Abstract
Topical microbicides that block the sexual transmission of HIV and herpes simplex virus 2 (HSV-2) are desperately needed to reduce the incidence of HIV infections worldwide. Previously we completed phase 3 testing of the carrageenan-based gel Carraguard. Although the trial did not show that Carraguard is effective in preventing HIV transmission during vaginal sex, it did show that Carraguard is safe when used weekly for up to 2 years. Moreover, Carraguard has in vitro activity against human papillomavirus (HPV) and HSV-2 and favorable physical and rheological properties, which makes it a useful vehicle to deliver antiviral agents such as zinc acetate. To that end, we previously reported that a prototype zinc acetate carrageenan gel protects macaques against vaginal challenge with combined simian-human immunodeficiency virus reverse transcriptase (SHIV-RT). Herein, we report the safety and efficacy of a series of zinc acetate and/or carrageenan gels. The gels protected mice (75 to 85% survival; P < 0.001) against high-dose (106-PFU) HSV-2 vaginal or rectal challenge. In contrast, zinc acetate formulated in HEC (hydroxyethylcellulose; or the Universal Placebo) failed to protect mice against the high-dose vaginal HSV-2 challenge (similar to aqueous zinc acetate solution and the placebo controls). The gels were found to be effective spreading gels, exhibited limited toxicity in vitro, caused minimal damage to the architecture of the cervicovaginal and rectal mucosae in vivo, and induced no increased susceptibility to HSV-2 infection in a mouse model. Our results provide a strong rationale to further optimize and evaluate the zinc acetate/carrageenan gels for their ability to block the sexual transmission of HIV and HSV-2.
INTRODUCTION
Microbicide research is focused primarily on the prevention of HIV. However, the introduction of an effective microbicide may decrease condom use (17), leading to the emergence of other sexually transmitted infections (STIs). Moreover, the presence of other STIs (e.g., herpes simplex virus 2 [HSV-2]) has been shown to increase the spread of HIV infection (2, 11, 18, 22). Thus, microbicides that contain either an active pharmaceutical ingredient (API) or a combination of APIs with activity against multiple STIs are needed. The recent results of the phase 2b CAPRISA-004 trial showed that a gel formulation containing the antiviral drug tenofovir might constitute a good approach for decreasing the transmission of HIV-1 and HSV-2 (1). However, there remains the underlying concern with drug resistance (particularly against drugs like tenofovir that are already used in the treatment of HIV), and so it would be advantageous to identify a formulation that is not only less likely to promote the emergence of drug-resistant viruses but also active against antiretroviral (ARV)-resistant viruses.
Studies have shown that zinc salts have antiviral activity against a broad range of viruses, including HIV and HSV (4, 7, 19, 20, 27, 29, 45, 54). Thus, topical application of formulations containing low-dose zinc salts represents a novel strategy for preventing the sexual transmission of HSV-2 and potentially HIV without the use of antiretroviral drugs.
Zinc is an indispensable element found in organisms and microorganisms. Its biological roles as a structural or functional factor include participation in signal transduction, gene expression, and metabolism of different biomolecules (6, 21, 41, 52). Moreover, zinc salts have been proven safe for human use; zinc acetate is an FDA-approved oral treatment for Wilson's disease.
In vitro assays are challenging due to the toxicity of zinc salts to cells. While there have been some concerns raised about adverse effects of zinc salts (used nasally in humans [3] or vaginally in mice [7]), it is important to point out that these problems have been encountered only with high doses of zinc salts (∼222 mM nasally and 200 mM vaginally). No zinc toxicity was seen in rabbits after daily vaginal dosing with 90 mM zinc acetate for 10 days (14) or after a 10-day treatment with zinc sulfate-loaded sponges (9). Topical formulations require a suitable delivery vehicle. Carrageenan-based gels have been shown to be safe and acceptable for topical use in humans. This favorable safety profile, as well as good physical/rheological properties, makes carrageenan-based gels a potentially useful vehicle for future microbicides (12, 13, 15, 25, 26, 35, 37, 44, 49, 50).
In addition, carrageenan has intrinsic antiviral activity. It has been shown to prevent human papillomavirus (HPV) infection in mice (43), while closer analysis of a subgroup of highly adherent subjects in the Carraguard phase 3 clinical trial suggested that the carrageenan gel reduced the prevalence of HPV (34). Carrageenan-based gels have been shown to prevent significant HSV-2 infection in the HSV-2 mouse model when challenged with low viral doses of HSV-2 (30, 32, 53). Thus, using a carrageenan gel as the vehicle potentially further broadens the formulation's spectrum of activity. Using a stringent macaque model, we recently showed that a prototype formulation containing zinc acetate in a carrageenan gel provided significant protection (70%, compared to a control carrageenan gel without zinc) against vaginal immunodeficiency virus infection when applied daily for 2 weeks or every other day for 4 weeks prior to virus challenge (23).
Therefore, we developed a zinc acetate-carrageenan gel as a broad-spectrum microbicide that could prevent HIV, HSV-2, and potentially HPV. To identify an optimized formulation, we prepared a series of formulations in which both the manufacturing method and buffer varied. These candidate formulations were subjected to a battery of experimental (both in vitro and in vivo) assays and computational assessment. Tests included the monitoring of physicochemical properties, prediction of vaginal coating, in vitro release of the zinc, effects on cell viability and cell monolayer integrity, in vivo evaluation of histological changes, and efficacy in both HSV-2 mouse rectal and vaginal challenge models. Herein, we demonstrate that the optimized formulation of zinc acetate in carrageenan is an effective spreading gel that is safe and highly effective against high-dose vaginal and rectal HSV-2 infection. Taken together with our macaque data, these studies are promising enough to warrant testing in humans to determine that zinc acetate in carrageenan is safe and effective.
MATERIALS AND METHODS
Formulation—preparative methods. (i) Carrageenan/zinc acetate gels.
The lambda/kappa carrageenan was provided by FMC Corporation (Philadelphia, PA). Hydroxyethyl cellulose (HEC) 250 HX Pharm was obtained from Ashland Aqualon Functional Ingredients (Wilmington, DE). All other chemicals were purchased from commercial suppliers. Three different procedures for preparing iso-osmolar carrageenan-based gels were evaluated.
(a) Method 1.
In step 1, a 1-liter mixing jar was filled with 347.5 ml of 10 mM pH 5.5 sodium citrate buffer and 4.0 g of NaCl. The solution was heated for 10 min at 75°C with stirring at 300 rpm using a Yamato LT 400 Lab-Stirrer. Fifteen grams of carrageenan was then added, and the mixture was stirred for 1 h at 75°C. In step 2, the formulation was cooled to 60°C. In step 3, a preformed solution of 1 g of methyl paraben in 100 ml of citrate buffer (previously heated at 60°C for 45 min) was added to the mixing jar, and the solution was allowed to cool to room temperature and stirred for an additional 1 h. In step 4, a premixed solution of zinc acetate dihydrate (2.5 g) in 10 mM citrate buffer (30 ml) was then added. In step 5, the solution was stirred for 2 h at room temperature.
(b) Method 2.
In method 2, the same procedure was followed as in method 1, except step 4 was combined with step 1, and the solution in step 5 was stirred for an additional hour.
(c) Method 3.
All components were combined and heated at 75°C for 1 h. The solution was allowed to cool to room temperature and then stirred for an additional 3 h.
Formulation composition.
Six additional carrageenan gels (three using method 1 and three using method 2) were prepared in which the 10 mM sodium citrate buffer was replaced with either 10 mM potassium acetate, 10 mM sodium acetate, or 10 mM potassium citrate. Using method 1 above, carrageenan gels containing 1.0, 0.5, 0.3, 0.1, and 0.03 wt% zinc acetate dihydrate were prepared. These gels were evaluated with the HSV-2 mouse model to determine the minimum effective dose of zinc acetate dihydrate.
HEC-zinc acetate gels. (i) Method 4: pH 4.5 sorbic acid preservative.
A 1,000-ml mixing jar was filled with 375 ml of water, 0.5 g of sorbic acid, and 2.5 g of zinc acetate dihydrate. While stirring the solution at 300 rpm with a Yamato LT 400 Lab-Stirrer, 14.0 g of HEC (2.7% HEC loading) was added in portions over 15 min. The mixture was stirred for 20 h at room temperature, and the pH was adjusted to 4.5 with 1 N HCl. Lower-viscosity formulations (to match the viscosity of the carrageenan-based gels) were prepared using 2.2% HEC loading.
(ii) Method 5: pH 6.9 methyl paraben preservative.
A 1,000-ml mixing jar was filled with 375 g of water and 2.5 g of zinc acetate dihydrate. While stirring the solution at 300 rpm with a Yamato LT 400 Lab-Stirrer, 14.0 g of HEC was added over 15 min. The mixture was stirred for 17 h at room temperature. A solution of methyl paraben in water was prepared by heating methyl paraben (1 g) in 100 ml of water to 60°C. The methyl paraben solution was added to the formulation, which was then stirred for 1 h at room temperature. The pH was adjusted using 1 N NaOH. Lower-viscosity formulations were prepared using 2.2% HEC loading.
Determination of methyl paraben content.
Methyl paraben content was measured via high-performance liquid chromatography (HPLC) on an Agilent 1100 integrated system equipped with an ACE 3 (150 by 4.6 mm) C18 column and UV detection at 260 nm. The mobile phase was 50:50 acetonitrile–0.2 M NH4Ac(pH 5), with an injection volume of 10 μl. The flow rate was 0.5 ml/min. One milliliter of test gel (exact mass recorded) was transferred to a 25-ml volumetric flask and diluted to volume with 40:60 (vol/vol) acetonitrile–5% dimethyl sulfoxide (DMSO)–95% H2O. The flask was then sonicated for 60 min (Branson model 1510). A portion of the solution was filtered through a 0.45-μm filter and injected directly into the high-performance liquid chromatograph. Methyl paraben concentration was determined using a five-point standard curve.
Determination of zinc content.
UV absorbance to obtain zinc content was measured at 555 nm using a Perkin-Elmer Lambda 2 UV/visible light spectrophotometer (47). Thirteen milligrams of test gel was mixed with 200 μl of 1 N HCl in a 2-ml conical centrifuge tube and vortexed for 5 min. One hundred microliters of this solution was transferred to a 15-ml centrifuge tube that contained 1 ml of 0.05 M Na2CO3–0.05 M NaHCO3 (85:15 [pH 9.5]), 2 ml of 100% ethanol, and 1.5 ml of PAN solution [0.1% 1-(2-pyridylazo-)-2-naphthol in ethanol]. The solution was vortexed for 10 s. Zinc content was determined using a five-point standard curve (lower level of quantitation is 100 ng/ml).
Analyses of gel spreading: rheology, modeling of gel spreading, and computation of an objective function that merited gel spreading performance.
Viscosity-versus-shear rate measurements were performed at 37°C in a TA model AR 1500ex rheometer (TA Instruments, New Castle, DE) using a 4° cone and 20-cm-diameter plate. Gels were tested undiluted and also after a 20% (vol/vol) dilution with human vaginal fluid simulant to mimic dilution by cervicovaginal fluids (40). Viscosity was obtained by controlled stress measurements that created a range of shear rates (10−3 to 102 s−1) characteristic of those experienced during vaginal spreading by a gel (28). Yield stress (undiluted or diluted gels) was obtained by measuring the residual stress at 37°C in a Brookfield model 5HB DV-III Ultra rheometer (Brookfield Engineering Laboratories, Middleboro, MA) (28). The rheological data were fit to a constitutive equation, the Carreau model, which characterized the relationship between the viscosity of the gel and its local shear rate (28). These results were input to computations of coated area versus time, using a biomechanical model of spreading of the gel (24). Here we used a gel volume of 3.5 ml.
To summarize the predicted vaginal spreading performance of the different gels, we employed an objective function, termed the “scoring function” (SF), that places merit on gel spreading (33). The principle of the SF used here is that spreading is optimal when gel coats the entire surface area of the vaginal canal (∼100 cm2), for which the value of the SF, termed the “score,” is unity. For predicted coated areas that are less than ∼100 cm2, the value of the SF is <1; if the predicted coated area exceeds ∼100 cm2—so that the gel leaks out of the vagina—the SF is also <1. Thus, SF can be expressed as a function of predicted coated area and has a maximum equal to unity when that coated area equals vaginal epithelial surface area. For further details, see the article by Mahalingam et al. (33).
Physiochemical properties (osmolality, pH, and simple viscosity).
Osmolality was measured on a Vapro vapor pressure osmometer 5520 (Wescor, Inc., Logan, UT) calibrated with Opti-mole 100-, 290-, and 1,000-mmol/kg osmolality standards. Ten microliters of gel was sampled. pH was determined using an Orion 4-Star Plus benchtop pH/ISE meter (Thermo Scientific) with an Orion 8235BN PerpHect Ross flat-surface pH probe calibrated using three points: pH 4.0, 7.0, and 10.0. To obtain a simple measure of gel viscosity, for use in initial gel screening and interpretation of differences in carrageenan configuration within gels, viscosity was measured at 35°C on a Brookfield DV-II+ programmable viscometer equipped with a circulating water bath (Brookfield Engineering Laboratories, Middleboro, MA) and spindle SC4-28. The spindle speed was 5.0 rpm.
Stability studies.
Twenty-five-gram aliquots of test gel were placed in 30-ml low-density polyethylene bottles that were stored under the following conditions: 30°C with 65% relative humidity, 40°C with 75% relative humidity, and 50°C with ambient humidity. Bottles were removed at scheduled times, and the gel was analyzed for methyl paraben content, osmolality, pH, viscosity, and zinc content.
Zinc release in vitro.
Zinc release at 37°C with 75% relative humidity was measured using a Franz cell with a 9-mm-diameter donor chamber and a 5-ml receptor chamber with type 3 flow porting (PermeGear, Inc., Hellertown, PA). A 1-kDa-cutoff membrane was used to separate the donor and receptor chambers. Two hundred-fifty milligrams of test gel was placed on the filter; the release medium was iso-osmolar 10 mM citrate buffer (pH 4.5). Two hundred microliters of receptor medium was withdrawn at 1, 2, 6, 18, and 24 h, and an equivalent volume of citrate buffer was replaced to maintain a constant volume. Zinc content was assayed as described above.
Cell lines and viruses.
Caco-2 cells were obtained through the American Type Culture Collection (ATCC; Rockville, MD). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and antibiotics at a final concentration of 50 U/ml of penicillin and 50 μg/ml streptomycin (Invitrogen). For the seeding of Caco-2 cells, cells were grown and differentiated using the BD BioCoat HTS Caco-2 assay system (BD Biosciences, Bedford, MA).
The HSV-2 strain G was obtained from the ATCC and propagated in Vero cells (ATCC) as described by McDermott et al. (36). The plaque formation assay on Vero cells was used to obtain the viral titer in PFU/ml, as described by Ashley (5). The aliquots of virus stock were stored at −80°C.
Synergistic effect of the combination.
The virucidal activity of each independent compound (lambda/kappa carrageenan and zinc acetate dihydrate) or the combination was determined as described by Arens et al. (4), with some modifications. Briefly, ∼104 PFU of the HSV-2 G strain was combined with different concentrations of each compound or their combination in 10 mM sodium acetate buffer (pH 6.2; 300 mosmol/kg) in a total volume of 200 μl. A mock-infected control containing only virus in 10 mM sodium acetate buffer (pH 6.2; 300 mosmol/kg) was also included. Each concentration or control was tested in triplicates. The assay was designed in a way that the ratio of carrageenan to zinc acetate dihydrate is approximately the 50% inhibitory concentration (IC50)/IC50 ratio for the two compounds. Eight different concentrations of carrageenan (0.6, 0.3, 0.15, 0.075, 0.037, 0.018, 0.009, and 0.0045 μg/ml) and zinc acetate dihydrate (600, 300, 150, 75, 37, 18, 9, and 4.5 μg/ml) were tested. The samples were incubated for 6 h at 37°C, in 5% CO2, with 98% humidity. After incubation, decimal dilutions of each sample were performed to determine the virus titer using the plaque assay as described by Ashley et al. (5). By performing these dilutions, the in vitro toxic effect of zinc was prevented. The percentage of virus inhibition was used to analyze the effect of the combination on the virucidal activity and to estimate the combination index (CI) values (8).
Toxicity and monolayer integrity testing of formulations.
The XTT assay, which is based on the reduction of the tetrazolium salt 2,3-bis (2-methyloxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (Sigma, St. Louis, MO), was used in Caco-2 cells to determine the CC50 value (concentration of the formulation that showed 50% viability compared to a cell control that contained only media). Various dilutions of each formulation were added to the cells and incubated at 37°C in 5% CO2 with 98% humidity for 2 or 6 h. Gynol II (a spermicidal gel containing Nonoxynol 9 and sodium carboxymethyl cellulose; Caldwell Consumer Health, LLC, Parsippany, NJ) was used as a positive control for cell death, and a carrageenan-based gel alone was used as a negative control. After the incubation, the monolayer was washed once with D-PBS (Invitrogen) and then replaced with fresh complete medium. The XTT assay was performed as previously described (15).
Changes in Caco-2 monolayer integrity were measured by transepithelial electrical resistance (TEER) using the 3-day BD BioCoat HTS Caco-2 assay system. Briefly, Caco-2 cells were grown on double-chamber 24-well plates until differentiation was reached at a TEER value higher than 300 Ω × cm2 as measured by an EVOM epithelial voltimeter (World Precision Instruments, Sarasota, FL). Neat formulations were added to the apical surface of the monolayer, and resistance readings were measured at 0, 2, 4, and 6 h. Cells in growth media, carrageenan, Dulbecco's phosphate-buffered saline (D-PBS), or Gynol II versus media without cells (blank) were included as controls. The epithelial resistance was calculated by subtracting the Ω × cm2 of the blank from the Ω × cm2 of the treated wells.
Vaginal HSV-2 challenge.
The vaginal HSV-2 challenge was performed on 8-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) as described previously (53). Mice received subcutaneously 100 μl of medroxyprogesterone acetate (Depo-Provera; Upjohn, Kalamazoo, MI) at 25 mg/ml in D-PBS (Invitrogen). One week later, 10 μl of the test formulation was inserted in the vagina 10 min before 10 μl of virus (106 PFU/mouse) was applied.
In each experiment, two placebo groups (D-PBS and HEC) were used in addition to the carrageenan vehicle-only gel to compare to the test formulations containing zinc acetate. Beginning on day 4 after inoculation, mice were examined and scored daily for 19 days total. Animals with signs of infection such as hind limb paralysis, redness, hair loss, swelling, and/or lesions in the vaginal area were scored as infected and euthanized.
Rectal HSV-2 challenge.
Eight-week-old female BALB/c mice (Charles River Laboratories) were fasted for 24 h prior to the rectal HSV-2 challenge assay, but food and water were available ad libitum following initial infection. At the time of formulation insertion and viral challenge, animals were anesthetized to both immobilize them and prevent defecation using an intraperitoneal (i.p.) injection of 100 μl of a solution containing 1 ml of dissolved ketamine (Fort Dodge Laboratories, Fort Dodge, IA), 500 μl of xylazine (Miles, Inc., Shawnee Mission, KS), and 6.75 ml PBS. Twenty microliters of test formulation was applied rectally, and after 10 min, mice were challenged rectally with 10 μl of 106 PFU of HSV-2. Mice were examined and scored daily for 19 days, starting on day 4 after inoculation. Any mice with symptoms of infection including swelling, hair loss, redness, hind limb paralysis, and/or lesions in the rectal area were scored as infected and euthanized.
HSV-2 increased-susceptibility model.
We followed the method described by Wilson et al. (51). Eight-week-old female BALB/c mice were treated with medroxyprogesterone acetate as described above. Ten microliters of gel formulations (versus D-PBS and Gynol II) was delivered intravaginally daily for 7 days, and 12 h after the last application, mice were challenged with 2 × 103 PFU of the HSV-2 G strain in a volume of 10 μl. Mice were examined and scored daily for 19 days as described in the “Vaginal HSV-2 challenge” section.
Histological evaluation of the cervicovaginal and rectal mucosae after single-dose gel application.
Eight-week-old female BALB/c mice were treated with medroxyprogesterone acetate or fasted and anesthetized before adding the formulations as described above for vaginal and rectal challenges, respectively. Mice treated with D-PBS or Gynol II were used as controls (positive and negative, respectively) to evaluate the normal architecture in the vaginal and rectal mucosae. Mice were sacrificed at 1, 6, and 24 h after gel applications, and the entire reproductive or lower rectal tracts were surgically excised. The tissues were fixed in 10% formalin (Sigma) and embedded in paraffin before preparing tissue sections of 4 to 6 μm. Morphological analysis was performed using hematoxylin and eosin (H&E) staining followed by examination of the stained tissue sections with a Zeiss Axioplan 2 upright microscope. The micrographs were obtained using a Spot Insight QE color digital camera and the Spotbasic software. For each experimental condition, six sections of two to three different animals were analyzed.
Ethics statement for animal procedures.
Protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Comparative Bioscience Center (CBC) at Rockefeller University. Animal care procedures were in compliance with the regulations detailed under the Animal Welfare Act (10) and in the Guide for the Care and Use of Laboratory Animals (39).
Statistical methods.
Each experiment was repeated at least twice. In those experiments in which different dilutions of a compound or formulation were tested, each dilution or control was tested in triplicate. The CC50 (cytotoxicity from the XTT assay) values and its 95% confidence intervals were calculated using a dose-response inhibition analysis on GraphPad Prism v5.0b software for Mac. Analyses of the combination effect were done according to the method described by Chou and Talalay (8). The CI values were estimated using the Calcusyn for Windows software (Biosoft, Cambridge, United Kingdom). The Fisher's exact test was used for statistical comparison of the percentages of survival in the HSV-2 mouse model, using GraphPad Prism version v5.0b (GraphPad Software, San Diego, CA). A Mann-Whitney test was performed to compare levels of zinc release from all of the formulations at different time points. P values of <0.05 were taken as statistically significant.
RESULTS
Synergistic effect of the zinc acetate-carrageenan combination in vitro and a dose-dependent prevention of vaginal HSV-2 infection of mice by zinc acetate and/or carrageenan gels.
Combining carrageenan with zinc acetate dihydrate resulted in a 3.7-fold reduction in the virucidal IC50 compared to carrageenan alone. The CI value for the combination is given in Table 1. A synergistic interaction is suggested by the CI values for the IC50 (0.41), IC75 (0.26), and IC90 (0.17). Carrageenan and zinc acetate dihydrate yield parallel lines in the median-effect plot, but the mixture was not parallel, indicating mutual nonexclusivity (8).
Table 1.
Carrageenan/zinc acetate anti-HSV-2 synergism by the Chou-Talalay methoda
| Compound(s) | CI value atb: |
Dmc | md | re | ||
|---|---|---|---|---|---|---|
| IC50 | IC75 | IC90 | ||||
| Carrageenan | NA | NA | NA | 0.036 | 0.77 | 0.966 |
| Zinc acetate dihydrate | NA | NA | NA | 67.379 | 0.67 | 0.885 |
| Carrageenan-zinc acetate dihydrate combination (1:1,000) | 0.41 | 0.26 | 0.17 | 0.009 | 1.06 | 0.994 |
Results were obtained using the average percentage of inhibition from two independent experiments.
CI is a quantitative measure of the degree of drug interaction. CI = 1, additive effect; CI < 1, synergism; CI > 1, antagonism. NA, not applicable.
Dm, median-effect dose (IC50).
m represents a measurement of the sigmoidicity of the dose-effect curve. m also represents the slope of the median-effect plot.
r is the linear correlation coefficient of the median-effect plot.
Previously, we had shown that carrageenan alone can reduce HSV-2 infection of mice in a low-dose (103- to 104-PFU/mouse), but not a high-dose (106-PFU/mouse), challenge model (R. A. Maguire et al., presented at the Microbicides Conference, Cape Town, South Africa, 23 to 26 April 2006) and that 1.5% zinc acetate dihydrate in carrageenan can reduce HSV-2 infection in mice in a high-dose model (R. A. Maguire, M. Thorne, and D. M. Phillips, U. S. patent application 2005/0261240 A1). To first determine if gels with reduced amounts of zinc maintained their anti-HSV-2 activity in mice, we tested gels containing different amounts of zinc for their ability to prevent significant vaginal HSV-2 infection after a high-dose challenge (106 PFU). Strikingly, formulations containing 1% (46 mM), 0.5% (23 mM), and 0.3% (14 mM) zinc acetate dihydrate provided significant protection against the high-dose challenge (75 to 85%) when compared to carrageenan or D-PBS (P = 0.0003 or P < 0.0001) (Fig. 1). There was no significant difference between the efficacies of the 1%, 0.5%, and 0.3% zinc acetate dihydrate formulations. Therefore, we chose to perform extended optimization studies on the intermediate 0.5% zinc acetate dihydrate gel, determining the stability, safety, and efficacy of an optimized formulation.
Fig 1.
Zinc acetate in carrageenan prevents high-dose HSV-2 infection. Medroxyprogesterone acetate-treated BALB/c mice were challenged with 106 PFU HSV-2 10 min after applying the indicated gel (or D-PBS) (20 animals per group). The percentages of infection over time, based on symptoms, are shown for each treatment group. Carrageenan-based gels containing more than 0.3% zinc acetate dihydrate were significantly more protective than formulations containing lower concentrations of zinc acetate dihydrate (P = 0.0003 to 0.01), the carrageenan vehicle alone (P = 0.0003 or P < 0.0001), or D-PBS (P < 0.0001).
Formulation optimization and stability testing of zinc acetate/carrageenan gels.
As shown in Table 2, we compared properties of carrageenan-based (formulations A to G) and HEC-based (formulations H to K) gels formulated with different methods and buffers to select the optimized formulation. Carrageenan-based gels prepared using method 1 (zinc acetate mixed with hydrated carrageenan at room temperature) were generally less viscous (by up to 40%) than gels prepared using method 2 or 3 (zinc acetate mixed with carrageenan at 75°C) and were relatively insensitive, as measured by viscosity, to the cation present in the buffer. However, method 1 gels were sensitive to the acid component in the buffer. Gels that contained citrate buffer were more viscous (10% to 23%) than gels that contained acetate buffer. Gels prepared according to method 2 were sensitive to the cation present in the buffer (data not shown). Specifically, gels containing potassium acetate or potassium citrate were 29% to 67% more viscous than gels prepared with sodium acetate or sodium citrate.
Table 2.
Summary of the formulations, describing the different preparative methods, buffers used, and physicochemical properties
| Formulation | Preparative methoda | Buffer | pH (mean ± SD)b | Viscosity (cP; mean ± SD)b | Preservativec |
|---|---|---|---|---|---|
| 3% carrageenan | NA | 10 mM PBS | 6.8 ± 0.06 | 27,700 ± 643 | MP |
| 2.7% HEC | NA | None | 4.6 ± 0.06 | 56,000 ± 718 | SA |
| 0.5% Zn (OAc)2 | NA | 10 mM sodium acetate | 7.0 ± 0.1 | NA | NA |
| Carageenan-based gelsd | |||||
| A | 1 | 10 mM sodium citrate | 5.4 ± 0.06 | 25,600 ± 621 | MP |
| B | 1 | 10 mM potassium citrate | 5.5 ± 0.06 | 24,400 ± 499 | MP |
| C | 1 | 10 mM sodium acetate | 5.9 ± 0.06 | 20,800 ± 609 | MP |
| D | 1 | 10 mM potassium acetate | 5.9 ± 0.06 | 22,900 ± 544 | MP |
| E | 2 | 10 mM potassium acetate | 5.5 ± 0.06 | 28,400 ± 589 | MP |
| G | 3 | 10 mM sodium acetate | 5.4 ± 0.1 | 39,400 ± 225 | MP |
| HEC-based gelse | |||||
| H | 5 | None | 6.9 ± 0.06 | 30,500 ± 322 | MP |
| I | 4 | None | 4.4 ± 0.1 | 29,400 ± 456 | SA |
| J | 5 | None | 6.9 ± 0.06 | 55,900 ± 523 | MP |
| K | 4 | None | 4.5 ± 0.1 | 56,400 ± 437 | SA |
The numbers correspond to the methods described in Materials and Methods. NA, not applicable.
Both viscosity and pH were analyzed in triplicate.
MP, 0.2% methyl paraben; SA, 0.1% sorbic acid.
Carrageenan-based gels containing 0.5% zinc acetate dihydrate.
HEC-based gels containing 0.5% zinc acetate dihydrate.
We made no attempt to determine the effect of buffer and cation on HEC viscosity; all HEC gels in Table 2 are unbuffered and contain 0.85% sodium chloride to increase tonicity. Lower-viscosity gels H and I contained 2.2% HEC, whereas the higher-viscosity gels J and K contained 2.7% HEC. However, we can say that the viscosity of HEC gels is insensitive to the pH of the formulation and preservative. Gels H and I (pH 6.9 and 4.4, respectively) had similar viscosities, as did gels J and K (pH 6.9 and 4.4, respectively).
Methyl paraben content, osmolality, and pH of the various zinc-containing gels remained relatively stable (varied by less than 20%) over the duration of the stability studies: 6 months at 30°C, 5 months at 40°C, and 1 month at 50°C. On the other hand, gel viscosity dropped significantly for some gels: as much as 33% at 30°C and as much as 75% at 40°C. As assessed by changes in viscosity, gels prepared via method 1 were generally more stable than gels prepared by method 2 or 3. In addition, gels that contained either potassium or sodium acetate buffer were more stable than those that contained either potassium or sodium citrate.
The anti-HSV-2 activity of zinc acetate requires formulation in carrageenan.
Although carrageenan represents a promising delivery vehicle for zinc acetate, we also explored the potential utility of HEC, since HEC-based gels such as the Universal Placebo and 1% tenofovir gel have been widely used in clinical trials and shown to be safe (48), and in the case of tenofovir gel, an effective vehicle.
Zinc release from the various formulations was measured over time (Fig. 2). The amount of zinc released from the carrageenan-based gels over 24 h ranged from 17 to 25% of the zinc loaded in the formulation; the HEC-based gels released 38% of the loaded zinc over 24 h. At all time points, the HEC-based gels released significantly more zinc (P < 0.01) than any of the carrageenan-based gels. The difference was smaller at early time points, but still significant, and became much larger after 5 h (around 30% versus almost 200%).
Fig 2.
HEC-based gels release significantly more zinc acetate than carrageenan-based gels. Carrageenan-based (A to G) and HEC-based (H to K) zinc acetate formulations (defined in Table 2) were examined for the release of zinc acetate at 37°C over a 24-h period (3 independent experiments per time point for each sample; mean values ± standard deviation [SD] are shown for each). The Mann-Whitney test was used to compare the zinc release from carrageenan- versus HEC-based gels, revealing that significantly more zinc was released from the HEC gels (P < 0.01).
We then tested the efficacy of these gels in preventing rectal or vaginal HSV-2 infection (Fig. 3) in mice. Zinc acetate formulated in carrageenan significantly protected mice against a high-dose HSV-2 vaginal challenge, as compared to carrageenan-only gel, a zinc acetate solution, or D-PBS (P = 0.04 to 0.001 or <0.001) (Fig. 3, upper panels). Remarkably, those formulations using HEC as a delivery vehicle for zinc acetate failed to protect the animals against vaginal infection, showing similar survival curves to HEC alone or D-PBS.
Fig 3.
Zinc acetate in HEC does not prevent vaginal or rectal HSV-2 infection in mice. Medroxyprogesterone acetate-treated (vaginal; A) or untreated (rectal; B) mice had the indicated gels (versus D-PBS or the zinc acetate solution) applied vaginally or rectally 10 min before being challenged with 106 PFU (via the respective routes). Survival was monitored for up to 20 days, and the survival curves are shown for each condition (n = 15 to 20 for each treatment group).
The top three carrageenan gels as defined by vaginal efficacy (formulations G, D, and E) and the HEC-based gels were also tested rectally (Fig. 3, lower panels). Just as for vaginal challenge, only the carrageenan-based zinc acetate gels protected mice against rectal infection with the high dose of HSV-2. Protection was highly significant when compared to the carrageenan- and HEC- and D-PBS-treated controls (P = 0.04 to 0.0003), as well as significantly different compared to the HEC-based formulations containing zinc acetate (P < 0.001).
Carrageenan-based gels are likely to spread throughout the vagina.
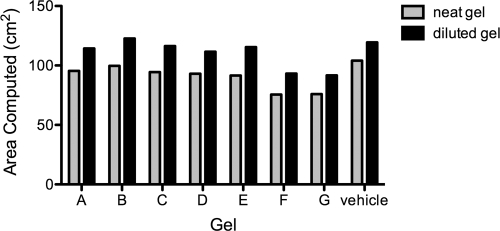
Rheological and computational analyses were performed to further characterize the carrageenan-based gels that were effective in the HSV-2 model. None of the gels exhibited a yield stress. However, as seen in Table 2, there was a 2-fold variation in the simple viscosity of various gels. More comprehensive rheological analyses also showed a considerable range of viscosity versus shear rate values, for both diluted and undiluted gels. In general, dilution with vaginal fluid simulant reduced the viscosity of the gels over the entire range of shear rates (data not shown). There was variability in the computationally predicted rates of gel spreading and coated area for both undiluted gels and gels diluted 20% with vaginal fluid simulant, the latter being higher (Fig. 4). However, the range of variation in predicted coated area was not nearly as great as that for the single values of simple viscosity (Table 2). Taken together, our data suggest that neat and diluted carrageenan-based gels have the potential to effectively spread in vivo, coating the entire vaginal surface area.
Fig 4.
Zinc-carrageenan gels as well as gels containing carrageenan alone are good spreading gels. Shown is the spreadability of different formulations containing 0.5% zinc acetate dihydrate in a carrageenan-based gel using the computed vaginal surface area with undiluted or diluted gel (20% vaginal simulant). Gel rheological measurements of viscosity versus shear rate were performed at 37°C in a TA model AR 1500ex rheometer (TA Instruments, New Castle, DE) using a 4° cone and 20-cm-diameter plate. The 20% dilution is a conservative upper bound limit of the extent of gel dilution over the first 5 min of vaginal spreading in vivo. According to the biomechanical model, optimal spreading is 100 cm2 using 3.5 ml of gel with a force of 1 pound-force (lbf) for 300 s. The experiment includes a control with 3% carrageenan (vehicle). The measurement of rheological properties and subsequent computations of coated area were performed in triplicate for each gel, undiluted and diluted (three independent rheometric experiments with subsequent computations of coated area performed for each gel). Coefficients of variation for the rheometric parameters, computed from the raw rheometric data and subsequent computations of coated area, were all less than 5%. The data represent median values.
Zinc-carrageenan gels exhibit little toxicity in vitro and in vivo and do not increase susceptibility to HSV-2 infection after repeated application.
A microbicide must cause little or no damage to the local tissue to avoid enhancing infection rates. Although the efficacy data suggested that the zinc-containing gels were not damaging local tissues, we examined the three lead carrageenan-based gels (G, D, and E) more thoroughly through in vitro and in vivo approaches to rule out any potential safety concerns. Initially, the impact of these gels on epithelial integrity was determined using the stringent Biocoat Caco-2 cell system in which TEER values are measured after exposure to neat gels for up to 6 h (Fig. 5). As expected, we found that Gynol completely destroyed the monolayer and the TEER values dropped to 0 within only 2 h of exposure. In contrast, TEER values for monolayers treated with gels D and E remained largely unaltered (comparable to carrageenan-treated monolayers), while the TEER values for monolayers treated with gel G were reduced about 50% (although electrical resistance remains above 400 Ω × cm2). The TEER levels of gel D- and G-treated monolayers decreased further after 4 and 6 h, but the values were still greater than those for Gynol-treated monolayers. Additionally, all formulations showed similar results in the cytotoxicity assay with CC50 values of >1/20 dilution of the formulation, although the lowest dilution tested (1/20) showed a trend toward decreased viability without reaching the CC50 value. Gynol showed CC50 values around 1/500 for both 2- and 6-h incubations (see the supplemental material).
Fig 5.
Epithelial monolayer integrity after exposure to neat zinc acetate-containing gels. Caco-2 cell monolayers were incubated with the indicated neat gels for 0, 2, 4, or 6 h before the TEER values were measured (three replicates for each gel at each time point). The mean ± SD TEER values are shown for each gel (from two independent experiments).
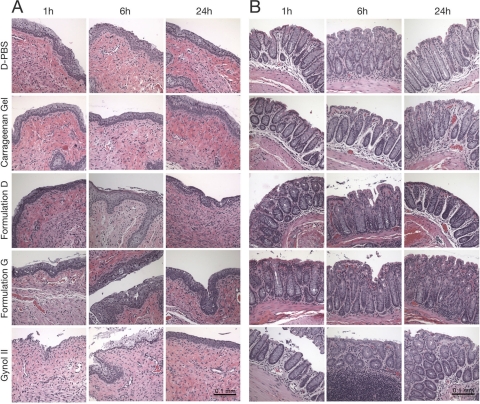
To further establish the biological relevance and utility of our TEER model, we studied the effect of selected zinc acetate/carrageenan formulations in vivo in mice. Histological analysis of tissues harvested 1, 6, and 24 h after vaginal or rectal application of formulations G and D (which showed the largest reductions in TEER at the later time points) revealed negligible damage to the vaginal and rectal epithelia and intact lamina propria at all of these time points (Fig. 6). This contrasts the dramatic damage detected within 1 to 6 h of treatment with Gynol, which is largely repaired by 24 h. Neither, D-PBS nor carrageenan gel damaged the architecture of the vaginal or rectal mucosa.
Fig 6.
Zinc-carrageenan formulations do not negatively impact the architecture of cervicovaginal and rectal epithelia in vivo. Eight-week-old female BALB/c mice were treated with medroxyprogesterone acetate or fasted and anesthetized before adding D-PBS, carrageenan-based gel, formulation D, formulation G, or Gynol II to evaluate the normal architecture in the cervicovaginal and rectal mucosae. Mice were sacrificed at 1, 6, and 24 h after gel applications, and the entire reproductive or rectal tract was surgically excised, fixed, and embedded in paraffin before preparing tissue sections for morphological analyses using H&E staining. Panel A (cervicovaginal mucosa) and panel B (rectal mucosa) represent a sample of the 6 sections of two or three animals that were analyzed per formulation. The original magnification is 20×, and the bar length represents 0.1 mm.
Additionally, we performed a functional assay for epithelial damage by examining whether repeated pretreatment with the gels could enhance an animal's susceptibility to low-dose HSV-2 challenge. Mice received gels D, E, and G (versus carrageenan, D-PBS, and Gynol) daily for 7 days prior to being challenged with 2 × 103 PFU of HSV-2 (a dose that infects only 50% of the animals). The viral challenge was performed 12 h after the last gel application, and HSV-2 infection was monitored for 20 days. Animals treated with zinc acetate-containing carrageenan gels were infected at a similar frequency to animals treated with D-PBS or carrageenan (Fig. 7). This contrasts with the significant enhancement of infection caused by pretreatment with Gynol (P = 0.0009 versus D-PBS; P = 0.0004 versus carrageenan). Therefore, zinc acetate-containing carrageenan gels appear safe and effective in this demanding mouse HSV-2 efficacy model. These data support the advancement of zinc acetate-carrageenan gels as candidate topical microbicides.
Fig 7.
Repeated treatment with zinc-carrageenan formulations does not increase HSV-2 susceptibility in the mouse model. The indicated gel formulations were delivered intravaginally daily for 7 days to medroxyprogesterone acetate-treated BALB/c mice (n = 20 to 24 per group). Twelve hours after the last gel was applied, mice were challenged with 2 × 103 PFU of HSV-2 strain G. Mice were examined and scored daily for 19 days. Percent survival over time is shown for each treatment group. There was not a significant difference between each individual gel (D, E, or G; P > 0.096) or the combination of the data from those three gels (P > 0.055) compared to D-PBS or carrageenan gel. A very significant difference (P = 0.0009 or P = 0.0004) was observed when comparing Gynol versus D-PBS or carrageenan, respectively.
DISCUSSION
This work was predicated on prior findings that zinc salts in solution, carrageenan gels, and zinc acetate-carrageenan gels exhibit anti-HSV-2 activity (4, 27, 30–32). Thus, it was logical to optimize a gel with properties suited for vaginal application as an anti-HSV-2 microbicide. In so doing, we integrated a variety of test methods: in vivo, in vitro, and in silico. Our underlying hypothesis was that the physical properties, stability, bioactivity, and safety of a zinc-carrageenan gel could be optimized for vaginal application. Our results confirmed this hypothesis. Specifically, zinc acetate in carrageenan (formulations D, E, and G) was effective in the HSV-2 mouse protection model and met or exceeded criteria for physicochemical properties, prediction of vaginal coating, in vitro release of the zinc, effects on cell viability and cell monolayer integrity, and in vivo evaluation of histological changes.
Carrageenan is a high-molecular-weight linear polysaccharide. The carrageenan used in this study was a 95:5 mixture of lambda and kappa carrageenans. As such, properties of the various formulations were dominated by the lambda component. Lambda and kappa carrageenans differ in the amount of 3,6-anhydrogalactose (3,6-AG) and sulfate ester present in the polymer chains. Kappa carrageenan contains approximately 34% 3,6-AG and 25% ester sulfate, whereas lambda carrageenan contains little or no 3,6-AG and ∼35% ester sulfate. Carrageenan strongly binds monovalent and divalent cations (positively charged ions such as zinc) to neutralize the negatively charged (anionic) ester sulfate groups. Kappa carrageenan is sensitive to different cations, whereas lambda carrageenan is not.
In contrast to carrageenan, HEC has no ionic groups that can bind cations. However, HEC can form weaker, nonionic (Lewis acid/base) bonds with zinc through oxygen atoms present in the polymer backbone. Gels prepared from both the lambda and kappa carrageenan mixture and HEC released zinc. The carrageenan-based formulations released 17% to 25% of the total zinc loaded after 24 h, whereas the HEC-based formulations released significantly more (P < 0.01): 32% to 38%. The projected vaginal dose of our gel, in humans, ranges from 3 to 4 ml. This would translate to 4 to 6 mg of zinc released per day; an 8- to 12-ml rectal dose would translate to 12 to 16 mg per day. If we take into consideration that only 20% of the zinc will be released from the gel in 24 h, then the maximum daily dose of zinc released into the vagina or rectum, but not necessarily absorbed systemically, will be much lower than the oral dietary allowances of 8 to 13 mg per day as recommended by the Food and Nutrition Board (16).
The carrageenan-based zinc gels were shear thinning: that is, viscosity decreases with increasing shear rate. Within the vagina, gels can experience shear rates spanning five orders of magnitude: precoital spreading (shear rates as low as 10−3 s−1) to coitus-induced spreading (shear rates as high as 102 s−1) (28, 46). The biomechanics of gel spreading and retention depends upon this entire range, in a multivariate and nonlinear way (24, 28, 46). A single value of viscosity, measured under standardized conditions, can prove valuable in monitoring differences in gel structure and stability (see below). However, no single value of viscosity can correlate with rates of spreading, for example. Thus, there is no simple correlation between the simple viscosity values in Table 2 and the predicted spreading areas in Fig. 4. This exemplifies the fact that single measurements of viscosity of a non-Newtonian shear-thinning fluid, such as a gel, do not correlate linearly with rates of flow and surface areas coated (whether predicted using biomechanical models, as performed here, or measured experimentally) and that comprehensive rheological evaluation of gels is needed to fully assess the properties of gels.
The incorporation of data on gel rheology, and the resulting predictions of vaginal spreading, into an algorithm that creates a summary measure of gel spreading performance (i.e., the SF) is a new approach with promise to improve the rational design of vaginal microbicide gels (33). Our use of this algorithm here enabled us to place merit and rank order the spreading performance of 10 different prototype carrageenan formulations, plus the carrageenan-alone gel (data presented in Fig. 4). The results showed that the carrageenan-alone gel performed very well, but 5 other formulations performed very nearly as well. This narrowed the set of candidate formulations; additional performance factors could be and are being applied to selection of the “best” formulations.
Since carrageenan alone can effectively impede infection in the HSV-2 mouse model when using a 104-PFU challenge dose per mouse, we performed the viral challenge using 106 PFU per mouse, a viral concentration that translates to ∼10,000 50% lethal doses (LD50s). At such a high viral challenge, carrageenan-based gels containing 0.3 to 1% of zinc acetate dihydrate were able to protect 75 to 85% of the mice, while the carrageenan-alone gel failed to protect them.
The HSV-2 efficacy testing, which compared carrageenan-based and HEC-based zinc acetate-containing gels, clearly revealed the benefit of using carrageenan as a drug delivery vehicle. This could be related to the fact carrageenan-plus-zinc formulations contained two proven antiviral components, while the HEC formulations contained only one, zinc acetate. However, there was significantly more zinc released from the HEC gels compared to the carrageenan formulations. This raises obvious questions regarding the possible mechanism(s) of antiviral action of the zinc acetate-carrageenan gels that we are currently trying to answer. For example, is it the free or carrageenan-bound zinc, or a combination of both, which is responsible for the highly effective antiviral activity in vivo?
Several papers have discussed the benefit of zinc as a broad-spectrum antimicrobial agent (4, 19, 20, 27, 29, 42, 45, 54). However, there are characteristics of zinc salts that render zinc assessment problematic in a number of in vitro assays. Most of the intracellular zinc is bound to proteins, and only an exceptionally small percentage is free or loosely bound (29). Just a small overload of free zinc, which the cells cannot buffer, will be toxic to them. Perhaps this is why we saw a decrease in viability and a drop in TEER values at the 4- and 6-h time points in those formulations containing zinc and is also the primary reason that our efficacy and safety studies have focused on the in vivo model; here, the dose that was formulated in carrageenan was highly efficacious in blocking HSV-2 infection, without severe signs of toxicity. Other experiments have shown that the efficacy of zinc salts as candidate topical microbicides in the vaginal HSV-2 model could be related to the sloughing of infected epithelial cells before the virus could enter peripheral neurons (7). In this cited study, a 200 mM dose of different zinc salts (including zinc acetate) was used. This concentration represents around 10 times more zinc than was used in our formulation.
To assess if our formulations could induce substantial vaginal epithelial disruption, we examined the architecture of the cervicovaginal and rectal mucosae of mice at 1, 6, and 24 h after gel application. The histological analyses of the samples treated with formulations D and G showed some fractures of the vaginal and rectal epithelia at times 1 and 6 h after gel application; but this did not compromise the lamina propia, as seen in Fig. 6A and B. Since the vehicle (carrageenan) showed no negative effect on the architecture of the epithelia, we hypothesize that zinc may be responsible for the subtle, transient changes in the epithelia. As mentioned before, Bourne et al. (7) suggested that the protection from HSV-2 infection seen in mice treated with solutions of zinc salt might be the result of epithelial sloughing and the subsequent lack of a site for primary replication of the virus. In our assay, we used highly stringent conditions: medroxyprogesterone acetate to thin the epithelia and an extremely high virus concentration (106 PFU/mouse, equivalent to ∼10,000 LD50s). These were sufficient to eradicate any protection from carrageenan, zinc acetate solutions, or zinc acetate in HEC. Moreover, although some cell sloughing is seen due to the fracture of the epithelia, most of the cells were still associated with the vaginal and rectal mucosae. Taking into consideration the reduction in the TEER values in vitro and knowing that previous publications using diluted formulations (compared to our neat gels) have related the decrease in TEER to increased susceptibility to HIV infection (38), we then decided to perform a more biologically relevant assay. In this assay, we examined if daily vaginal administration for 7 consecutive days could enhance HSV-2 susceptibility in mice. Several laboratories have recently used this model as a way to predict safety (51). The model looks at increased HSV-2 susceptibility (presumably due to disruption of the epithelium) and may also provide a biomarker for a similar effect on HIV susceptibility. Based on our results, the zinc-carrageenan formulations have no deleterious effect. All of these factors suggest that inducing epithelial sloughing may not be the mode of action of the zinc acetate/carrageenan gels. Moreover our published data on a zinc-carrageenan (PC-707) and nonnucleoside reverse transcriptase inhibitor (NNRTI)-zinc-carrageenan (PC-1005) gels, where the gels are administered daily vaginally for 14 consecutive days, show that significant protection is achieved even when challenging the macaques with combined simian-human immunodeficiency virus reverse transcriptase (SHIV-RT) 8 or 24 h after the last gel application. In this study, no locally adverse effects were seen, including no impact on vaginal pH or significant changes in the levels of cytokines or chemokines in vaginal fluid. It is important to mention that the zinc formulation used in this study contains a lower concentration of zinc acetate dihydrate (0.3%), compared to the 0.5% concentration in gel D, G, or E in our present study. The other constituents and physical characteristics (carrageenan, buffer composition, and rheology) are similar.
The CAPRISA-004 trial showed that a gel containing an ARV drug (tenofovir) may effectively protect against both HIV and HSV-2 transmission (1). This result constitutes the first proof of concept that a microbicide gel administered vaginally may impede sexually transmitted infections. Our zinc acetate-carrageenan gels have the potential to block HIV and HSV-2 and even HPV. In addition, our formulation is iso-osmolar (instead of hyperosmolar like the 1% tenofovir gel) and the use of a non-ARV molecule could be an important advantage in terms of selection of resistance and the possibility of being available over the counter. It is likely that the introduction of a tenofovir gel, if proven effective to prevent HIV and HSV-2, will help to reduce the number of new infections. However, it is critical to look for broad-spectrum, non-ARV-based microbicides and/or combinations of APIs that could minimize the selection of resistance due to a monotherapy approach.
Zinc acetate-carrageenan gels optimized for use as vaginal microbicides inhibit HSV-2 infection in mice and prevent immunodeficiency virus infection (after 14 days of repeated vaginal use) in macaques (23). These gels offer significant promise for use as broad-spectrum microbicides.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant U19-AI065412, the Swedish Ministry of Foreign Affairs, and the Swedish International Development Cooperation Agency. This paper was also made possible by the generous support of the American people through the United States Agency for International Development (USAID), Bureau for Global Health, Office of Population and Reproductive Health, under the terms of award no. GPO-A-00-04-00019. M.R. is a 2002 Elizabeth Glaser Scientist.
The contents of this paper are the sole responsibility of the Population Council and do not necessarily reflect the views of USAID or the United States Government.
We acknowledge the assistance from members of Rockefeller University's Bioimaging Facility and core director A. North with the bioimaging analysis.
Footnotes
Published ahead of print 7 November 2011
Supplemental material for this article may be found at http://aac.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Abdool Karim Q, et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abu-Raddad LJ, et al. 2008. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 3:e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander TH, Davidson TM. 2006. Intranasal zinc and anosmia: the zinc-induced anosmia syndrome. Laryngoscope 116:217–220 [DOI] [PubMed] [Google Scholar]
- 4. Arens M, Travis S. 2000. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J. Clin. Microbiol. 38:1758–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ashley RL. 1995. Herpes simplex viruses, p 375-395 In Schmidt NJ, Emmons RW. (ed), Diagnostic procedures for viral, rickettsial, and chlamydial infections, 7th ed American Public Health Association, Washington, DC [Google Scholar]
- 6. Berg JM, Shi Y. 1996. The galvanization of biology: a growing appreciation for the roles of zinc. Science 271:1081–1085 [DOI] [PubMed] [Google Scholar]
- 7. Bourne N, et al. 2005. Efficacy and toxicity of zinc salts as candidate topical microbicides against vaginal herpes simplex virus type 2 infection. Antimicrob. Agents Chemother. 49:1181–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chou TC. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58:621–681 [DOI] [PubMed] [Google Scholar]
- 9. Chvapil M, et al. 1979. Reaction of vaginal tissue of rabbits to inserted sponges made of various materials. J. Biomed. Mater. Res. 13:1–13 [DOI] [PubMed] [Google Scholar]
- 10. Code of Federal Regulations Title 9 Accessed 14 November 2011 Animal Welfare Act and Regulation. Chapter 1. Subchapter A. Animals and animal products. http://www.nal.usda.gov/awic/pubs/Rodents/rodents.htm
- 11. Corey L. 2007. Herpes simplex virus type 2 and HIV-1: the dialogue between the 2 organisms continues. J. Infect. Dis. 195:1242–1244 [DOI] [PubMed] [Google Scholar]
- 12. Crostarosa F, et al. 2009. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PLoS One 4:e8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cummins JE, Jr, et al. 2007. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob. Agents Chemother. 51:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fahim MS, Wang M. 1996. Zinc acetate and lyophilized aloe barbadensis as vaginal contraceptive. Contraception 53:231–236 [DOI] [PubMed] [Google Scholar]
- 15. Fernández-Romero JA, et al. 2007. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex. Transm. Dis. 34:9–14 [DOI] [PubMed] [Google Scholar]
- 16. Food and Nutrient Board, Institute of Medicine 2001. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academy Press, Washington, DC: [PubMed] [Google Scholar]
- 17. Foss AM, Vickerman PT, Heise L, Watts CH. 2003. Shifts in condom use following microbicide introduction: should we be concerned? AIDS 17:1227–1237 [DOI] [PubMed] [Google Scholar]
- 18. Freeman EE, et al. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83 [DOI] [PubMed] [Google Scholar]
- 19. Geist FC, Bateman JA, Hayden FG. 1987. In vitro activity of zinc salts against human rhinoviruses. Antimicrob. Agents Chemother. 31:622–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haraguchi Y, Sakurai H, Hussain S, Anner BM, Hoshino H. 1999. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res. 43:123–133 [DOI] [PubMed] [Google Scholar]
- 21. Hirano T, et al. 2008. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv. Immunol. 97:149–176 [DOI] [PubMed] [Google Scholar]
- 22. Kapiga SH, et al. 2007. The role of herpes simplex virus type 2 and other genital infections in the acquisition of HIV-1 among high-risk women in northern Tanzania. J. Infect. Dis. 195:1260–1269 [DOI] [PubMed] [Google Scholar]
- 23. Kenney J, et al. 2011. An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques. PLoS One 6:e15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kieweg SL, Katz DF. 2006. Squeezing flows of vaginal gel formulations relevant to microbicide drug delivery. J. Biomech. Eng. 128:540–553 [DOI] [PubMed] [Google Scholar]
- 25. Kilmarx PH, et al. 2008. A randomized, placebo-controlled trial to assess the safety and acceptability of use of Carraguard vaginal gel by heterosexual couples in Thailand. Sex. Transm. Dis. 35:226–232 [DOI] [PubMed] [Google Scholar]
- 26. Kilmarx PH, et al. 2006. Safety and acceptability of the candidate microbicide Carraguard in Thai Women: findings from a phase II clinical trial. J. Acquir. Immune. Defic. Syndr. 43:327–334 [DOI] [PubMed] [Google Scholar]
- 27. Kumel G, Schrader S, Zentgraf H, Daus H, Brendel M. 1990. The mechanism of the antiherpetic activity of zinc sulphate. J. Gen. Virol. 71:2989–2997 [DOI] [PubMed] [Google Scholar]
- 28. Lai BE, et al. 2008. Dilution of microbicide gels with vaginal fluid and semen simulants: effect on rheological properties and coating flow. J. Pharm. Sci. 97:1030–1038 [DOI] [PubMed] [Google Scholar]
- 29. Lazarczyk M, Favre M. 2008. Role of Zn2+ ions in host-virus interactions. J. Virol. 82:11486–11494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maguire RA, Bergman N, Phillips DM. 2001. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytotoxicity, antibacterial properties, and sperm immobilization. Sex. Transm. Dis. 28:259–265 [DOI] [PubMed] [Google Scholar]
- 31. Reference deleted.
- 32. Maguire RA, Zacharopoulos VR, Phillips DM. 1998. Carrageenan-based nonoxynol-9 spermicides for prevention of sexually transmitted infections. Sex. Transm. Dis. 25:494–500 [DOI] [PubMed] [Google Scholar]
- 33. Mahalingam A, et al. 2010. Design of a semisolid vaginal microbicide gel by relating composition to properties and performance. Pharm. Res. 27:2478–2491 [DOI] [PubMed] [Google Scholar]
- 34. Marais D, et al. 22 August 2011, posting date The effectiveness of Carraguard, a vaginal microbicide, in protecting women against high-risk human papillomavirus infection. Antiviral Ther. doi:10.3851/IMP1890 [DOI] [PubMed] [Google Scholar]
- 35. Martin S, et al. 2010. Carraguard acceptability among men and women in a couples study in Thailand. J. Womens Health (Larchmt.). 19:1561–1567 [DOI] [PubMed] [Google Scholar]
- 36. McDermott MR, et al. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 51:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McLean CA, et al. 2010. HIV genital shedding and safety of Carraguard use by HIV-infected women: a crossover trial in Thailand. AIDS 24:717–722 [DOI] [PubMed] [Google Scholar]
- 38. Mesquita PM, et al. 2009. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J. Infect. Dis. 200:599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. National Research Council 2010. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC [Google Scholar]
- 40. Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95 [DOI] [PubMed] [Google Scholar]
- 41. Prasad AS. 2008. Zinc in human health: effect of zinc on immune cells. Mol. Med. 14:353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prasad AS, Beck FW, Bao B, Snell D, Fitzgerald JT. 2008. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J. Infect. Dis. 197:795–802 [DOI] [PubMed] [Google Scholar]
- 43. Roberts JN, et al. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 13:857–861 [DOI] [PubMed] [Google Scholar]
- 44. Skoler-Karpoff S, et al. 2008. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 372:1977–1987 [DOI] [PubMed] [Google Scholar]
- 45. Suara RO, Crowe JE., Jr 2004. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 48:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szeri AJ, Park SC, Verguet S, Weiss A, Katz DF. 2008. A model of transluminal flow of an anti-HIV microbicide vehicle: combined elastic squeezing and gravitational sliding. Phys. Fluids 20:83101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thanasarakhan W, Liawruangrath S, Wangkarn S, Liawruangrath B. 2007. Sequential injection spectrophotometric determination of zinc(II) in pharmaceuticals based on zinc(II)-PAN in non-ionic surfactant medium. Talanta 71:1849–1855 [DOI] [PubMed] [Google Scholar]
- 48. Tien D, et al. 2005. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res. Hum. Retroviruses 21:845–853 [DOI] [PubMed] [Google Scholar]
- 49. Turville SG, et al. 2008. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS One 3:e3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Whitehead SJ, et al. 2006. Acceptability of Carraguard vaginal gel use among Thai couples. AIDS 20:2141–2148 [DOI] [PubMed] [Google Scholar]
- 51. Wilson SS, et al. 2009. Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: expansion of a murine model of microbicide safety. Antivir. Ther. 14:1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamasaki S, et al. 2007. Zinc is a novel intracellular second messenger. J. Cell Biol. 177:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zacharopoulos VR, Phillips DM. 1997. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin. Diagn. Lab. Immunol. 4:465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang ZY, et al. 1991. Zinc inhibition of renin and the protease from human immunodeficiency virus type 1. Biochemistry 30:8717–8721 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.