Abstract
Liposomal amphotericin B (LAMB) and caspofungin (CAS) are important antifungal agents in allogeneic hematopoietic stem cell transplant (aHSCT) recipients. Little is known, however, about the pharmacokinetics (PK) of both agents and their combination in this population. The PK of LAMB and CAS and the potential for PK interactions between both agents were investigated within a risk-stratified, randomized phase II clinical trial in 53 adult aHSCT recipients with granulocytopenia and refractory fever. Patients received either LAMB (n = 17; 3 mg/kg once a day [QD]), CAS (n = 19; 50 mg QD; day 1, 70 mg), or the combination of both (CAS-LAMB; n = 17) for a median duration of 10 to 13 days (range, 4 to 28 days) until defervescence and granulocyte recovery. PK sampling was performed on days 1 and 4. Drug concentrations in plasma (LAMB, 405 samples; CAS, 458 samples) were quantified by high-pressure liquid chromatography and were analyzed using population pharmacokinetic modeling. CAS concentration data best fitted a two-compartment model with a proportional error model and interindividual variability (IIV) for clearance (CL) and central volume of distribution (V1) (CL, 0.462 liter/h ± 25%; V1, 8.33 liters ± 29%; intercompartmental clearance [Q], 1.25 liters/h; peripheral volume of distribution [V2], 3.59 liters). Concentration data for LAMB best fitted a two-compartment model with a proportional error model and IIV for all parameters (CL, 1.22 liters/h ± 64%; V1, 19.2 liters ± 38%; Q, 2.18 liters/h ± 47%; V2, 52.8 liters ± 84%). Internal model validation showed predictability and robustness of both models. None of the covariates tested (LAMB or CAS comedication, gender, body weight, age, body surface area, serum bilirubin, and creatinine clearance) further improved the models. In summary, the disposition of LAMB and CAS was best described by two-compartment models. Drug exposures in aHSCT patients were comparable to those in other populations, and no PK interactions were observed between the two compounds.
INTRODUCTION
Invasive opportunistic fungal infections are important causes of morbidity and mortality following allogeneic hematopoietic stem cell transplantation (aHSCT). Infection rates range between 10 and 25%, and overall mortality is 35 to 50% for invasive candidiasis and 65 to 90% for invasive aspergillosis and infections by other filamentous fungi (5, 14).
Both liposomal amphotericin B (LAMB) and caspofungin (CAS) are important agents in the management of invasive fungal infections in aHSCT recipients. Although compromised by its potential for nephrotoxicity, LAMB is a standard agent with broad-spectrum fungicidal antifungal activity (12) and first-line indications against major opportunistic fungal infections (8, 24, 35). CAS has favorable pharmacokinetic properties (15), documented efficacy against Candida and Aspergillus infections, and an excellent safety profile (26, 28, 32, 40). Despite limited clinical data, both agents are also used in combination in patients with fulminant or refractory infections, infections in compartments that are difficult to treat, and infections by pathogens with decreased antifungal susceptibility (2, 6, 23, 26, 33).
In order to provide a foundation for further systematic clinical evaluation, we investigated the safety and pharmacokinetics (PK) of LAMB, CAS, and the combination of both in a formal risk-stratified, randomized, phase II clinical trial in persistently febrile, granulocytopenic adult aHSCT recipients immunosuppressed with cyclosporine (13). Here we report a detailed population pharmacokinetic analysis of this trial that provides evidence for the absence of clinically relevant differences in drug exposures in comparison to other populations, the lack of impact of gender, body weight, body surface area, age, serum bilirubin, or creatinine clearance (CLCR) on the pharmacokinetics of both agents, and the absence of pharmacokinetic interactions between the agents in combination in this special population.
(These results were presented in preliminary form at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 12 to 15 September 2009 [42].)
MATERIALS AND METHODS
Study design overview.
The study was designed as an open, prospective, randomized multicenter phase II clinical trial conducted to investigate the safety and pharmacokinetics (PK) of CAS, LAMB, and the combination (CAS-LAMB) in 75 adult aHSCT recipients with granulocytopenia and refractory fever despite adequate antibacterial therapy. After giving informed consent, eligible patients were stratified according to donor status (HLA matched/related versus HLA mismatched/unrelated) and randomized into one of three treatment arms: CAS at 50 mg once a day (QD) with a loading dose of 70 mg on day 1, LAMB at 3 mg/kg QD, or the combination of CAS and LAMB at similar dosages. Treatment was to continue until neutrophil engraftment (absolute neutrophil count of ≥500/μl for three consecutive days) and defervescence (<38°C for 72 h) or diagnosis of probable or proven (2) invasive fungal infection, or occurrence of a nonhematological grade III/IV adverse event (NCI-CTCAE) judged to be causally related to study drug medication. Safety and the absence of breakthrough infections were monitored daily during treatment, at the end of treatment (EOT), and 14 days after EOT. Serial PK samples were collected on days 1 and 4 and twice weekly thereafter. Antifungal efficacy and survival were assessed at 14 days after EOT. The primary endpoint of the study was determined by the number of toxicity-related premature study drug discontinuations as well as clinical and laboratory adverse events during treatment. PK assessments and the evaluation of treatment success were used to determine secondary endpoints (13).
Patient eligibility.
The study was performed at five German study sites under a protocol approved by the institutions' internal review boards. Written informed consent was obtained from each patient prior to study entry. Study-specific inclusion criteria were an age of ≥18 years, granulocytopenia (absolute neutrophil count [ANC] of ≤500/μl) following aHSCT, persisting or new fever (oral temperature of ≥38°C) despite adequate antibacterial therapy for ≥36 to 48 h considered to require empirical antifungal therapy, and immunosuppression with cyclosporine.
Study-specific exclusion criteria at entry were the presence of a probable or proven invasive fungal infection according to the Mycoses Study Group/European Organization for Research and Treatment of Cancer (MSG/EORTC) criteria published in 2002 (2); active veno-occlusive disease; hemodynamic instability; estimated life expectancy of <5 days; serum bilirubin >3 times, serum glutamic oxalacetic transaminase and/or serum glutamic pyruvic transaminase (SGOT/SGPT) >3 times, alkaline phosphatase >5 times, and serum creatinine >2 times the upper limit of normal values; and comedication with rifampin, phenytoin, carbamazepine, phenobarbital, and dexamethasone.
Study drug treatment.
Eligible patients were stratified according to donor status (HLA matched and/or related versus HLA mismatched and/or unrelated) and randomized to receive either CAS 50 mg QD with a loading dose of 70 mg on day 1, LAMB 3 mg/kg QD, or the combination of CAS and LAMB (CAS-LAMB) at similar dosages. Treatment with CAS and/or LAMB was to continue until neutrophil engraftment and defervescence, diagnosis of probable or proven invasive fungal infection, or occurrence of an NCI-CTCAE judged to be causally related to study drug medication (13). CAS was provided by Merck, Sharp and Dohme (Haar, Germany) as the approved Cancidas product, and LAMB was provided by Gilead Sciences (Martinsried, Germany) as the approved AmBisome product. Both compounds were prepared and administered according to the manufacturer's recommendations. The infusion time was 60 min for each drug, with the process of drug administration being delineated in a study-specific standard procedure. In the CAS-LAMB arm, the order of drug administration was LAMB followed by CAS. To allow PK comparison, no dose adjustment of CAS was made in individuals with a weight of >80 kg.
Pharmacokinetic sampling and recording of covariates.
The duration of treatment with CAS and/or LAMB was determined by individual clinical outcomes. Based on clinical trial experience concerning the duration of drug administration in the setting of empirical therapy (40, 41), serial plasma sampling was performed on days 1 and 4 for up to 24 h after dosing at prespecified time intervals (immediately before administration and at 0.5 to 1.5 h, 1.5 to 3 h, 3 to 5 h, 5 to 11 h, and 22 to 23 h after administration) and thereafter at random time points twice weekly until the end of treatment. Blood specimens (5 ml) were collected through a separate line in heparinized tubes and immediately centrifuged for 10 min at 1,500 × g. Separated plasma was stored at −80°C until it was assayed.
For analysis of covariates, the age, gender, and height of each patient were recorded on day 1; weight, serum bilirubin, and creatinine were recorded daily. CLCR was calculated using the formula by Cockcroft and Gault (7), and the body surface area (BSA) was calculated using the formula of Mosteller (29). Missing covariates (for 481 CAS administrations, 1 weight, 24 serum bilirubin, and 3 serum creatinine values; for 400 LAMB administrations, 24 serum bilirubin and 2 serum creatinine values) were replaced as follows: for day 1 or the last day, the value from day 2 or the day before, respectively, was used; for all other days, the mean of the day before and the day after was used.
Analytical methods.
Concentrations of CAS and total amphotericin B were measured based on previously published high-pressure liquid chromatography (HPLC) methods (1, 34) and were validated according to Guidance for Industry—Bioanalytical Method Validation (38). Standard curves were linear across the calibration range (r2 >0.99; 0.15 to 10 mg/liter for CAS; 0.1 to 15 mg/liter for amphotericin B). The lower limits of quantitation were 0.15 mg/liter for CAS and 0.1 mg/liter for amphotericin B. Accuracies were within ±11.7%, and intraday and interday variability (precision) ranged from 5.8 to 11.3% and 2.2 to 10%, respectively.
Caspofungin acetate reference standard and the internal standard for CAS were provided by Merck Research Laboratories (Rahway, NJ). The assay was performed using 150 μl plasma diluted with 600 μl of a 40:60 (vol/vol) mixture of 0.1% trifluoroacetic acid in water adjusted to pH 3 with triethylamine and acetonitrile. After addition of the internal standard, the samples were subjected to solid-phase extraction (SPE) on Cyano 100 columns (Mallinckrodt Baker Inc., Phillipsburg, NJ) preconditioned with water and methanol. After washing with water and methanol, the analytes were eluted with 500 μl of a methanolic solution containing 865 μl 28% ammonium hydroxide solution and 25 μl 0.05% trifluoroacetic acid per ml. The eluates were evaporated under nitrogen and then dissolved with a 40:60 (vol/vol) mixture of 0.1% trifluoroacetic acid in water adjusted to pH 3 with triethylamine and acetonitrile. A 75-μl portion of this solution was separated by HPLC on a Lichrospher analytical column (100 CN, 250-4, 5 μm; Merck, Darmstadt, Germany) at a flow rate of 1.5 ml/min using a binary gradient consisting of 0.1% trifluoroacetic acid in water adjusted to pH 3 with triethylamine and acetonitrile. CAS and the internal standard were detected by fluorimetric detection with an excitation wavelength of 224 nm and an emission wavelength of 302 nm.
Amphotericin B reference standard was purchased from the United States Pharmacopeia (USP; Rockville, MD). The internal standard 4-dimethylaminobenzaldehyde was obtained from Sigma GmbH (Deisenhofen, Germany). The assay was performed using 100 μl plasma diluted with 150 μl of a 25:75 (vol/vol) mixture of acetonitrile and methanol containing 250 mg/liter of the internal standard. The samples were shaken and heated at 70°C to facilitate protein precipitation and the release of amphotericin B from the liposomes. After centrifugation at 14,000 rpm, 100 μl of the supernatant was injected onto the HPLC system. Separation was performed on a Lichrocart LiChrospher analytical column (250-4, 100 RP18e; Merck) at a flow rate of 1 ml/min. The mobile phase consisted of 63.5:36.5 (vol/vol) 10 mM ammonium acetate buffer (pH 7.4) and acetonitrile. Amphotericin B and the internal standard were detected at a wavelength of 405 nm.
Population pharmacokinetic analysis.
Population pharmacokinetic (PopPK) analysis was performed by nonlinear mixed-effects modeling using NONMEM (version 7.1.0, level 1.0; ICON Development Solutions, Ellicott City, MD). The objective function value (OFV), a measurement of goodness of fit estimated by NONMEM (equal to a −2 log likelihood value of data), as well as graphical model diagnostics within Xpose 4.3.2 (20) and Pirana 2.4.0 (22), was used to search for appropriate models. For hierarchical models, an OFV drop of 10.83 units, indicating an improved fit at the P = 0.001 level for 1 degree of freedom, was considered statistically significant.
In the first step, the structural PopPK model was developed using the first-order conditional estimate (FOCE) method with interaction. One-, two-, and three-compartment models were tested. For amphotericin B, a two-compartment model with Michaelis-Menten elimination, a time-dependent change of clearance (CL) according to Boddy et al. (4) as well as a mixture model was tested. Exponentially scaled interindividual variability (IIV) was assessed for CL and central volume of distribution (V1). Further, IIV was tested on intercompartmental clearance (Q) and peripheral volume of distribution (V2). Interoccasion variability (IOV) was tested for CL and V1 on two occasions (day 1 and day 4). For assessing the residual variability additive, proportional and combined error models were examined.
Thereafter, the influence of the covariates comedication (for CAS, comedication with LAMB, and for LAMB, comedication with CAS), gender, weight (linear, allometric scaling [18] with fixed scaling factor of 0.75 on CL or estimated scaling factor), age, and BSA on the PK of CAS and LAMB were investigated. Serum bilirubin and CLCR were considered in the model in two different ways: first, based on the day 1 value, and second, by using a linear function between all values. Relationships were investigated by visual inspection of individual parameter values versus covariates and based on physiological rationale and clinical meaning (weight was tested as a potential covariate for all PK parameters; the other covariates were tested only for CL and V1). Inclusion of covariates was tested at a significance level (P) of 0.001. For continuous covariates, the following equation was tested, taking weight as a covariate of CL: CLi = θpop(1 + θ2(WT − WTmedian)), where CLi is the individual value of CL, θpop is the typical value of CL in the population, θ2 is the fractional change in the CL for each unit change in weight from the median value of weight, and WTmedian is the median weight of the total population.
Categorical covariates were tested according to the following equation: CLi = θpop · θ2, where θ2 is 1 when the value of the group is 1.
The fit of the PopPK models was assessed by visual inspection using goodness-of-fit (GOF) plots (observations versus population or individual predictions) and separate plots of conditional weighted residual versus population predictions or time after dose (TAD).
In order to verify that the model predicted both the central tendency and the variability in the observed data, a visual predictive check (VPC) as an internal model evaluation method was performed using the Pearl-speaks-NONMEM (PsN) (25) toolkit and Xpose (20). The robustness of the model was assessed by performing a nonparametric bootstrap analysis (n = 1000) using the PsN toolkit (25).
Statistical comparisons were performed with SAS version 9.1.3 Service Pack 4 (SAS, Heidelberg, Germany).
RESULTS
Patients.
Fifty-seven patients were recruited. For the analysis of safety and efficacy, two erroneously registered and randomized patients (CAS cohort) were removed (13). For the PK analyses, 4 of 57 patients were removed: one of the patients who had been erroneously randomized to the CAS cohort despite lack of concomitant immunosuppression with cyclosporine was also removed in this PK analysis, as cyclosporine is known to affect the disposition of CAS (15, 21, 31); a second patient who had been erroneously randomized to CAS despite SGOT values exceeding 3 times the upper limit of normal with available concentration data was included in this PopPK analysis; three of the 20 patients randomized to LAMB could not be included for PopPK analysis due to the administration of less than one complete dose (n = 1) or missing concentration data (n = 2). The majority of patients included in this trial (70%) had either acute lymphatic leukemia (ALL) or acute myeloid leukemia (AML) as an underlying condition, and two-thirds had received an HLA-mismatched and/or unrelated stem cell product (13).
Population pharmacokinetics of caspofungin.
Nineteen patients from the CAS cohort and 17 patients out of the CAS-LAMB cohort received between 5 and 28 (median, 13) infusions of CAS according to the protocol (70 mg on day 1, followed by 50 mg QD). Covariates, total numbers of infusions, and numbers of PK samples are listed in Table 1.
Table 1.
Demographic data and covariates in the 53 patients included in the pharmacokinetic analysesa
| Variable | CAS arm (n = 19) | LAMB arm (n = 17) | CAS-LAMB arm (n = 17) | |
|---|---|---|---|---|
| Gender (male/female) | 11/8 | 11/6 | 10/7 | |
| Age (yr) | 43.4 (20.1, 57.6)b | 38.9 (18.2, 59.5)b | 47.9 (20.1, 61.4) | |
| Weight (kg) | 71.2 (56.0, 99.2)b | 72.3 (44.0, 105.3)b | 79.5 (53.6, 99.1) | |
| Body surface area (m2) | 1.84 (1.61, 2.21)b | 1.90 (1.37, 2.35)b | 1.92 (1.56, 2.24) | |
| Serum bilirubin (mg/dl) | ||||
| Day 1 | 1.1 (0.3, 5.1)b | 1.1 (0.4, 4.9)b | 1.2 (0.4, 2.5) | |
| Day 4 | 1.0 (0.2, 4.9)c | 1.0 (0.4, 4.8)c | 1.2 (0.5, 3.7) | |
| CLCR (ml/min) | ||||
| Day 1 | 125 (73.4, 350)b | 146 (67.9, 250)b | 136 (91.8, 189) | |
| Day 4 | 131.9 (90.9, 225)c | 111 (61.8, 235)d | 116.9 (83.9, 239) | |
| No. of infusions | 239 | 164 | 242 | 236 |
| No. of PK samples | ||||
| Day 1 | 79 | 77 | 81 | 78 |
| Day 4 | 98 | 78 | 88 | 85 |
| After day 4 | 62 | 27 | 50 | 60 |
| Total | 239 | 182 | 219 | 223 |
Continuous data are presented as medians, with ranges in parentheses. CLCR was determined according to the formula of Cockcroft and Gault (7), and body surface area was calculated according to the formula of Mosteller (29).
No statistically significant difference versus CAS-LAMB (paired t test, P > 0.05).
No statistically significant difference between day 1 and day 4 values (paired t test, P > 0.05).
Statistically significant difference between day 1 and day 4 (paired t test, P < 0.01).
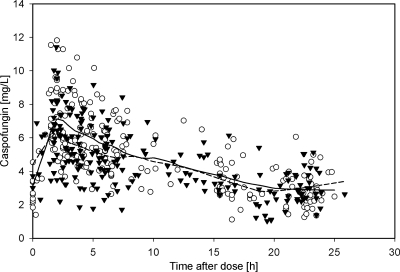
Covariates on day 1 were comparable in the CAS and the CAS-LAMB cohorts, and no significant change in covariates during treatment was observed (paired t test, P = 0.05). On day 1, 35% of the 458 plasma samples were obtained. On day 4, 41% were collected, and the remaining samples were collected on days 2 and 3 or between days 5 and 28 (Table 1). While a deep compartment with a terminal half-life of 40 to 50 h has been reported for CAS (11, 36), only 11 samples with a TAD of >30 h were collected. As these data were too sparse to be modeled and as it was not the primary objective of the study to describe this deep compartment, data with a TAD of >30 h were excluded from further analysis. Further, two samples were excluded due to uncertainties regarding the exact time of their sampling, and four data points were considered erroneous due to implausibly high or low concentrations. Thus, the final data set consisted of 441 plasma samples from 36 patients. The corresponding plasma concentration-time data for CAS are shown in Fig. 1.
Fig 1.
Observed plasma concentrations of caspofungin as a function of time after dose. Open circles represent the CAS arm; black triangles represent the CAS-LAMB arm. The solid line is the LOESS curve for the CAS arm; the dashed line is the LOESS curve for the CAS-LAMB arm.
The PopPK of CAS was best described by a linear two-compartment model with IIV in CL and V1, covariance between CL and V1, and a proportional error model. Neither IIV in Q or V2 nor alternative error or compartment models improved the model further. Inclusion of IOV or serum bilirubin significantly reduced the OFV; however, as IIV of CL and V1 were not reduced, parameter estimates did not change and unexplained variability (ETA)-versus-covariate plots did not improve after inclusion of serum bilirubin, IOV or serum bilirubin were not included in the final model. Of note, neither weight (linearly modeled on CL and/or V1 or on all PK-parameters as well as allometric scaling) nor comedication with LAMB influenced the PK of CAS (decrease in OFV with LAMB comedication as the covariate for CL, −0.912; decrease with comedication as the covariate for V1, −0.430).
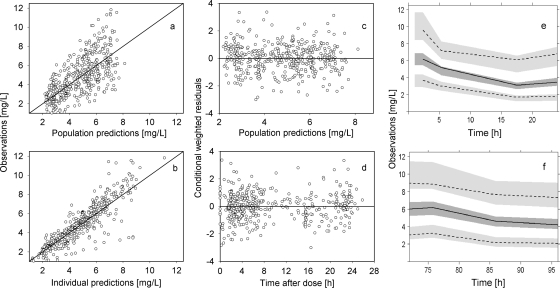
The parameter estimates of the final PopPK model are listed in Table 2. All parameters were reliably estimated, as the relative standard errors were <50%. GOF plots are shown in Fig. 2a and b. No trend was observed in the conditional weighted residuals versus population predictions or versus TAD plots, indicating no model misspecification (Fig. 2c and d). Evaluating the model for its predictability, the VPC did not show any bias as the median and the 5th and 95th percentiles of the observed CAS concentrations were not under- or overpredicted by the model simulations (Fig. 2e and f). Robustness of the final model was shown by the bootstrap results (Table 2). Based on the final PopPK model, we predicted the levels for the data collected more than 30 h after the last administration, which were excluded during model building: data with a TAD of 30 h to 88 h were well predicted, while predictions for later time points were inadequate. These results were accepted, as the PopPK model was not intended to describe data in this time interval. Simulations of steady-state kinetics showed an area under the curve (AUC) of 112 ± 28.4 mg · h/liter, a maximum concentration (Cmax) of 8.47 ± 2.73 mg/liter, and a trough concentration (Cmin) of 2.86 ± 1.13 mg/liter (mean values ± standard deviations [SD]).
Table 2.
Population-based pharmacokinetic parameter estimatesa
| Model parameter | Caspofungin final model | Amphotericin B final model | Amphotericin B mixture model |
|---|---|---|---|
| CL (liters/h) | 0.462 (4%) [0.462 (0.423–0.503)] | 1.22 (16%) [1.21 (0.84–1.57)] | 0.637 (17%) [0.70 (0.29–1.31)] |
| V1 (liters) | 8.33 (11%) [8.11 (6.09–9.79)] | 19.2 (9%) [19.12 (15.6–23.2)] | 18.6 (12%) [18.7 (15–23)] |
| Q (liters/h) | 1.25 (48%) [1.45 (0.59–3.14)] | 2.18 (13%) [2.18 (1.7–2.75)] | 2.27 (12%) [2.2 (1.76–2.69)] |
| V2 (liters) | 3.59 (22%) [3.8 (2.45–5.71)] | 52.8 (29%) [55 (29–103)] | 49.2 (33%) [55.5 (27.6–109)] |
| Proportional error (%) | 21 (9%) [21 (17–25)] | 27 (7%) [26 (22–30)] | |
| IIV (%) for: | |||
| CL | 25 (15%) [25 (18–33)] | 64 (19%) [65 (44–92)] | 35 (70%) [31 (9–63)] |
| V1 | 29 (18%) [30 (21–44)] | 38 (25%) [38 (20–53)] | 41 (26%) [40 (21–58)] |
| Q | 47 (25%) [44 (17–63)] | 37 (49) [36 (9–63)] | |
| V2 | 84 (14%) [82 (55–105)] | 95 (13%) [91 (58–117)] | |
| Factor on CL in subpopulation 2 | 3.0 (21%) [3.4 (1.0–6.8)] | ||
| Fraction in subpopulation 1 | 0.56 (28%) [0.51 (0.07–0.87)] |
Values in parentheses are relative standard errors of the estimates; values in brackets are means and 95% confidence intervals from bootstrap analysis.
Fig 2.
Final population pharmacokinetic model of caspofungin. (a and b) Observations versus population (a) or individual (b) predictions. The line of identity is shown. (c and d) Conditional weighted residuals versus population predictions (c) or time after dose (d). (e and f) Visual predictive check showing observations and model predictions on day 1 (e) and day 4 (f). The median (solid line) and the 5th and 95th percentiles (dashed black lines) for the observed data with 95% confidence intervals (gray fields) based on simulations are shown.
Population pharmacokinetics of liposomal amphotericin B.
Seventeen patients in the LAMB cohort and 17 patients in the CAS-LAMB cohort received 4 to 28 (median, 10) infusions of LAMB according to the protocol (135 mg to 300 mg [median, 234 mg] or 2.67 mg/kg to 3.46 mg/kg [median, 3.0 mg/kg]). Covariates, total numbers of infusions, and numbers of PK samples are listed in Table 1.
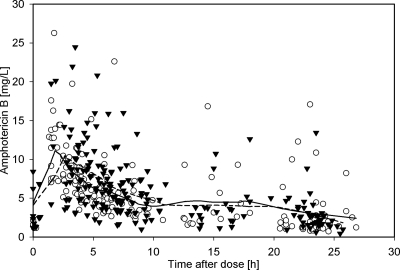
Covariates on day 1 were comparable in the LAMB and the CAS-LAMB cohorts. However, there was a significant increase in serum creatinine combined with a significant decrease of CLCR during treatment (day 1 versus day 4, paired t test, P = 0.01). On day 1, 38% of the 405 plasma samples were obtained. On day 4, 40% were collected and the remaining samples were collected on days 2 and 3 or between days 5 and 25. As with CAS, a deep compartment with a long terminal half-life of 100 to 150 h has been reported for LAMB (3, 10). As the number of samples (n = 12) with a TAD of >30 h was too low to be used for prediction of such a compartment, these samples were excluded from further analysis. One further sample was excluded due to uncertainties regarding the exact time of its sampling, and six data points were considered to be erroneous due to implausibly high or low concentrations. Moreover, the data points (n = 10) for one patient were completely excluded as concentration data from day 1 were much higher than data from day 4 and a dosing error had to be considered. Thus, the final data set consisted of 376 plasma samples from 33 patients. The plasma concentration-time data for amphotericin B after administration of LAMB are shown in Fig. 3.
Fig 3.
Observed plasma concentrations of amphotericin B after administration of liposomal amphotericin B as a function of time after dose. Open circles represent the LAMB arm; black triangles represent the CAS-LAMB arm. The solid line is the LOESS curve for the LAMB arm; the dashed line is the LOESS curve for the CAS-LAMB arm.
The PopPK of amphotericin B after administration of LAMB was best described by a linear two-compartment model with IIV in CL, V1, Q, and V2 and a proportional error model. Inclusion of covariance or IOV resulted in unstable runs. Neither allometric scaling nor inclusion of weight as a covariate in CL and/or V1 improved the model. Linear modeling of weight as a covariate for all PK parameters improved the model; however, the relative standard error (RSE) of the parameter estimate was inadequate. IIVs of CL and V1 were not reduced, and bootstrap analysis could not show robustness of the model. Therefore, weight was not included as a covariate in the model. Comedication with CAS did not influence the PK of amphotericin B (decrease in OFV with CAS comedication as a covariate for CL, −0.13, and as a covariate for V1, −0.023). None of the other covariates improved the PK model.
Visual inspection of individual concentration-time data showed 20 patients (10 of the LAMB and 10 of the CAS-LAMB cohort) with almost overlapping profiles on day 1 and day 4 whereas 13 patients (6 of the LAMB and 7 of the CAS-LAMB cohort) showed much higher concentrations on day 4 than day 1. Neither a three-compartment model nor Michaelis-Menten PK or modeling of time-dependent PK according to the model of Boddy et al. (4) could explain these findings. A mixture model with two subpopulations for CL indicated subgroups of similar sizes, with CL differing by a factor of three and IIV of CL reduced from 64% to 35% (Table 2). However, allocation to the subgroups based on the PopPK model or visual inspection differed. Bootstrap analyses showed a wide range for the factor on CL in subgroup 2 (0.07 to 0.87), and about 40% of the runs were unsuccessful. Based on these results and the small population of only 34 patients included in the present study, the mixture model was not regarded as the final PopPK model.
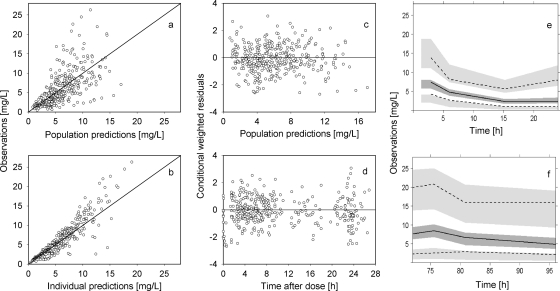
The parameter estimates of the final model are listed in Table 2. PK parameters were reliably estimated. The GOF plots are shown in Fig. 4a and b. Population predictions versus observations were wide but roughly symmetrically distributed around the line of identity; only a few high observed plasma concentrations were individually underpredicted by the model. A regular distribution of the conditional weighted residuals versus population prediction or versus TAD plots around the line of identity was observed without indicating a model misspecification (Fig. 4c and d). Based on simulations, the VPC did not show a distinct bias, and the median and 5th and 95th percentiles of the observed amphotericin B concentrations were not under- or overpredicted (Fig. 4e and f). The mean values obtained by bootstrap analysis were comparable to the parameter estimates from the original data set, indicating that the accuracy and robustness of the final model was acceptable. PK data with a TAD of >30 h, which were excluded during PK modeling, were well predicted based on the final PopPK model. The mean (± SD) values of the derived PK parameters at steady state were 228 ± 159 mg·h/liter for AUC, 18.0 ± 8.6 mg/liter for Cmax, and 6.5 ± 5.8 mg/liter for Cmin.
Fig 4.
Final population pharmacokinetic model of amphotericin B after administration of liposomal amphotericin B. (a and b) Observations versus population (a) or individual (b) predictions. The line of identity is shown. (c and d) Conditional weighted residuals versus population predictions (c) or time after dose (d). (e and f) Visual predictive check showing observations and model predictions on day 1 (e) and day 4 (f). The median (solid line) and the 5th and 95th percentiles (dashed black lines) for the observed data with 95% confidence intervals (gray fields) based on simulations are shown.
DISCUSSION
Information on the pharmacokinetics of therapeutics in a relevant target population is essential for their safe and effective use. In order to provide a foundation for further clinical investigations of antifungal combination therapy, we selected the setting of empirical therapy to study the safety and PK of CAS, LAMB, and the combination of both in a risk-stratified, randomized phase II clinical trial in adult aHSCT recipients receiving immunosuppression with cyclosporine (13). Apart from the assessments of safety, drug exposure, and treatment success, the data also allowed a more detailed PK modeling of CAS and LAMB to further explore dose intensity, impact of covariates, and the PK interaction of both agents in this population.
Concentration-time data for CAS were best described by a two-compartment PK model. IIVs of CL (25%) and V1 (29%) were low. While in the present study half-lives were estimated to be 1.34 h (α) and 18.5 h (β), an additional γ phase has been observed by others at low concentrations after 48 h postdosing (11, 36). However, it was not the primary objective of the study to describe this terminal phase in the clinical setting of critically ill patients after bone marrow transplantation, and the number of data was too low to model such an additional compartment.
In accordance with published data (9, 11, 21), the present PopPK analysis demonstrated that no dosage adjustments of CAS are necessary on the basis of gender, age (range, 20.1 to 61.4 years), or renal impairment as assessed by the CLCR (range, 73.4 to 350 ml/min). For patients with moderate hepatic insufficiency (11, 27), a dosage reduction to 35 mg daily following the 70-mg loading dose is recommended. Increases of serum bilirubin to >3 times, of SGOT/SGPT to >3 times, and of alkaline phosphatase to >5 times the upper limit of normal values were defined as study-specific exclusion criteria. Serum bilirubin (range of baseline values, 0.3 to 5.1 mg/dl) as potential covariate for CL or V1 improved the model. However, as IIV was not reduced, parameter estimates did not change and ETA-versus-covariate plots did not improve after inclusion of serum bilirubin, serum bilirubin was not included in the final model. Of note, in contrast to the FDA label information (11), the European Public Assessment Report (9) recommends dose adjustment for patients weighing >80 kg. Patients in the present study showed a wide range of weights (53.6 kg to 99.2 kg), and 15 of the 36 patients receiving CAS had a weight exceeding 80 kg. However, neither linear nor allometric scaling of weight as a potential covariate in the PK parameters improved the PopPK model, and thus the results did not support to the need for dose adjustment according to weight in the adult aHSCT population.
The PopPK analysis clearly showed that the PK of CAS is not altered by the concomitant administration of LAMB. Mean peak plasma concentrations at steady state were within the range of published data (8.47 ± 2.73 mg/liter; mean values in the literature, 7.39 to 10.0 mg/liter), and trough concentrations were higher than published data (mean, 2.86 ± 1.13 mg/liter; range, 0.39 to 8.61 mg/liter; mean values in the literature, 1.41 to 2.4 mg/liter) (27, 30, 36, 37, 39). Based on the lower fluctuation of CAS concentrations during daily treatment, total drug exposure of CAS was in the upper range of reported data (112 ± 28.4 mg · h/liter; mean values in the literature: 83.17 to 107.59 mg · h/liter) (27, 30, 36, 37, 39) (see Table SA in the supplemental material). In the present study, all patients received comedication with cyclosporine. Cyclosporine is known to moderately increase the AUC of CAS by about 35% (15, 21, 31), thus explaining the moderately higher AUC in our patients. Although the critical CAS PK parameter for efficacy in patients is unknown, dosing in the present study ensured that drug exposures considered to be effective (22) were achieved.
Following administration of LAMB, two-thirds of the patients had comparable plasma concentration-time profiles after the 1st and 4th doses, whereas the remaining proportion of patients had significantly increased plasma concentrations after repeated dosing. As patients were evenly distributed in the LAMB and CAS-LAMB arms, a potential influence of comedication of CAS can be excluded. Walsh et al. (41) reported a more than 50% decrease in CL after a mean duration of treatment of 7.6 ± 1.1 days combined with a significant increase in drug exposure in patients with neutropenic fever. A potential explanation is the existence of at least one saturable pathway involved in the disposition of amphotericin B (41), including but not limited to saturable reticuloendothelial uptake mechanisms.
In the present data set, the PopPK of LAMB was best described by a linear two-compartment model. Neither a three-compartment model, Michaelis-Menten kinetics, nor time-dependent modeling of PK parameters according to Boddy et al. (4) improved the fit of the data further or accounted for a possible time-dependent or saturable PK of amphotericin B after multiple-dose administration of LAMB. A mixture model with two subpopulations for CL indicated subgroups which were similar in size, but the CL values between the subgroups differed by a factor of 3. The physiological rationale for these subpopulations is unclear. Possible explanations might be a saturable elimination pathway, disease status, or differences in parenteral nutrition. However, due to these uncertainties and due to the small population (only 34 patients) in the present study, the mixture model was not regarded as the final PopPK model.
Hong et al. (19) also used a two-compartment model to describe the PK of amphotericin B in pediatric patients with malignant diseases after multiple-dose administration of LAMB. These investigators found a high IOV for CL and V1, with an occasion being defined as period of time in which patients were administered LAMB on a daily basis and the interval between two occasions being at least 2 weeks. However, inclusion of IOV in our PopPK model did not lead to any improvement in the results of the PopPK analysis, and IOV was therefore not included in the PopPK model. Of note, none of the tested covariates (CAS comedication, gender, weight, age, BSA, serum bilirubin, and CLCR) improved the model, and the data clearly show that the PK of LAMB is not altered by concomitant administration of CAS.
The CL values of 1.22 liters/h as well as peak plasma concentrations of 18.0 mg/liter obtained in the present study are within the range of data reported for other populations; the terminal half-life of 54.3 h is comparable to results obtained by Hong et al. (59.4 h) but longer than those found in other studies (3, 10, 16, 41) (see Table SB in the supplemental material). While the IIVs of 38% for V1 and up to 84% for V2 observed in our study may appear high, the results of previous studies suggested that LAMB PK is characterized by significant interpatient variability: For example, based on a PopPK analysis, Hong et al. (19) estimated IIVs of 10% for CL, 77% for Q, and 74% for V2 combined with IOV of 46% for CL and 56% for V1. IIV reported by Bekersky et al. (3) was about 40% to 75%. Finally, using a dose of 2.5 mg/kg, Walsh et al. (41) found an IIV for CL of 68%. The high PK variability of LAMB may be founded in differences in the uptake of the liposomal carrier with bound drug into nonblood compartments or in the dissolution of the drug from the liposomal carriers with consequences for its disposition in the blood; further potential factors include differences in disease status and inflammatory molecules, the composition of plasma proteins, and solutions used for concomitant parenteral nutrition (3). Interestingly, liposomal encapsulation of daunorubicin with liposomes of similar structure (Daunoxome) resulted in a substantial reduction of IIV in the PK parameters (17).
In conclusion, PopPK models were developed for CAS and LAMB in adult aHSCT recipients. There were no clinically relevant differences in drug exposures relative to other populations. The PK of CAS was not altered by coadministration of LAMB and vice versa. Covariates, including age, gender, weight, BSA, and serum bilirubin, and CLCR as parameters of hepatic and renal function, respectively, did not influence the PK of either compound. As reported in detail elsewhere (13), except for a higher frequency of hypokalemia with the combination, CAS-LAMB combination therapy was as safe as monotherapy with CAS and LAMB, and there were no apparent differences in the occurrence of breakthrough invasive fungal infections and survival between the three treatments.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sonja Baier, Sibylle Gammelin, João Paulo Vieira Pinheiro, Gerlinde Benninger-Doering, Juergen Grebe, Achim Heinecke, and the entire staff of the Centre for Clinical Trials Muenster (BMBF 01KN0705/BMBF 01KN1105) for their excellent administrative and logistic support.
This clinical trial was initiated and conducted under the sponsorship of the Centre for Clinical Trials Muenster (BMBF 01KN0705), University Hospital Muenster, Muenster, Germany. The study was supported by clinical research grants of Gilead Sciences, Martinsried, Munich, and of Merck, Sharp & Dohme, Haar, Germany. Gilead Sciences and Merck, Sharp & Dohme had no role in the design of the study, data capture, or data analysis or in the writing and submission of this article.
A.H.G. has received research grants and has served as consultant and speaker to Gilead Sciences and to Merck, Sharp & Dohme. H.O., G.S., and W.J.H. have served as consultants to and speakers for Gilead Sciences and Merck, Sharp & Dohme. G.W. is supported be the German Federal Ministry of Research and Education (BMBF 01KN0705/BMBF 01KN1105). O.A.C. is supported by the German Federal Ministry of Research and Education (BMBF 01KN0706) and has received research grants from, is an advisor to, and served at the speakers' bureau of Gilead Sciences and Merck. All other authors have no competing interest to declare.
Footnotes
Published ahead of print 14 November 2011
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Alak A, Moy S, Bekersky I. 1996. A high-performance liquid chromatographic assay for the determination of amphotericin B serum concentrations after the administration of AmBisome, a liposomal amphotericin B formulation. Ther. Drug Monit. 18:604–609 [DOI] [PubMed] [Google Scholar]
- 2. Ascioglu S, et al. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7–14 [DOI] [PubMed] [Google Scholar]
- 3. Bekersky I, et al. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boddy AV, Cole M, Pearson AD, Idle JR. 1995. The kinetics of the auto-induction of ifosfamide metabolism during continuous infusion. Cancer Chemother. Pharmacol. 36:53–60 [DOI] [PubMed] [Google Scholar]
- 5. Bow EJ. 2009. Invasive fungal infection in haematopoietic stem cell transplant recipients: epidemiology from the transplant physician's viewpoint. Mycopathologia 168:283–297 [DOI] [PubMed] [Google Scholar]
- 6. Caillot D, et al. 2007. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies—a randomized pilot study (Combistrat trial). Cancer 110:2740–2746 [DOI] [PubMed] [Google Scholar]
- 7. Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 8. Cornely OA, et al. 2007. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin. Infect. Dis. 44:1289–1297 [DOI] [PubMed] [Google Scholar]
- 9. EMEA 2008. European public assessment report: Cancidas. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000379/WC500021025.pdf
- 10. Food and Drug Administration 2008. Ambisome (amphotericin B) liposome for injection. FDA label information, Aug. 10, 2008. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050740s016lbl.pdf
- 11. Food and Drug Administration 2010. Caspofungin. CANCIDAS. FDA Label information, Oct 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021227s028lbl.pdf
- 12. Groll AH, Piscitelli SC, Walsh TJ. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343–500 [DOI] [PubMed] [Google Scholar]
- 13. Groll AH, et al. 2010. Randomized comparison of safety and pharmacokinetics of caspofungin, liposomal amphotericin B, and the combination of both in allogeneic hematopoietic stem cell recipients. Antimicrob. Agents Chemother. 54:4143–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Groll AH, Tragiannidis A. 2009. Recent advances in antifungal prevention and treatment. Semin. Hematol. 46:212–229 [DOI] [PubMed] [Google Scholar]
- 15. Groll AH, Walsh TJ. 2001. Caspofungin: pharmacology, safety and therapeutic potential in superficial and invasive fungal infections. Expert Opin. Investig. Drugs 10:1545–1558 [DOI] [PubMed] [Google Scholar]
- 16. Heinemann V, et al. 1997. Pharmacokinetics of liposomal amphotericin B (Ambisome) in critically ill patients. Antimicrob. Agents Chemother. 41:1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hempel G, Reinhardt D, Creutzig U, Boos J. 2003. Population pharmacokinetics of liposomal daunorubicin in children. Br. J. Clin. Pharmacol. 56:370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holford NH. 1996. A size standard for pharmacokinetics. Clin. Pharmacokinet. 30:329–332 [DOI] [PubMed] [Google Scholar]
- 19. Hong Y, et al. 2006. Population pharmacokinetics of liposomal amphotericin B in pediatric patients with malignant diseases. Antimicrob. Agents Chemother. 50:935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jonsson EN, Karlsson MO. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51–64 [DOI] [PubMed] [Google Scholar]
- 21. Keating G. M., Jarvis B. 2001. Caspofungin. Drugs 61:1121–1129 [DOI] [PubMed] [Google Scholar]
- 22. Keizer RJ, van Benten M, Beijnen JH, Schellens JHM, Huitema ADR. 2011. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput. Methods Programs Biomed. 101:72–79 [DOI] [PubMed] [Google Scholar]
- 23. Kontoyiannis DP, et al. 2003. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer 98:292–299 [DOI] [PubMed] [Google Scholar]
- 24. Kuse ER, et al. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519–1527 [DOI] [PubMed] [Google Scholar]
- 25. Lindbom L, Pihlgren P, Jonsson N. 2005. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 79:241–257 [DOI] [PubMed] [Google Scholar]
- 26. Maertens J, et al. 2006. Multicenter, noncomparative study of caspofungin in combination with other antifungals as salvage therapy in adults with invasive aspergillosis. Cancer 107:2888–2897 [DOI] [PubMed] [Google Scholar]
- 27. Mistry GC, et al. 2007. Single- and multiple-dose administration of caspofungin in patients with hepatic insufficiency: implications for safety and dosing recommendations. J. Clin. Pharmacol. 47:951–961 [DOI] [PubMed] [Google Scholar]
- 28. Mora-Duarte J, et al. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020–2029 [DOI] [PubMed] [Google Scholar]
- 29. Mosteller RD. 1987. Simplified calculation of body-surface area. N. Engl. J. Med. 317:1098. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen TH, et al. 2007. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J. Antimicrob. Chemother. 60:100–106 [DOI] [PubMed] [Google Scholar]
- 31. Pacetti SA, Gelone SP. 2003. Caspofungin acetate for treatment of invasive fungal infections. Ann. Pharmacother. 37:90–98 [DOI] [PubMed] [Google Scholar]
- 32. Pappas PG, et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883–893 [DOI] [PubMed] [Google Scholar]
- 33. Reed C, et al. 2008. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect. Dis. 47:364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz M, Kline W, Matuszewski B. 1997. Determination of a cyclic hexapeptide (L-743 872), a novel pneumocandin antifungal agent, in human plasma and urine by high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 351:299–307 [Google Scholar]
- 35. Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J, Ibrahim AS. 2009. Recent advances in the management of mucormycosis: from bench to bedside. Clin. Infect. Dis. 48:1743–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stone JA, et al. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stone JA, et al. 2004. Potential for interactions between caspofungin and nelfinavir or rifampin. Antimicrob. Agents Chemother. 48:4306–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. U.S. Department of Health Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine 2001. Guidance for industry—bioanalytical method validation. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf
- 39. Walsh TJ, et al. 2005. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother. 49:4536–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walsh TJ, et al. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 351:1391–1402 [DOI] [PubMed] [Google Scholar]
- 41. Walsh TJ, et al. 1998. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob. Agents Chemother. 42:2391–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wuerthein G, et al. 2009. Population Pharmacokinetics of liposomal amphotericin B, caspofungin and the combination of both in allegeneic hematopoietic stem cell recipients. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother, abstr. A1-591 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.