Abstract
Voriconazole is a first-line agent for the treatment of invasive fungal infections. The pharmacology of voriconazole is characterized by extensive interindividual variability and nonlinear pharmacokinetics. The population pharmacokinetics of voriconazole in 64 adults is described. The patient population consisted of 21 healthy volunteers, who received a range of intravenous (i.v.) and oral voriconazole regimens, and 43 patients with proven or probable invasive aspergillosis, who received the currently licensed dosage. Voriconazole concentrations were measured using high-performance liquid chromatography (HPLC). The pharmacokinetic data were modeled using a nonparametric methodology and with a nonlinear pharmacokinetic structural model. The extent and consequences of pharmacokinetic variability were explored using Monte Carlo simulation. The relationship between drug exposure and clinical response was explored using logistic regression. Optimal sampling times were identified using D-optimal design. The fit of the nonlinear model was acceptable. Data from the healthy volunteers provided robust estimates for Km and the maximum rate of enzyme activity (Vmax). The Bayesian parameter estimates were more variable and statistically different in patients than in volunteers. There was a linear relationship between the trough concentration and area under the concentration-time curve (AUC0-12). There was no relationship between the AUC0-12 and clinical response. The original parameter values were readily recapitulated using Monte Carlo simulation. Initial i.v. dosing resulted in higher AUC0-12 and trough concentrations compared with oral dosing. Sample collection times of 1, 2, 3, 4, 8, and 12 h after an i.v. infusion are maximally informative times for future pharmacokinetic studies.
INTRODUCTION
Voriconazole is an orally bioavailable triazole with potent activity against a range of medically important fungal pathogens. Voriconazole is a first-line agent for the treatment of invasive aspergillosis (13), disseminated candidiasis (9) and invasive infections caused by less common fungal pathogens (6). Recent studies suggest that voriconazole can be used for the prevention of invasive fungal infections in immunocompromised patients (7, 14). Despite extensive clinical trial data, there is a persistent debate relating to issues surrounding the optimal clinical use of voriconazole. Population pharmacokinetics provides a summary of the pharmacokinetic behavior in patient populations and is a critical tool for the identification of a range of strategies that may be important for the optimal clinical use of voriconazole.
There has been a progressive understanding of the relationship between voriconazole drug exposure and clinical outcome. Studies in a number of disparate populations and settings consistently allude to clinically relevant exposure-effect and exposure-toxicity relationships. In some studies these relationships have been quantified using a range of modeling techniques (8, 10, 12). The pharmacokinetics of voriconazole is known to be highly inherently variable. Voriconazole also exhibits nonlinear (Michaelis-Menten) pharmacokinetic behavior, which represents a challenge for precise dosage adjustment in clinical settings, and the consistent achievement of systemic drug concentrations that are therapeutic and nontoxic.
This study describes the population pharmacokinetics of voriconazole in adults. The populations of interest include patients with invasive aspergillosis who were enrolled in a phase III clinical trial (3) and healthy volunteers studied in the early phases of drug development. The latter were included to buttress the relatively sparse data obtained from patients and to enable robust estimates for the two parameters that describe nonlinear pharmacokinetics (Vmax [maximum rate of enzyme activity] and Km). This population pharmacokinetic model will enable further exploration of exposure-response relationships, insights into pharmacokinetic sampling, clinical trial design, therapeutic drug monitoring, and dosage adjustment for individual patients.
MATERIALS AND METHODS
Patients.
To enable a robust estimate of the parameters describing the nonlinear pharmacokinetics of voriconazole, the following phase I data from 21 healthy volunteers receiving the following voriconazole regimens were used. (i) Cohort 1 (n = 14) received 6 mg/kg voriconazole intravenously (i.v.) for 2 doses followed by 3 mg/kg i.v. every 12 h (q12h) for 1 week, followed by 200 mg q12h orally. After a washout period of 1 week, these same patients received i.v. voriconazole 6 mg/kg i.v. for 2 doses followed by 5 mg/kg i.v. q12h, followed by 400 mg q12h orally. (ii) Cohort 2 (n = 7) received 6 mg/kg voriconazole for 2 doses followed by 4 mg/kg q12h, followed by 300 mg q12h for 1 week. The mean weight ± standard deviation of these patients was 76.82 ± 7.07 kg (range, 66 to 91 kg). All patients were Caucasian males aged 19 to 38 years who consumed fewer than 21 units of alcohol per week. For both cohorts, pharmacokinetic data were obtained as a trough concentration after the end of each day. A full pharmacokinetic profile was obtained after i.v. dosing between 144 and 168 h postinitiation of therapy with samples obtained at 144.25, 144.50, 144.75, 145, 145.25, 145.5, 146, 147, 148, 150, 152, 154, 156, and 168 h. A similar design was used to characterize the pharmacokinetics of oral voriconazole, with intensive sampling between 312 and 336 h (i.e., after 1 week of oral therapy). The same design and sampling strategy was used following redosing after the washout period.
The data from these 21 healthy volunteers were combined with those from 43 patients with invasive aspergillosis, who comprised a subset of patients receiving voriconazole who were enrolled in a phase III clinical trial conducted by Herbrecht et al. (3). These patients received voriconazole 6 mg/kg i.v for two doses, followed by 4 mg/kg i.v. (for a variable time), followed by oral voriconazole 200 mg q12h. The average age ± standard deviation and the average weight ± standard deviation for these 43 patients were 51.23 ± 14.48 years and 71.37 ± 13.54 kg, respectively.
Voriconazole concentrations were measured using a well-validated high-performance liquid chromatography (HPLC) assay. The limit of quantification was 0.01 mg/liter, and the ranges of imprecision and inaccuracy were 3.3 to 13.1% and −2.3 to 7.9%, respectively.
Population pharmacokinetic modeling.
The pharmacokinetic data from the healthy volunteers (n = 21) and patients (n = 43) were comodeled using the BIG version of the population pharmacokinetic program nonparametric adaptive grid (BIG NPAG) (5). Voriconazole is well known to exhibit nonlinear pharmacokinetics, and only a structural model that incorporated nonlinear terms for clearance was fitted to the data (i.e., a linear structural model was not investigated). The following structural pharmacokinetic model described by three ordinary differential equations was used:
| (1) |
| (2) |
| (3) |
where X(1), X(2) and X(3) represent the amount of voriconazole (in milligrams) in the gut, central compartment, and peripheral compartment, respectively; Ka is the first-order rate constant connecting the gut with the central compartment; Vmax is the maximum rate of enzyme activity of voriconazole (mg/h); Km is the concentration of voriconazole in the central compartment at which clearance is half-maximal; Kcp and Kpc are the first-order intercompartmental rate constants; and R(1) represents the i.v. infusion of voriconazole. Two further parameters were incorporated into this model: (i) a lag function and (ii) an estimate of bioavailability, which was possible because of concomitant oral and i.v. input. Equations 1, 2, and 3 describe the rates of change of the amount of voriconazole in the gut, central compartment, and peripheral compartment, respectively. The data were weighted by the inverse of the estimated assay variance. The fit of the model to the data was assessed using the log likelihood ratio, mean weighted error (a measure of bias) and bias-adjusted mean weighted squared error (a measure of precision). A visual inspection and the identification of the coefficient of determination of a linear regression of the observed-versus-predicted values were also performed. Bayesian estimates for the parameters for the individual volunteers and patients were obtained. A comparison was made between volunteers and patients to further understand the impact of disease on the population pharmacokinetic parameters. The relationship between the Bayesian posterior parameter estimates and weight was explored.
Monte Carlo simulations were performed using the program ADAPT 5 (1). The mean parameter values and their associated variances were inserted into subroutine PRIOR of ADAPT 5. The currently licensed i.v. regimen of 6 mg/kg i.v. for 2 dosages followed by 4 mg/kg i.v q12h was used for the simulations. The absolute dosage of voriconazole was calculated using the mean weight of the study population. A series of 5,000-patient simulations were performed. Both normal and log-normal parameter distributions were explored and distinguished on the basis of their ability to recapitulate the parameter values of the starting population. For oral dosing, the intersubject variability in bioavailability (F) was incorporated into the simulations via the output equations. The AUC0-12 was calculated using integration.
Statistical methods.
Differences in the Bayesian estimates parameter values between healthy volunteers and patients were compared using a Mann-Whitney test (SYSTAT version 11). Logistic regression was used to investigate the relationship between drug exposure and the response to voriconazole at the end of treatment (SYSTAT version 11). The latter was determined using the global assessment by the Data Review Committee (DRC). The estimated AUC0-12 in individual patients was determined using the Bayesian estimates for the pharmacokinetic parameters. The AUC0-12 was calculated using the Bayesian estimates for the individual patient's parameter values and the specific regimen that that particular patient received. The clinical, radiological, and mycological responses to therapy for these patients have been reported elsewhere (3).
Optimal sampling.
To provide a further insight into the portions of the dosing interval that are information rich in terms of obtaining blood samples to delineate pharmacokinetic parameter estimates, the method originally described by Tam et al. (11) was used. The multiple model file from the output of BIG NPAG was generated. This file contains a summary of the “support points” and their associated probabilities that describes the behavior of voriconazole in the volunteer and patient population. Using the SAMPLE module of ADAPT 5 (1), a standard intravenous voriconazole regimen (6 mg/kg for two dosages followed by 4 mg/kg) was “administered” to each support point. Five optimal sampling times (corresponding to the five model parameters required to describe the behavior of intravenous voriconazole, namely Vmax, Km, volume, Kcp, and Kpc) were obtained. These times were obtained on day 3 of therapy, between 48 and 60 h postinitiation of voriconazole. These times were then plotted on a histogram after being multiplied by their associated probability. The histogram contained 15-min intervals.
RESULTS
Pharmacokinetic data from healthy volunteers.
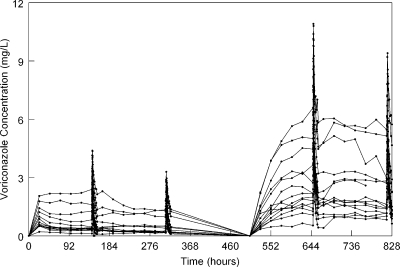
The con-centration-time profiles of voriconazole for cohort 1 (n = 14 patients) receiving various sequential regimens of i.v. and oral voriconazole are shown in Fig. 1. This figure highlights the extent of pharmacokinetic variability inherent to voriconazole, even in a relatively homogeneous population and well-controlled conditions.
Fig 1.
Voriconazole concentration-time profile of 14 healthy volunteers receiving 6 mg/kg i.v. for 2 doses followed by 3 mg/kg i.v. q12h for 1 week, followed by 200 mg q12h orally. After a washout period of 1 week, these same patients received i.v. voriconazole 6 mg/kg i.v. for 2 doses followed by 5 mg/kg i.v. q12h, followed by 400 mg q12h orally. Intensive sampling was performed at the end of weeks 1 and 2 and then weeks 4 and 5.
Population pharmacokinetic model.
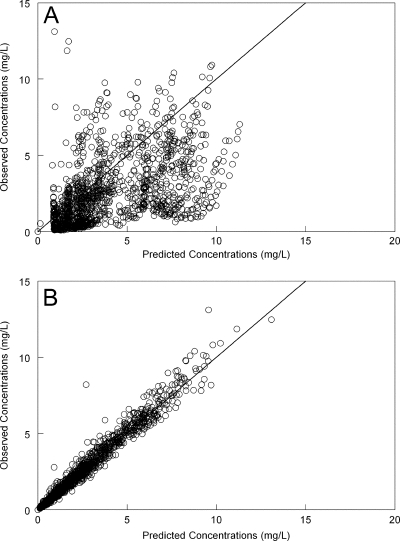
The fit of the nonlinear population pharmacokinetic model to the data was acceptable. The estimates for the mean and median parameter values along with their standard deviations are summarized in Table 1. There was a degree of bias when the population model was used to predict individual concentrations (the model tended to slightly overpredict observed concentrations [Fig. 2A]). The individual predicted concentrations from the Bayesian posterior estimates for each patient closely approximated observed concentrations (Fig. 2B). Overall, the use of the population parameter means gave better Bayesian predictions than the medians, but the differences were small and either value could have been reasonably used. The coefficient of determination for the linear regression of the observed-predicted values after the Bayesian step was 0.96, with an intercept and slope that approximated 0 and 1, respectively (Fig. 2B). There was no relationship between any of the Bayesian estimates for volume and clearance versus weight.
Table 1.
Parameters from the population pharmacokinetic analysis
| Parametera | Result |
||
|---|---|---|---|
| Mean | Median | SD | |
| Ka (h−1) | 9.77 | 3.85 | 10.30 |
| Vmax (mg/h) | 37.67 | 37.38 | 11.19 |
| Km (mg/liter) | 2.07 | 2.28 | 1.11 |
| Vol (liter) | 149.11 | 76.65 | 173.49 |
| Kcp (h−1) | 2.01 | 0.44 | 3.33 |
| Kpc (h−1) | 9.35 | 2.14 | 11.68 |
| F | 0.86 | 0.91 | 0.13 |
| Lag (h) | 1.12 | 0.77 | 0.99 |
Ka is the first-order rate constant that connects the gut with the central compartment, Vmax is the maximum rate of enzyme activity of voriconazole, Km is the concentration of voriconazole where clearance is half maximal, Kcp and Kpc are the first-order intercompartmental rate constants connecting the central and peripheral compartments, F is the bioavailability, and Lag is the absorption time.
Fig 2.
Observed-predicted values. Panel A, population-predicted values versus observed voriconazole concentrations; panel B, individual-predicted values versus observed voriconazole concentrations. The solid line is the line of identity (observed = predicted).
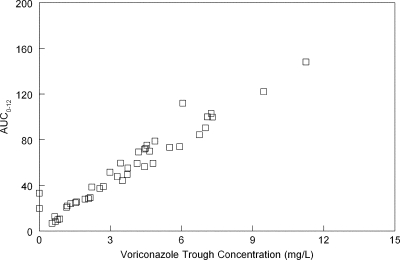
The Bayesian estimates and the original voriconazole regimen used for each of the 43 patients in this analysis were used to calculate the AUC0-12 at the end of the first week. All of these patients initially received i.v. therapy. The trough concentrations for each of these individual patients were calculated. The AUC0-12 and the trough concentration after the first week of therapy (Fig. 3) were highly positively correlated (Spearman coefficient, 0.977; 43 observations; P < 0.01; Fig. 3). In this data set, there were only 7/43 (16.3%) patients with trough concentrations less than 1 mg/liter. There was no relationship between the estimated AUC0-12 (at the end of the first week) and the ultimate clinical outcome of voriconazole therapy as determined by the Data Review Committee (DRC) in the study of Herbrecht et al. (3) (odds ratio, 0.997; 95% confidence interval [CI], 0.987 to 1.008; P = 0.652).
Fig 3.
Relationship between the AUC0-12 and the trough concentration at the end of the first week of voriconazole for 43 patients receiving voriconazole (6 mg/kg i.v. for 2 doses followed by 4 mg/kg i.v.). The values are highly positively correlated (Spearman coefficient, 0.977; 43 observations; P < 0.01). Only 7/43 patients had trough concentrations of <1 mg/liter.
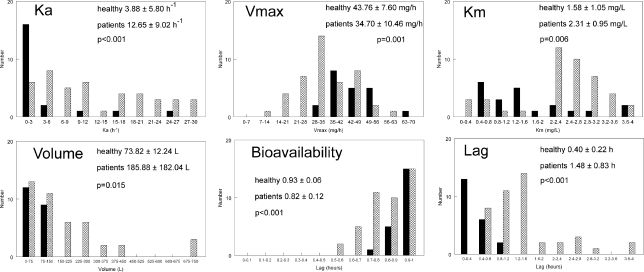
The Bayesian estimates for the population pharmacokinetic parameters in healthy volunteers and patients are shown in Fig. 4. The parameter values for healthy volunteers and patients were statistically different (Fig. 4). Most notably, the volume of patients tended to be much higher than that of healthy volunteers. Moreover, the parameter estimates in patients were more variable than those of healthy volunteers.
Fig 4.
Histogram of the Bayesian estimates for the parameter values in healthy patients (solid black bars; n = 21) and patients (gray hatched bars; n = 43). Ka is the first-order rate constant that connects the gut with the central compartment, Vmax is the maximum rate of enzyme activity of voriconazole, Km is the concentration of voriconazole where clearance is half maximal, Kcp and Kpc are the first-order intercompartmental rate constants connecting the central and peripheral compartments, F is the bioavailability, and Lag is the absorption time.
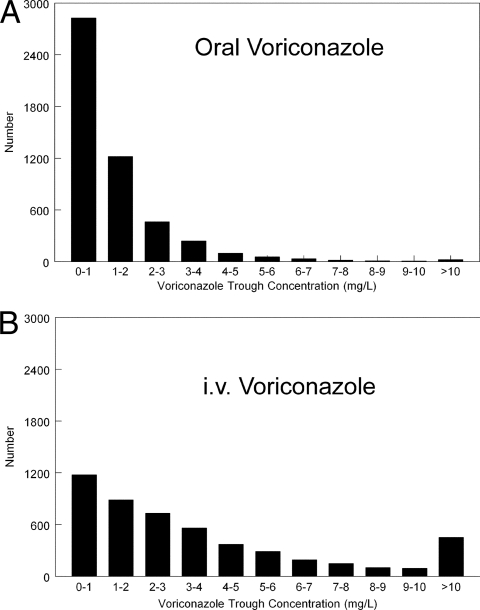
The original parameter values and their dispersions were recapitulated using Monte Carlo simulation. The trough concentrations after the first week for patients receiving oral (400 mg q12h for two doses followed by 200 mg q12h) and i.v. (6 mg/kg q12h for two doses followed by 4 mg q12h) therapy for 1 week are shown in Fig. 5. The AUC0-12 values for i.v. and oral therapy (median ± standard deviation [SD]) were 45.24 ± 76.09 versus 17.99 ± 21.57 mg.h/liter, respectively. The median trough concentrations (± SD) following i.v. and oral therapy were 2.54 ± 5.88 and 0.83 ± 1.64 mg/liter, respectively. The proportions of patients receiving i.v. and oral therapy with trough concentrations of ≤1 mg/liter were 1,180/5,000 (23.6%) and 2,633/5,000 (56.7%), respectively. The proportions of the same simulated patients with trough concentrations of ≥6 mg/liter were 985/5,000 (19.7%) and 93/5,000 (1.9%), respectively. The proportions of patients receiving i.v. or oral voriconazole with concentrations outside this therapeutic range (i.e., <1 or >6 mg/liter) were 2,165/5,000 (43.3%) and 2,926/5,000 (58.5%), respectively.
Fig 5.
Monte Carlo simulations from the population pharmacokinetic model for oral (A) and i.v. (B) therapy. Intravenous (i.v.) therapy results in higher measures of drug exposure compared with oral therapy.
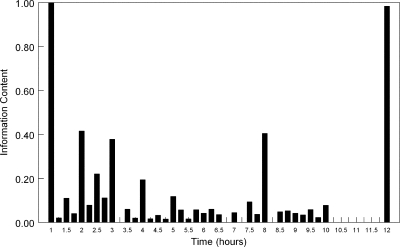
The multiple model file from the output of BIG NPAG contained 42 support points that were used to determine optimal sampling times. Each support point demanded a sample taken at the end of the i.v. infusion (i.e., 49 h after treatment initiation) and 41 support points also required a trough concentration (i.e., 60 h after treatment initiation). The remaining three sampling times were scattered throughout the dosing interval. The frequency of these sampling times for the population are summarized in Fig. 6. This histogram enables information-rich sampling times to be determined for voriconazole. A reasonable sampling strategy would be to obtain samples at 1, 2, 3, 4, 8, and 12 h postdosing. A larger number of times would be possible and would potentially capture more information (i.e., those patients with “less common” pharmacokinetics), but this obviates the whole purpose of optimal sampling strategies (as previously argued by Tam et al. (11).
Fig 6.
Optimal sampling times for patients receiving a standard i.v. regimen of voriconazole. These times have been determined within the 5th dosing interval (49 to 60 h after initiation of therapy). Reasonable sampling times are 1, 2, 3, 4, 8, and 12 h after initiation of therapy.
DISCUSSION
In comparison to those of many anti-infective agents, the pharmacokinetics of voriconazole are relatively well characterized. Somewhat surprisingly, however, there are no population pharmacokinetic models for adult patients. Two models have been developed for children (4, 8), and these have been important to identify optimal dosing strategies in this population. The absence of models for adults primarily relates to the absence of robust pharmacokinetic data from patients with invasive fungal infections. A further obstacle has been the inherently variable pharmacokinetics of voriconazole. This agent is well known to exhibit Michaelis-Menten (nonlinear) pharmacokinetics, which is related to saturable clearance mechanisms. Robust population pharmacokinetics models represent an important step for the optimal use of voriconazole in critically ill patients.
A robust estimate of the degree of nonlinearity in individual patients and/or populations requires the use of more than one dosage—a single fixed regimen may not enable the extent of nonlinearity to be accurately estimated. While a degree of variation in absolute dosage is achieved using weight-based i.v. dosing, the variations in dosage may not be large enough to trigger and therefore describe nonlinear pharmacokinetics. In the current analyses, this problem was circumvented by comodeling a rich data set from healthy volunteers in whom a range of dosages had been studied and for some of whom dosage escalation had also been performed. This approach provided rich information that enabled robust estimates to be obtained for Vmax and Km, which are the two parameters describing nonlinear clearance. A potential disadvantage of this approach is the mixing of populations (i.e., healthy volunteers and patients) who may handle drug differently. Nevertheless, in the absence of informative data from patients, this is a necessary first step and provides a foundation for the future studies that are required to further characterize the pharmacokinetics of voriconazole.
The fit of the model to the data was acceptable. The population pharmacokinetic model enables the well-recognized and clinically relevant interpatient variability to be quantified. Such estimates are vital for predicting drug behavior in populations of patients. This model can now be used to provide a greater understanding of the utility of voriconazole (e.g., estimating the proportion of patients with subtherapeutic or toxic concentrations and providing decision support for setting in vitro susceptibility breakpoints). The intrinsic pharmacokinetic variability of voriconazole is reflected throughout these analyses—it is evident in the estimates for coefficient of variation for some of the parameters, the Monte Carlo simulations, the Bayesian estimates for the parameter values, and the optimal sampling times. The overall estimate for bioavailability (86%) is higher than in other studies in children (4, 8) but lower than previously described in healthy volunteers. Similarly, the estimate for Km (2.07 mg/liter) in this study is lower than has been previously described in children (range, 3.03 to 7.84 mg/liter) (4, 8), consistent with the observation that pharmacokinetics of voriconazole in adults are more likely to be nonlinear than in children. The reasons for the extent of observed pharmacokinetic variability in this study are not readily apparent (i.e., the pharmacokinetic variability is not accounted for by covariates). While the CYP2C19 genotype clearly has an impact upon the clearance of voriconazole, this information was not available for the patients in this study (2). The greater extent of pharmacokinetic variability in patients than in healthy volunteers (Fig. 4) likely reflects a degree a range of factors such as physiological derangement, drug-drug interactions, and altered gut motility.
One advantage of population pharmacokinetic modeling is the ability to obtain estimates for population pharmacokinetic parameter values for individual patients using relatively sparse data. These parameter values can be used to describe the behavior of a drug in that individual and to estimate measures of drug exposure such as the trough concentration and AUC0-12. Unlike previous studies, these results did not indicate any relationship between drug exposure at the end of the first week and the ultimate clinical outcome. There are a number of possible explanations for this: (i) the sample size is relatively small for patients with a syndrome whose ultimate outcome is affected by many factors, and (ii) all patients in this study initially received i.v. therapy, which produced drug exposures that were probably associated with near-maximal antifungal effect (there were only 7/43 patients with trough concentrations of <1 mg/liter at the end of the first week [Fig. 2]).
The population pharmacokinetic model suggests that therapeutic drug monitoring for voriconazole may be an important adjunct to the routine use of voriconazole. Other studies have suggested that trough concentrations in the range between 1 and 6 mg/liter may be associated with optimal clinical outcomes (10). The Monte Carlo simulations in this study (Fig. 5) demonstrate that a relatively small proportion of the population receiving a fixed dosing regimen falls into this concentration range after 1 week of i.v. or oral therapy. Therapeutic drug monitoring with dosage adjustment may be used to achieve therapeutic concentrations and thereby optimize clinical efficacy. Further research is required to address how this can be achieved in a timely and optimally precise manner. The Monte Carlo simulations also highlight the potential peril of using oral dosing as initial therapy. If an initial oral regimen is deemed appropriate on clinical grounds, an oral loading dosage should be used and consideration given to therapeutic drug monitoring.
This study contains the first description of optimal sampling times that are maximally informative and that facilitate the further description of voriconazole pharmacokinetics. In busy clinical settings, the best sampling strategy remains unclear, but the following are relevant: (i) a single trough concentration is very difficult to interpret because some patients whose serum concentrations are persistently greater than Km will rapidly accumulate drug and are at increased risk of developing toxicity; (ii) the best dosing interval for sampling is uncertain, but a reasonable approach is to sample at the end of day 2 and then again in the first week of therapy; (iii) a distinction should be made between drawing samples to optimize therapy (e.g., ensuring that trough concentrations are within a prespecified range) and drawing samples to enable the pharmacokinetics of an individual patient to be described. The latter is a more challenging problem because of the nonlinear pharmacokinetics of voriconazole. If robust estimates of Km and Vmax in an individual patient are required, then a range of dosages must be studied (i.e., an attempt must be made to trigger nonlinear pharmacokinetics in that patient). Obviously, this may be difficult to achieve in a patient with a life-threatening infection. The optimal sampling histogram also highlights the difficulties in sampling strategies to ensure that the pharmacokinetics are adequately described in a population receiving an inherently variable compound. Here, there is a tension between obtaining enough samples to ensure that pharmacokinetic idiosyncrasies can be described and intensive sampling that is potentially intrusive and unnecessary.
Voriconazole remains a first-line treatment for the prevention and treatment of invasive fungal infections. This population pharmacokinetic model summarizes the behavior of voriconazole in adult patients. Initiatives are now required to capitalize on the insights and information contained within these analyses so that outcomes for patients with rapidly life-threatening fungal infections are further optimized.
ACKNOWLEDGMENTS
I am supported by a National Institute of Health Research (NIHR) Clinician Scientist Fellowship.
The studies on which this work is based were originally sponsored by Pfizer Inc., but the present analysis was done by me, and I received no compensation from Pfizer.
Footnotes
Published ahead of print 7 November 2011
REFERENCES
- 1. D'Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA [Google Scholar]
- 2. FDA Antiviral Drugs Advisory Committee 2001. Briefing document for voriconazole (oral and intravenous formulations). www.fda.gov/ohrms/dockets/AC/01/briefing/3792b2_01_Pfizer.pdf
- 3. Herbrecht R, et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 4. Karlsson MO, Lutsar I, Milligan PA. 2009. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob. Agents Chemother. 53:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leary R, Jelliffe R, Schumitzky A, van Guilder M. 2001. An adaptive grid, non-parametric approach to pharmacokinetic and dynamic (PK/PD) models, p 389–394 In Proceedings, Fourteenth IEEE Computer Society, Bethesda MD [Google Scholar]
- 6. Lortholary O, et al. 2010. International retrospective analysis of 73 cases of invasive fusariosis treated with voriconazole. Antimicrob. Agents Chemother. 54:4446–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marks DI, et al. 2011. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br. J. Haematol 155:318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neely M, Rushing T, Kovacs A, Jelliffe R, Hoffman J. 2010. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin. Infect. Dis. 50:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pappas PG, et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pascual A, et al. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211 [DOI] [PubMed] [Google Scholar]
- 11. Tam VH, Preston SL, Drusano GL. 2003. Optimal sampling schedule design for populations of patients. Antimicrob. Agents Chemother. 47:2888–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Troke PF, Hockey HP, Hope WW. 2011. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob. Agents Chemother. 55:4782–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh TJ, et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 14. Wingard JR, et al. 2010. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 116:5111–5118 [DOI] [PMC free article] [PubMed] [Google Scholar]