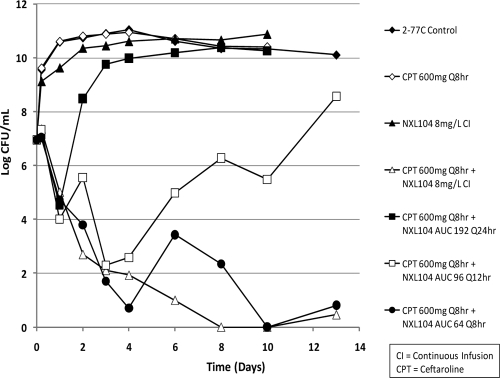
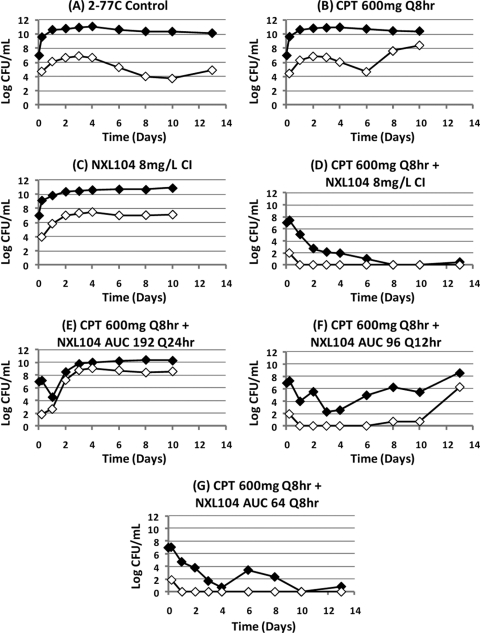
The experimental arms for this dose fractionation hollow-fiber study were as follows: (arm A) control; (arm B) NXL104 as an 8-mg/liter continuous infusion (continuous infusion produces an AUC24 of 192 mg · h/liter); (arm C) ceftaroline at 600 mg q8h alone; (arm D) ceftaroline at 600 mg q8h plus NXL104 as an 8-mg/liter continuous infusion (as in arm B); (arm E) ceftaroline at 600 mg q8h plus NXL104 (AUC 192 mg · h/liter per 24 h); (arm F) ceftaroline at 600 mg q8h plus NXL104 (AUC 96 mg · h/liter per 12 h); and (arm G) ceftaroline at 600 mg q8h plus NXL104 (AUC 64 mg · h/liter per 8 h).
Treatment was planned for 10 days. For experimental arms that had not failed by day 10, treatment was continued to day 13. The combination of ceftaroline and NXL104 was effective against this AmpC-producing bacterium when ceftaroline at 600 mg q8h was administered together with NXL104 either as a continuous infusion at 8 mg/liter or when the free 24-h AUC generated with this regimen (192 mg · h/liter) was given as three divided doses q8h (Fig. 10). Administration of NXL104 as a single dose once daily or as one-half the total daily dose every 12 h resulted in treatment failure. Failure was due to emergence of resistance (Fig. 11). Similar to the results of the dose fractionation study with the KPC-2-producing K. pneumoniae strain, the results of this dose fractionation study suggests that “time > MIC” is the pharmacodynamic index linked with treatment success through prevention of resistance.
Fig 10.
Dose fractionation study for ceftaroline plus NXL104 on E. cloacae 2-77C, a strain stably derepressed for the production of an AmpC β-lactamase. The effect of each regimen on the total bacterial population is shown.
Fig 11.
Effect of each regimen in the dose fractionation study on the total E. cloacae 2-77C bacterial population (black diamond) and the less-susceptible bacterial subpopulation (≥3× the MIC, white diamond).
Susceptibility studies were conducted for strains that were isolated from treatment arms that had failed. The ceftaroline/NXL104 MICs for the parent strain were 0.75/4 mg/liter when susceptibility testing was conducted using serial 2-fold dilutions for both drugs. Treatment failure was due to amplification of strains with ceftaroline/NXL104 MICs of 1.5/8 mg/liter.
For susceptibility studies in which the concentration of NXL104 was fixed at 4 mg/liter and serial 2-fold dilutions of ceftaroline were used, the MICs for the parent strain were 0.375/4 mg/liter for the ceftaroline/NXL104 combination. In the experimental arms in which treatment failed, for mutants that were amplified with suboptimal regimens the ceftaroline/NXL104 MICs ranged from 1.5/4 to 6.0/4 mg/liter.
DISCUSSION
Although β-lactamase inhibition has been used clinically for decades, little data have been generated for the optimization of bactericidal effect with regard to the pharmacodynamics of the inhibitors. Only Strayer et al. (10) have attempted to identify the dynamically linked index for an inhibitor, specifically, tazobactam. Unfortunately, only 6- and 8-h dose fractionations were performed, so the results were inconclusive. It was our hypothesis that NXL104 could inhibit even the worst set of new β-lactamases. To this end, we studied an organism (K. pneumoniae 27-908M) that elaborated a KPC-2 β-lactamase in addition to two other enzymes. This isolate had an MIC of 2,048 mg/liter for ceftaroline. We felt that identifying the dynamically linked index and exposure target for a fixed dose of ceftaroline in combination with different regimens of NXL104 in this isolate would identify a dose and schedule for NXL104 that would be able to inhibit the vast majority of other enzymes. We also studied an isolate of K. pneumoniae (strain 24-1318A) elaborating a CTX-M-15 enzyme, as well as a strain of E. cloacae elaborating a stably derepressed AmpC enzyme. In both, the ceftaroline plus NXL104 regimen that inhibited the isolate with the KPC-2 enzyme also inhibited the enzymes of these other isolates and generated good organism cell kill.
For the K. pneumoniae isolate that expressed the KPC-2, SHV-27, and TEM-1 enzymes, the two dose-ranging studies demonstrated an exposure-response relationship for incremental dosages of NXL104 when examined in combination with a fixed ceftaroline regimen. Ceftaroline monotherapy was ineffective against K. pneumoniae 27-908M (Kpc-2 enzyme-bearing strain), and the isogenic strain in which the KPC-2 plasmid was “cured.” For K. pneumoniae 27-908M, simulated regimens of ceftaroline at 600 mg q8h, when given alone and in combination with continuous infusions of NXL104 at ≤4 mg/liter, failed because of the inactivity of these exposures as monotherapies and as combination regimens. Ceftaroline in combination with a continuous infusion of 6 mg of NXL104/liter failed in one trial but succeeded in the second trial. Regimens of ceftaroline at 600 mg q8h in combination with continuous infusions of ≥8 mg of NXL104/liter consistently killed the bacterial population and did not amplify the more resistant bacterial subpopulations.
In the dose fractionation study for NXL104, simulated concentration-time profiles for ceftaroline at 600 mg q8h were examined in combination with different schedules of administration of NXL104. Each schedule of administration of NXL104 generated approximately the same 24-h AUC associated with giving this compound as a continuous infusion at a concentration of 8 mg/liter (AUC0-24 = 192 mg · h/liter). The pharmacodynamic parameters associated with ceftaroline and the fractionated regimens of NXL104 are shown in Table 1.
For the arms in which ceftaroline was administered in combination with different schedules of administration of NXL104 (total daily AUC of 192 mg · h/liter for NXL104), giving the total daily dose as a single dose once daily failed by day 2 of treatment. The regimen in which one-half of the total daily dose was given every 12 h in combination with ceftaroline failed on day 4 of therapy (Fig. 7). Although the total population returned to baseline values (ca. 107 CFU/ml), no organisms grew on antibiotic-containing agar plates infused with 3× the baseline MIC value for ceftaroline plus 4 mg of NXL104/liter. We suggest (but cannot prove) that mutants were not captured because they had MIC values around twice the baseline, but less than three times the baseline.
In contrast, against the KPC-2-producing K. pneumoniae strain, the regimens of ceftaroline in combination with the more fractionated schedules of administration (NXL104 given as three divided doses every 8 h and the total daily dose given over 24 h as a continuous infusion) were successful. These regimens reduced the total K. pneumoniae 27-908M population for the duration of the 10-day experiment and did not amplify the less-susceptible bacterial subpopulations. Since the more fractionated regimens maximize “time > threshold,” the dose fractionation study demonstrated that time-above-MIC (threshold) is the pharmacodynamic index linked with efficacy and with prevention of the emergence of resistance during therapy. Based on the pharmacodynamic values presented in Table 1, a time-above-MIC value for NXL104 exceeding 14.8 h and at most 19.2 h was needed to optimize treatment outcomes for regimens in which ceftaroline at 600 mg q8h is used in combination with this β-lactamase inhibitor.
There are a number of conclusions that may be drawn from these hollow-fiber studies for the KPC-2-producing K. pneumoniae strain.
First, monotherapies consisting of simulated regimens of ceftaroline at 600 mg q8h alone and in combination with continuous infusions of 2 and 4 mg of NXL104/liter were clearly ineffective against K. pneumoniae 27-908M, a strain that expresses a KPC-2 β-lactamase, as well as SHV-27 and TEM-1. Monotherapy with ceftaroline at 600 mg q8h was also ineffective against the isogenic K. pneumoniae strain that was “cured” of the KPC-2-containing plasmid. This suggests that the expression of blaSHV-27 or another unidentified β-lactamase by this bacterium can lead to treatment failure with ceftaroline alone.
Second, in dose-ranging studies, an exposure-response effect was seen for NXL104 when it is used as a continuous infusion in combination with a fixed regimen of ceftaroline at 600 mg q8h. A continuous infusion of 8 mg of NXL104/liter was required to achieve a persistent reduction in the density of K. pneumoniae 27-908M and to prevent the emergence of resistance for the duration of the 10-day hollow-fiber system experiment.
Third, dose fractionation studies demonstrate that “time > threshold” is the pharmacodynamic index that predicts the efficacy of NXL104.
We also examined two other isolates—K. pneumoniae 24-1318A with a CTX-M-15 β-lactamase and an E. cloacae strain, 2-77C, with a stably derepressed AmpC β-lactamase—in dose fractionation studies. In both instances, the results were consistent with “time > threshold” as being the index most closely linked to an optimal bacteriological effect.
For the Klebsiella strain bearing CTX-M-15 (strain 24-1318A), it should be noted that a regimen of 900 mg of ceftaroline plus 900 mg of NXL104 q12h (AUC0-24 = 222.0 of NXL104) was examined and was successful, which would seem to indicate that AUC24 is linked to outcome. However, when we examine Fig. 9, there is no major difference among any of the combination regimens examined (regimens D to G). Further, the MICs for this pathogen with a 2:1 dilution scheme were 1.0 and 0.5 mg/liter. The measured drug concentrations at h 11 were 3.47 mg/liter for ceftaroline and 2.06 mg/liter for NXL104, indicating that both drugs were above the MICs for the drugs when tested together for the full 12-h interval. This shows that, for this isolate, we are at or near the top of the exposure response curve.
For the Enterobacter isolate, Fig. 10 and 11 demonstrate that the response is concordant with that seen earlier, with the failure of the regimen with resistance with NXL104 administered daily. With every 12-h administration, failure with resistance emergence occurs late (day 8 or later). With 8-hourly administration or administration by continuous infusion, no emergence of resistance was observed, even out to day 13.
Of note, Borgonovi et al. (3) examined NXL104 in combination with ceftazidime. These authors concluded that, as seen in our own investigation, “time > threshold” is the dynamically linked index. In the Borgonovi study, a conclusion drawn was that a critical drug concentration of NXL104 was 0.5 mg/liter. The abstract does not provide the process by which this conclusion was drawn. Further, it should be noted that, in contrast to our set of experiments, the bacterial inoculum was quite low, i.e., between 105 and 106 CFU/ml, and thus >1.5 logs lower than the 107- to 108-CFU/ml inoculum used in our studies. Further, drug administration was only for 24 h, with outgrowth monitored for approximately another 24 h. Over this time frame, no resistance emergence was seen. This is quite concordant with the results of the present experiments, where there is no early resistance emergence in regimens containing both ceftaroline and NXL104. Experiments need to be run longer to clinically applicable durations in order to identify regimens that that will both kill organisms and suppress resistant subpopulation amplification.
It is important to recognize that the hollow-fiber infection model is a very stringent test for a β-lactamase inhibitor. For Gram-negative pathogens, the β-lactamase enzyme exists to a great degree in the periplasmic space. As organisms die a natural or a drug-induced death, much of this β-lactamase may leak out into the surrounding medium. It cannot pass through the pores and so is trapped in the peripheral compartment. This resembles a high-density infection in this regard, like ventilator-associated pneumonia (VAP) or a large intra-abdominal abscess. Using ceftaroline alone in these experiments, we observed significant hydrolysis of this cephalosporin. The addition of NXL104 prevented antibiotic hydrolysis.
Washing the inoculum, as we did after the first experiment, caused a blunting of the early ceftaroline hydrolysis, but this rapidly caught up to that seen in the earlier experiment. Again, addition of the inhibitor markedly changed the hydrolysis of ceftaroline.
When all of the experiments in which there was dose fractionation that were conducted in our project are examined, it is possible to identify a target trough concentration of NXL104. This is seen in Table 2. When one examines the ceftaroline/NXL104 concentrations required to achieve an MIC for the bacterial isolate examined for both of the susceptibility methods (serial dilution of ceftaroline in the presence of a fixed NXL104 concentration of 4 mg/liter or a serial dilution of both agents), it is apparent by inspection that the ceftaroline concentrations in the hollow-fiber studies are always in excess of the required number at the trough level. Thus, it is the NXL104 concentration that drives the outcome. For K. pneumoniae 27-908M, the KPC-2-bearing isolate, the calculated trough NXL104 concentration when administered daily is 0.12 mg/liter, which fails. For the 12-hourly administration, the trough value for NXL104 is 1.4 to 1.5 mg/liter, which also fails. On an 8-hourly schedule, the trough value is 2.5 to 2.9 mg/liter, which succeeds.
Table 2.
Concentrations of ceftaroline and NXL104 at trough values associated with microbiological success and resistance suppression or treatment failure for several bacterial strains
| Regimen | Trough (mg/liter)a |
Outcome | |
|---|---|---|---|
| CPT | NXL104 | ||
| K. pneumoniae 27-908M bearing KPC-2b | |||
| NXL104 AUC = 192, q24h | 3.2 | 0.12 | Fail |
| NXL104 AUC = 96, q12h | 3.2 | 1.4-1.5 | Fail |
| NXL104 AUC = 64, q8h | 3.2 | 2.5-2.9 | Succeed |
| K. pneumoniae 24-1318A bearing CTX-M-15c | |||
| NXL104 AUC = 32, q8h | 3.2 | 1.1-1.2 | Succeed |
| NXL104 AUC = 108, q12h | 2.2 | 1.3-1.4 | Succeedd |
| E. cloacae 2-77C bearing stably derepressed AmpCe | |||
| NXL104 AUC = 192, q24h | 3.2 | 0.16 | Fail |
| NXL104 AUC = 96, q12h | 3.2 | 1.5-1.6 | Fail |
| NXL104 AUC = 64, q8h | 3.2 | 2.9-3.4 | Succeed |
The ceftaroline (CPT) trough values indicated are nominal values. The actual values (measured at h 7) were always higher. The NXL104 concentrations were the result of modeling all the data for each regimen and simulating the 8-h NXL104 concentrations for the first 24 h.
The MICs using serial dilutions of CPT and NXL104 fixed at 4 mg/liter were 0.75/4.0 mg/liter; the MICs using serial dilutions of both agents were 0.75/4.0 mg/liter.
The MICs using serial dilutions of CPT and NXL104 fixed at 4 mg/liter were 0.12/4.0 mg/liter; the MICs using serial dilutions of both agents were 1.0/0.5 mg/liter.
This regimen was CPT at 900 mg, q12h, and NXL104 at 900 mg, q12h.
The MICs using serial dilutions of CPT and NXL104 fixed at 4 mg/liter were 0.75/4.0 mg/liter; the MICs using serial dilutions of both agents were 2.0/1.0 mg/liter.
For the K. pneumoniae isolate 24-1318A, bearing the CTX-M-15 enzyme, it should be pointed out that none of the regimens that had both ceftaroline and NXL104 failed. Ceftaroline concentrations exceeded the MIC values when performed by either approach. However, even the lowest NXL104 trough concentration (1.1 to 1.2 mg/liter) succeeded. Likewise, for 12-hourly administration with higher exposures for both ceftaroline and NXL104, ceftaroline troughs always exceeded the MIC and the NXL104 trough was 1.3 to 1.4 mg/liter.
The E. cloacae isolate 2-77C with the stably derepressed AmpC β-lactamase also always had ceftaroline trough concentrations in excess of the MIC. For NXL104, the daily and 12-hourly administration schedules failed, with NXL104 trough concentrations of 0.16 mg/liter and 1.5 to 1.6 mg/liter, respectively. The 8-hourly administration schedule attained trough concentrations of 2.9 to 3.5 mg/liter and succeeded.
When one examines all of the data, it is clear that the enzymes differ from one another, requiring differing NXL104 trough concentrations for success. For the KPC-2 isolate, NXL104 troughs of >1.4 to 1.5 mg/liter and ≤2.5 to 2.9 mg/liter were required. For the stably derepressed AmpC isolate, NXL104 troughs of >1.5 to 1.6 mg/liter and ≤2.9 to 3.5 mg/liter were required for microbiological success and resistance suppression. For the K. pneumoniae isolate bearing the CTX-M-15 enzyme, much less NXL104 is required at the trough level. Indeed, it succeeded at the lowest value tested, at 1.1 to 1.4 mg/liter. So, for enzymes that are more difficult to inhibit, NXL104 trough concentrations should be targeted at >1.6 mg/liter and at least 2.5 mg/liter. Given these results, it would be optimal to pair ceftaroline and NXL104 on an 8-hourly administration schedule for circumstances where highly resistant pathogens are likely to be encountered. This will allow optimal bacterial cell killing (highest likelihood of successful clinical outcome), as well as the suppression of resistance emergence.
ACKNOWLEDGMENT
This study was supported by Cerexa (Oakland, CA), a wholly owned subsidiary of Forest Laboratories, Inc. (New York, NY).
Footnotes
Published ahead of print 24 October 2011
REFERENCES
- 1. Bilello JA, Bauer G, Dudley MN, Cole GA, Drusano GL. 1994. Effect of 2′,3′-didehydro-3′-deoxythymidine in an in vitro hollow fiber pharmacodynamic model system correlates with results of dose-ranging clinical studies. Antimicrob. Agents Chemother. 38:1386–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blaser J, Stone BB, Groner MC, Zinner SH. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borgonovi M, et al. 2008. Importance of NXL104 pharmacokinetics (PK) in the pharmacodynamics (PD) of ceftazidime+NXL104 combinations in an in vitro hollow fiber infection model, abstr. A-023. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother./46th Meet. Infect. Dis. Soc. Am [Google Scholar]
- 4. Drusano GL, et al. 2001. Hollow fiber unit evaluation of a new human immunodeficiency virus type 1 protease inhibitor, BMS-232632, for determination of the linked pharmacodynamic variable. J. Infect. Dis. 183:1126–1129 [DOI] [PubMed] [Google Scholar]
- 5. Drusano GL, et al. 2002. Pharmacodynamics of abacavir in an in vitro hollow fiber model system. Antimicrob. Agents Chemother. 46:464–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gumbo T, et al. 2004. Selection of a moxifloxacin dose that suppresses Mycobacterium tuberculosis resistance by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642–1651 [DOI] [PubMed] [Google Scholar]
- 7. Jacoby G. 2009. 2009. AmpC β-lactamases. Clin. Microbiol. Rev. 22:664–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mendona̧ N, Leitão J, Manageiro V, Ferreira E, Caniça M. 2007. Spread of extended-spectrum beta-lactamase CTX-M-producing Escherichia coli clinical isolates in community and nosocomial environments in Portugal. Antimicrob. Agents Chemother. 51:1946–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith Moland E, et al. 2003. Plasmid-mediated, carbapenem-hydrolyzing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J. Antimicrob. Chemother. 51:711–714 [DOI] [PubMed] [Google Scholar]
- 10. Strayer AH, et al. 1994. Pharmacodynamics of piperacillin alone and in combination with tazobactam against piperacillin-resistant and -susceptible organisms in an in vitro model of infection. Antimicrob. Agents Chemother. 38:2351–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]