Abstract
Cystic fibrosis (CF) is characterized by a chronic neutrophilic inflammatory response resulting in airway remodeling and progressive loss of lung function. Doxycycline is a tetracycline antibiotic that inhibits matrix metalloproteinase 9, a protease known to be associated with the severity of lung disease in CF. The pharmacokinetics of doxycycline was investigated during the course of a clinical trial to evaluate the short-term efficacy and safety in adults with CF. Plasma samples were obtained from 14 patients following a single intravenous dose and after 2 and 4 weeks of oral administration of doses ranging from 40 to 200 mg daily. The data were analyzed using noncompartmental and compartmental pharmacokinetics. The maximum concentration of drug in serum (Cmax) and area under the concentration-time curve from 0 h to infinity (AUC0-∞) values ranged from 1.0 to 3.16 mg/liter and 15.2 to 47.8 mg/liter × h, respectively, following single intravenous doses of 40 to 200 mg. Cmax and time to maximum concentration of drug in serum (Tmax) values following multiple-dose oral administration ranged from 1.15 to 3.04 mg/liter and 1.50 to 2.33 h, respectively, on day 14 and 1.48 to 3.57 mg/liter and 1.00 to 2.17 on day 28. Predose sputum/plasma concentration ratios on days 14 and 28 ranged from 0.33 to 1.1 (mean, 0.71 ± 0.33), indicating moderate pulmonary penetration. A 2-compartment model best described the combined intravenous and oral data. Absorption was slow and delayed (absorption rate constant [Ka], 0.414 h−1; lag time, 0.484 h) but complete (bioavailability [F], 1.16). The distribution and elimination half-lives were 0.557 and 18.1 h, respectively. Based on these data, the plasma concentrations at the highest dose, 200 mg/day, are in the range reported to produce anti-inflammatory effects in vivo and should be evaluated in clinical trials.
INTRODUCTION
Cystic fibrosis (CF) is a genetic disorder characterized by a defect in chloride transport within the apical membranes of airway epithelial cells. Impaired chloride secretion and excessive sodium reabsorption cause a reduction in airway surface liquid, leading to chronic obstruction, infection, and inflammation. The current treatment includes therapies to improve mucociliary clearance and antibiotics to combat chronic infections involving Pseudomonas aeruginosa. However, despite these therapies, the relentless cycle of airway obstruction, infection, and inflammation results in bronchiectasis, progressive loss of lung function, and eventual respiratory failure.
Recent studies highlight proteases as central mediators of the progressive destruction of the airways in patients with CF and offer a potential therapeutic target. In particular, levels of matrix metalloproteinase 9 (MMP-9) in the airways are significantly associated with matrix breakdown products (i.e., elastin, glycosaminoglycans, and collagen) in bronchoalveolar lavage fluid, as well as the severity of pulmonary function (9, 20). These data indicate that the intense neutrophilic inflammatory response within the airways of patients with CF results in release of proteolytic enzymes (e.g., elastase and MMP-9), which cause destruction of the airways and progressive loss of lung function.
Doxycycline, a tetracycline antibiotic, exhibits immune-modulating activities that have been exploited in the treatment of several inflammatory conditions mediated by MMP-9. Doxycycline has been demonstrated to improve inflammatory biomarkers in patients with abdominal aortic aneurysms and to prevent acute coronary syndromes and periodontitis (1–4). In addition, doxycycline has shown beneficial effects in experimental models of asthma (7, 15), acute lung injury (5), pulmonary fibrosis (6), and emphysema (18). The mechanism of the anti-inflammatory effect of doxycycline appears to be mediated by inhibition of the mitogen-activated protein kinase (MAPK) and Smad pathways (11, 13). The effective concentrations for the anti-inflammatory activity are in the range of 3 to 10 μg/ml (2, 11, 13, 14). Positive results from these studies suggest a potential role for doxycycline in reducing airway inflammation and protease activity in patients with CF; however, the pharmacokinetics of doxycycline in this population has not been previously described. There are several factors that may alter the pharmacokinetics in this population, including pancreatic insufficiency, which is known to affect the absorption of fat-soluble compounds; reduced adipose mass, which may impact the volume of distribution; and reduced lung penetration, given the mucus-impacted airways. The purpose of this investigation was to characterize the pharmacokinetics of doxycycline in a short-term single- and multiple-dose-ranging study in stable adult patients with cystic fibrosis.
MATERIALS AND METHODS
Subjects.
A total of 20 patients with cystic fibrosis were enrolled in the clinical trial, 14 of whom participated in the pharmacokinetics portion of the study. The patients were clinically stable, with no evidence of acute pulmonary exacerbation, based on pulmonary function tests and a review of pulmonary signs and symptoms. The study was conducted at the Clinical Trials Unit at the University of Southern California (USC) University Hospital. Study participants were screened to determine eligibility within 1 week prior to enrollment. Subjects were deemed eligible if they satisfied the following entry criteria: age, >18 years; clinically stable (forced expiratory volume in 1 s [FEV1] within 10% of baseline); FEV1, >40% of the predicted value; no hypersensitivity to doxycycline; not receiving systemic antibiotics within 4 weeks of baseline, doxycycline within 60 days of baseline, or concomitant anti-inflammatory therapies, such as nonsteroidal anti-inflammatory drugs (NSAIDs) or systemic corticosteroids; and no recent history of gastrointestinal bleeding or ulceration. Patients receiving oral azithromycin were allowed to continue the therapy during the course of the study. All participants provided written informed consent to participate in the trial. The study protocol was approved by the local Institutional Review Board prior to study initiation.
Study design.
The study was designed to evaluate the short-term efficacy, safety, and pharmacokinetics of doxycycline in adult patients with cystic fibrosis. Subjects were stratified based on pulmonary function into two groups: mild (FEV1, >70% of predicted) and moderate (FEV1, 40 to 70% of predicted) lung disease. The subjects were then randomized in block to receive no study drug or daily doxycycline at 40 mg, 100 mg, or 200 mg for 28 days.
The subjects fasted overnight prior to each study visit. Two days prior to initiation of daily oral dosing, the patients received a single dose of doxycycline at 40 mg, 100 mg, or 200 mg intravenously (i.v.), corresponding to the respective oral dosage regimen they were randomized to receive. The 40-mg and 100-mg doses were infused over 1 h, and the 200-mg dose was infused over 2 h, according to the manufacturer's recommendations. Serial blood samples (5 ml) for determination of doxycycline concentrations were obtained before and at 0.5, 1, 2, 4, 12, 24, and 48 h after the initiation of the single i.v. infusion of doxycycline. Once daily oral dosing of doxycycline commenced immediately following the 48-hour blood sample and continued until day 28. Additional blood samples were obtained before and 0.5, 1, and 2 h after oral doses administered on days 14 and 28. Blood samples were drawn in EDTA tubes, kept on ice, and centrifuged within 2 h of collection at 2,500 × g and 4°C for 15 min. Plasma was harvested and stored at −70°C until analysis.
Sputum samples were obtained by induction with 35 ml of 3% hypertonic saline, which was administered via a large-volume ultrasonic nebulizer system (Ultrasonic 2000 nebulizer system; Nouvag, Lake Hughes, CA) on days 14 and 28 in a subset of patients. The subjects were instructed to cough sputum into a sterile specimen cup every 2 min for 12 min. The sputum samples were stored at −70°C until analysis.
Analytical methods.
Total doxycycline concentrations in plasma and sputum were quantified by validated assay procedures using reverse-phase chromatography with UV detection as previously described (19). Briefly, plasma samples (500 μl) were spiked with the internal standard (500 μl of piroxicam at 5 μg/ml in acetonitrile). The samples were centrifuged at 13,000 × g for 15 min. The supernatants (800 μl) were evaporated to dryness under a steady stream of dry air and reconstituted with 200 μl of the mobile phase (2.75% acetic acid-20% methanol-25% acetonitrile) and placed in autosampler vials. A 100-μl aliquot of the supernatant was injected onto a high-performance liquid chromatography (HPLC) column (Zorbax SB-C18; 4.6 by 250 cm; 5 μm). Separation was achieved by isocratic solvent elution at a flow rate of 1.5 ml/min. The peak heights were quantified using an Agilent Series 1100 liquid chromatography system (Agilent Technologies, Santa Clara, CA) equipped with a binary pump, degasser, autosampler, thermostated column compartment, and variable-wavelength detector. The linear standard curve range was 0.1 to 10 μg/ml, with a lower limit of quantitation of 0.1 μg/ml. Low-quality (0.75-μg/ml) and high-quality (7.5-μg/ml) control samples were used during routine analysis. The interassay precision in plasma ranged from 4.4% to 9.5% for calibration standards. The precision and accuracy in plasma ranged from 2.4% to 2.7% and 92.5% to 107% for quality control samples, respectively. The interassay precision in sputum ranged from 1.2% to 17% for calibration standards. The precision and accuracy in sputum ranged from 2.1% to 2.8% and 93.9% to 105%, respectively, for quality control samples.
Pharmacokinetic evaluation.
The plasma concentration-time data on doxycycline for each subject were analyzed using standard noncompartmental methods. The maximum concentration of drug in serum (Cmax) and time to maximum concentration of drug in serum (Tmax) were obtained directly from the observed data. The area under the curve from 0 h to infinity (AUC0-∞) was calculated using the linear trapezoidal rule, with the terminal area estimated from the last observed concentration divided by the terminal slope of the log-linear phase. Compartmental pharmacokinetic analysis was performed using maximum-likelihood estimation via the expectation maximization (EM) algorithm with sampling using ADAPT 5 software (Biomedical Simulation Resource, University of Southern California, Los Angeles, CA). Model discrimination was determined by a likelihood ratio test. A two-compartment model with first-order absorption with a lag time was applied to the plasma concentration-time data incorporating both the single-dose intravenous and multiple-dose oral administration periods. The parameters estimated in the compartmental analysis included total clearance (CLt), volume of the central compartment (Vc), distributional clearance (Q), volume of the peripheral compartment (Vp), distributional rate constant (α), elimination rate constant (β), distributional half-life (T1/2α), elimination half-life (T1/2β), lag time (TLag), absorption rate constant (Ka), and bioavailability (F).
Statistical analysis.
Subject characteristics and pharmacokinetic parameters are expressed as mean and standard deviation. A nonlinear power model was used to assess dose proportionality: parameter = a × doseb, where a is a constant, b is the proportionality constant, and parameter is Cmax or AUC0-∞ (12). Log transformation of the equation results in a linear equation: log a + b × log dose. The constant b was determined from linear regression of the log-transformed parameter and dose data. The relationship is considered dose proportional if b is equal to 1 and the 95% confidence intervals include unity. Log transformation and linear regression were performed using GraphPad Prism version 5.0c for Mac (GraphPad Software, San Diego, CA).
RESULTS
Fourteen adult patients with CF were randomized to receive 200 mg (n = 6), 100 mg (n = 5), and 40 mg (n = 3) of the drug. Two patients withdrew from the multiple-dose phase (one each from the 40- mg and 100-mg groups), one due to an acute pulmonary exacerbation requiring hospitalization and the second due to inability to meet study visit requirements. The demographic and clinical characteristics are summarized in Table 1. The study participants were well nourished, as evidenced by the normal body mass index. With the exception of one patient, the CFTR (cystic fibrosis transmembrane conductance regulator) genotypes were either homozygous or heterozygous for ΔF508. All patients had normal renal and hepatic function based on laboratory studies.
Table 1.
Subject characteristics
| Characteristica | Mean value (SD) |
|---|---|
| Age (yr) | 32.1 (12.3) |
| Gender (M/F) | 8/6 |
| Ht (cm) | 168 (11.6) |
| Wt (kg) | 66.2 (13.2) |
| Body mass index (kg/m2) | 23.6 (3.71) |
| No. with CFTR genotype: | |
| ΔF508/ΔF508 | 6 |
| ΔF508/other | 7 |
| Other/other | 1 |
| GFR (ml/min/1.73 m2) | 115 (21.9) |
| Total bilirubin | 0.4 (0.2) |
| AST | 26.3 (11.1) |
| ALT | 26.8 (14.7) |
M, male; F, female; GFR, glomerular filtration rate; AST, aspartate transaminase; ALT, alanine aminotransferase.
Pharmacokinetics.
The results of the noncompartmental pharmacokinetic analysis are presented in Table 2. The average Cmax following single intravenous doses increased from 1.0 to 3.16, and the AUC0-∞ increased from 15.2 to 47.8 as the dose increased from 40 to 200 mg. The doxycycline dose level increased in the ratio of 1.0:2.5:5, whereas the mean Cmax, and AUC0-∞ values increased in the ratios of 1.0:2.6:3.2 and 1.0:1.6:3.1, respectively. The exponents of the power model used to determine dose proportionality were 0.75 and 0.74 for Cmax and AUC0-∞, respectively, indicating less than a dose-proportional relationship. The 95% confidence intervals (0.51 to 0.99 and 0.55 to 0.94) around the mean approached unity, suggesting dose proportionality. One potential explanation for the less than proportional relationship with Cmax could be the longer infusion time for the 200-mg doses, allowing more time for distribution to occur, resulting in a lower Cmax. This is supported by the approximate doubling of the AUC0-∞ with the increased dose of 100 mg to 200 mg.
Table 2.
Noncompartmental pharmakokinetics
| Dose (mg) | Single-dose intravenous, day 1 [mean (SD)] |
Multiple-dose oral [mean (SD)]a |
||||
|---|---|---|---|---|---|---|
| Day 14 |
Day 28 |
|||||
| Cmax | AUC0-∞ | Cmax | Tmax | Cmax | Tmax | |
| 40 | 1.00 (0.63) | 15.2 (3.12) | 1.15 (0.09) | 1.50 (0.71) | 1.48 (0.53) | 1.00 (0.00) |
| 100 | 2.57 (1.45) | 23.8 (7.42) | 1.85 (1.05) | 2.25 (0.50) | 2.06 (0.48) | 1.75 (0.50) |
| 200 | 3.16 (0.90) | 47.8 (12.6) | 3.04 (0.66) | 2.33 (0.82) | 3.57 (1.12) | 2.17 (0.41) |
Secondary peaks were noted in 3/25 (12%).
The plasma concentration-time profiles of doxycycline during multiple-dose oral administration demonstrated relatively consistent absorption patterns, with several (12%) instances of secondary peaks. The Tmax ranged from 1 to 2.3 h and appeared to be longer at higher doses (100 mg and 200 mg versus 40 mg). The Cmax values appeared to be slightly higher on day 28; however, the differences were not statistically significant. Following repeated dosing, the mean doxycycline Cmax increased in the ratio of 1.0:1.6:2.64 at 14 days and 1.0:1.4:2.4 at 28 days. The exponents of the power model for Cmax were 0.63 and 0.56 on days 14 and 28, respectively, indicating a less than dose-proportional relationship. The 95% confidence intervals around the mean exponents included unity at day 14 and approached unity on day 28, suggesting dose proportionality. The smaller number of patients receiving the 40-mg dose (n = 2) could influence the determination of dose proportionality.
Sputum concentrations were also measured in a subset of patients who were able to produce sufficient sputum (Table 3). Sputum concentrations ranged from 0.15 to 1.9 mg/liter (mean, 0.78 mg/liter) and were higher in patients receiving 200 mg versus 100 mg a day. The concentrations did not differ significantly between days 14 and 28, indicating a steady state had been achieved. Sputum penetration was determined by a ratio of the predose sputum and plasma doxycycline concentrations on days 14 and 28. The mean (SD) sputum penetration ratio was 0.71 ± 0.33, indicating doxycycline concentrations in pulmonary secretions are lower than in plasma.
Table 3.
Predose doxycycline concentrations in plasma and sputum (n = 4)
| Subject | Day | Dose (mg) | Sputuma (mg/liter) | Plasmaa (mg/liter) | Ratiob |
|---|---|---|---|---|---|
| 1 | 14 | 100 | 0.30 | 0.33 | 0.89 |
| 28 | 0.68 | 0.59 | 1.2 | ||
| 2 | 14 | 100 | 0.25 | 0.46 | 0.55 |
| 28 | 0.15 | 0.44 | 0.33 | ||
| 3 | 14 | 200 | 0.73 | 1.5 | 0.49 |
| 28 | 0.78 | 1.9 | 0.40 | ||
| 4 | 14 | 200 | 1.5 | 1.6 | 1.1 |
| 28 | 1.9 | 1.3 | 0.69 |
Predose concentrations on days 14 and 28.
Mean sputum penetration ratio (SD) = 0.71 (0.33).
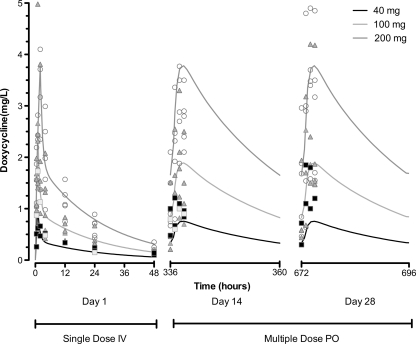
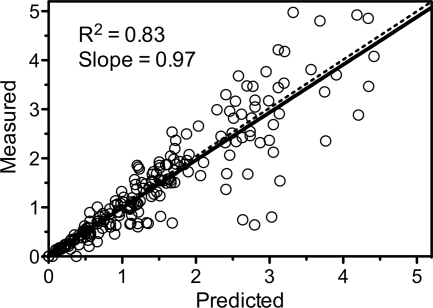
The results of the compartmental pharmacokinetic analysis are depicted in Table 4 and Fig. 1 and 2. A two-compartment model with first-order absorption incorporating a lag time best described the plasma concentration-time data. The plot of predicted versus measured concentrations (Fig. 1) showed good agreement (r2 = 0.83; slope = 0.97). The absorption was characterized by a lag time and a relatively low and variable absorption rate. However, the extent of absorption was complete, with a mean bioavailability of 1.16. The disposition is characterized by a relatively quick distribution (T1/2α = 0.56 h) followed by a more prolonged elimination phase (T1/2β = 18.1 h).
Table 4.
Compartmental pharmacokinetic parameters
| Parameter | Mean value (SD) |
|---|---|
| CLt (liters/h) | 3.70 (0.995) |
| Vc (liters) | 27.9 (6.44) |
| Q (liters /h) | 22.1 (11.4) |
| Vp (liters) | 61.4 (24.2) |
| α (h−1) | 1.25 (0.579) |
| β (h−1) | 0.038 (0.008) |
| T1/2, α (h) | 0.557 (0.259) |
| T1/2, β (h) | 18.1 (3.74) |
| TLag (h) | 0.484 (0.304) |
| Ka (h−1) | 0.414 (0.796) |
| F | 1.16 (0.299) |
Fig 1.
Time course of doxycycline plasma concentrations. PO, oral administration. ■, 40 mg;  , 100 mg; ○, 200 mg.
, 100 mg; ○, 200 mg.
Fig 2.
Measured versus individual model-predicted concentrations. Solid line, linear regression through the points; dashed line, line of unity.
DISCUSSION
The primary findings from this study are that doxycycline exhibits excellent oral bioavailability and moderate pulmonary penetration upon multiple dosing in patients with CF. Overall, the pharmacokinetics of doxycycline in patients with CF are consistent with prior studies in non-CF populations (16, 17). The Cmax and Tmax following a 7-day treatment course of doxycycline at 200 mg orally once daily for Plasmodium falciparum malaria were 4.4 μg/ml and 3 h, respectively (16). These values are similar to those in the current study (3.04 and 3.57 μg/ml and 2.33 and 2.17 h on days 14 and 28, respectively).
The absorption rate is variable but complete. The data are consistent with published data, demonstrating a mean bioavailability of 95% (21). The average bioavailability in this trial exceeds 100%, indicating possible enterohepatic recirculation. This is also suggested by the appearance of multiple peaks observed for several patients. Doxycycline is known to be eliminated through biliary excretion and reabsorbed into the systemic circulation (17).
Sputum penetration in this CF cohort was moderate, with levels approximately 70% of plasma. The sputum/plasma ratio exceeds values from prior published data, which range from 17 to 55%. The higher rate of penetration may be related to the longer drug administration period in this study, leading to greater accumulation. The sputum penetration ratio was noted to increase from 7.5 to 46% from the first to the seventh day of treatment in an earlier study (8). In addition, it is also possible that the degree of airway inflammation is greater in patients with CF, resulting in greater delivery of doxycycline to the airways through cellular partitioning within neutrophils that migrate to the airways.
The concentrations achieved with standard doses approach the therapeutic target for anti-inflammatory effects established in prior studies. Based on in vitro studies and in vivo data in patients with abdominal aortic aneurysms, the total doxycycline concentration necessary to exert an anti-inflammatory response is approximately 3 to 10 μg/ml (2, 11, 13, 14). Doxycycline exhibits high protein binding (88%), which limits the biological activity of the agent (10). However, doxycycline at doses comparable to those used in humans demonstrated a significant reduction in airway neutrophils and MMP-9 in bronchoalveolar lavage fluid in a murine model of neutrophilic airway inflammation (5). In addition, clinical trials demonstrated a significant reduction in inflammatory biomarkers in serum (e.g., MMP-9 and C-reactive protein [CRP]) during long-term administration with standard dosing of doxycycline (metalloproteinase inhibition with low-dose doxycycline to prevent acute coronary syndromes [MIDAS] and atherosclerotic disease). These reductions may be related to accumulation within inflammatory cells (intracellular/extracellular ratio, 7.5) (22).
There are some limitations to our study data. The interassay precision of the sputum concentrations was relatively high (17%) at the lowest standard, 0.1 μg/ml. However, the measured data for 7/8 samples were well above this value, which would minimize the effect on the measured concentrations. Second, the study results apply to well-nourished adult CF patients with mild to moderate pulmonary disease. This population was chosen because the beneficial effects of prior anti-inflammatory therapies (e.g., corticosteroids and ibuprofen) in slowing the progression of pulmonary disease were significant for patients with mild lung disease.
In conclusion, doxycycline exhibits favorable oral absorption characteristics, with an overall pharmacokinetic profile similar to those in other patient populations. The concentrations achieved in plasma and sputum approach the levels necessary for anti-inflammatory effects. These results suggest that further exploration of doxycycline as a potential anti-inflammatory therapy in patients with CF is warranted.
ACKNOWLEDGMENTS
This research was supported by grants from the Webb Foundation, the American Fondation for Pharmaceutical Education, and the NIH/NCRR SC CTSI (grant no. UL1 RR03986).
Footnotes
Published ahead of print 24 October 2011
REFERENCES
- 1. Abdul-Hussien H, et al. 2009. Doxycycline therapy for abdominal aneurysm: improved proteolytic balance through reduced neutrophil content. J. Vasc. Surg. 49:741–749 [DOI] [PubMed] [Google Scholar]
- 2. Baxter BT, et al. 2002. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J. Vasc. Surg. 36:1–12 [DOI] [PubMed] [Google Scholar]
- 3. Brown DL, et al. 2004. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler. Thromb. Vasc. Biol. 24:733–738 [DOI] [PubMed] [Google Scholar]
- 4. Ciancio S, Ashley R. 1998. Safety and efficacy of sub-antimicrobial-dose doxycycline therapy in patients with adult periodontitis. Adv. Dent. Res. 12:27–31 [DOI] [PubMed] [Google Scholar]
- 5. Fujita M, et al. 2007. Doxycycline attenuated lung injury by its biological effect apart from its antimicrobial function. Pulm. Pharmacol. Ther. 20:669–675 [DOI] [PubMed] [Google Scholar]
- 6. Fujita M, et al. 2006. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrob. Agents Chemother. 50:739–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gueders MM, et al. 2008. A novel formulation of inhaled doxycycline reduces allergen-induced inflammation, hyperresponsiveness and remodeling by matrix metalloproteinases and cytokines modulation in a mouse model of asthma. Biochem. Pharmacol. 75:514–526 [DOI] [PubMed] [Google Scholar]
- 8. Hartnett BJ, Marlin GE. 1976. Doxycycline in serum and bronchial secretions. Thorax 31:144–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hilliard TN, et al. 2007. Airway remodelling in children with cystic fibrosis. Thorax 62:1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Houin G, et al. 1983. The effects of chronic renal insufficiency on the pharmacokinetics of doxycycline in man. Br. J. Clin. Pharmacol. 16:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoyt J, Ballering J, Numanami H, Hayden J, Robbins R. 2006. Doxycycline modulates nitric oxide production in murine lung epithelial cells. J. Immunol. 176:567–572 [DOI] [PubMed] [Google Scholar]
- 12. Hummel J, McKendrick S, Brindley C, French R. 2009. Exploratory assessment of dose proportionality: review of current approaches and proposal for a practical criterion. Pharm. Stat. 8:38–49 [DOI] [PubMed] [Google Scholar]
- 13. Kim HS, Luo L, Pfugfelder SC, Li DQ. 2005. Doxycycline inhibits TGF-B1 induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 46:840–848 [DOI] [PubMed] [Google Scholar]
- 14. Krakauer T, Buckley M. 2003. Doxycycline is anti-inflammatory and inhibits staphylococcal exotoxin-induced cytokines and chemokines. Antimicrob. Agents Chemother. 47:3630–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee KS, Jin SM, Kim SS, Lee YC. 2004. Doxycycline reduces airway inflammation and hyperresponsiveness in a murine model of toluene diisocyanate-induced asthma. J. Allergy Clin. Immunol. 113:902–909 [DOI] [PubMed] [Google Scholar]
- 16. Newton PN, et al. 2005. Pharmacokinetics of oral doxycycline during combination treatment of severe Falciparum malaria. Antimicrob. Agents Chemother. 49:1622–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pedersen PV, Miller R. 1980. Pharmacokinetics of doxycycline reabsorption. J. Pharm. Sci. 69:204–207 [DOI] [PubMed] [Google Scholar]
- 18. Rossiter HB, Scadeng M, Tang K, Wagner PD, Breen EC. 2008. Doxycycline treatment prevents alveolar destruction in VEGF-deficient mouse lung. J. Cell Biochem. 104:525–535 [DOI] [PubMed] [Google Scholar]
- 19. Ruz N, et al. 2004. Rapid and simple determination of doxycycline in serum by high-performance liquid chromatography. Application to particulate drug delivery systems. J. Chromatogr. A 1031:295–301 [DOI] [PubMed] [Google Scholar]
- 20. Sagel S, Kapsner R, Osberg I. 2005. Induced sputum matrix metalloproteinase-9 correlates with lung function and airway inflammation in children with cystic fibrosis. Pediatr. Pulmonol. 39:224–232 [DOI] [PubMed] [Google Scholar]
- 21. Saivin S, Houin G. 1988. Clinical pharmacokinetics of doxycycline and minocycline. Clin. pharmacokinet. 15:355–366 [DOI] [PubMed] [Google Scholar]
- 22. Walters JD. 2006. Characterization of minocycline transport by human neutrophils. J. Periodontol. 77:1964–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]